Abstract
Enhanced inflammation and reduced apoptosis sustain the growth of endometriotic lesions. Alterations in the expression of estrogen receptor (ER) α and β accompany the conversion of resident endometrial cells within the normal uterine environment to ectopic lesions located in extra-uterine sites. Recent studies highlighted in this focused review linked ERβ to dysregulation of apoptotic and inflammatory networks involving novel interacting partners in endometriosis. The elucidation of these non-genomic actions of ERβ using human cells and mouse models is an important step in understanding key regulatory pathways that are disrupted leading to disease establishment and progression.
Keywords: endometriosis, estrogen receptor-beta, inflammation, non-genomic, apoptosome
Estrogens are key mediators of endometrial homeostasis, hence, any dysregulation in their synthesis, metabolism, and/or activities irrefutably leads to a broad range of endometrial pathologies. Two major estrogen receptor proteins, estrogen receptor-alpha (ERα) and estrogen receptor-beta (ERβ) which are encoded by distinct genes (White et al., 1987; Kuiper et al., 1996), mediate the actions of estrogens in target cells. The significant homologies in DNA (94%) and ligand (59%)-binding domains between these ERs enable both proteins to bind estrogens with equal affinity and to transcriptionally regulate common subsets of ER-responsive genes. ERα and ERβ display distinct spatial, temporal and physiological expression. Genetic deletions support most roles for ERα in the uterus and for ERβ to prevail in the ovary (Hamilton et al., 2014). While ligand-bound ERα is requisite for mitogenesis of uterine cells, ERβ is considered to inhibit ERα-dependent cell proliferation in part, through its ability to form ERα/ERβ heterodimers with different ligand specificity, interacting partners, and transcriptional targets than ERα homodimers (Pace et al., 1997).
Endometriosis is an estrogen-dependent gynecological disorder, defined as the growth of endometrial stroma and glands in extra-uterine sites such as in the peritoneum and the ovary (Burney & Giudice, 2012). The disease affects 1 in 10 reproductive age women and can lead to debilitating pelvic pain, dysmenorrhea, and reduced fertility. While benign, endometriosis is a chronic condition that requires long-term treatment throughout a woman’s reproductive years. The goals of treatment for endometriosis include hormonal suppression of active endometriosis or surgical excision/ablation of visible lesions (Vercellini et al., 2014). However, medical therapies are not without side effects and surgical removal of the ovaries and uterus, while considered a definitive management for the condition, is not without associated morbidity.
In recent years, ERβ has emerged as an important player in the pathogenesis of endometriosis. Human endometriotic lesions, whether of ovarian or peritoneal locations, display higher ERβ expression when compared to normal human endometrial cells; this was not shown for ERα (Bulun et al., 2012). The reversal in ERβ to ERα ratio in lesions (>>1), relative to normal endometrial cells (<<1) has been similarly demonstrated in many animal models of endometriosis (Fazleabas et al., 2003; Greaves et al., 2014; Heard et al., 2014) and raises the intriguing question of how the conventionally anti-proliferative ERβ in the endometrium, in the context of endogenous estrogens, dismantles its customary role and orchestrates a pro-proliferative/anti-apoptotic/pro-inflammatory response to drive disease pathogenesis. In a recent issue of Cell (Han et al., 2015), an elegant study by O’Malley and co-workers unequivocally demonstrated that ERβ is required for the progression of endometriosis in mice and defined the apoptosome and inflammasome as endogenous targets of non-genomic ERβ action. Katzenellenbogen and colleagues in a related study published in Science Translational Medicine (Zhao et al., 2015) described two ER antagonists, one specific for ERα (chloroindazole, CLI) and the other specific for ERβ (oxabicycloheptane sulfonate, OBHS), that individually function to inhibit the estrogen-inflammatory axis to suppress endometriosis in mice. These two studies collectively define a novel role for ERβ in the upper echelon of the inflammatory regulatory hierarchy. Importantly, as inflammation is well-considered as one of the major contributors to the development and progression of endometriosis, the study findings offer promise for novel therapeutic strategies that may be relevant to endometriosis and other reproductive and non-reproductive diseases associated with chronic inflammation.
To uncover the underlying mechanism(s) by which ERβ promotes endometriosis, O’Malley and his group used ovariectomized+E2-pelleted immunocompetent mouse models in which endometriosis was surgically-induced through auto transplantation of ERβ over-expressing (gain-of-ERβ-function) and ERβ null (loss-of-ERβ-function) endometrial tissues in the peritoneal cavity. Ectopic lesions generated from ERβ over-expressing (ERβ-OE) endometrium had larger volumes while those from ERβ null endometrium had smaller volumes, than did wildtype (WT; control) ectopic lesions. Consistent with these observations, the use of the ERβ selective antagonist PHTTP (a pyrazolol [1.5-α] pyrimidine-based ligand) suppressed ectopic lesion growth relative to vehicle alone. The inhibitory effect of PHTPP on lesion growth was accompanied by the loss of recruitment of CD163-positive monocyte/macrophage cells that normally infiltrate lesions. Conversely, the ERβ specific agonist ERB-041 enhanced the growth of mouse ectopic lesions compared with vehicle alone. Because these noted changes in lesion growth occurred in the absence of perturbations in ERα, enhanced ERβ expression and activity appear sufficient to promote endometriosis progression. In an earlier study (Harris et al., 2005), athymic nude mice surgically implanted with human endometriotic lesions showed lesion regression when administered ERB-041, a response clearly contrasting with that obtained with the immunocompetent mouse model in the Cell study. Intriguingly, ectopic lesions formed in control and ERB-041-treated nude mice did not express ERβ, a major departure from lesions of women and those generated in other animal models of endometriosis, in which ERβ is the predominant ER isoform. While the molecular basis underlying the differential responses of the two mouse models to ERB-401 remains unknown, these results provide support for the complex interactions between the immune system and ER-mediated signaling in the development and progression of endometriosis. Additionally, these results highlight innate limitations of the animal models utilized for many endometriosis studies, including those described in the two highlighted papers in this review (e.g. the need to implant estrogen pellets, which most likely does not reflect/only approximates the situation in humans) and underscore the continuing need for the development of more relevant models to fully understand the human disease.
How might ERβ alter lesion biology distinct from its role in non-diseased endometrial cells? The authors analyzed by mass spectroscopy, flag-tagged ERβ-containing protein complexes that were immunoprecipitated from eutopic endometria of endometrial ERβ-overexpressing mice. Further confirmation by Western blotting revealed that a majority of proteins interacting with ERβ are involved in inflammation and apoptosis signaling. Apoptosis plays an important role in inflammation and in the resolution of inflammatory reactions, and the two are irrefutably linked since cell death signaling initiated by tumor necrosis factor-alpha (TNF-α) activates inflammasomes to initiate IL-1β driven inflammation (Vince & Silke, 2016). An attractive candidate identified in the study as an ERβ-interacting protein is apoptosis signal-regulating kinase-1 (ASK-1). ASK-1 is a component of TNF-α-induced apoptosis complex 1, whose formation is required for TNF-α-induced apoptosis. Serine/threonine kinase receptor-associated protein (STRAP) and 14-3-3 protein were also identified to interact with ERβ in the same screen. Interestingly, STRAP and 14-3-3 proteins have been previously demonstrated to bind ASK-1 in a tripartite complex (Jung et al., 2010). The formation of this complex interferes with the functional association between ASK-1 and the TNF receptor-associated factor 2 in response to TNF-α signaling, resulting in the inhibition of TNF-α-induced apoptosis. To validate this model, the authors demonstrated: 1) lower status of phosphorylated ASK-1 (phospho-Thr845 ASK-1), without accompanying changes in total ASK-1 protein levels, indicating loss of ASK-1 activation and hence, function, in ERβ-OE ectopic lesions compared with WT ectopic lesions; 2) conversely, increased levels of phosphorylated ASK-1 in ERβ-null ectopic lesions compared with WT ectopic lesions; and 3) lower mitochondrial cytochrome c levels in ERβ-OE ectopic lesions, consistent with the disruption of TNF-α-induced ASK-1 activation that normally leads to caspase 9 activation. The link between ERβ and caspase 9 was further illuminated by demonstrating that ERβ-OE ectopic lesions had lower caspase 9 levels and lacked detectable interactions between caspase 9 and apoptotic peptidase-interacting factor; by contrast, the latter interaction was easily detected in ERβ-null ectopic lesions. Taken together, these novel findings identify TNF-α-induced apoptosis as a key regulatory pathway disrupted by cytoplasmic-based ERβ to promote lesion survival. The cytoplasmic-localized ERβ anti-apoptotic action likely occurs in conjunction with nuclear-localized ERβ transcriptional activation of gene targets such as the serum and glucocorticoid-regulated kinase, which by phosphorylating the pro-apoptotic FOXO3, inhibits apoptosis (Monsivais et al., 2016). However, the relative contribution of nuclear vs. cytoplasmic actions of ERβ to lesion survival is unknown.
The intriguing idea that ERβ multitasks outside of the nucleus was further demonstrated in other experiments from the same study. The authors identified caspase 1 and the NLR family pyrin-domain-containing 3 as additional ERβ-interacting proteins. These findings are significant in the context of the inflammatory process, given the requisite participation of caspase 1 and NLR in the processing of pro-IL-1β to the mature bioactive IL-1β, a key regulator of adhesion and proliferation in endometrial cells (Kao et al., 2011). In this regard, ERβ-OE lesions had higher IL-1β and cleaved caspase 1 levels than did control ectopic lesions and conversely, ERβ-null lesions had lower levels of both components. Given that primary human endometriotic stromal cells also showed elevated levels of IL-1β and increased anti-apoptosis signaling when treated with TNF-α, the results suggest that the key ERβ-mediated events elucidated in these mouse models of endometriosis are relevant to the human disease.
Interestingly, the authors found, using immortalized human endometrial epithelial cells expressing Myc-tagged human ERα genes that TNF-α treatment of these cells did not elicit comparable anti-apoptotic responses and an increase in IL-1β expression noted for the same human cells expressing ERβ. More intriguingly, co-transfection of ERα and ERβ in these cells resulted in ERα inhibition of ERβ-mediated IL-1β production, suggesting that ERα may assume a negative regulatory role in ERβ-mediated promotion of inflammation. Whether this effect of ERα reflects its competitive displacement of ERβ from its interacting proteins, or its sequestering of ERβ through formation of ERα/ERβ heterodimers is unknown. This result, while baffling, sheds new light on the contrasting activities of ERβ and ERα in endometriosis and must be reconciled with previous findings that ERα is an equally active player in the pathology of this disease in mice (Burns et al., 2012). In the study by Katzenellenbogen and colleagues (Zhao et al., 2015), specific ERα (CLI) and ERβ (OBHS) antagonists exhibited ER-dependent anti-inflammatory activities in a mouse model of endometriosis and in human endometriotic stromal cells. In these models, CLI and OBBHS equally inhibited estrogen-dependent processes including cell proliferation, cyst formation, vascularization, cytokine production, macrophage infiltration and lesion growth. A previous study has reported the presence of both ERα and ERβ in peritoneal fluid macrophages and shown that both ERs display higher expression in peritoneal fluid macrophages of women with endometriosis when compared to women without endometriosis (Montagna et al. 2008). Interestingly, this same study showed that only ERα expression was positively correlated with increased pro-inflammatory cytokine levels in peritoneal fluids of women with endometriosis. ERβ levels while higher in peritoneal fluid macrophages of women with endometriosis, were correlated with expression of proinflammatory cytokines, irrespective of disease status, suggesting a role for macrophage ERβ in basal pro-inflammatory cytokine production and for macrophage ERα as a likely ‘driver’ of increased inflammation seen in endometriosis. Since mouse ectopic lesions, similar to human lesions display higher levels of ERβ than ERα, one intriguing question raised by the Katzenellenbogen study pertains to the unexpected comparable levels of inhibition of estrogen-dependent responses with disruption of ERα signaling to that mediated by the more highly-expressed ERβ. Perhaps the significant contribution of recruited macrophages expressing higher ERα and hence, equally higher production of pro-inflammatory cytokines may underlie the favorable response of lesions to ERα antagonists. It is also tempting to speculate that the cellular locations of the respective actions of ERα and ERβ may be at play, given that in O’Malley’s study, ERβ disruption of inflammasome and apoptosome functions takes place outside of the nucleus, invoking non-genomic actions and involving novel interactions with proteins not previously identified to interact with ERβ. If the latter is true, then a follow-up question is whether ERα interacts (or not) with some of the same proteins that were identified for ERβ. Yet another question is whether ERβ preferentially acts outside of the nucleus in endometriotic lesions and if this may underlie the reversal of its (generally good) fortune of being the better half to ERα in controlling mitogenesis. The answer is not likely going to be straightforward given other nuclear actions attributed to ERβ in ectopic lesions, one of which is its transcriptional regulation of ERα in endometriotic stromal cells (Trukhacheva et al., 2009). Time (and more in-depth scrutiny) will tell.
The molecular details of complex mechanisms have gained much ground from careful dissection of context-dependent cross-talk among seemingly unrelated molecules. For an enigmatic condition such as endometriosis, the identification of novel partners elucidated here for ERβ begs the question of whether progesterone receptors (whose expression and function are equally compromised in endometriosis) and other steroid receptor co-activators or co-repressors may similarly assume new extra-nuclear (cytoplasmic) roles to sustain ectopic growth. A tipoff to this possibility comes from the recent discovery of a new 70 kDa (truncated) Steroid Receptor Co-Activator 1 (SRC-1) isoform, which similar to ERβ, was found to have little expression in normal endometrium, but displayed significant expression in endometriotic lesions (Han et al., 2012). Moreover, the truncated SRC-1 protein, similar to ERβ, was demonstrated to be essential in the initial stages of endometriosis establishment. In the current Cell paper, SRC-1 isoform was shown to form a complex with ERβ and caspase 8, inhibiting the latter from interacting with its usual partner Fas-associated via death domain protein to generate apoptosis complex II that augments TNF-α-induced apoptosis. Whether the truncated SRC-1 only partners with ERβ or exhibits a more expansive repertoire of interacting proteins to promote endometriosis remains to be explored.
The present studies provide fundamental insights into the adaptive functions of ERβ in inflammation and apoptosis (Figure 1). Fine-tuning our awareness of the different networks orchestrated by steroid hormone receptors and their changing partners in normal and endometriotic cells may offer the much-needed therapeutic opportunities to address the development and progression of endometriosis.
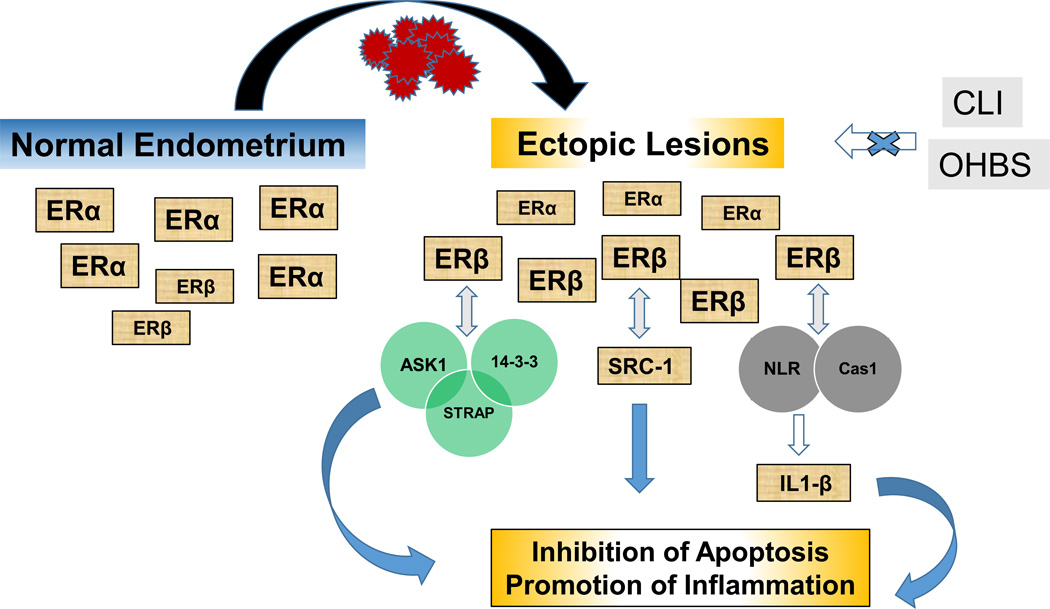
Figure 1.
Proposed model for Estrogen receptor-β regulation of inflammation and apoptosis in endometriotic lesions. Normal endometrium and ectopic lesions display distinct levels of estrogen receptor (ER)-α and –β. The direct interactions of ERβ with proteins associated with inflammation and apoptosis in ectopic lesions (Han et al., 2015) provide novel, non-genomic mechanisms for ERβ-mediated pathogenesis of endometriosis. CLI and OBHS are respectively, specific ERα and ERβ antagonists shown to inhibit ER-mediated promotion of inflammation underlying endometriosis progression (Zhao et al., 2015). ASK-1, STRAP, 14-3-3, NLR, Cas1, and IL-1β are defined in the text.
Acknowledgments
Funding
This work was supported in part by the National Institutes of Health (RO1CA136493, UL1TR000039) and the Arkansas Biosciences Institute Intramural Grant Program.
Footnotes
Declaration of interest
The authors declare no potential conflicts of interest.
References
- Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, Tokunaga H, Su EJ. Role of estrogen receptor-β in endometriosis. Semin Reprod Med. 2012;30:39–45. doi: 10.1055/s-0031-1299596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–519. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KA, Rodriguez KF, Hewitt SC, Janardhan KS, Young SL, Korach KS. Role of estrogen receptor signaling required for endometriosis-like lesion establishment in a mouse model. Endocrinology. 2012;153:3960–3971. doi: 10.1210/en.2012-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Chai D, Langoi D, Bulun SE. Steroid receptor and aromatase expression in baboon endometriotic lesions. Fertil Steril. 2003;80(Suppl 2):820–827. doi: 10.1016/s0015-0282(03)00982-8. [DOI] [PubMed] [Google Scholar]
- Greaves E, Cousins FL, Murray A, Esnal-Zufiaurre A, Fassbender A, Horne AW, Saunders PT. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am J Pathol. 2014;184:1930–1939. doi: 10.1016/j.ajpath.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KJ, Arao Y, Korach KS. Estrogen hormone physiology: reproductive findings from estrogen receptor mutant mice. Reprod Biol. 2014;14:3–8. doi: 10.1016/j.repbio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Hawkins SM, Begum K, Jung SY, Kovanci E, Qin J, Lydon JP, DeMayo FJ, O'Malley BW. A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nat Med. 2012;18:1102–1111. doi: 10.1038/nm.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Jung SY, Wu S-P, Hawkins SM, Park MJ, Kyo S, Qin J, Lydon JP, Tsai SY, Tsai M-J, et al. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell. 2015;163:960–974. doi: 10.1016/j.cell.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HA, Bruner-Tran KL, Zhang X, Osteen KG, Lyttle CRA. Selective estrogen receptor-beta agonist causes lesion regression in an experimentally induced model of endometriosis. Hum Reprod. 2005;20:936–941. doi: 10.1093/humrep/deh711. [DOI] [PubMed] [Google Scholar]
- Heard ME, Simmons CD, Simmen FA, Simmen RC. Krüppel-like factor 9 deficiency in uterine endometrial cells promotes ectopic lesion establishment associated with activated notch and hedgehog signaling in a mouse model of endometriosis. Endocrinology. 2014;155:1532–1546. doi: 10.1210/en.2013-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Seong HA, Manoharan R, Ha H. Serine-threonine kinase receptor-associated protein inhibits apoptosis signal-regulating kinase 1 function through direct interaction. J Biol Chem. 2010;285:54–70. doi: 10.1074/jbc.M109.045229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao AP, Wang KH, Long CY, Chai CY, Tsai CF, Hsieh TH, Hsu CY, Chang CC, Lee JN, Tsai EM. Interleukin-1β induces cyclooxygenase-2 expression and promotes the invasive ability of human mesenchymal stem cells derived from ovarian endometrioma. Fertil Steril. 2011;96:678–684. doi: 10.1016/j.fertnstert.2011.06.041. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsivais D, Dyson MT, Yin P, Navarro A, Coon JS, 5th, Pavone ME, Bulun SE. Estrogen receptor β regulates endometriotic cell survival through serum and glucocorticoid-regulated kinase activation. Fertil Steril. 2016;105:1266–1273. doi: 10.1016/j.fertnstert.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna P, Capellino S, Villaggio B, Remorgida V, Ragni N, Cutolo M, Ferrero S. Peritoneal fluid macrophages in endometriosis: correlation between the expression of estrogen receptors and inflammation. Fertil Steril. 2008;90:156–164. doi: 10.1016/j.fertnstert.2006.11.200. [DOI] [PubMed] [Google Scholar]
- Pace P, Taylor J, Suntharalingam S, Coombes RC, Ali S. Human estrogen receptor beta binds DNA in a manner similar to and dimerizes with estrogen receptor alpha. J Biol Chem. 1997;272:25832–25838. doi: 10.1074/jbc.272.41.25832. [DOI] [PubMed] [Google Scholar]
- Trukhacheva E, Lin Z, Reierstad S, Cheng YH, Milad M, Bulun SE. Estrogen receptor (ER) beta regulates ERalpha expression in stromal cells derived from ovarian endometriosis. J Clin Endocrinol Metab. 2009;94:615–622. doi: 10.1210/jc.2008-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- Vince JE, Silke J. The intersection of cell death and inflammasome activation. Cell Mol Life Sci. 2016 doi: 10.1007/s00018-016-2205-2. PMID: 27066895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Lees JA, Needham M, Ham J, Parker M. Structural organization and expression of the mouse estrogen receptor. Mol Endocrinol. 1987;1:735–744. doi: 10.1210/mend-1-10-735. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Gong P, Chen Y, Nwachukwu JC, Srinivasan S, Ko C, Bagchi MK, Taylor RN, Korach KS, Nettles KW. Dual suppression of estrogenic and inflammatory activities for targeting of endometriosis. Sci Transl Med. 2015;7:271. doi: 10.1126/scitranslmed.3010626. [DOI] [PMC free article] [PubMed] [Google Scholar]