Abstract
Purpose
Palmar-plantar erythrodysethesia (PPE) is a common chemotherapy and anti-VEGF multi-kinase inhibitor class-related toxicity that often results in debilitating skin changes and often limits the use of active anti-cancer regimens. Mechanistic and anecdotal clinical evidence suggested that topical application of sildenafil cream may help reduce the severity of PPE. Therefore, we conducted a randomized, double-blind, placebo-controlled pilot study to evaluate the feasibility, safety and efficacy of topical sildenafil cream for the treatment of PPE.
Methods
Eligible subjects were required to have grade 1–3 PPE associated with either capecitabine or sunitinib. Subjects were randomized to receive 1% topical sildenafil cream to the left extremities or right extremities and placebo cream on the opposite extremity. 0.5 mL of cream was applied to each affected hand/foot two times per day. The primary endpoint was improvement in PPE grading at any point on study. Clinical assessments were evaluated by NCI-CTC 4.0 grading and patient self-reported pain.
Results
Ten subjects were enrolled; 9 were evaluable for safety and efficacy. Five of nine subjects reported some improvement in foot pain and 3 of 8 subjects for hand pain improvement. One of these subjects noted specific improvement in tactile function. No treatment-related toxicities were observed.
Conclusions
In this limited, single center study, topical cream containing 1% sildenafil is feasible to administer, is well-tolerated, and may mitigate PPE-related symptoms due to anti-cancer therapeutic agents. Further validation is necessary.
Keywords: Sildenafil, Palmar Plantar Erythrodysesthesia, Capecitabine, Sunitinib
Introduction
Palmar-plantar erythrodysethesia (PPE), also known as hand foot syndrome (HFS), is a common adverse event of many anticancer agents, including intravenous (IV) 5FU, capecitabine (Oral 5-FU, Xeloda™), ara-C (Cytosar-U®), liposomal doxorubicin (Doxil™), and the multi-kinase inhibitors (MKIs) sorafenib (Nexavar™), sunitinib (Sutent™). Although PPE is rarely life-threatening, the skin changes are often painful and debilitating and can impair activities of daily function. PPE is among the most common reasons for dose reduction and/or discontinuation of these agents. For example, capecitabine is associated with any grade PPE in over 50% of patients and approximately 10–15% of patients have grade 3 (severe) PPE [1–3]. Sunitinib is associated with any grade PPE in approximately 14–21% of patients, and grade 3 PPE is seen in approximately 4–5.5% of patients [4, 5]. Regorafenib (Stivarga®) is associated with any grade PPE in approximately 50% of patients, and grade 3 PPE is seen in approximately 17% of patients [6, 7]. Cabozantinib (Cometriq™) is associated with any grade PPE in approximately 50% of patients and grade 3 PPE is seen in approximately 13 % of patients [8].
The mechanisms underlying PPE remain poorly understood, due to the lack of preclinical models and the difficulty of obtaining biopsies of inflamed tissues in patients. Limited dermato-pathologic studies have reported inflammatory and vascular changes consistent with a wound healing response [3, 9–11]. The common association of PPE with sunitinib, regorafenib, sorafenib, and cabozantinib, as well as pazopanib and axitinib, is particularly interesting. These agents inhibit multiple VEGF and PDGF receptors which are critical for endothelial cell proliferation and survival and are markedly up-regulated in wound healing [12, 13]. Thus, it is hypothesized that inhibition of these targets may impair wound healing in dermal capillary endothelium [14]. Likewise, vascular targeting may also be involved in the mechanisms of PPE related to infusional 5-fluoruracil, capecitabine and liposomal doxorubicin, three other chemotherapy drugs commonly associated with PPE. 5-FU is known to cause vasospasm and has potent anti-angiogenic properties [15]. Capecitabine, an oral pro-drug of 5-FU, is activated by the enzyme thymidine phosphorylase, also known as platelet derived endothelial cell growth factor, another potent angiogenic factor up-regulated in tumor and wound tissues [2, 9, 16]. Liposomal formulations of doxorubicin preferentially exit the vasculature in areas of increased vascular permeability, such as in tumors and inflamed tissues [17]. Thus, all agents commonly associated with PPE have known vascular and endothelial cell toxicities.
Many endothelial cell functions, including, vasodilation, proliferation, and survival are mediated by nitric oxide (NO) signaling which is critical for many wound healing responses [18, 19]. NO works primarily via up-regulation of cyclic guanosine monophosphate (cGMP), which in turn is regulated by cGMP-specific phosphodiesterases (PDEs), such as PDE5. In vascular beds that express PDE5, inhibition of PDE5 prevents the degradation of cGMP, thereby augmenting and prolonging the effect of NO [20]. Intriguingly, nitric oxide is down-regulated by anti-VEGF therapy [12, 13]. Sildenafil is a highly selective PDE5 inhibitor and is approved by the Food and Drug Administration (FDA) for the treatment of erectile dysfunction and for the treatment of pulmonary hypertension [21]. Based upon its effects on endothelial cell nitric oxide, sildenafil has been shown to improve wound healing in a variety of preclinical wound models [22–26]. Topical sildenafil has also been reported to improve clinical wound healing in the setting of vascular compromise, such as digital ulceration related to Raynaud’s syndrome [27–30], and complicated anal fissures [31, 32].
Attempts to prevent and/or treat PPE related to chemotherapeutic agents have included various emollients, Cox2 inhibitors, pyridoxine, DPD (dihydropyrimidine dehydrogenase) inhibitors, and corticosteroids, among other approaches. However, few of these have been validated with randomized controlled trials [33–35]. As a result, current standard care for PPE includes use of emollients and modifying daily activities to reduce friction and heat exposure in addition to dose interruption and/or reduction of the anti-cancer agent [36–38].
At our institution, a patient with capecitabine associated Raynaud’s and concurrent PPE was treated with topical sildenafil, with an improvement of several dermal ulcers associated with her Raynaud’s. The patient’s PPE near these ulcers also improved with topical sildenafil. When topical sildenafil was more broadly applied to her PPE, these affected areas also improved. Two additional patients with refractory PPE were subsequently treated with topical sildenafil, one with PPE related to capecitabine and one with PPE related to sunitinib. In all three patients, treatment was initiated on only one hand or foot at a time and their PPE-related symptoms markedly improved as a result of topical sildenafil treatment. To further evaluate these anecdotal findings, we conducted a pilot, randomized, double-blind, placebo-controlled study to evaluate the safety, efficacy and feasibility of topical sildenafil cream for the treatment of PPE.
Patients and Methods
Patient Selection
Eligible patients were required to have grade 1–3 PPE related to capecitabine or sunitinib, where these agents were administered as part of the patients’ standard anti-cancer therapy. Additional eligibility requirements included: age ≥18 years, Karnofsky performance status (KPS) performance status ≥70%. Adequate organ and marrow function was defined as: absolute neutrophil count (ANC) ≥1,000/µl; platelets ≥75,000/µl; total bilirubin ≤ 1.5 times the upper limit of normal (ULN); aspartate aminotransferase/alanine aminotransferase (AST/ALT) ≤5 times ULN; creatinine clearance ≥40 mL/min/1.73 m2. Additional eligibility parameters included: absence of pregnancy; absence of hypersensitivity or intolerance to sildenafil or other related products; no current use of sildenafil or other related products; no resting hypotension (blood pressure (BP) <90/50 mmHg) or hypertension (BP >170/110 mmHg); no cardiac failure or coronary artery disease causing unstable angina; no history of myocardial infarction, stroke or life-threatening arrhythmia; no known retinitis pigmentosa; no concomitant use of organic nitrates, strong cytochrome P450 3CYPA4 inducers or inhibitors or other treatments for PPE other than standard emollients. Other acute or chronic inflammatory conditions or infections of the hands or feet that would complicate safety, application of topical creams, or study endpoints were prohibited. Any other serious medical conditions that might have significantly affected patient safety or toxicity assessment were also prohibited.
Study Design
The primary endpoint was to assess the number of subjects who achieved at least one grade improvement in PPE according to National Cancer Institute Common Toxicity Criteria Adverse Events (NCI CTCATE) version 4.0 at any time during protocol treatment. Subject cream assignment was un-blinded at the 8 or 9 week time period or when subjects came off study however subjects with a 2 grade improvement were eligible for early unblinding. Each subject served as her/his own control and were eligible to receive topical sildenafil for up to a maximum of 6 months.
The doses and schedules of sunitinib and capecitabine were left to the discretion of the treating physician.
Figure 1 illustrates the study schema. Patient extremities (right hand/foot vs. left hand/foot) were randomized to treatment with topical sildenafil or topical placebo cream.
Figure 1.
Subjects were instructed to apply active 1% sildenafil cream to either the right or left extremity (hand/foot) and placebo cream to the opposite extremity. New allotments of topical sildenafil and placebo creams were dispensed at every visit. Subjects were instructed to apply 0.5 mL of cream to each affected extremity two times per day. Non-latex waterproof gloves were supplied to help rub the cream into the hand or foot being treated and minimize contamination of creams; separate gloves were used for every application (each hand or foot, each time point)
This was a single-center study (NCT01219023) approved by the Duke Institutional Review Board (IRB) and followed the guidelines of the Helsinki Declaration. All patients provided informed written consent prior to any study-related procedure and were treated at Duke University Medical Center. Subject accrual took place from August 2010 to February 2011.
Patient Evaluation
All patients completed a routine medical history, baseline physical examination and clinical assessment prior to receiving study cream. Safety and efficacy assessments were performed at each standard of care visit and as clinically indicated. These assessments included vital signs, routine complete blood count (CBC) and a biochemistry profile. General symptom management and supportive care were provided as clinically indicated to ensure optimal patient compliance.
Subjects were given diary cards and asked to record study cream application, visual skin changes and daily hand and foot pain scores using a scale of 1–10, 0 (none) to 10 (severe), at rest and stressed (i.e. clenching fists for hands and standing for feet, each for at least 5 seconds).
Use of standard emollients was encouraged as part of standard care for PPE. Patients were instructed to apply the emollient at least approximately 2 hours after administration of topical sildenafil to allow for as much sildenafil absorption as possible prior to emollient treatment. Patients were instructed to gently wash affected extremities with soap and water or commercially available skin wipes prior to each application of topical study cream. NCI CTCAE version 4.0 was used to grade adverse events [39].
Statistical Analysis
PPE grade and any treatment-related adverse events were assessed at each visit.
Time of day and use of affected limbs is known to affect pain from PPE; in addition, some patients experience different levels of pain in their affected extremities. To preliminarily evaluate patterns (levels) of pain reporting, mean placebo pain scores for all the patient extremities were plotted by day comparing three different parameters: 1) amount of pressure, stressed versus rested, 2) type of extremity, hands versus feet and 3) time of day, AM versus PM. Placebo pain scores were used as a baseline measurement to detect possible differences in pain reporting under the different parameters. Each parameter was individually assessed while controlling for the other two.
Treatment efficacy was assessed by using the differences in pain scores between placebo and active study cream, for each patient, averaged over the first 40 days on study and plotted by extremity (hands and feet) while stressed and while rested (AM). The 40 day landmark time point was selected to capture maximum efficacy on treatment and to minimize confounding related to changes in chemotherapy related to toxicity or disease progression. Difference in pain score was defined as placebo minus active score; thus, efficacy of the treatment was reflected by a positive difference. In all cases, mean differences greater than zero indicate that the study drug cream was effective; mean differences less than or equal to zero indicate that the study drug cream was ineffective. The Wilcoxon Signed Rank test was used to test the hypothesis of no improvement in pain score differences.
Study Cream Formulation
Topical sildenafil cream was compounded by the Duke compounding facility at Duke University Hospital using commercially available 100 milligram (mg) sildenafil citrate tablets (Viagra®, Pfizer) finely ground via a Strand grinder (high-speed grinder) and added to cold cream unscented (Medisca) using an unguator, a high speed mixing device set for the cream setting. To make 1% sildenafil citrate cream, enough tablet debris was used to make 10 mg of sildenafil citrate per gram of cold cream. The placebo was made by adding Blue Dye number 2 to 70 grams of lactose powder to match the appearance of the active cream. Twelve grams of this mixture was added to 88 grams of Cold Cream using an Unguator. The active study drug cream and color-matched placebo was packaged into 20 mL Unguator jars with AcuCap adapters (Medisca) or Topi-Click® metered dispensing units to ensure proper dispensing of study cream.
Results
Patient demographics are summarized in Table 1. A total of 10 patients were enrolled; 9 were evaluable for safety and efficacy. One patient was unable to apply any study cream due to intercurrent illness and was considered inevaluable. The median age was 58.5 years (range 43–74). Two subjects received capecitabine monotherapy, four subjects received capecitabine plus bevacizumab, and three patients received sunitinib. At baseline, nine subjects had grade 2 PPE and one subject had grade 1 PPE. Median duration of blinded study drug treatment was 77 days (range 36–88). All nine treated patients reported pain scores on a scale from 0 (none) to 10 (severe) from day 1 of protocol therapy through last day of study cream application.
Table 1.
Patient Characteristics
| Characteristics | No. of patients |
|---|---|
| Total Patients | 10 |
| Gender | |
| Male | 3 |
| Female | 7 |
| Age (Years) | |
| Median | 58.5 |
| Range | 43–74 |
| Karnofsky performance status, (%) | |
| Median | 90 |
| Range | 80–100 |
| Baseline PPE Grade | |
| 1 | 1 |
| 2 | 9 |
| 3 | 0 |
| Current Cancer Therapy | |
| Capecitabine | 2 |
| Capecitabine plus bevacizumab | 4 |
| Sunitinib | 4 |
| Primary Tumor Type | |
| Colorectal | 4 |
| Renal | 2 |
| Breast | 1 |
| Pancreatic | 1 |
| GIST | 1 |
| Angiosarcoma | 1 |
Patterns of pain reporting
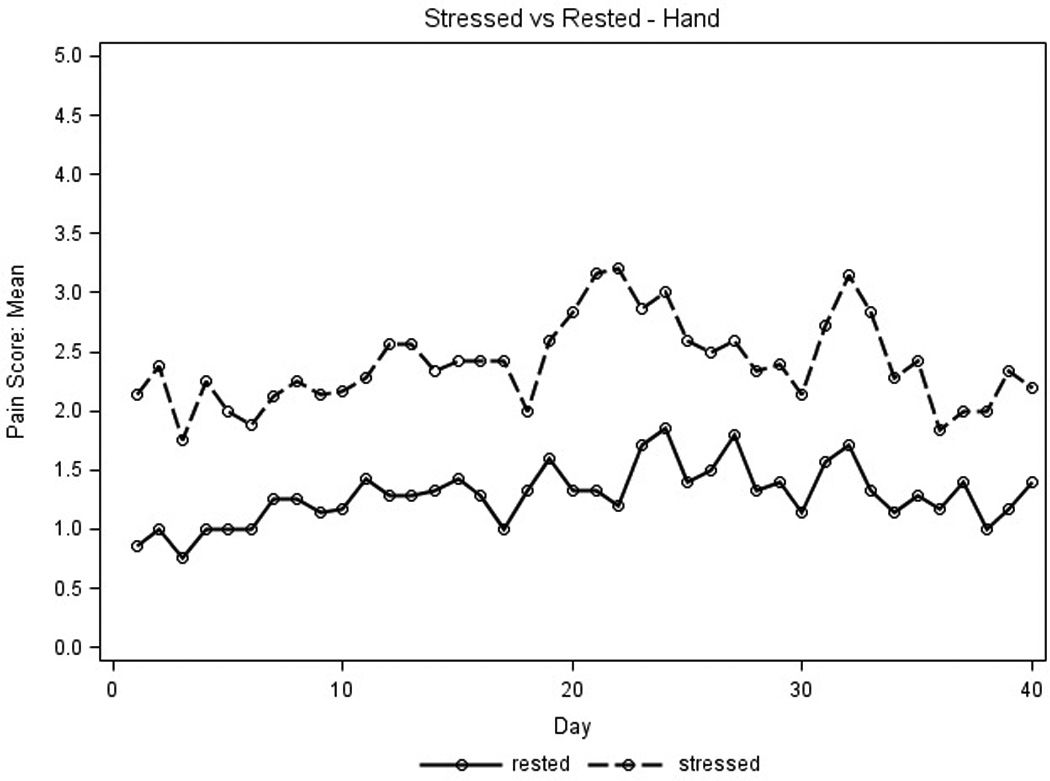
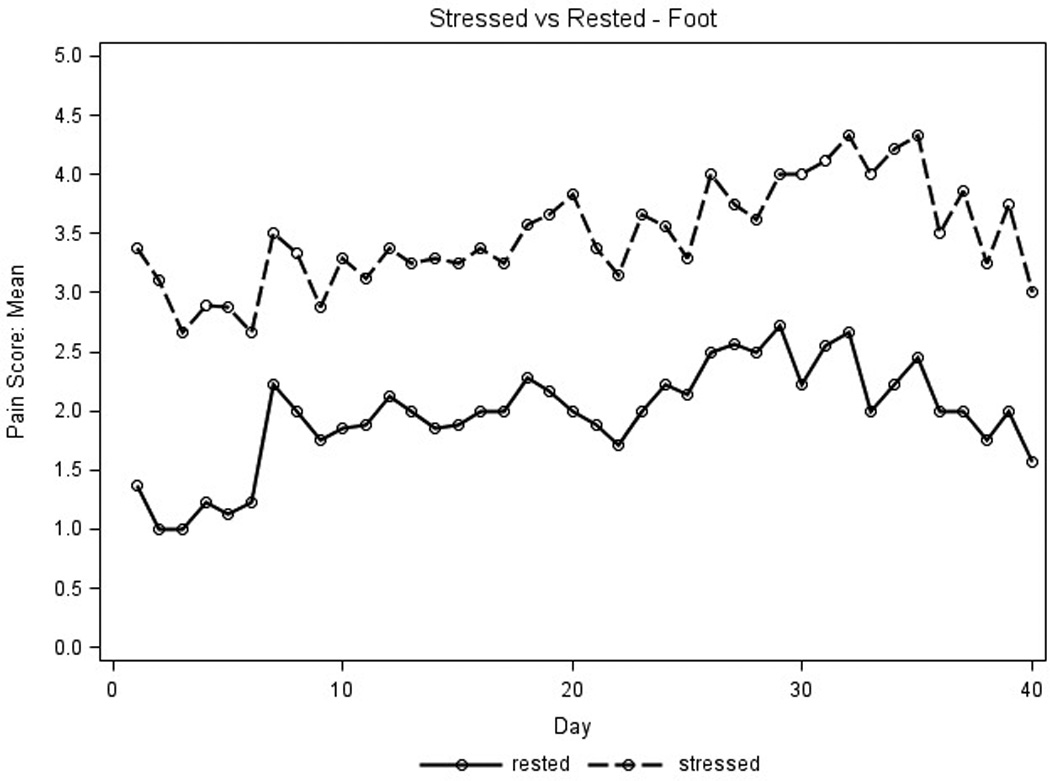
Placebo pain scores were used to detect any differences in parameters that influenced pain reporting and identify which parameters would maximize the ability to evaluate the active study cream. Average placebo pain scores for the first 40 days for stressed extremities were higher than those for extremities at rest (Figure 2A) and average pain scores for feet were higher than those for hands (Figure 2B). No differences were seen for average pain scores measured in the AM and PM (data not shown). Thus, the placebo pain scores were highest on stressed extremities regardless of time of day and were subsequently used to assess treatment efficacy.
Figure 2. Patterns of Pain Reporting.
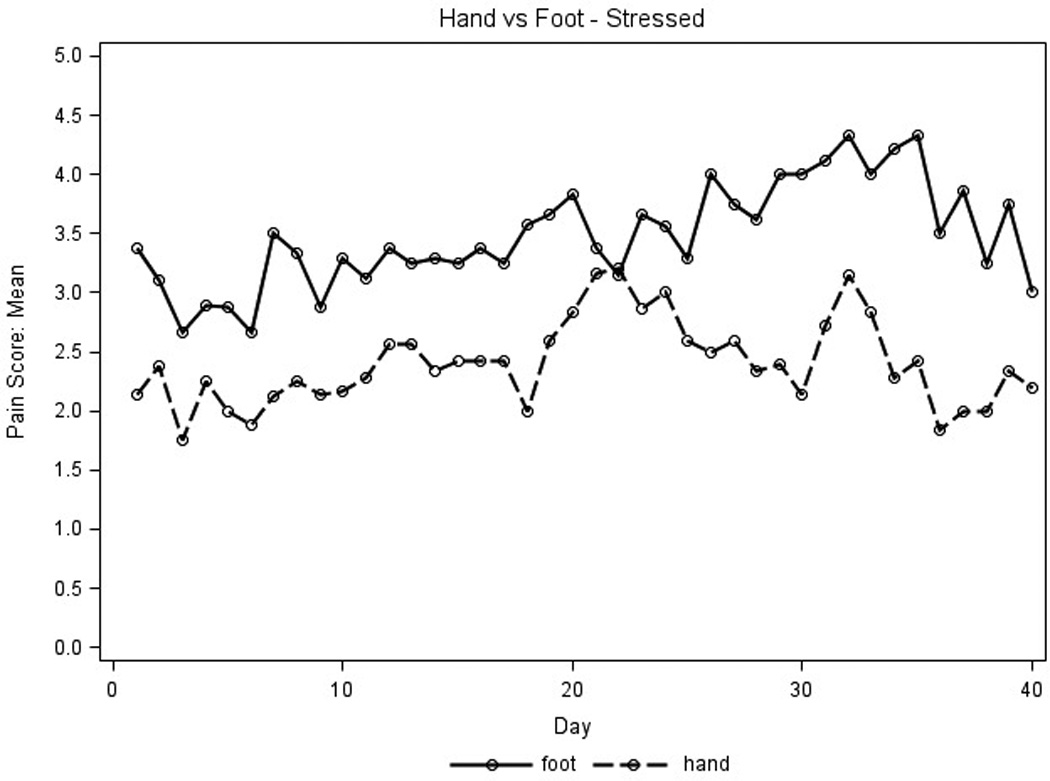
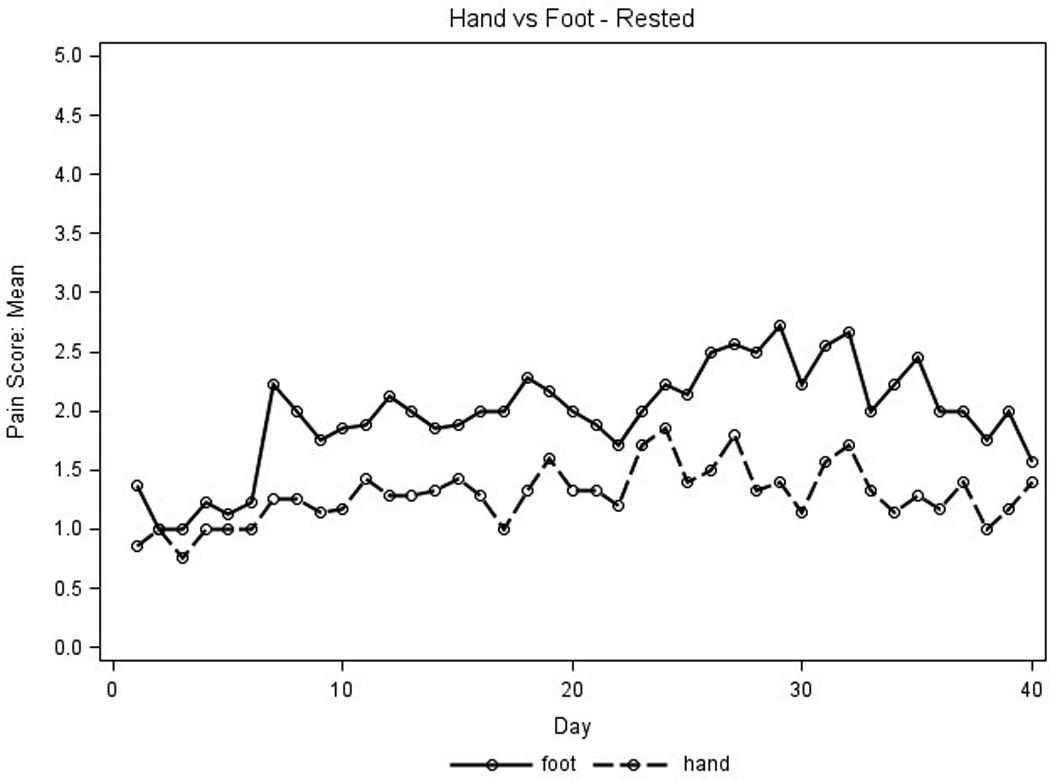
Placebo pain scores were graphed to assess parameters that influenced pain reporting. X axis represents days on treatment; Y axis represents mean placebo pain scores for all patient extremities (n=9 for hands, n=8 for feet). Pain scores used a scale of 1–10, 0 (none) to 10 (severe). A.) For the amount of pressure, the solid line represents pain scores at rest, the dotted line represents pain scores when stressed. B.) For type of extremity, the solid line represents feet pain scores; the dotted line represents hand pain scores.
Efficacy and Safety
There were no significant changes in the NCI CTC grade of PPE with active treatment. Mean differences in pain scores for stressed extremities in the AM per subject are illustrated in Figure 3; results in the PM were comparable (data not shown). Three of eight patients (0.37) reported some pain relief on active drug applied to hands, both stressed and rested (exact 95% binomial confidence interval, 0.09, 0.76). Similarly, five of nine patients (0.55) reported some pain relief of stressed feet (exact 95% binomial confidence interval 0.21 to 0.86). Differences in mean pain scores were not significant. Figure 4 illustrates daily pain scores for one individual with pain improvement on treatment for feet. Another patient reported an unexpected grade 2 improvement in PPE pain between the placebo and active study cream.
Figure 3. Evaluation of Study Cream Efficacy.
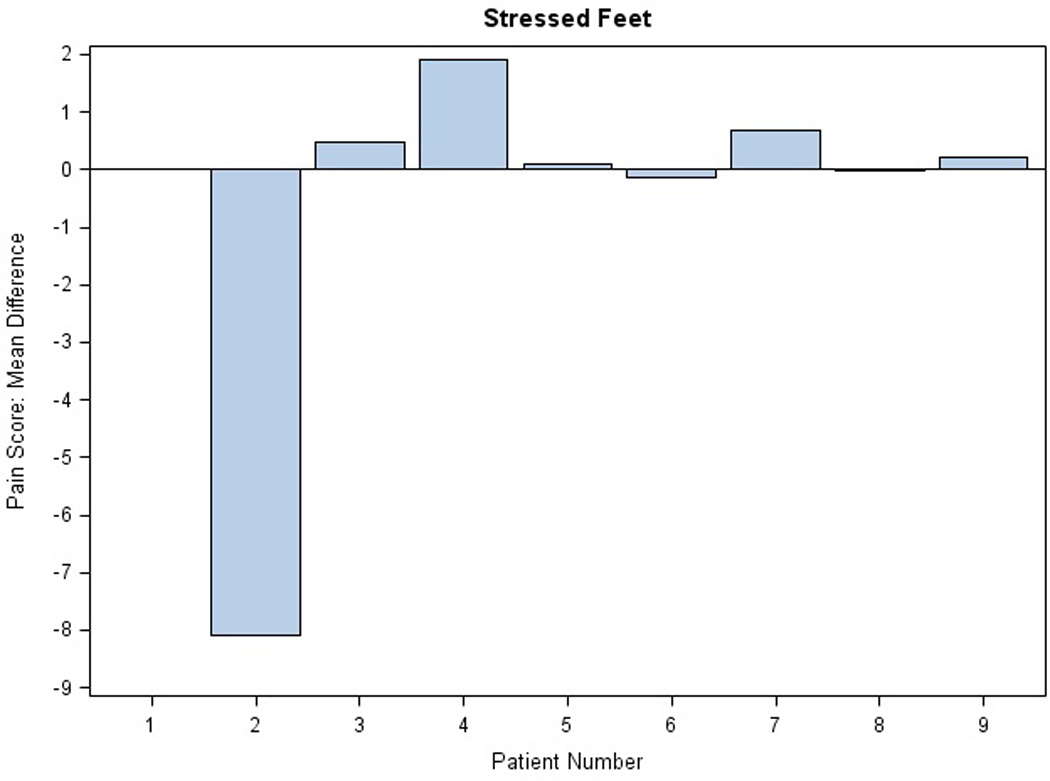
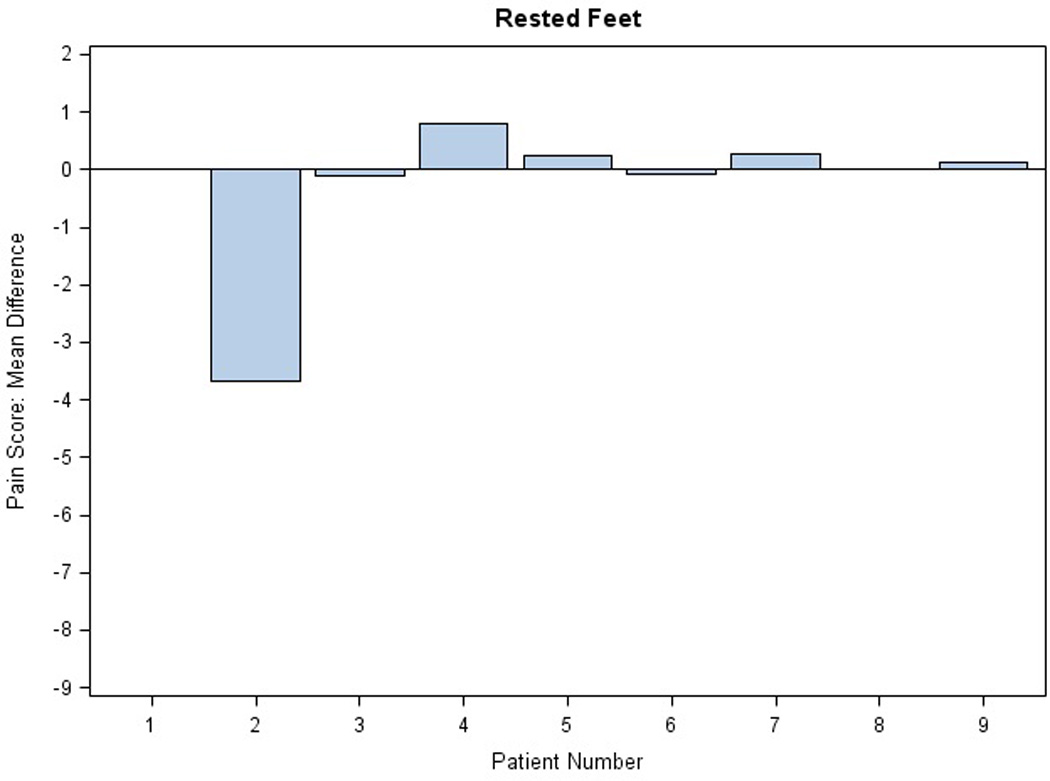
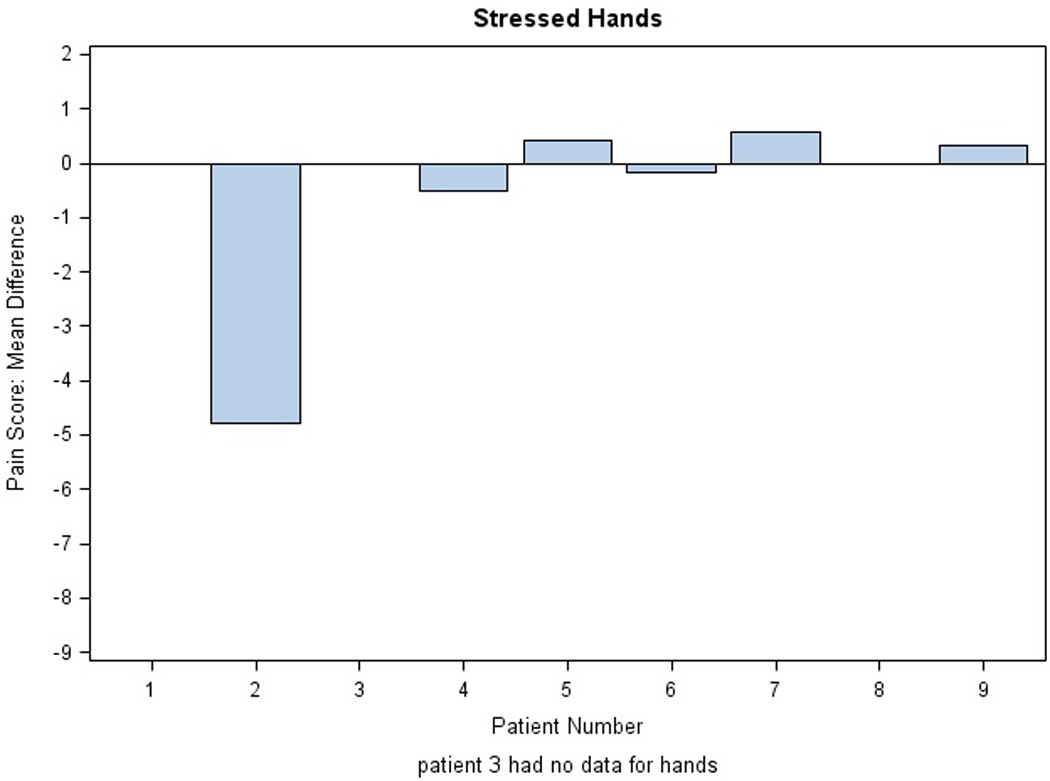
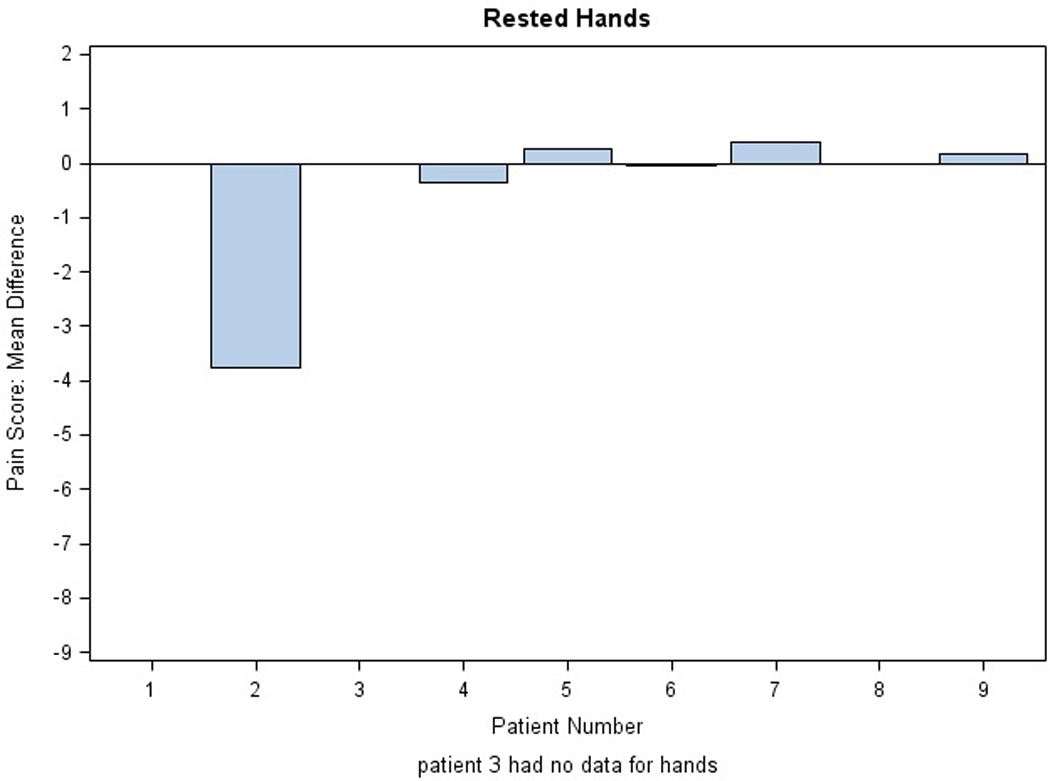
Mean differences in pain scores are shown for each extremity in the AM: A) stressed and rested feet and B) stressed and rested hands. Each bar represents one individual. The average of the difference was calculated across the first 40 days on protocol. Difference in pain score was defined as placebo minus active score; thus, efficacy of the treatment is reflected by a positive difference.
Figure 4. Pain Improvement for Patient 7.
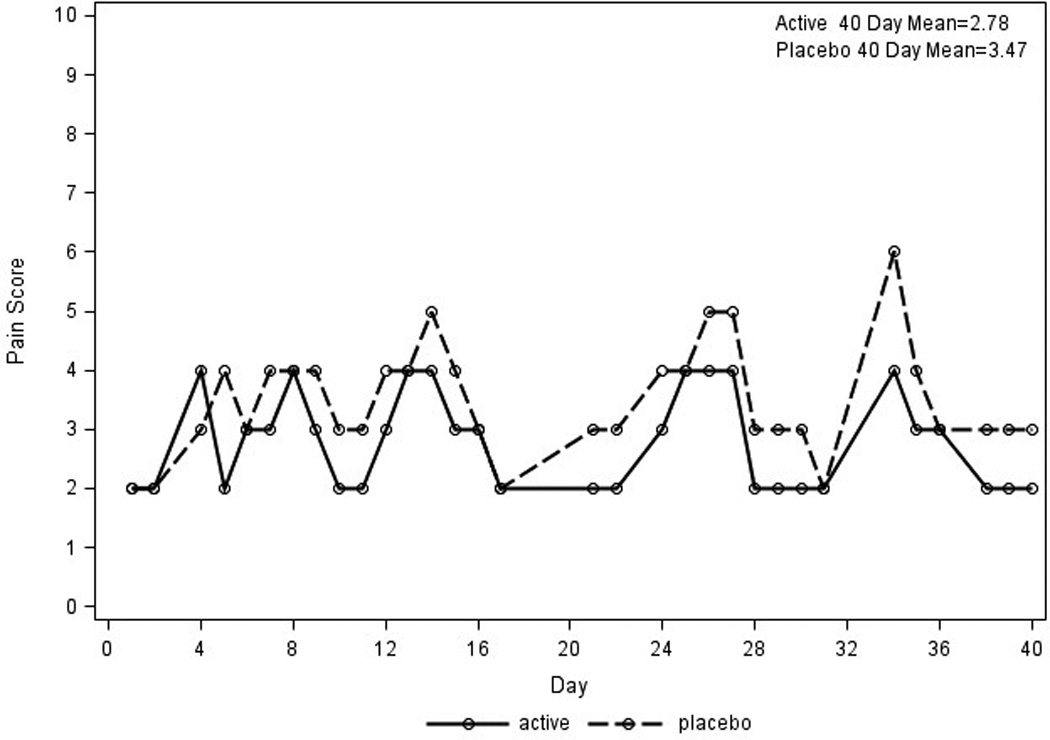
Daily pain scores are plotted for patient 7 with pain improvement on treatment for stressed feet. X axis represents days; Y axis represents daily pain scores The solid line represents pain scores on the foot with study cream; the dotted line represents the pain score for the foot receiving placebo cream.
Reported improvements in skin-related symptoms included mild reductions in erythema, dryness and cracking. Additionally, one subject specifically reported an improvement in tactile function.
No treatment-related toxicities were observed.
Discussion
PPE is a common chemotherapeutic-related toxicity often associated with debilitating skin changes resulting in dose adjustment and/or interruption which may potentially limit active anti-cancer regimens. PPE has also been noted for the majority of orally available VEGF inhibitors. In addition the rates of PPE associated with chemotherapy appear to increase with the addition of bevacizumab [40–43].
Our study was prompted by three marked responses in patients treated with topical sildenafil for PPE before this study. One of these responses, in a patient with recurrent PPE related to sunitinib, was dramatic, with essentially normalization of grade 3 PPE within two weeks. The treatment utilized for these patients was sildenafil [citrate or other salt] active pharmaceutical ingredient mixed in A-Mantle cream. Due to regulatory considerations, the current study used sildenafil derived from sildenafil citrate (Viagra®) tablets and used cold cream as the vehicle. The current study was designed to be pilot in nature, in large part due to resource constraints, particularly around the cost of drug formulation.
Our study evaluated patients with pre-existing grade PPE related to either capecitabine or sunitinib. Our study also took advantage of the usually highly symmetrical involvement of PPE and was double-blinded, which allowed each patient to serve as his or her own control. While no improvement in PPE grading was reported for the active study cream, a trend was noted for relative improvement in pain. Interestingly, improvements in skin texture were seen in 2 patients and one patient noted significant improvement in tactile functioning. The NCI grading system may under-estimate modest to moderate differences in pain as experienced by the patient. The 2 grade improvement in PPE symptoms associated with placebo treatment in one patient was unexpected and no reversal of treatment application was identified.
The treatment effect seen with the current formulation of topical sildenafil appears less robust than that seen in our earlier anecdotal findings. The current findings may better reflect the effect of topical sildenafil on PPE. However, these differences may also reflect the effect of differing formulations. Our initial anecdotal experience used sildenafil [salt] active pharmaceutical ingredient in A-Mantle cream. Amantyl cream is commonly used to increase drug penetration into the skin. The current clinical trial used ground Viagra® tablets and commercial cold cream. It is possible that the excipients and fillers in the pills and the barrier properties of cold cream may have affected drug absorption.
Our study was not designed to assess whether PPE related to different agents would respond better to topical sildenafil. The focus on PPE related to capecitabine and sunitinib was pragmatic based upon local treatment usage of agents causing PPE. Interestingly, modest improvements in PPE related pain were noted in patients taking capecitabine or sunitinib. The effect of topical sildenafil in PPE related to other agents is not known. Similarly, the effect of prophylactic treatment with topical sildenafil is not known. However, it is possible that earlier and more pro-active use of topical sildenafil may reduce the severity of PPE that emerges on treatment more readily than it can reverse moderate to severe PPE once it is established. Lastly, the use of oral sildenafil for the treatment of PPE is not known. However, we are not aware of case reports of this activity and oral sildenafil at its standard dose of 50mg per day had no effect on the PPE of our anecdotal patient who had a dramatic response to topical sildenafil. The ability of topical formulations to deliver higher and more sustained levels of local drug concentrations may help explain this finding.
In summary, topical sildenafil cream may improve PPE toxicity related to several common anti-cancer agents. Future studies with sildenafil in combination with alternative topical formulations are warranted.
Acknowledgments
We gratefully acknowledge the invaluable contributions of the patients and their families. We specifically acknowledge the contribution of Neal Kaplan and Drs. Bruce Burnett and Erin O’Reilly of Duke Translational Medicine Institute regulatory who provided guidance and support with the preparation of the IND application and are funded in part by Duke University's CTSA Grant 1 UL1 RR024128 from NCRR/NIH. This research was supported by National Institute of Health Grant 5K24-CA113755-05 (H Hurwitz).
Footnotes
Conflict of Interest
The authors have no financial conflicts of interest to disclose and have full control of all primary data. The authors agree to allow the journal to review the data if requested.
References
- 1.Hoffman LaRoche Laboratories Inc. Capecitabine Prescribing Information. 2010. [Google Scholar]
- 2.Walko CM, Lindley C. Capecitabine: a review. Clin Ther. 2005;27:23–44. doi: 10.1016/j.clinthera.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Milano G, Etienne-Grimaldi MC, Mari M, Lassalle S, Formento JL, Francoual M, Lacour JP, Hofman P. Candidate mechanisms for capecitabine-related hand-foot syndrome. Br J Clin Pharmacol. 2008;66:88–95. doi: 10.1111/j.1365-2125.2008.03159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfizer Labs. Sunitinib malate Prescribing Information. 2010. [Google Scholar]
- 5.Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, Garbe C, Hauschild A, Puzanov I, Alexandrescu DT, Anderson RT, Wood L, Dutcher JP. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–1011. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 6.Bayer Healthcare Pharmaceuticals Inc. Stivarga Prescribing Information. 2013. [Google Scholar]
- 7.Grothey A, Sobrero AF, Siena S, Falcone A, Ychou M, Lenz H, Yoshino T, Cihon F, Wagner A, Van Cutsem E Team; obotCS. Results of a phase III randomized, double-blind, placebo-controlled, multicenter trial (CORRECT) of regorafenib plus best supportive care (BSC) versus placebo plus BSC in patients (pts) with metastatic colorectal cancer (mCRC) who have progressed after standard therapies. J Clin Oncol. 2012;30(suppl 4) abstr LBA385. [Google Scholar]
- 8.Exelixis I. Cometriq Prescribing Information. 2012. [Google Scholar]
- 9.Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. European Journal of Cancer. 1998;34:1274–1281. doi: 10.1016/s0959-8049(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 10.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 11.Wood J, Scott E, Thomas AL. Novel VEGF signalling inhibitors: how helpful are biomarkers in their early development? Expert Opin Investig Drugs. 2009;18:1701–1714. doi: 10.1517/14728220903336466. [DOI] [PubMed] [Google Scholar]
- 12.Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol. 1998;274:H1054–H1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- 13.Tsurumi Y, Murohara T, Krasinski K, Chen D, Witzenbichler B, Kearney M, Couffinhal T, Isner JM. Reciprocal relation between VEGF and NO in the regulation of endothelial integrity. Nat Med. 1997;3:879–886. doi: 10.1038/nm0897-879. [DOI] [PubMed] [Google Scholar]
- 14.Chu D, Lacouture ME, Fillos T, Wu S. Risk of hand-foot skin reaction with sorafenib: a systematic review and meta-analysis. Acta Oncol. 2008;47:176–186. doi: 10.1080/02841860701765675. [DOI] [PubMed] [Google Scholar]
- 15.Daher I, Yeh ET. Vascular complications of selected cancer therapies. Nature Reviews Cardiology. 2008;5:797–805. doi: 10.1038/ncpcardio1375. [DOI] [PubMed] [Google Scholar]
- 16.Nash GF, Walsh DC, Kakkar AK. The role of the coagulation system in tumour angiogenesis. Lancet Oncol. 2001;2:608–613. doi: 10.1016/s1470-2045(01)00518-6. [DOI] [PubMed] [Google Scholar]
- 17.Lorusso D, Di Stefano A, Carone V, Fagotti A, Pisconti S, Scambia G. Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia ('hand-foot' syndrome) Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2007;18:1159–1164. doi: 10.1093/annonc/mdl477. [DOI] [PubMed] [Google Scholar]
- 18.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 20.Kass DA, Takimoto E, Nagayama T, Champion HC. Phosphodiesterase regulation of nitric oxide signaling. Cardiovasc Res. 2007;75:303–314. doi: 10.1016/j.cardiores.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Pfizer Labs. Sildenafil Prescribing Information. 2009. [Google Scholar]
- 22.Ayten R, Cetinkaya Z, Girgin M, Ozercan I, Ustundag B, Aygen E. The effects of intraperitoneal sildenafil administration on healing of left colonic anastomoses and intra-abdominal adhesion formation in the presence of intra-abdominal infection. Dis Colon Rectum. 2008;51:1837–1841. doi: 10.1007/s10350-008-9398-x. [DOI] [PubMed] [Google Scholar]
- 23.Luo JD, Chen AF. Nitric oxide: a newly discovered function on wound healing. Acta Pharmacol Sin. 2005;26:259–264. doi: 10.1111/j.1745-7254.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 24.Salcido RS. Viagra and wound healing: the NO connection. Adv Skin Wound Care. 2008;21:106, 108–109. doi: 10.1097/01.ASW.0000305426.55671.42. [DOI] [PubMed] [Google Scholar]
- 25.Sarifakioglu N, Gokrem S, Ates L, Akbuga UB, Aslan G. The influence of sildenafil on random skin flap survival in rats: an experimental study. Br J Plast Surg. 2004;57:769–772. doi: 10.1016/j.bjps.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Shabani M, Pulfer SK, Bulgrin JP, Smith DJ. Enhancement of wound repair with a topically applied nitric oxide-releasing polymer. Wound Repair Regen. 1996;4:353–362. doi: 10.1046/j.1524-475X.1996.40312.x. [DOI] [PubMed] [Google Scholar]
- 27.Colglazier CL, Sutej PG, O'Rourke KS. Severe refractory fingertip ulcerations in a patient with scleroderma: successful treatment with sildenafil. J Rheumatol. 2005;32:2440–2442. [PubMed] [Google Scholar]
- 28.Fries R, Shariat K, von Wilmowsky H, Bohm M. Sildenafil in the treatment of Raynaud's phenomenon resistant to vasodilatory therapy. Circulation. 2005;112:2980–2985. doi: 10.1161/CIRCULATIONAHA.104.523324. [DOI] [PubMed] [Google Scholar]
- 29.Gore J, Silver R. Oral sildenafil for the treatment of Raynaud's phenomenon and digital ulcers secondary to systemic sclerosis. Ann Rheum Dis. 2005;64:1387. doi: 10.1136/ard.2004.034488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamata Y, Kamimura T, Iwamoto M, Minota S. Comparable effects of sildenafil citrate and alprostadil on severe Raynaud's phenomenon in a patient with systemic sclerosis. Clin Exp Dermatol. 2005;30:451. doi: 10.1111/j.1365-2230.2005.01797.x. [DOI] [PubMed] [Google Scholar]
- 31.Moghimi M, Ghosdosi I. Topical Sildenafil (Viagra) in the Treatment of Chronic Anal Fissure: A Randomized Double Blind Controlled Trial International. Journal of Pharmacology. 2006;2:608–612. [Google Scholar]
- 32.Torrabadella L, Salgado G, Burns RW, Berman IR. Manometric study of topical sildenafil (Viagra) in patients with chronic anal fissure: sildenafil reduces anal resting tone. Dis Colon Rectum. 2004;47:733–738. doi: 10.1007/s10350-003-0110-x. [DOI] [PubMed] [Google Scholar]
- 33.Kang YK, Lee SS, Yoon DH, Lee SY, Chun YJ, Kim MS, Ryu MH, Chang HM, Lee JL, Kim TW. Pyridoxine is not effective to prevent hand-foot syndrome associated with capecitabine therapy: results of a randomized, double-blind, placebo-controlled study. J Clin Oncol. 2010;28:3824–3829. doi: 10.1200/JCO.2010.29.1807. [DOI] [PubMed] [Google Scholar]
- 34.Wolf SL, Qin R, Menon SP, Rowland KM, Jr, Thomas S, Delaune R, Christian D, Pajon ER, Jr, Satele DV, Berenberg JL, Loprinzi CL. Placebo-controlled trial to determine the effectiveness of a urea/lactic acid-based topical keratolytic agent for prevention of capecitabine-induced hand-foot syndrome: North Central Cancer Treatment Group Study N05C5. J Clin Oncol. 2010;28:5182–5187. doi: 10.1200/JCO.2010.31.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92:308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- 36.Lassere Y, Hoff P. Management of hand-foot syndrome in patients treated with capecitabine (Xeloda) Eur J Oncol Nurs. 2004;8(Suppl 1):S31–S40. doi: 10.1016/j.ejon.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Anderson R, Jatoi A, Robert C, Wood LS, Keating KN, Lacouture ME. Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs) The Oncologist. 2009;14:291–302. doi: 10.1634/theoncologist.2008-0237. [DOI] [PubMed] [Google Scholar]
- 38.Gressett SM, Stanford BL, Hardwicke F. Management of hand-foot syndrome induced by capecitabine. J Oncol Pharm Pract. 2006;12:131–141. doi: 10.1177/1078155206069242. [DOI] [PubMed] [Google Scholar]
- 39.Common Terminology Criteria for Adverse Effects Version 4.0. [Google Scholar]
- 40.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 41.Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, Harbeck N, Steger GG, Schneeweiss A, Wardley AM, Chlistalla A, Romieu G. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 42.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 43.Azad NS, Aragon-Ching JB, Dahut WL, Gutierrez M, Figg WD, Jain L, Steinberg SM, Turner ML, Kohn EC, Kong HH. Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:1411–1416. doi: 10.1158/1078-0432.CCR-08-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]


