Abstract
Objective
Investigate the association of chronic depressive symptomatology (chrDS) with cortical atrophy rates and conversion to Alzheimer’s dementia (AD) over three years in mild cognitive impairment (MCI).
Design
Prospective elderly cohort study.
Setting
Multicenter, clinic-based cohort.
Participants
MCI participants were selected from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) repository, based on availability of both serial structural MRI and chrDS endorsed on three depression-related items from the Neuropsychiatric Inventory Questionnaire (chrDS n=32 or no depressive symptoms n=62) throughout follow-up. Clinical and laboratory investigations were performed every six months during the first two years and yearly thereafter (median time of follow-up three years, interquartile range 1.5 to 4.0 years).
Measurements
Cortical atrophy rates in 16 predefined fronto-temporo-parietal regions affected in major depression and AD, and the rate of incident AD at follow-up.
Results
ChrDS in a single domain amnestic MCI sample were associated with accelerated cortical atrophy in the frontal lobe and anterior cingulate, but not with atrophy rates in temporomedial or other AD-affected regions. During follow-up 38 participants (42.7%) developed AD. Participants with chrDS had 60% shorter conversion time to AD than those without depressive symptoms. This association remained significant in survival models adjusted for temporo-medial atrophy rates and showed the same trend in models adjusted for frontal cortical atrophy rate, which all increased the risk of AD.
Conclusions
Our results suggest that chrDS associated with progressive atrophy of frontal regions may represent an additional risk factor for conversion to dementia in MCI as opposite to representing typical prodromal AD symptomatology.
Keywords: Depressive Symptoms, Mild Cognitive Impairment, Alzheimer’s Dementia, MRI, cohort study
INTRODUCTION
The amnestic forms of mild cognitive impairment (MCI), the most frequent cognitive impairment in elderly, may progress to Alzheimer’s dementia (AD) with variable rates due to different risk factors (1). Neuropsychiatric symptoms of depression are reported in 35–85% of elderly with MCI (2). Evidence has accumulated showing additional risk to convert to AD in MCI associated with symptoms of depression (3) and apathy (4). Depressive symptomatology not of the severity or frequency to meet criteria for depression, are the most commonly reported neuropsychiatric symptoms in MCI (5, 6). Clinical studies have also associated the persistence of less severe depressive symptoms to major depression, either as representing partially remitted major depression (7) or as increasing the risk of major depression in late life (LLD) (8). These studies did not, however, evaluate cognition. Some studies suggest that LLD may increase the risk of AD (9). Lately, even depressive symptoms in MCI have been conceptualized as representing either a co-occurring risk factor for AD or symptoms of an underlying neurodegenerative process (10).
Neuroimaging brain studies of depressive symptomatology in MCI hold significant promise to clarify the role that these symptoms may play in conversion to AD. To date, however, few studies have been conducted for this purpose. Nonetheless, neuroimaging studies of late life depression (LLD) have found cortical atrophy in frontal regions, most consistently in the anterior cingulate cortex (ACC), prefrontal and orbitofrontal cortex (11). Hippocampal atrophy has been shown in LLD with cognitive decline (12). A more distributed pattern of atrophy in frontotemporal as well as parietooccipital cortices has also been recently reported in LLD (13). In contrast to the rather non-specific findings in LLD, accelerated brain atrophy in hippocampus and entorhinal areas has been established as a reliable biomarker of amnestic MCI and conversion to AD (14). Atypical structural changes in frontoparietal cortices in MCI and early stage AD have also been reported but less consistently (15).
In a study of depressive symptoms assessed at baseline in relation to longitudinal brain changes in MCI, depressive symptoms were associated with accelerated cortical atrophy in ACC (16). A more recent study in MCI found that chronic, but not baseline depressive symptoms were associated with bilateral frontoparietotemporal white matter (WM) atrophy and consequently with an increased risk to develop AD (17). Although these studies focused on different brain structures, both suggest that cortical changes associated to depressive symptomatology may reflect progressive AD neuropathology. Moreover, these studies highlight that chronic depressive symptomatology (chrDS) in MCI may reflect different brain pathology than isolated episodes of depressive symptoms, yet the neurobiological underpinnings of these changes are not completely elucidated.
In order to better understand the relationship between chrDS and the putative AD-pathology in MCI, our main objective was to investigate the association of chrDS in MCI with rates of cortical atrophy in predefined regions associated with LLD (frontal lobe, anterior cingulate, temporal lobe, hippocampus, precuneus and cuneus) (11–13) and early stage AD (temporal lobe, parahippocampus, hippocampus, entorhinal cortex, posterior cingulate and precuneus) (14, 15). Since structural changes associated with chrDS in MCI are not clearly established, we also examined rates of cortical atrophy throughout the brain in relation to chrDS in exploratory analyses. Furthermore, to clarify the relationship between chrDS and conversion to AD in MCI, our secondary objective was to investigate the rate of incident AD in individuals with MCI and chrDS compared to those with no depressive symptoms (no DS). We hypothesized that: 1) chrDS will be associated with accelerated atrophy in frontal lobe, ACC and hippocampus; and 2) chrDS will be associated with an accelerated rate of incident AD.
METHODS
1. Study design and participants
Data used in this article were downloaded from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu) during August, 2012, except revised data on WM hyperintensities (WMH) downloaded January, 2014. The ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies and non-profit organizations. The overall goal of ADNI is standardization and validation of imaging and other biological markers for effective AD clinical trials (18). For up-to-date information on ADNI, see www.adni-info.org.
From ADNI we identified 94 participants with amnestic MCI, who underwent brain MRI at baseline and at follow-up (median number of scans 4.0, interquartile range (hereafter reported as ‘range’) 3.0 to 6.0), and provided consistent symptomatology on the informant-based Neuropsychiatric Inventory Questionnaire (NPI-Q) (19) at least on two occasions (median number of assessments 6.0, range 4.0 to 7.0). Participants received clinical, cognitive and laboratory investigations including brain MRI every six months for the first two years and yearly thereafter between 2005–2012, after the initial screening (median time of follow-up three years, range 1.5 to 4.0 years). The amnestic MCI diagnosis (single and multiple domains) was based on Petersen’s criteria (20). Inclusion and exclusion criteria for ADNI have been previously reported (21).
The procedures for this study were approved by Institutional Review Boards of all participating institutions. Informed written consent was obtained from all participants at each site.
2. Neuropsychiatric assessment
Neuropsychiatric symptoms were assessed with NPI-Q (19). This approach was dictated by informant reports being a valid source of information in psychiatric settings, corroborated with previous observation that the self-reported scale Geriatric Depression Scale (GDS) in MCI individuals could not capture depressive symptomatology predictive of AD (6). Using the self-administered questionnaire, a knowledgeable informant reported the presence (yes/no) of 12 behavioral symptoms (delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, aberrant motor behavior and neurovegetative disturbances of sleep/nighttime behavior and appetite/eating) over the last month. Informants were asked to rate the symptoms as 1=mild, 2=moderate, 3=severe. Participants were classified as “chrDS” if symptoms were endorsed by the knowledgeable informant at each available NPI-Q assessment on at least one of the three pre-defined depression-related items, as used previously (22): Depression/Dysphoria; Apathy/Indifference and Appetite/Eating Disturbances. The first two items converge on ‘depressed mood’ and ‘diminished interest’, which are mandatory criteria for depression episodes according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), whereas neurovegetative items are often encountered in depressed elderly and may overshadow the cardinal symptoms (23). We did not include though ‘Sleep/nighttime behavior disturbances’ to avoid bias toward caregivers’ appraisal of nocturnal wondering, confusion and other types of disruptive behavior as opposite to sleep pattern disturbances expected in early stage AD (i.e, insomnia and excessive day sleepiness), a well-recognized ambiguity in the assessment of sleep disturbances in AD (24). Participants were classified as “no DS” if the informant did not endorse depressive symptoms at any assessment. Those with sporadic endorsement of any of the three depressive symptoms were excluded.
3. Incident AD diagnosis
AD was diagnosed at follow-up by a consensus panel of experts based on the National Institute of Neurological and Communicative Disorders and Stroke – Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria (25). The diagnostic procedures have been described in ADNI General Procedures Manual (adni.loni.usc.edu/wp-content/uploads/2010/09/ADNI_GeneralProceduresManual.pdf).
4. Image acquisition and analysis
A standardized 1.5 Tesla MRI protocol was used to acquire images using General Electric, Philips and Siemens scanners (adni.loni.usc.edu/methods/documents/mri-protocols/). The ADNI MRI quality control was performed at the Mayo Clinic (26). For the cortical atrophy we used automated measures of cortical thickness, except for hippocampus for which volumetric measurements were available. All measures were computed using FreeSurfer software longitudinal processing framework (http://surfer.nmr.mgh.harvard.edu/fswiki, package version 4.4) (27, 28). We used cortical thickness of eight predefined brain regions and their subregions that have been associated with LLD (11–13): frontal lobe (medial and lateral orbitofrontal, rostral and caudal middle frontal and superior frontal), anterior cingulate (rostral and caudal anterior cingulate), temporal lobe, precuneus and cuneus; and with incipient AD (14, 15): posterior cingulate, temporal lobe, parahippocampus, entorhinal cortex, precuneus. Thickness of left and right hemispheres were averaged, and summed when indicated, to a unique value for each cortical region of interest. Automatically extracted bilateral hippocampal volumes were also averaged to a unique value. Additionally, we analyzed nineteen exploratory regions throughout the rest of the brain (on the medial surface: frontal pole, paracentral, isthmus cingulate, pericalcarine, lingual, fusiform, and temporal pole; on the lateral surface: pars opercularis, pars triangularis, pars orbitalis, insula, supramarginal, precentral, postcentral, transverse temporal, bank superior temporal, inferior parietal, superior parietal and lateral occipital). For WM lesions visualized as hyperintensities (WMH) we used volumetric data based on proton density (PD), T1- and T2-weighted images warped to a standardized elderly template as described at https://ida.loni.usc.edu/pages/access/studyData. For data consistency with the cortical gray matter processing, we have also analyzed WM hypointensities computed by FreeSurfer (27, 29). The results were similar and thus we chose to present the widely accepted WMH data.
5. Statistical analyses
Differences in demographic characteristics by chrDS category (no DS versus chrDS) were analyzed using Mann-Whitney tests for continuous measures (age, years of education, WMH volumes, and Mini Mental Status Examination (MMSE), informant reported NPI-Q and self-reported Geriatric Depression Scale (GDS) scores), and median values with interquartile range were reported. Differences in proportions were tested using Fisher’s Exact test (sex, APOE genotype dichotomized as carrier of at least one ε4 allele vs. non-carrier, and presence of multiple domain impairments). A generalized linear mixed effects regression model (GLME) with a binomial outcome and Wald t-test derived p-values was used to test the demographics, APOE status and chrDS category effects on missing data. We then modeled repeated measures of cortical thickness in nine predefined regions and WMH by chrDS category using linear mixed effects regression (LME) with a random intercept and slope. Post-hoc analyses were run in seven subregions within the frontal lobes and ACC (as above). In exploratory analyses we examined additional 19 regions (as above). ChrDS category, time of observation and the interaction between chrDS category and time were included as predictors as well as the covariates age, sex and APOE status. There was no longitudinal association between WMH and rates of cortical atrophy and thus WMH were not used as covariates in the LME. LME models were assessed by an analysis of the residuals. P-values from Wald t-tests were adjusted for multiple comparisons by false discovery rate (FDR) correction. The number of tests for FDR correction corresponds to the number of a priori regions (n=10), sub-regions (n=7) and exploratory regions (n=19). Parametric survival models, assuming a Weibull distribution, were used to model the effect of chrDS on mean time to conversion to AD. In post-hoc analyses we also adjusted for rates of atrophy. Five hundred bootstrap samples were used to account for the initial step of estimating the subjects’ atrophy rates and to estimate the variance of the effect of atrophy and chrDS in the post-hoc survival models. All p-values were two-tailed and considered significant at the 0.05 level, including FDR corrected p-values. The statistical analyses were conducted with the R package (v. 2.8.1, The R Foundation for Statistical Computing).
RESULTS
The baseline characteristics of the sample, categorized by the presence or absence of chrDS, are presented in Table 1. There were no differences between groups regarding sociodemographics (age, sex, years of education), APOE status, MMSE score and WMH burden. Only four individuals exhibited multiple domains amnestic MCI in this sample, with no difference between groups. As expected, the chrDS group had higher GDS scores and higher scores on the remaining 9-items of NPI-Q than those without chrDS. In the GLME model, no association was found between missing data and demographics, chrDS category and the interaction chrDS category by time of observation (results not shown). However, participants carrying APOE ε4 allele were more likely to have missing data at follow-up (β-estimate=1.053, standard error (SE)=0.519, Wald t=2.029, df=89, and Wald p=0.045).
Table 1.
Baseline characteristics of the amnestic MCI sample categorized by chrDS
| Characteristics | No DS n=62 |
ChrDS n=32 |
P-values |
|---|---|---|---|
| Women, n (%) | 21 (33.9) | 8 (25.0) | 0.482 |
| Age, median (range) | 75.2 (68.5 to 80.5) | 76.9 (71.2 to 79.4) | 0.500 |
| Years of education, median (range) | 16.0 (14.0 to 18.0) | 16.0 (14.0 to 18.0) | 0.502 |
| MMSE, median (range) | 27.0 (26.0 to 29.0) | 27.0 (25.0 to 29.0) | 0.774 |
| GDS score, median (range) | 1.0 (0.0 to 2.0) | 2.0 (1.0 to 3.0) | 0.018 |
| 9-item NPI-Q score1, median (range) | 0.0 (0.0 to 0.7) | 2.0 (1.0 to 3.0) | <0.001 |
| WMH, median (range) | 0.2 (0.1 to 0.5) | 0.2 (0.1 to 0.6) | 0.593 |
| APOE ε4 carriers, n (%) | 31 (50.0) | 19 (59.4) | 0.513 |
| Amnestic multiple domains, n (%)2 | 2 (3.3) | 2 (6.5) | 0.601 |
| AD at follow-up3, n (%) | 20 (33.3) | 18 (62.1) | 0.008 |
Neuropsychiatric Interview Questionnaire (NPI-Q) score includes the 9 items that were not used to define chronic depressive symptomatology (chrDS).
Missing data on type of MCI in one individual in each group.
Incident AD cases that were used in the parametric survival model are indicated (no data available for diagnosis at follow-up in two individuals with chrDS and three with no DS) and p-value according to Wald significance test from the parametric survival model adjusted for sex, age, education and APOE.
The median value and interquartile range are reported for continous measurements. Differences in median values were tested using Mann-Whitney test. Differences in proportions were tested using Fisher’s Exact test.
MCI Mild Cognitive Impairment; MMSE Mini Mental State Examination; GDS Geriatric Depression Scale; WMH White Matter Hyperintensities; AD Alzheimer’s Dementia
Rate of cortical atrophy related to chrDS
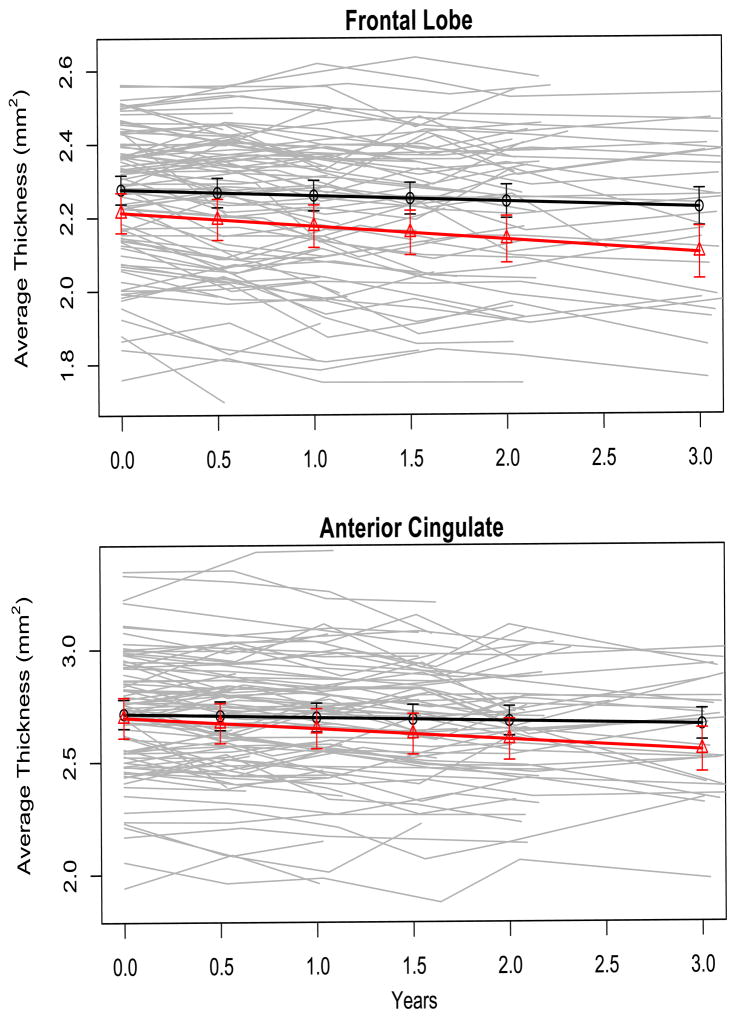
ChrDS was associated with an accelerated rate of atrophy in the frontal cortex and ACC, but not in other main regions of interest based on LME models assessed by Wald test (Figure 1). The associations remained significant after correction for multiple comparisons (Table 2). Post-hoc LME analyses with subregions of interest in the frontal lobes and ACC regressed on the interaction chrDS category by time of observation showed robust associations, except a trend in the lateral orbitofrontal cortex. The exploratory LME analyses showed no associations between chrDS and rate of atrophy in auxiliary cortical regions (Table 3).
Figure 1. Cortical atrophy rate in frontal and anterior cingulate regions related to chronic Depressive Symptomatology (chrDS).
Cortical thickness (mm2 on y-axis) at follow-up (years on x-axis) by chrDS category in the two cortical areas for which atrophy rates were significantly associated with chrDS in linear mixed effects regression models with Wald significance testing. Different y-axis scales are due to inherent different thicknesses and intercepts. Solid black line represents no depressive symptomatology and solid red line represents chrDS.
Table 2.
The relationship between chrDS in MCI and cortical atrophy in regions of interest
| Cortical regions | Interaction chrDS * time | ||||
|---|---|---|---|---|---|
| β estimate | SE | t-value | P-value | FDR-adjusted p-value | |
| Main ROI | |||||
| Frontal lobe | −0.020 | 0.008 | −2.457 | 0.011 | 0.050 |
| Anterior cingulate | −0.032 | 0.010 | −3.103 | 0.002 | 0.020 |
| Posterior cingulate | −0.017 | 0.011 | −1.579 | 0.115 | 0.290 |
| Temporal lobe | −0.017 | 0.011 | −1.577 | 0.116 | 0.290 |
| ERC | 0.004 | 0.020 | 0.214 | 0.831 | 0.940 |
| Parahippocampus | −0.010 | 0.011 | −0.925 | 0.356 | 0.590 |
| Hippocampus | −2.457 | 13.732 | −0.179 | 0.858 | 0.940 |
| Precuneus | −0.011 | 0.008 | −1.385 | 0.167 | 0.330 |
| Cuneus | 0.000 | 0.006 | −0.076 | 0.939 | 0.940 |
| Post-hoc ROI | |||||
| Caudal ACC | −0.036 | 0.012 | −2.917 | 0.004 | 0.030 |
| Rostral ACC | −0.030 | 0.012 | −2.546 | 0.011 | 0.030 |
| SuperiorFrontal | −0.020 | 0.010 | −2.068 | 0.039 | 0.050 |
| Rostral Middle Frontal | −0.018 | 0.007 | −2.388 | 0.018 | 0.030 |
| Caudal Middle Frontal | −0.018 | 0.009 | −2.045 | 0.042 | 0.050 |
| Medial Orbitofrontal | −0.024 | 0.009 | −2.597 | 0.010 | 0.030 |
| Lateral Orbitofrontal | −0.019 | 0.011 | −1.702 | 0.090 | 0.090 |
Longitudinal cortical thickness was regressed on age, sex, APOE status, chrDS category and time interval from baseline and the interaction chrDS * time using linear mixed models (df=302). Wald t-test P-values ≤ 0.05 were considered significant, including after FDR correction. ChrDS Chronic Depressive Symptoms. MCI Mild Cognitive Impairment. ERC Entorhinal Cortex. ACC Anterior Cingulate Cortex.
Table 3.
The relation between chrDS in MCI and cortical atrophy in auxiliary brain regions
| Auxiliary cortical regions | Interaction chrDS * time | ||||
|---|---|---|---|---|---|
| β estimate | SE | t-value | P-value | FDR-adjusted p-value | |
| Medial surface | |||||
| Frontal Pole | −0.026 | 0.016 | −1.610 | 0.108 | 0.530 |
| Paracentral | −0.012 | 0.009 | −1.314 | 0.190 | 0.530 |
| Isthmus Cingulate | −0.009 | 0.011 | −0.759 | 0.448 | 0.570 |
| Pericalcarine | 0.005 | 0.005 | 1.100 | 0.272 | 0.530 |
| Lingual | −0.004 | 0.004 | −1.009 | 0.314 | 0.540 |
| Fusiform | −0.012 | 0.011 | −1.102 | 0.271 | 0.530 |
| Temporal Pole | −0.048 | 0.025 | −1.917 | 0.056 | 0.530 |
| Lateral surface | |||||
| Pars Opercularis | −0.008 | 0.010 | −0.839 | 0.402 | 0.570 |
| Pars Triangularis | −0.014 | 0.010 | −1.352 | 0.177 | 0.530 |
| Pars Orbitalis | −0.013 | 0.011 | −1.120 | 0.263 | 0.530 |
| Insula | −0.011 | 0.008 | −1.358 | 0.176 | 0.530 |
| Supramarginal | −0.009 | 0.008 | −0.924 | 0.244 | 0.530 |
| Precentral | −0.015 | 0.009 | −1.590 | 0.085 | 0.530 |
| Postcentral | −0.005 | 0.007 | −0.756 | 0.450 | 0.570 |
| Transverse Temporal | −0.002 | 0.014 | −0.120 | 0.904 | 0.900 |
| Bank Superior Temporal | 0.001 | 0.010 | 0.153 | 0.879 | 0.900 |
| Inferior Parietal | −0.006 | 0.009 | −0.618 | 0.537 | 0.640 |
| Superior Parietal | −0.002 | 0.008 | −0.245 | 0.807 | 0.900 |
| Lateral Occipital | −0.006 | 0.006 | −1.080 | 0.281 | 0.530 |
Longitudinal cortical thickness in exploratory regions was regressed on age, sex, APOE status, chrDS category, time interval from baseline and the interaction chrDS * time using linear mixed models (df=302). Wald t-test P-values ≤ 0.05 were considered significant, including after FDR correction. ChrDS Chronic Depressive Symptoms. MCI amnestic Mild Cognitive Impairment.
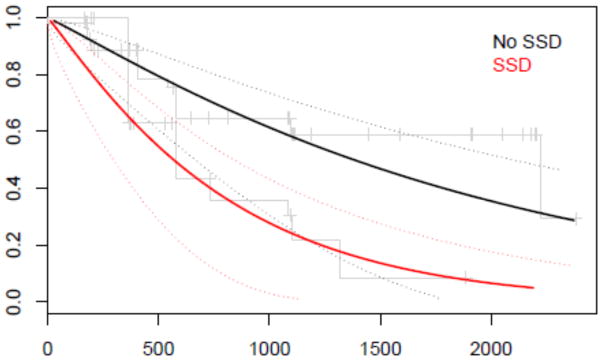
Incident AD related to chrDS
Incident AD was more frequent in chrDS than in no chrDS group (62.1% versus 33.3%). The adjusted survival model estimated 60% shorter conversion time to AD in chrDS compared to those without chrDS (β-estimate= −0.865, SE=0.326, Wald t=−2.652, df=83 and Wald p=0.008) (Figure 2). The same trend remained after adjustment for frontal atrophy rate (β-estimate=−0.533 with bootstrap estimated SEbootstrap=0.314, tbootstrap=−1.698, pbootstrap=0.093). However, the association chrDS and incident AD was removed after adjustment for ACC atrophy rate (β-estimate=−0.252, SEbootstrap=0.426, tbootstrap=−0.593, pbootstrap=0.555). ChrDS remained significantly associated with incident AD when further adjustments were made for hippocampal and entorhinal atrophy rates (rate of incident AD by chrDS category: β-estimate= −0.726, SEbootstrap=0.348, tbootstrap=−2.087, pbootstrap=0.040; and β-estimate= −0.699, SEbootstrap=0.325, tbootstrap=−2.148, pbootstrap=0.035, respectively). In the adjusted models the risk of incident AD was increased with progressive atrophy of frontal cortex (β-estimate=34.604, SEbootstrap=15.552, tbootstrap=2.225, pbootstrap=0.029), but not by atrophy rate in ACC (β-estimate=29.384, SEbootstrap=19.850, tbootstrap=1.480, pbootstrap=0.143). Hippocampal and entorhinal atrophy rates were associated with incident AD regardless of chrDS category (β-estimate=0.014, SEbootstrap=0.006, tbootstrap=2.391, pbootstrap=0.019; β-estimate=11.278, SEbootstrap=5.030, tbootstrap=2.242, pbootstrap=0.028, respectively).
Figure 2. Parametric survival model by chrDS category with AD as outcome.
Proportion of participants (y-axis) who developed Alzheimer’s dementia at follow-up (‘Days of follow-up’ on x-axis) by chrDS category according to the parametric survival model with Wald significance testing using age, sex, years of education and APOE status as covariates. chrDS Chronic Depressive Symptomatology. AD Alzheimer’s dementia. Solid black line represents no depressive symptomatology and solid red line represents chrDS.
DISCUSSION
The primary findings of the study were: 1) chrDS was associated with accelerated cortical atrophy in frontal cortex and ACC; 2) chrDS was not associated with rates of cortical atrophy in other brain regions; 3) chrDS was associated with an increased rate of conversion to AD; 4) the association between chrDS and incident AD was independent of cortical atrophy rates in the temporal regions, but not of cortical atrophy rates in frontal regions. Taken together these findings suggest that chrDS are associated with structural brain changes that may contribute to more rapid conversion to dementia in MCI. The biological underpinnings of our findings are discussed below.
As hypothesized, data supported a relationship between chrDS and atrophy rates in frontal cortex and ACC. This pattern of atrophy, previously encountered in LLD and possibly related to damage in the frontosubcortical neuronal circuits (30), could develop concomitantly with the ongoing neurodegenerative process in MCI. Although our sample expressed a very mild neuropsychiatric phenotype, due to major depression being an exclusion criterion in ADNI, chrDS seem to reflect a LLD atrophy pattern as confirmed in post-hoc analyses of subregions of interest. Nevertheless, neuroimaging studies of biomarkers of AD such as amyloid and tau protein aggregation in association with neuropsychiatric symptomatology support the hypothesis of depressive symptomatology in elderly with MCI reflecting a common neurodegenerative pathway (31). It has been suggested that changes in the serotonergic system driven by neurodegeneration in adjacent cholinergic system may induce depression and accelerate cognitive impairment (32). Thus, our findings could also be explained by a progression of the underlying MCI neurodegenerative pathology, as previously suggested (16, 17). We cannot exclude, however, yet another explanation, namely that chrDS in MCI may represent an atypical neurodegenerative pattern with additional frontal and ACC atrophy, a line of thought supported by studies of atypical AD (33). Furthermore, without the pathological diagnosis of dementia we cannot exclude the presence of other neurodegenerative pathology that may affect frontal lobes (e.g. Lewy body degeneration, variants of frontal lobe dementias) (34).
Contrary to our hypothesis, our second main finding showed that chrDS was not related to accelerated atrophy in the hippocampus. In fact, we found no relationship between chrDS and rates of atrophy in the temporoparietal regions that may be affected by neurodegeneration in MCI (14, 15). The lack of association between chrDS and atrophy rates in typical AD-related regions in our MCI sample suggests that chrDS does not reflect or exacerbate the underlying AD pathology. Previous neuroimaging studies have also related hippocampal atrophy to LLD (12). In neuroimaging studies of LLD, however, dementia was not an outcome. Thus the cognitive decline with underlying hippocampal atrophy as part of a long preclinical stage of dementia could explain previous results (35). In our sample, hippocampal atrophy rate was associated with incident AD as discussed below.
The third main finding that chrDS was associated with incident AD expands on the previous findings using the ADNI repository that showed a trend between non-memory GDS items and development of dementia on a 2-year follow-up (6). However, the association of chrDS with incident AD was mediated by rates of frontal cortical atrophy. This effect may also be explained by the alternative explanations posited in relation to our findings above, namely that chrDS may reflect a) co-occurring frontosubcortical pathology, b) progression of underlying AD-pathology or c) an atypical neurodegenerative process. Longitudinal studies have suggested that chrDS in MCI may be a risk factor for AD by reducing the brain reserve and making the brain susceptible to additional neuronal damage (36, 37), although one large population study could not confirm an increased risk of dementia in individuals with MCI and depressive symptomatology (38). Other studies support the hypothesis that chrDS and cognitive impairment may share underlying neurodegenerative pathology (17). However, our last main finding showed that chrDS predicted the rate of incident AD after adjusting for rates of atrophy in hippocampal and entorhinal cortices. Moreover, hippocampal and entorhinal atrophy rates were associated with incident AD in the adjusted survival analyses. This further suggests that frontal atrophy-associated chrDS in MCI may accelerate conversion to AD by an additional process to the neurodegeneration-associated hippocampal and entorhinal atrophy.
The strength of this study consists in a long follow-up of a clinically well-described, homogenous MCI sample. Some methodological issues remain, however, to be discussed. First, the clinical significance of a complex depressive symptomatology that does not fulfill criteria for minor or major depression, with respect to treatment and functional consequences remains open. Instead we have focused our analyses on evaluating longitudinal trajectories of brain atrophy in cognitively impaired patients who experience chronic depressive symptomatology frequently encountered in MCI. We used an inclusive definition of chrDS, as previously suggested by other investigators (22), to acknowledge the clinical observations of a large spectrum of depressive symptomatology in MCI (5, 6). Our participants classified as chrDS had higher scores in GDS at baseline than those without chrDS, which supports our classification. Low GDS scores at baseline in this MCI sample, considering that GDS scores >5 were used as exclusion criteria for major depression (21), may reflect insight in memory problems (6). Although an overlap of other neuropsychiatric symptoms cannot be excluded, by using only those participants with depressive symptoms that were endorsed across NPI-Q assessments we have ensured consistency of chrDS categories over three years of follow-up. The small sample size restricted the analyses of overlapping symptomatology. Moreover, the analysis of other putative biological markers of neurodegeneration, e.g, brain amyloid burden, was not possible due to insufficient data. Despite this drawback, the associations of chrDS and cortical atrophy rates were robust in all main and post-hoc analyses with FDR corrections. Another drawback of this small amnestic MCI sample was that it did not allow meaningful analyses of chrDS by subgroups with single vs multiple impairments. Secondly, the informant-based NPI-Q used to define chrDS was designed as a neuropsychiatric screening instrument for dementia. Although this may minimize the symptomatology in individuals with high cognitive functioning, we ensured similar levels of functionality between groups by focusing on a MCI sample. Moreover, informant reports are valid sources of information in psychiatric settings, especially in people with cognitive impairments when the accuracy of self-reported scales may be reduced (6). Thirdly, our sample had a high level of education. Therefore, the results may lack generalizability to elderly populations in the community. Also, due to evidence that chrDS in elderly may reflect the presence of cerebrovascular disease (39) we have also analyzed WMH in relation to chrDS, but did not find an association at baseline or longitudinally (results for the latter not shown). Further, participants with APOE ε4 were more likely to have missing data at follow-up, which may have biased the sample toward less susceptibility for AD. This could have reduced the effect size of the associations with AD. AD incidence rate of 43% over three years was though expected in a high risk MCI group (40).
In conclusion, we have shown that chrDS in MCI is related to the structural integrity of the frontal regions, most likely due to dysfunctional frontosubcortical circuitry as in LLD or due to an atypical frontal neurodegenerative pattern. Also, chrDS-associated frontal atrophy may hasten the cognitive decline to dementia. These findings that are stemming from an amnestic MCI single domain at baseline, suggest chrDS as an early marker of frontal cortical atrophy, which in turn may represent an additional risk factor for conversion to dementia in MCI, rather than a progressive stage of the AD neurodegenerative process. Further exploration of chrDS and its underlying biological mechanisms is therefore warranted.
Acknowledgments
Study funding: Supported by NIH (U01 AG024904), NIMH R01 0977669 and NIMH K08 MH081065.
Study funding: Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for NeuroImaging at the University of Southern California.
The current study was supported by postdoctoral fellowship to Simona Sacuiu from The Swedish Brain Foundation, The Swedish Society of Medicine SLS-323431 and The Göteborg Medical Society GLS-324681; and by grants NIMH R01 0977669 and NIMH K08 MH081065 to Scott Mackin.
Footnotes
No disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Simona Sacuiu, Center for Imaging of Neurodegenerative Diseases (CIND) at the Veterans Affairs Medical Center (VAMC) San Francisco and Department of Radiology and Biomedical Imaging (DRBI) at University of California San Francisco (UCSF), CA, USA and Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Sweden.
Philip S. Insel, CIND at VAMC San Francisco, CA, USA.
Susanne Mueller, CIND at VAMC San Francisco and DRBI at UCSF, CA, USA.
Duygu Tosun, CIND at VAMC San Francisco and DRBI at UCSF, CA, USA.
Niklas Mattsson, CIND at VAMC San Francisco and DRBI at UCSF, CA, USA and Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Sweden.
Clifford R. Jack, Jr, Department of Radiology, Mayo Clinic, Rochester, MN, USA.
Charles DeCarli, Department of Neurology and Center for Neuroscience, University of California at Davis, Sacramento, CA, USA.
Ronald Petersen, Mayo Alzheimer’s Disease Research Center and Mayo Clinic College of Medicine, Rochester, MN, USA.
Paul S. Aisen, Department of Neurosciences, University of California, San Diego, CA, USA.
Michael W. Weiner, CIND at VAMC San Francisco and DRBI at UCSF, CA, USA;.
R. Scott Mackin, CIND at VAMC and Department of Psychiatry at UCSF, CA, USA.
References
- 1.Amieva H, Letenneur L, Dartigues JF, et al. Annual rate and predictors of conversion to dementia in subjects presenting mild cognitive impairment criteria defined according to a population-based study. Dement Geriatr Cogn Disord. 2004;18:87–93. doi: 10.1159/000077815. [DOI] [PubMed] [Google Scholar]
- 2.Penna S. Cognitive and emotional dysfunction in mild cognitive impairment. Clin Geriatr Med. 2013;29:773–789. doi: 10.1016/j.cger.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Johnson LA, Hall JR, O’Bryant SE. A depressive endophenotype of mild cognitive impairment and Alzheimer’s disease. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0068848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richard E, Schmand B, Eikelenboom P, et al. Symptoms of apathy are associated with progression from mild cognitive impairment to Alzheimer’s disease in non-depressed subjects. Dement Geriatr Cogn Disord. 2012;33:204–209. doi: 10.1159/000338239. [DOI] [PubMed] [Google Scholar]
- 5.Geda YE, Roberts RO, Knopman DS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry. 2008;65:1193–1198. doi: 10.1001/archpsyc.65.10.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackin RS, Insel P, Aisen PS, Geda YE, Weiner MW. Longitudinal stability of subsyndromal symptoms of depression in individuals with mild cognitive impairment: relationship to conversion to dementia after 3 years. Int J Geriatr Psychiatry. 2011;27:355–363. doi: 10.1002/gps.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50:97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- 8.Lyness JM, Heo M, Datto CJ, et al. Outcomes of minor and subsyndromal depression among elderly patients in primary care settings. Ann Intern Med. 2006;144:496–504. doi: 10.7326/0003-4819-144-7-200604040-00008. [DOI] [PubMed] [Google Scholar]
- 9.Butters MA, Young JB, Lopez O, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10:345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steffens DC. Depressive symptoms and mild cognitive impairment in the elderly: an ominous combination. Biol Psychiatry. 2012;71:762–764. doi: 10.1016/j.biopsych.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bora E, Harrison BJ, Davey CG, Yucel M, Pantelis C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol Med. 2012;42:671–681. doi: 10.1017/S0033291711001668. [DOI] [PubMed] [Google Scholar]
- 12.Sawyer K, Corsentino E, Sachs-Ericsson N, Steffens DC. Depression, hippocampal volume changes, and cognitive decline in a clinical sample of older depressed outpatients and non-depressed controls. Aging Ment Health. 2012;16:753–762. doi: 10.1080/13607863.2012.678478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackin RS, Tosun D, Mueller SG, et al. Patterns of reduced cortical thickness in late-life depression and relationship to psychotherapeutic response. Am J Geriatr Psychiatry. 2013;21:794–802. doi: 10.1016/j.jagp.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen RC, Jack CR., Jr Imaging and biomarkers in early Alzheimer’s disease and mild cognitive impairment. Clin Pharmacol Ther. 2009;86:438–441. doi: 10.1038/clpt.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apostolova LG, Thompson PM. Mapping progressive brain structural changes in early Alzheimer’s disease and mild cognitive impairment. Neuropsychologia. 2008;46:1597–1612. doi: 10.1016/j.neuropsychologia.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahodne LB, Gongvatana A, Cohen RA, Ott BR, Tremont G. Are apathy and depression independently associated with longitudinal trajectories of cortical atrophy in mild cognitive impairment? Am J Geriatr Psychiatry. 2013;21:1098–1106. doi: 10.1016/j.jagp.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee GJ, Lu PH, Hua X, et al. Depressive symptoms in mild cognitive impairment predict greater atrophy in Alzheimer’s disease-related regions. Biol Psychiatry. 2012;71:814–821. doi: 10.1016/j.biopsych.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1:55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 20.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 21.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyness JM, Kim J, Tang W, et al. The clinical significance of subsyndromal depression in older primary care patients. Am J Geriatr Psychiatry. 2007;15:214–223. doi: 10.1097/01.JGP.0000235763.50230.83. [DOI] [PubMed] [Google Scholar]
- 23.Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry. 200:275–281. doi: 10.1192/bjp.bp.111.095950. [DOI] [PubMed] [Google Scholar]
- 24.Yesavage JA, Friedman L, Ancoli-Israel S, et al. Development of diagnostic criteria for defining sleep disturbance in Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2003;16:131–139. doi: 10.1177/0891988703255684. [DOI] [PubMed] [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 28.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salat DH, Greve DN, Pacheco JL, et al. Regional white matter volume differences in nondemented aging and Alzheimer’s disease. Neuroimage. 2009;44:1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexopoulos GS. Frontostriatal and limbic dysfunction in late-life depression. Am J Geriatr Psychiatry. 2002;10:687–695. [PubMed] [Google Scholar]
- 31.Lavretsky H, Siddarth P, Kepe V, et al. Depression and anxiety symptoms are associated with cerebral FDDNP-PET binding in middle-aged and older nondemented adults. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2009;17:493–502. doi: 10.1097/jgp.0b013e3181953b82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dagyte G, Den Boer JA, Trentani A. The cholinergic system and depression. Behavioural brain research. 2011;221:574–582. doi: 10.1016/j.bbr.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 33.Duker AP, Espay AJ, Wszolek ZK, Rademakers R, Dickson DW, Kelley BJ. Atypical motor and behavioral presentations of Alzheimer disease: a case-based approach. Neurologist. 2012;18:266–272. doi: 10.1097/NRL.0b013e3182675376. [DOI] [PubMed] [Google Scholar]
- 34.Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau) J Mol Neurosci. 2011;45:384–389. doi: 10.1007/s12031-011-9589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steffens DC, Payne ME, Greenberg DL, et al. Hippocampal volume and incident dementia in geriatric depression. Am J Geriatr Psychiatry. 2002;10:62–71. [PubMed] [Google Scholar]
- 36.Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75:27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, Au R. Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology. 2010;75:35–41. doi: 10.1212/WNL.0b013e3181e62138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panza F, Capurso C, D’Introno A, et al. Impact of depressive symptoms on the rate of progression to dementia in patients affected by mild cognitive impairment. The Italian Longitudinal Study on Aging. Int J Geriatr Psychiatry. 2008;23:726–734. doi: 10.1002/gps.1967. [DOI] [PubMed] [Google Scholar]
- 39.Lee JY, Insel P, Mackin RS, et al. Different associations of white matter lesions with depression and cognition. BMC Neurol. 2013;12:83. doi: 10.1186/1471-2377-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vos SJ, van Rossum IA, Verhey F, et al. Prediction of Alzheimer disease in subjects with amnestic and nonamnestic MCI. Neurology. 2013;80:1124–1132. doi: 10.1212/WNL.0b013e318288690c. [DOI] [PubMed] [Google Scholar]