Abstract
In addition to the global use of organophosphate (OP) pesticides for agriculture, OP nerve agents and pesticides have been employed on battlefields and by terrorists (e.g., the recent sarin attack in Syria). These occurrences highlight the need for an effective countermeasure against OP exposure. Human butyrylcholinesterase (HuBChE) is a leading candidate, but injection of the high doses required for protection present pharmacokinetic challenges. An aerosolized recombinant from (aer-rHuBChE) that can neutralize inhaled OPs at their portal of entry has been assessed for its efficacy to protect macaques against respiratory toxicity following inhalation exposure to the pesticide paraoxon (aer-Px). While protection in macaques has been demonstrated using a Microsprayer delivery device, administration to humans will likely employ a vibrating mesh nebulizer (VMN). Compared to the 50–70% lung deposition achieved in adult humans with a VMN, deposition in macaques is < 5%, an initial major obstacle to demonstrating protection. Such problems have been partly overcome by using a more efficient modified VMN and proportionally higher doses, which together generate an effective rHuBChE pulmonary bioshield and protect against high levels of inhaled Px.
Keywords: pulmonary, butyrylcholinesterase, protection, inhaled neurotoxins, macaques
Introduction
The lung’s large absorptive surface area and its thin air-to-blood barrier, as well as recent innovations in device design and drug formulation, have recently led drug manufacturers to consider the pulmonary route of delivery as an alternative to injection therapy for small biologics/drugs that are targeted to the systemic circulation.1,2 Intrapulmonary delivery of aerosolized medications is currently the preferred route of therapy for a number of respiratory diseases where the lung is the target organ, including asthma, pulmonary infections, and chronic obstructive pulmonary disease, as well as treatments for cystic fibrosis1–4 (e.g., aer-recombinant human deoxyribonuclease (Pulmozyme)). A second successful approach involves targeted vectored vaccine delivery of aerosolized vaccines (e.g., the aerosolized Ebola vaccine in macaques, which has been shown to activate immune T and B cells in the respiratory system, resulting in full protection against the virus).5
In this article, we describe another new pulmonary delivery strategy that utilizes prophylactic deposition of a high–molecular weight organophosphate bioscavenger in the lungs in order to neutralize subsequently inhaled neurotoxins in situ, thereby preventing their entry into the systemic circulation. Specifically, pretreatment with an aerosolized recombinant human butyrylcholinesterase (aer-rHuBChE) has been used to generate a “pulmonary BChE bioshield” in macaques and mice, which binds and sequesters inhaled organophosphates (OPs) in the form of pesticides and nerve agents, thereby preventing/reducing their exit from the lungs and their interaction with their physiological targets in the brain and blood (Fig. 1). Importantly, lungs represent the portal of entry of many OPs.
Figure 1.
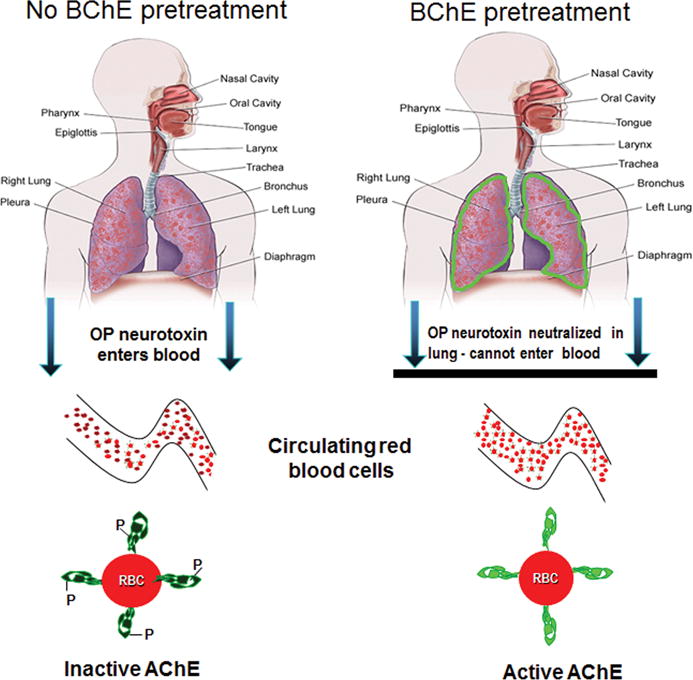
Protection by aer-rHuBChE against inhaled organophosphate neurotoxins. (Left) In untreated individuals, inhaled OPs transit the lung epithelium and enter the blood, resulting in inactivation/inhibition of acetylcholinesterase (dark green) on red blood cells (RBCs). (Right) Pulmonary delivery of aer- rMaBChE generates a pulmonary bioshield (green) in the lungs, which neutralizes subsequently inhaled OPs in situ and prevents their entry into the blood and their inactivation of circulating RBC-ACh (light green).
Butyrylcholinesterase
Plasma-derived BChE currently represents the most advanced pretreatment for protection against OP exposures,6–8 which may take the form of threats to agricultural workers, military personnel, first responders, and civilians in the case of deliberate contamination of the environment and critical water supplies. Structurally, BChE (also known as EC3.1.1.8, pseudocholinesterase, nonspecific cholinesterase) is a serine esterase (molecular weight (MW) = 345,000) comprising four identical subunits containing 574 amino acids, held together by noncovalent bonds, and 36 carbohydrate chains (23.9% by weight).9 The efficacy of BChE prophylaxis, in terms of survivability and prevention of cognitive impairment, has been clearly demonstrated in many animal models against multiple LD50s of nerve agents and pesticides,10–12 and more recently, the ability of HuBChE delivered by intramuscular (IM) injection has been shown to protect against lethal inhaled sarin in minipigs.13 In Turkey, frozen plasma (containing 3000–5700 units of BChE) given as an alternative or adjunctive treatment with atropine and oximes, has been shown to prevent mortality and intermediate syndrome in acutely insecticide-exposed and hospitalized individuals.14
However, because of the limited availability and cost of producing sufficient quantities of plasma-derived HuBChE, focus has switched to the development of a recombinant (r) BChE countermeasure. To date, rBChE has been successfully produced in goat milk, mammalian cells, and plants.15–17 However, all forms exhibit poor plasma stability, presumably due to host cell–specific glycosylation and must undergo posttranslational modification (e.g., PEGylation18) or be expressed in genetically modified host cells19
Previous animal protection studies using BChE as a pretreatment for OP toxicity have delivered the scavenger molecules to the blood by parenteral administration. The dogma underlying this mode of delivery is that, to function as an effective bioscavenger, BChE must circulate in the blood at high concentrations for prolonged periods, in order to detoxify OPs before they can reach their AChE targets on red blood cells (AChE-RBC) and in neuromuscular junctions and other cholinergic synapses. Specifically, highly toxic OPs must be scavenged to a level below their median lethal dose within one blood-circulation time to prevent toxicity.20 However, efficient and user-friendly delivery options are limited. BChE has a high MW and its ability to scavenge OPs follows a 1:1 stoichiometry21 requiring a very high dose of > 6 mg/kg (> 420 mg/adult). Transdermal patches are not capable of delivering large, complex glycoproteins, while IM injections using autoinjectors may elicit variable pharmacokinetics22 and will require several painful injections, which may not be popular among civilian agricultural workers or children. As an example, pain, inconvenience, and disruption have led to noncompliance in many diabetes patients who require daily multiple injections. Finally, intravenous (IV) injections, although the most efficient means of rapidly increasing scavenger levels in the blood, are not easily manageable in the field.
To optimize the scavenging efficacy of large rBChE molecules, an alternate delivery approach for treating OP exposure involves creating a protective “BChE pulmonary bioshield” by intrapulmonary delivery of sufficient tetrameric aer-rHuBChE into the lungs to scavenge incoming (inhaled) OPs in situ, thereby preventing OP entry into the systemic circulation and thus preventing their inhibition of their AChE and BChE in the blood and the nervous system (Fig. 1).
This takes advantage of the fact that (1) inhalation of vapors and particles is the predominant form of exposure to insecticides and G-type nerve agents and serves as a major means of intoxication because of rapid accesses of the OP to the blood; (2) inhaled rBChE molecules will be retained in the lung, being too large to transit the lung endothelium; and (3) levels of rBChE can be easily maintained in the lungs in a user-friendly way by maintenance “puffs.”
Organophosphate pesticides and nerve agents
While nerve agents are much more potent than pesticides and may vary widely in clinical course, all cause toxicity by inhibiting the activity of AChE and BChE.23,24 At higher doses, such OP cholinesterase inhibitors cause sweating, dizziness, vomiting, diarrhea, convulsions, cardiac arrest, respiratory arrest, and, in extreme cases, death. Because of this critical targeting of cholinesterases, any efficacious pretreatment candidate for preventing/treating OP poisoning will be a molecule that can bind and competitively scavenge Ops, thus preventing a cholinergic crisis.
OP pesticides are used worldwide to control agricultural, household and structural pests. Although the more toxic agents have been banned in many countries, > 5 billion lb are still used annually; potentially exposing ~1.8 billion civilians.25–27 In addition, pesticide use has been one of the only two exposures consistently identified by Gulf War epidemiological studies to be significantly associated with the multisymptom illness described as Gulf War syndrome.28 In clinical studies conducted since the end of the war, pesticides are associated with neurocognitive deficits and neuroendocrine alterations in returning veterans. More recently, Islamist terrorists are thought to have used pesticides to attack schools in Afghanistan from 2010–2013, injuring > 2000 girls,29 and deliberate OP pesticide contamination of the environment and critical water supplies by terrorists has also become an ever-increasing threat, for both civilian and military personnel.
OP nerve agents are related chemical warfare agents (CWA), first synthesized before and during World War II primarily for military use, and represent the most neurotoxic synthetic compounds produced.30–32 To date, nerve agents have been used by Iraq against Iran in 1984–1986, against the Kurdish population in 1988, and by Aum Shinrikyo in Japan in the 1990s. The recent large-scale use of the nerve agent sarin in Syria, with many civilian deaths, has highlighted the need for an effective, affordable, and user-friendly countermeasure against nerve agent exposure. Chemical agents are classified as “nonpersistent.” and “persistent.” Nonpersistent agents are those that are volatile and hence evaporate quickly (e.g., sarin (GB)), while persistent agents predominantly remain on terrain, materiel, or equipment for days, weeks, or months, depending on the environmental conditions (e.g., VX).
OP exposure, physical nature of toxic material, and lung deposition
The majority of OPs are lipophilic, not ionized, and are absorbed rapidly following inhalation or ingestion.30–32 Respiratory OP exposure by inhalation of airborne toxic material, in the form of gas, vapor, and aerosols, including mists, fumes, smoke and dusts, are particularly hazardous because particles enter the bloodstream more quickly via the lungs and cause serious damage. Volatility and particle size and the type of dispersion device are important primary factors in determining the airborne persistence. Particles (such as those in mists, fumes, smoke, and dusts) present a more complex distribution pattern because the particle size affects its deposition at various levels of the airways. Under low-pressure dispersion devices, droplet size is too large to remain airborne. However, when high pressure, ultralow-volume application (ULV) or fogging equipment is used, respiratory exposure is increased owing to the production of mist- or fog-size particles. Predictably, weather conditions play a role in the severity of exposure by controlling whether the aerosol cloud stays near the ground or is carried on air currents for a considerable distance. For example, in warm environments, heavier-than-air gases may also exhibit increased rates of vaporization, making inhalational toxicity more likely. By contrast, increased humidity may increase particle size by hygroscopic effects, resulting in decreased respiratory exposure due to the precipitation of larger particles before inhalation or preferential collection in the upper airways, which have better clearance mechanisms.
In this context, factors within the lungs, such as sedimentation and impact rates, also control particle deposition in the exposed individual; heavier particles settle in the nasopharynx or upper airways, whereas lighter or smaller particles may reach more peripheral airways. Once they have impacted, particles are susceptible to a variety of respiratory defense mechanisms that determine the efficiency of particle removal, ultimately determining their adverse effects. In a similar way, the particle size generated by different pulmonary delivery devices determines the distribution of deposition of the rHuBChE pretreatment in the lung and thus its protective efficacy.
Pulmonary drug delivery
Pulmonary delivery has the advantage of providing a higher proportion of the dose to the lungs than parenteral methods, with lower systemic exposure and the elimination of first-pass metabolism.33 By comparison, a typical drug delivered intravenously may result in < 2% of the dose reaching the pulmonary epithelium. As noted, aerosol delivery has been an effective route of administration of drugs, both topically and systemically, demonstrated using a variety of in vivo and in vitro models.4 Historically however, pulmonary delivery has been considered to be inconsistent, owing to larger inter- and intra-subject variability. This was particularly true with the early aerosol devices, which only deposited in the range of 10% of the nominal or emitted dose. Several more efficient devices/procedures for pulmonary administration have now been developed.35
Instillation via the trachea is the most common method for pulmonary drug delivery in rodents and small animal, permitting a highly quantifiable, direct drug delivery into the lung with minimal loss in the nose, throat, and upper airway. Nevertheless, this route of administration results in a large proportion of aerosol depositing in the central airways, with unpredictable deposition to specific peripheral lung regions due to the effects of gravity.36 However, instillation is not a viable option for pulmonary administration for mammals greater than 1–2 kg, and is not feasible for treatment of humans in the ambulatory setting. Thus, a transition from instillation to aerosol delivery is warranted.
The Microsprayer™ (Penn Century, PA) is a mechanical device that delivers liquid or powder aerosols directly to the trachea without creating significant sheer stress. The Microsprayer device represents a significant improvement to delivery by instillation, because of the median particle sizes of >20 μm and deposition predominantly in the larger, central airway of the upper respiratory tract.37 Importantly, the Microsprayer delivers approximately 60% of the nominal dose to the lungs of rhesus macaques, compared to 1% with a jet nebulizer.4
Aerosol device selection is critical to assure effective dosing. There are a wide variety of aerosol-generating devices that are used in clinical medicine. Many of them, such as dry powder inhalers (DPIs), pressurized metered dose inhalers (pMDIs), and soft-mist liquid inhalers (SMLIs), are limited by design to relatively small dose volumes. The DPI and pMDI devices have pulmonary efficiency ranging from 10% to 30%, making delivery of large volumes of formulation to the lungs problematic.
For larger dose volumes, liquid nebulization is the mode of choice. Liquid aerosol generators include pneumatic jet nebulizers (JNs), ultrasonic nebulizers (USNs). and vibrating mesh nebulizers (VMN). Each has its strengths and limitations.
Jet nebulizers have been most commonly used over the last 80 years, requiring a flow of pressurized gas to generate aerosol continuously. Consequently, the aerosol produced is diluted in the volume of gas required to produce the aerosol. This and the pattern of nebulization reduces the proportion of emitted dose that can be inhaled by the subject. Typical jet nebulizer pulmonary delivery is < 1% with infants and small animals < 2 kg and 10–15% in larger children and adults. Breath-actuated jet nebulizers have been introduced, which can increase inhaled dose, but at the cost of increased dosing time.38,39
Electronic nebulizers include the USN and VMN. Neither require compressed gas to generate an aerosol and can provide a greater inhaled dose to adult subjects. The USN vibrates a piezo at approximately 3 mHz, to disrupt the surface of the formulation and form a standing wave that produces aerosol particles at its crest. In the process, considerable heat is generated, > 20 °C in 10 min, which has been cause of speculation that proteins might be compromised during the nebulization process. More recently, the VMN has been introduced, using a piezo to vibrate a domed plate with > 1000 funnel shaped apertures at 128 kHz. The mesh then pumps a liquid formulation through the apertures (https://www.youtube.com/watch?v=J5GOPTE6bEo), with the particle size dictated in large part by the exit diameter of the apertures. The VMN is capable of producing particles in the 1.9–3.0 micron (MMAD) range, which is optimal for pulmonary deposition in humans.
The vibrating mesh produces little or no heat and has very low residual dose volumes remaining at the end of administration. While sheer forces exist, they have not been identified as an issue in administration of a wide range of formulations, including proteins. In clinical aerosol medicine, it has been established that deposition is lower in small infants and toddlers than larger children and adults owing to a combination of factors, including airway size and breathing parameters. Using JNs and pMDIs, less than 1% deposition has been achieved in both 1- to 4-kg infants40,41 and nonhuman primates of comparable weight. By comparison, VMN typically achieves 1–5% deposition in non-ventilated monkeys, which increases to 12–15% in ventilated 1- to 2-kg animals.41
Increased lung deposition of an anti-ricin antibody to 13% has also been achieved in macaques by Marchand et al.42 using an Aeroneb Lab® VMN, adapted by the addition of a reservoir inhalation chamber connecting the nebulizer to the mask. This combination device resulted in a respirable fraction greater than 85% and an aerosol MMAD of 2.1 ± 0.4 μm at an output rate of 0.12 mL/min. However, while the adaptor increased lung deposition in these studies, γ scintigraphy using 99mtechnetium indicated that the antibody was predominantly distributed in the head and stomach, highlighting the challenges associated with high drug deposition in lungs of macaques using aerosol inhalation devices (Fig. 2). Recent information on respiration characteristics on sedated macaques using optimally fitting masks will further increase the deposition rate in macaques.42
Figure 2.
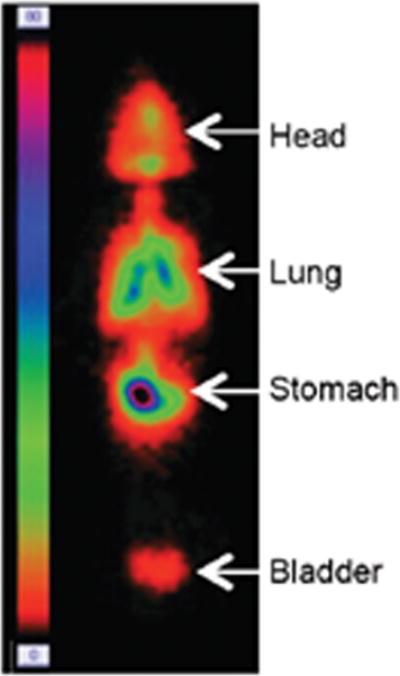
Distribution of formulation admixed with 99mtechnetium in macaques administered via aerosol with vibrating mesh nebulizer and chamber with mask, with 13% of dose deposited in lungs. Republished with permission from Ref. 42.
In contrast to the low-drug-deposition macaques, VMN technology has been shown to achieve lung doses of 50–70% in adults, underscoring the challenges encountered in certain cases when animal models are used to provide the required supporting data for an aerosolized human treatment.42 Consequently, the limited exposure of aerosol available to the smaller mammals, including macaques, while necessary to show proof of concept, is not representative of the intended use for administration to larger humans in the field. The lower dose efficiency in the macaques is challenging in terms of both the large dosing volumes and the time required for administration. The 4- to 6-fold greater efficiency in lung delivery to larger children and adults suggests that lower nominal doses and shorter administration times will be the ultimate basis for field delivery.
Protection studies
Owing to the ability of the Microsprayer to deliver high nominal doses to the lungs of rhesus macaques, the initial macaque protection studies assessed the protective efficacy of Chinese hamster ovary (CHO)–derived aer-rBChE against the toxic OP pesticide paraoxon (Px), employing a Microsprayer for pulmonary delivery of both the antidote and the OP.37 In order to avoid unnecessary deaths of macaques, protection was initially measured by the ability of aer-rBChE to prevent inhibition of blood cholinesterase activity following a 15 μg/kg dose of aer-Px that resulted in 63% inhibition. In these studies, aer-rMaBChE pretreatments of 4.8–9 mg/kg in homologous macaques demonstrated very good dose-dependent protection against aer-Px toxicity given 1 h later. Importantly, no immunogenicity was observed in this homologous macaque model, even after several administrations, and posttranslational modification of the rBChE was not required.
In subsequent studies, CHO-derived aer-rHuBChE was tested as a pretreatment in terms of its capacity to protect against aer-Px and its duration of protection. Similar to aer-rMaBChE, unmodified aer-rHuBChE at 8.5–9 mg/kg administered at 1, 16, 24, and 40 h before 15 μg/kg of Px was almost fully protective (90–100% AChE activity), with only incremental increases in AChE and BChE inhibition as the period between pretreatment and OP was extended. Since these successful studies using a 15 μg/kg exposure of Px, higher doses (36 μg/kg) given 72 h following rHuBChE pretreatment resulted in only 20% inhibition, indicating that the rHuBChE pulmonary bioshield appears to remain intact for at least 3 days (Fig. 3). In addition, preliminary studies suggest that administration of postexposure oxime can act synergistically to further enhance protection. Importantly, protected macaques did not exhibit abnormal cytokine/chemokine production in their bronchiolavage (BAL) as a result of delivery/retention of rHuBChE in the lungs.
Figure 3.
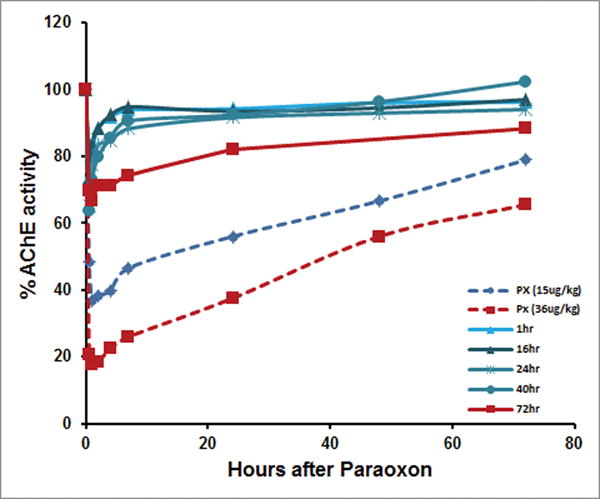
Protection by prophylactic aer-rHuBChE against aer-Px as measured by inhibition of RBC-AChE. Macaques were pretreated with 9 mg/kg and exposed to aer-Px (15 μg/kg) 1, 16, 24, and 40 h later (solid blue lines). Untreated control macaques were exposed to 15 μg/kg aer-Px only (dashed blue line). Macaques were pretreated with 8 mg/kg and exposed to 36 μg/kg aer-Px 72 h later (solid red), while control untreated macaques were exposed to 36 μg/kg aer-Px only (dashed red). Each line represents an average of two macaques. 100% activity represents total protection. Adapted from Ref. 37.
Although the successful protection studies using a Microsprayer provided critical results, animals require sedation for administration, and the large aer-rBChE particles (> 26 microns) remain predominantly in the upper airways (Fig. 4). Thus, more recent studies have investigated whether a protective rHuBChE pulmonary bioshield can be generated in young sedated macaques via a mask connected to a VMN similar to that used by humans and whether these 1- to 5-micron particles will extend the bioshield to the lower airways. To date, by using the combination aerosol VMN device43 to enhance lung deposition as well as greatly increasing the dose delivered, sufficient rHuBChE has been delivered to demonstrate protection. Thus, delivery of 40 mg/kg using a vibrating mesh Aerogen nebulizer/adaptor device has resulted in the deposition of ~ 6 mg/kg in the lungs of macaques, resulting in high levels of protection against a deposited dose of Px (45 μg/kg) known to induce tremors in untreated animals. As such, it now seems possible to overcome the challenges associated with low deposition and to generate the protection data required by the Food and Drug Administration’s Animal Rule for approval of an OP prophylactic pretreatment for use in humans, where a ratio of drug dose to deposited dose is less than 2:1, compared to the challenging 7:1 ratio in macaques.
Figure 4.
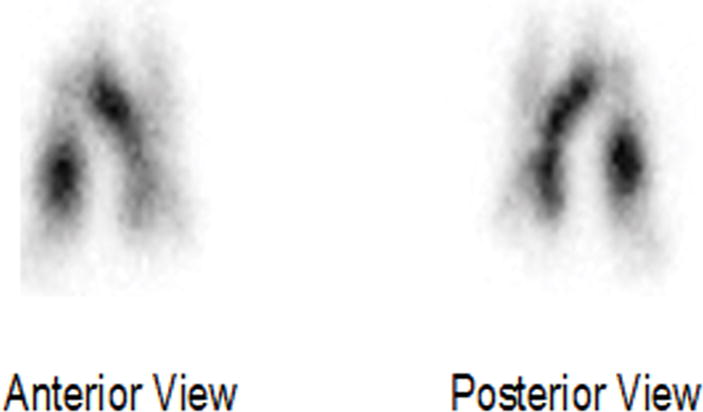
Distribution of rMaBChE (9 mg/kg) admixed with 99mtechnetium in the right and left lobes of the lung of rhesus macaques following delivery with a Microsprayer.37
In this context, mesh nebulizers have been shown to be effective aerosol-producing devices for delivering large amounts of biopharmaceuticals, including rHuBChE, while limiting protein instability during nebulization. Such VMN technology has been adopted by the World Health Organization in their inhaled measles vaccine program for use in field-based mass vaccinations in India,44 this bodes well for the use of similar nebulizer technology for the delivery of rHuBChE to humans.
References
- 1.Liu X, Jin L, Upham JW, et al. The developments of models for the evaluation of pulmonary drug disposition. Expert Opin Drug Metab. 2013;9:487–505. doi: 10.1517/17425255.2013.754009. [DOI] [PubMed] [Google Scholar]
- 2.Frederiksen B, Pressler T, Hansen A, et al. Effect of aerosolized rhDNase (Pulmozyme) on pulmonary colonization in patients with cystic fibrosis. Acta Paediatr. 2006;95(9):1070–1074. doi: 10.1080/08035250600752466. [DOI] [PubMed] [Google Scholar]
- 3.Laube BL. The expanding role of aerosols in systemic drug delivery, gene therapy and vaccination: an update. Transl Respir Med. 2014 Jan 13;2:3. doi: 10.1186/2213-0802-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck SE, Laube BL, Barberena CI, et al. Deposition and expression of aerosolized rAAV vectors in the lungs of Rhesus macaques. Mol Ther. 2002;6(4):546–554. doi: 10.1006/mthe.2002.0698. [DOI] [PubMed] [Google Scholar]
- 5.Meyer M, Garron T, Lubaki NM, et al. Aerosolized Ebola vaccine protects primates and elicits lung-resident T cell responses. J Clin Invest. 2015;125(8):3241–3255. doi: 10.1172/JCI81532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doctor BP, Maxwell DM, Ashani Y, et al. New approaches to Medical Protection against Chemical Warfare Nerve Agents. In: Somani SM, Romano JA, editors. Chemical Warfare Nerve Agents Toxicity at Low Levels. CRC Press; New York: 2001. pp. 191–214. [Google Scholar]
- 7.Lenz DE, Broomfield CA, Maxwell DM, Cerasoli DM. Nerve Agent Bioscavengers: Protection against High- and Low- Dose Organophosphorus Exposure. In: Somani SM, Romano JA, editors. Chemical Warfare Nerve Agents Toxicity at Low Levels. CRC Press; New York: 2001. pp. 215–243. [Google Scholar]
- 8.Saxena A, Sun W, Fedorko JM, et al. Prophylaxis with human serum butyrylcholinesterase protects guinea pigs exposed to multiple lethal doses of soman or VX. Biochem Pharmacol. 2011;81(1):164–169. doi: 10.1016/j.bcp.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Lockridge O. Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. Pharmacol Ther. 1990;47:35–60. doi: 10.1016/0163-7258(90)90044-3. [DOI] [PubMed] [Google Scholar]
- 10.Lenz DE, Maxwell DM, Koplovitz I, et al. Protection against soman or VX poisoning by human butyrylcholinesterase in guinea pigs and cynomolgus monkeys. Chem Biol Interact. 2005;157–158:205–210. doi: 10.1016/j.cbi.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Saxena A, Sun W, Dabisch PA, et al. Pretreatment with human serum butyrylcholinesterase alone prevents cardiac abnormalities, seizures, and death in Gottingen minipigs exposed to sarin vapor. Biochem Pharmacol. 2011;82(12):1984–1993. doi: 10.1016/j.bcp.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg YJ, Gearhart J, Mao L, et al. Protection against paraoxon toxicity by an intravenous pretreatment with polyethyleneglycol-conjugated recombinant butyrylcholinesterase in macaques. Chem Biol Interact. 2014;210:20–25. doi: 10.1016/j.cbi.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saxena A, Hasting NB, Sun W, et al. Prophylaxis with human serum butyrylcholinesterase protects Göttingen minipigs exposed to a lethal high-dose of sarin vapor. Chem Biol Interact. 2015;238:161–169. doi: 10.1016/j.cbi.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Guven M, Sungur M, Eser B, et al. The effects of fresh frozen plasma on cholinesterase levels and outcomes in patients with organophosphate poisoning. J Toxicol Clin Toxicol. 2004;42:617–623. doi: 10.1081/clt-200026967. [DOI] [PubMed] [Google Scholar]
- 15.Huang YJ, Huang Y, Baldassarre H, et al. Recombinant human butyrylcholinesterase from milk of transgenic animals to protect against organophosphate poisoning. Proc Natl Acad Sci USA. 2007;104(34):13603–13608. doi: 10.1073/pnas.0702756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg YJ, Saxena A, Sun W, et al. Demonstration of in vivo stability and lack of immunogenicity of a polyethyleneglycol-conjugated recombinant CHO-derived butyrylcholinesterase bioscavenger using a homologous macaque model. Chem Biol Interact. 2010;187(1–3):279–286. doi: 10.1016/j.cbi.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 17.Geyer BC, Kannan L, Garnaud PE, et al. Plant-derived human butyrylcholinesterase, but not an organophosphorous-compound hydrolyzing variant thereof, protects rodents against nerve agents. Proc Natl Acad Sci USA. 2010;107(47):20251–20256. doi: 10.1073/pnas.1009021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen O, Kronman C, Chitlaru T, et al. Effect of chemical modification of recombinant human acetylcholinesterase by polyethylene glycol on its circulatory longevity. Biochem J. 2001;357(Pt 3):795–802. doi: 10.1042/0264-6021:3570795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider JD, Castilho A, Neumann L, et al. Expression of human butyryl-cholinesterase with an engineered glycosylation profile resembling the plasma-derived orthologue. Biotechnol J. 2014;9:501–10. doi: 10.1002/biot.201300229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashani Y, Pistinner S. Estimation of the upper limit of human butyryl-cholinesterase dose required for protection against organophosphates toxicity: a mathematically based toxicokinetic model. Toxicol Sci. 2004;77(2):358–367. doi: 10.1093/toxsci/kfh012. [DOI] [PubMed] [Google Scholar]
- 21.Raveh L, Grauer E, Grunwald J, et al. The stoichiometry of protection against soman and VX toxicity in monkeys pretreated with human butyrylcholinesterase. Toxicol Appl Pharmacol. 1997;145(1):43–53. doi: 10.1006/taap.1997.8160. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg YJ, Jiang X, Mao L, et al. Development of a prophylactic butyrylcholinesterase bioscavenger to protect against insecticide toxicity using a homologous macaque model. In: Soloneski S, Larramendy M, editors. Insecticides Basic and Others Applications. First. InTech Press; Croatia: 2012. pp. 79–100. [Google Scholar]
- 23.Millard CB, Kryger G, Ordentlich A, et al. Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level. Biochemistry. 1999;38(22):7032–7039. doi: 10.1021/bi982678l. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberry TL, Mallender WD, Thomas PJ, et al. A steric blockade model for inhibition of acetylcholinesterase by peripheral site ligands and substrate. Chem Biol Interact. 1999;119–120:85–97. doi: 10.1016/s0009-2797(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 25.Alavanja MC. Pesticides Use and Exposure Extensive Worldwide. Rev Environ Health. 2009;24(4):303–309. doi: 10.1515/reveh.2009.24.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litchfield MH. Estimates of acute pesticide poisoning in agricultural workers in less developed countries. Toxicol Rev. 2005;24(4):271–278. doi: 10.2165/00139709-200524040-00006. Review. [DOI] [PubMed] [Google Scholar]
- 27.Jeyaratnam J. Acute pesticide poisoning: a major global health problem. World Health Stat Q. 1990;43(3):139–144. [PubMed] [Google Scholar]
- 28.Cao JL, Varnell AL, Cooper DC. Gulf War Syndrome: A role for organophosphate induced plasticity of locus coeruleus neurons. Nature Precedings. 2011 hdl:10101/npre.2011.6057.1. [Google Scholar]
- 29.Johnston WM. Chemical weapon terrorism in Iraq and Afghanistan. 2015 http://www.johnstonsarchive.net/terrorism/wmdterrorism-1.html. Accessed April 22, 2016.
- 30.Sidell FR, Takafuji ET, Franz DR. Overview: Medical Aspects of Chemical and Biological Warfare. In: Zajtchuk R, Bellamy RF, editors. Textbook of Military Medicine. 1997. pp. 1–7. [Google Scholar]
- 31.Smart J. Medical Aspects of Chemical and Biological Warfare. In: Zajtchuk R, Bellamy RF, editors. Textbook of Military Medicine. 1997. pp. 9–86. [Google Scholar]
- 32.Wiener SW. Nerve Agents: A comprehensive review. Intensive Care Med. 2004;19:22–37. doi: 10.1177/0885066603258659. [DOI] [PubMed] [Google Scholar]
- 33.Geller DE, Pitlick WH, Nardella PA, et al. Pharmacokinetics and bioavailability of aerosolized tobramycin in cystic fibrosis. Chest. 2002;122(1):219–26. doi: 10.1378/chest.122.1.219. [DOI] [PubMed] [Google Scholar]
- 34.Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv Drug Deliv Rev. 2006;58(9–10):1030–1060. doi: 10.1016/j.addr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Ari A, Fink JB. Differential medical aerosol device and interface selection in patients during spontaneous, conventional mechanical and noninvasive ventilation. J Aerosol Med Pulm Drug Deliv. 2015;29(2):95–106. doi: 10.1089/jamp.2015.1266. [DOI] [PubMed] [Google Scholar]
- 36.Colthorpe P, Farr SJ, Taylor G. The pharmacokinetics of pulmonary delivered insuling: a comparison of intratracheal and aerosol administration to the rabbit. Pharm Res. 1992;9(6):764–8. doi: 10.1023/a:1015851521551. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg YJ, Laube B, Mao L, et al. Pulmonary delivery of an aerosolized recombinant butyrylcholinesterase pretreatment protects against aerosolized paraoxon insecticide. Chem Biol Interact. 2013;203:167–171. doi: 10.1016/j.cbi.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ari A, Fink JB. Aerosol therapy in children: challenges and solutions. Expert Rev Respir Med. 2013;7(6):665–672. doi: 10.1586/17476348.2013.847369. [DOI] [PubMed] [Google Scholar]
- 39.Lin HL, Harwood RJ, Fink JB, et al. In vitro comparison of aerosol delivery using different face masks and flow rates with a high-flow humidity system. Respir Care. 2014;60(9):1215–9. doi: 10.4187/respcare.03595. [DOI] [PubMed] [Google Scholar]
- 40.Fok TF, Monkman S, Dolovich M, et al. Efficiency of aerosol medication delivery from a metered dose inhaler versus jet nebulizer in infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 1996;21(5):301–309. doi: 10.1002/(SICI)1099-0496(199605)21:5<301::AID-PPUL5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 41.Dubus JC, Vecellio L, de Monte M, et al. Aerosol deposition in neonatal ventilation. Pediatric Res. 2005;58(1):10–14. doi: 10.1203/01.PDR.0000156244.84422.55. [DOI] [PubMed] [Google Scholar]
- 42.Marchand D, Parent C, Respaud R, et al. Nebullization of antibodies against ricin poisoning by inhalation: Drug and Development. Respiratory Drug Delivery 2014. 2014;3:605–608. [Google Scholar]
- 43.MacLoughlin RJ, van Aergongen G, Fink JB, et al. Optimization and dose estimation of aerosol delivery to non-human primates. J Aerosol Med Pulm Drug Deliv. 2016;29(0):1–7. doi: 10.1089/jamp.2015.1250. [DOI] [PubMed] [Google Scholar]
- 44.Low N, Bavdekar A, Jeyaseelan L, et al. A randomized, controlled trial of an aerosolized vaccine against measles. N Engl J Med. 2015;372(16):1519–2529. doi: 10.1056/NEJMoa1407417. [DOI] [PubMed] [Google Scholar]


