Abstract
The combined effects of low-dose or high-dose alpha particles and depleted uranium (DU) in Zebrafish (Danio rerio) embryos were studied. Three schemes were examined—(i) [ILUL]: 0.44 mGy alpha-particle dose + 10 µg/l DU exposure, (ii) [IHUH]: 4.4 mGy alpha-particle dose + 100 µg/l DU exposure and (iii) [IHUL]: 4.4 mGy alpha-particle dose + 10 µg/l DU exposure—in which Zebrafish embryos were irradiated with alpha particles at 5 h post fertilization (hpf) and/or exposed to uranium at 5–6 hpf. The results were also compared with our previous work, which studied the effects of [ILUH]: 0.44 mGy alpha-particle dose + 100 µg/l DU exposure. When the Zebrafish embryos developed to 24 hpf, the apoptotic signals in the entire embryos, used as the biological endpoint for this study, were quantified. Our results showed that [ILUL] and [IHUL] led to antagonistic effects, whereas [IHUH] led to an additive effect. The effect found for the previously studied case of [ILUH] was difficult to define because it was synergistic with reference to the 100 µg/l DU exposure, but it was antagonistic with reference to the 0.44 mGy alpha-particle dose. All the findings regarding the four different schemes showed that the combined effects critically depended on the dose response to each individual stressor. We also qualitatively explained these findings in terms of promotion of early death of cells predisposed to spontaneous transformation by alpha particles, interacting with the delay in cell death resulting from various concentrations of DU exposure.
INTRODUCTION
Organisms living in the environment are simultaneously exposed to various stressors (e.g. ionizing radiation, heavy metals, etc.), and a good understanding of the combined effects is necessary for realistic risk assessments [1–7]. The combined effects can be more complicated than the simple sum of the effects from individual stressors. To date, the most commonly identified combined effects have included additive, synergistic and antagonistic effects. Interestingly, most investigations of combined effects were based on high-dose (HD) effects of individual stressors [3, 5, 8–13]. However, evidence has been accumulated showing that HD effects can be different from low-dose (LD) effects. For example, an hormetic response, characterized as a biphasic dose–response relationship exhibiting a low-dose stimulation and a high-dose inhibition, has been widely accepted as a universal phenomenon for various stressors [14–15].
In the present study, the combined effects of LD or HD exposure to uranium metal [in the form of depleted uranium (DU)] and exposure to alpha particles were examined. People are exposed to a range of doses of uranium, which occurs naturally in the environment, and its distribution may be influenced by human activities, such as the coal and phosphate industries, military application or nuclear fuel cycle. The man-made enrichment of 235U (which is the only naturally fissile isotope in the isotopic composition of uranium relative to the other isotopes, i.e. 238U and 234U) is a critical process in nuclear power generation and nuclear weapon production, and creates mixtures of U isotopes that may be more or less radioactive in terms of both alpha and gamma radiation types (see Ref. [16] for details). We note that DU is a byproduct of the enrichment process.
Interest in the chemical toxicity of DU has increased since the Gulf War because DU was extensively used in various armor munitions as the strengthening material [17]. High natural groundwater uranium concentrations found at some locations, e.g. in the USA [18] and Finland [19], may also contribute to the human population exposure. Alpha-particle irradiation is also ubiquitous in our natural environment, e.g. from the decay of radon progeny [20]. Extensive previous research has investigated the biological effects of radon on humans [21–22], and these studies have indicated that radon progeny in air could lead to health issues for humans, particularly lung cancers [23–25]. Besides affecting the human respiratory tract, radon can also be dissolved in blood and move within the human body through the blood circulatory system. It is now well established that alpha particles from radon progeny contribute the largest natural radiation dose to human beings [22, 23]. Taken together, it becomes apparent that people and animals that live in areas with high natural uranium concentrations in groundwater [18, 19] will experience exposures to both alpha particles and DU. Hence, the combined effects from DU and alpha-particle irradiation have real-life relevance.
Exposure to uranium compounds in which the uranium isotopic composition is natural or depleted can produce effects in mammalian organs and organ systems (such as the kidney, the central nervous system, the lung and the liver), largely depending on the chemical dose [26]. There have been reports showing that the kidney is the most sensitive organ to uranyl compounds and is the critical target for acute uranium toxicity [27–29]. Uranium may interact with DNA in a variety of ways. Stearns et al. [30] reported the formation of uranium–DNA adducts and mutations in CHO EM9 mammalian cells after directly exposing the cells to a DU compound. Uranium could also react with DNA through hydrolysis of the DNA phosphate backbone [31]. Yazzie et al. [31] demonstrated that DU in uranyl acetate caused DNA single-strand breaks in the presence of vitamin C. Another study suggested that the formation of hydroxyl radicals were responsible for oxidative DNA damage in the presence of reactions with uranyl ion, hydrogen peroxide and ascorbate [32]. Moreover, DU induced genomic instabilities, such as delayed reproductive death and micronuclei formation in cells [33].
Recently, our group examined the combined effects from HD DU (concentration = 100 μg/l) and LD alpha-particle irradiation (dose = 0.44 mGy) on embryos of the Zebrafish, Danio rerio [34]. We demonstrated that the LD effect induced by the first stressor (alpha-particle irradiation) in terms of a reduction in the number of apoptotic signals, when combined with the HD effect of the second stressor (DU) in terms of an increase in the number of apoptotic signals, led to apparently even more apoptotic signals than the HD effect of the second stressor. We proposed to explain this combined effect by suggesting the alpha particle–induced early death of cells predisposed to spontaneous transformation [14] and DU-induced delayed cell death [33]. We further commented that explorations with different combinations of LD or HD exposures to alpha particles and DU would allow studies of the various manifestations of the combined effect of these two stressors and thus help elucidate the general mechanisms underlying these various manifestations.
Therefore, the present work aimed to further study the combined effects from LD or HD exposures to alpha particles and DU, i.e. [LD of alpha particles + LD of DU], [HD of alpha particles + HD of DU] and [HD of alpha particles + LD of DU]. We continued our use of embryos of the Zebrafish in our study of the combined effects. Our group had also previously employed the embryos of the Zebrafish for studying combined effects resulting from alpha particles and cadmium [35–37]. In recent years, there have been a growing number of research projects using Zebrafish or their embryos to investigate the toxicity of various environmental stressors. One of the major reasons for this is that the Zebrafish and human genomes share considerable homology, including conservation of most DNA repair–related genes [38].
In summary, the present work showed that [LD of alpha particles + LD of DU] led to an antagonistic effect, [HD of alpha particles + HD of DU] led to an additive effect and [HD of alpha particles + LD of DU] led to an antagonistic effect. In relation, the previously studied theme [LD of alpha particles + HD of DU] [34] led to a new undefined combined effect.
MATERIALS AND METHODS
Ethics statement
The animal studies were approved by the Department of Health, Government of the Hong Kong Special Administrative Region, Reference No. (13–7) in DH/HA&P/8/2/5 Pt.1, and were performed in accordance with the guidelines. The embryos were anesthetized using 0.0016 M tricaine before their apoptotic signals were counted under a fluorescent microscope.
Experimental animals
Adult male and female Zebrafish were mixed and kept in fish tanks where the water was kept at 28°C. To maintain a good and stable production of embryos, the fish were maintained with a light–dark cycle of 14 h of light followed by 10 h of dark periods. A specially designed plastic embryo collector was used to collect embryos when the 14 h photoperiod began. To ensure the synchronization of their developmental stages, the embryos were collected within a period of 30 min. All collected embryos were then kept in deionized water and in an incubator maintained at 28°C until they reached 4 h post fertilization (hpf). At 4 hpf, corresponding to the sphere stage of the blastula period, healthy and well developed embryos were selected under a stereomicroscope, and were then transferred into a new Petri dish with a thin agarose gel layer at the bottom filled with E3 medium (5 mM NaCl, 0.33 mM MgSO4, 0.33 mM CaCl2, 0.17 mM KCl and 0.1% methylene blue), for dechorionation.
Exposure protocols
The present work aimed to study the outcomes of various experimental schemes: [IXUY],which involved (up to 240 s irradiation period resulting in an alpha-particle dose level ‘X’ at 5 hpf) + (1 h exposure period to a waterborne uranium concentration level ‘Y’ from 5 to 6 hpf), where ‘X’ could be either ‘L’, representing a LD of alpha-particle irradiation (0.44 mGy), or ‘H’, representing a HD of alpha-particle irradiation (4.4 mGy); ‘Y’ could be either ‘L’, representing a LD exposure to uranium (10 µg/l), or ‘H’ representing a HD exposure to uranium (100 µg/l).
Using the same nomenclature system, the experimental scheme adopted in our previous study [34] would be [ILUH]. In the present work, the three different experimental schemes were:
Condition 1: [ILUL] (i.e. LD of alpha particles + LD of DU)
Condition 2: [IHUH] (i.e. HD of alpha particles + HD of DU)
Condition 3: [IHUL] (i.e. HD of alpha particles + LD of DU)
A total of three to four replicate experiments were performed for each condition. For each replicate experiment, 40 dechorionated embryos were employed. These embryos were separated into four groups, each having 10 embryos, and accommodated in four separate Petri dishes lined with a thin layer of biocompatible agarose on the bottom. The four groups were as follows.
The Control group
In this group, ‘[C]’, the embryos were dechorionated and received no further treatment.
Alpha-particle–irradiated and uranium-dosed group
In this group, [IXUY], the dechorionated embryos received level X alpha-particle irradiation at 5 hpf + level Y uranium exposure for 1 h (from 5 to 6 hpf), as described above.
Alpha-particle–irradiated groups
In this group, [IX], the dechorionated embryos received level X alpha-particle irradiation at 5 hpf, as described above.
Uranium-dosed group
In this group, [UY], the dechorionated embryos received level Y uranium exposure for 1 h (from 5 to 6 hpf), as described above.
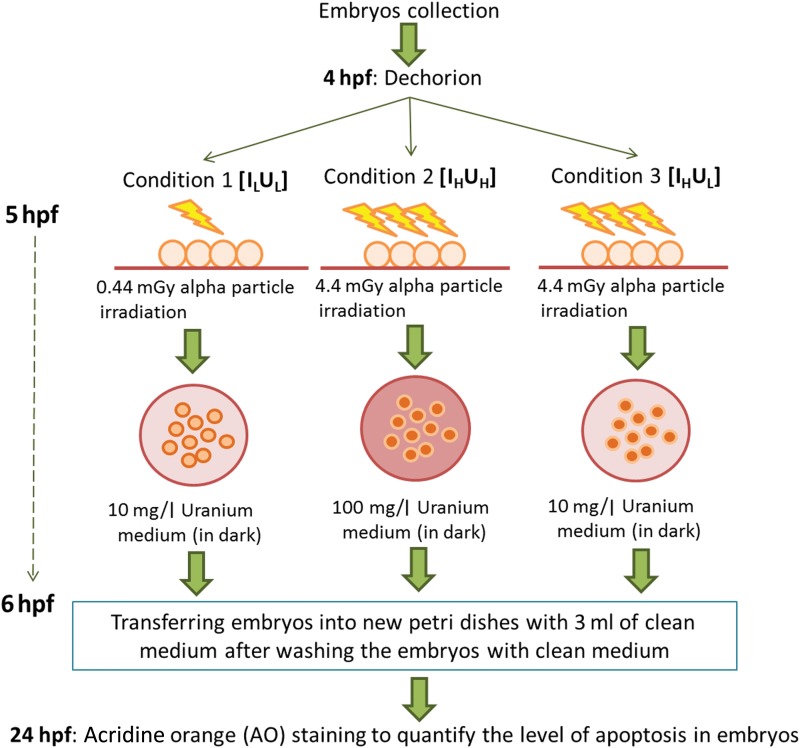
A volume of 3 ml of medium was used in each Petri dish. Since the DNA repair mechanism in Zebrafish embryos would start to operate after the cleavage stages (0.7–2.2 hpf) [39], exposures of embryos to alpha-particle radiation and/or DU started at 5 hpf, which was well after the cleavage stages. The embryos in the IX and UY groups were exposed to alpha-particle radiation or uranium, respectively, whereas the embryos in the IXUY group were exposed to both alpha-particle radiation and DU. The experimental flow for the treatment procedures of embryos in the IXUY group for the three different experimental schemes (Conditions 1–3) is shown in Fig. 1.
Fig. 1.
Experimental flow for treatment procedures of embryos. The experimental flow for the treatment procedures of embryos in the alpha-particle–irradiated and uranium-dosed group (IXUY) for the three different experimental schemes (Conditions 1 to 3).
Alpha-particle irradiation
In the present experiment, the setup for alpha-particle irradiation largely followed that designed by Yum et al. [40]. A biocompatible Mylar film (Dupont, Hong Kong) with a thickness of 3.5 μm was used as the support substrate during irradiation. The Mylar film was glued using an epoxy (Araldite Rapid, England) to the bottom of a Petri dish that had a hole with a diameter of 35 mm at the center. An 241Am source with alpha-particle energy of 5.49 MeV under vacuum and an activity of 4.26 kBq was employed in the present work. At 5 hpf, the embryos in the IX and IXUY groups were transferred onto the substrate in the irradiation dish and irradiated with alpha particles for 24 or 240 s, which corresponded to absorbed doses of ∼0.44 and ∼4.4 mGy, respectively. According to Yum et al. [41], the LD effect (∼0.44 mGy) and HD effect (∼4.4 mGy) of alpha particles in Zebrafish embryos were opposite in terms of the changes in the number of apoptotic signals within the embryos.
The two groups of embryos, (IL or IH) and (ILUL or IHUH or IHUL), were irradiated with alpha particles coming from below through the Mylar film, instead of coming from above (to avoid the problem of having different travelling distances of alpha particles in the medium before reaching the embryos). All embryos were orientated in such a way that all the cells of the embryos faced downwards towards the Mylar film so that the alpha particles would be directed towards the cells. Similar treatment and experimental procedures were followed for embryos in the UY group, except that the 241Am source was not used; this was referred to as ‘sham-irradiation’. After irradiation or sham-irradiation for the IXUY and UY groups, respectively, the embryos were then exposed to uranium.
Uranium exposure
In the present project, uranium exposure was achieved using uranyl acetate UO2(CH3COO)2·2H2O (Electron Microscopy Sciences). For each set of experiments, a new uranium stock solution was prepared to avoid uncertainties in the uranium concentration in different sets of experiments due to potential precipitation. Although making the stock solution in an acid like HCl could have kept the uranium concentration more stable, the acidic conditions could have affected the development of the Zebrafish embryos. To ensure that all the uranyl acetate was dissolved, the stock solution was prepared one day before performing each set of experiments. The uranyl acetate stock solution, with a concentration of between 0.15 and 0.30 g/l, was prepared by dissolving the uranyl acetate with MilliQ water. Since uranyl acetate was sensitive to light and would precipitate if exposed, all stock solution was kept at 4°C in the dark. On the day of the experiment, the stock solution was then further diluted to the desired concentration, which was either 10 µg/l (for Conditions 1 and 3) or 100 µg/l (for Condition 2) of uranium (in this study).
A volume of 3 ml of uranyl acetate working solution, which was sufficient to cover all embryos accommodated in each dish, was prepared in two new Petri dishes. Immediately after the IXUY and UY groups were irradiated and sham irradiated with alpha particles, respectively, the embryos were removed carefully with a glass dropper from the medium and transferred to a uranium solution to receive the uranium dosage. The embryos were kept in the uranium solution for 1 h in dark. After 1 h, the embryos were removed from the uranium solution. After washing with 6 ml of clean medium, the embryos were transferred to new Petri dishes with 3 ml of clean medium. All four groups of embryos (C, IXUY, IX and UY) were then returned to the 28°C incubator for further development.
Considering the specific activity of DU, the ranges in water of the alpha particles emitted by the relevant uranium isotopes and daughters, as well as the volume of the sensitive cells in the 5-hpf Zebrafish embryos, etc., the radiation dose received by the Zebrafish embryos due to DU exposure was many orders of magnitude lower than the radiation dose due to alpha particles emitted from the 241Am source described above. As such, the radiation dose from DU exposures could be safely neglected.
Quantification of apoptosis by vital dye staining
In the present project, apoptosis was chosen as the biological endpoint. Since the apoptotic activity in Zebrafish embryos was high before 24 hpf (due to organogenesis processes [42]), and pigment development on the Zebrafish embryos after 24 hpf presented problems for observing the signals from the apoptotic cells [43], we chose to examine the apoptotic signals in Zebrafish embryos at 24 hpf in the present work. When the Zebrafish embryos had developed to 24 hpf, the apoptotic signals in the entire embryos were quantified. Such an endpoint has been widely adopted for quantifying radiation effects in Zebrafish embryos [43, 44]. The staining procedure was as previously described by Choi et al. [45]. The four groups of embryos in the present study were transferred into a medium containing 2 µg/ml of a vital dye acridine orange (AO; Sigma, St Louis, MO, USA) (which has been commonly employed to quantify the level of apoptosis in Zebrafish embryos [46–48]) to stain in the dark for 60 min; they were then thoroughly washed twice in the culture medium. AO is a nucleic-acid selective fluorescent cationic dye. AO can cross the plasma membrane of viable and early apoptotic cells [49] and can interact with DNA and RNA by intercalation or electrostatic attractions, respectively. Hence, after AO staining, all nuclei of viable and early apoptotic cells would appear in green under a fluorescent microscope; apoptotic cells give much more intense green fluorescence as a result of chromatin condensation, which is a hallmark event for a cell undergoing apoptosis [50, 51]. In the present study, ‘apoptosis signals’ referred to the observed numbers of cells that were undergoing apoptosis. The apoptotic signals of the embryos were then counted under a fluorescent microscope after anesthetizing the embryo with 0.0016 M tricaine (Sigma, St Louis, MO, USA). For every single embryo, three images focusing on different sections of the embryos were captured under the fluorescent microscope (using a filter for green fluorescence and a magnification of ×40), and these three images were then combined into one signal image for quantification of apoptosis signals. All images were captured using identical exposure parameters. Only bright green spots within the embryos were scored. The computer program Particle Counting 2.0 (developed by J. Zhang) was employed to count the apoptotic signals in an embryo.
Data analysis
For each experimental scheme (i.e. Conditions 1 to 3 described in the section ‘Experiment protocols’), three to four sets of experiments, each with 40 Zebrafish embryos, were carried out on different days. The number of apoptotic signals for each whole Zebrafish embryo was counted as described above. For each condition, NC was interpreted as the average background apoptotic signal for the embryos in the corresponding set of experiments, and thus the net apoptotic signals for the IXUY, IX and UY groups could then be described as NIXUY Net = (NIXUY – NC), NIX Net = (NIX – NC) and NUY Net = (NUY – NC), respectively. Thus the normalized net apoptotic signal for these groups of the embryos could be determined by NIXUY † = [NIXUY Net/NC], NIX † = [NIX Net/NC] and NUY † = [NUY Net/NC]. The normalized net data for the all replicates were then grouped for further analyses. The data for each group (IXUY, IX and UY) were analyzed in turn as follows. As a first step, the statistical significance of the differences between the four conditions and the control (adding up to five conditions) was assessed using one-way analysis of variance (ANOVA). Cases with P values ≤0.05 were considered to correspond to statistically significant differences between at least two of the compared conditions. In such cases, post-hoc t-tests were then performed to assess the statistical significance of the difference between the control and each studied condition. A P value ≤0.005 (i.e. 0.05/10, where 10 was the total number of possible combinations of two-condition comparisons out of five conditions) was considered to correspond to a statistically significant difference between the control group and the studied condition. The combined effects due to the exposure to both alpha-particle radiation and uranium was characterized by comparing the IXUY group with the corresponding ‘reference’ groups through the Student's t-test, where the ‘reference’ groups were constructed based on the additive approach discussed in the following section. Cases with P values ≤0.05 were considered to correspond to statistically significant differences between the compared groups.
RESULTS
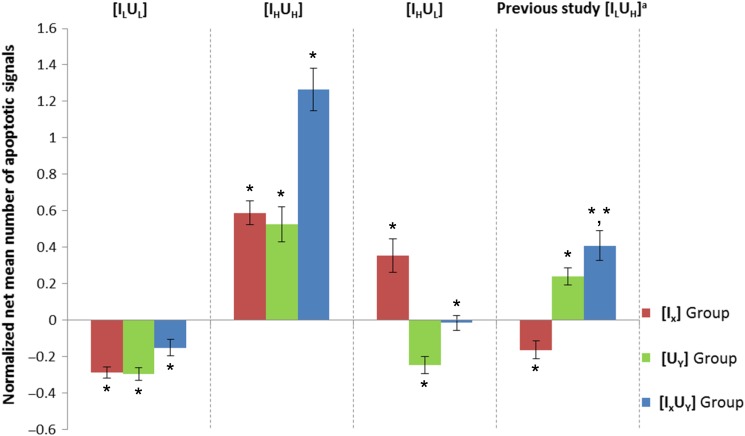
Three different experimental schemes, i.e. Condition 1 [ILUL], Condition 2 [IHUH] and Condition 3 [IHUL], were examined in the present work. The normalized net mean number of apoptotic signals (N ± SE) obtained from embryos in the C, IXUY, IX and UY groups in the three to four replicates of each experimental scheme have been plotted in Fig. 2 and recorded in Table 1 (where the results are represented as N ± SE, where SE is the standard error of the mean). Student's t-tests were used to determine the significance of the extent to which the IXUY, IX and UY groups differed from the corresponding C group of embryos under each set of experimental conditions. Cases with P ≤ 0.05 were considered statistically significant. The normalized net mean number of apoptotic signals (N ± SE) obtained in our previous study was also included for reference (in Fig. 2 and Table 1). In addition, results from one-way ANOVA for the five conditions (Control + Conditions 1–4) for each group (IXUY, IX and UY) and results from post-hoc t-tests (if P values were ≤0.05 from the one-way ANOVA) between the control and each studied Condition (1–4) were recorded in Table 2.
Fig. 2.
Normalized net mean numbers of apoptotic signals obtained for different groups of embryos. The normalized net mean numbers of apoptotic signals (N ± SE) obtained for embryos in the C, IXUY, IX and UY groups for the three to four replicates of the three experimental schemes (Condition 1 [ILUL], Condition 2 [IHUH] and Condition 3 [IHUL]). The results (for [ILUH]) from our previous study [34] were also included for comparison. *Cases with P ≤ 0.05 were considered statistically significant using Student's t-test by comparing with the corresponding C group of embryos under each experimental condition. aData extracted from Ref. [34].
Table 1.
Basal toxicity of DU and alpha particle exposures on embryos under each experiment condition
| Condition 1 [ILUL] | Na = 4 | NILUL† | NIL† | NUL† |
| –0.15 ± 0.04 | –0.28 ± 0.03 | –0.29 ± 0.03 | ||
| nb = 36 | n = 32 | n = 37 | ||
| Pc = 0.008* | P = 2.96 × 10−7* | P = 3.61 × 10−7* | ||
| Condition 2 [IHUH] | N = 4 | NIHUH† | NIH† | NUH† |
| 1.27 ± 0.12 | 0.59 ± 0.06 | 0.53 ± 0.09 | ||
| n = 37 | n = 37 | n = 33 | ||
| P = 1.70 × 10−13* | P = 1.26 × 10−10* | P = 3.32 × 10−6* | ||
| Condition 3 [IHUL] | N = 3 | NIHUL† | NIH† | NUL† |
| –0.01 ± 0.04 | 0.36 ± 0.09 | –0.25 ± 0.05 | ||
| n = 29 | n = 27 | n = 27 | ||
| P = 0.424 | P = 8.83 × 10−4* | P = 5.88 × 10−4* | ||
| Previous study [ILUH]d | N = 3 | NILUH† | NIL† | NUH† |
| 0.41 ± 0.08 | –0.16 ± 0.05 | 0.24 ± 0.05 | ||
| n = 30 | n = 27 | n = 30 | ||
| P = 7.65 × 10−5* | P = 0.019* | P = 0.001* |
aN = number of replicates involved. bn = total number of embryos employed in that experimental group under each experimental condition. cP = P value obtained using Student's t-test by comparing with the corresponding C group of embryos under each experimental condition. dReference data obtained in Ref. [34]. *Cases with P ≤ 0.05 were considered statistically significant.
Table 2.
Results from one-way ANOVA on the five conditions (Control + Conditions 1 to 4) for each group (IXUY, IX and UY), and results from post-hoc t-tests (if P values ≤ 0.05 from the one-way ANOVA) between the control and each studied Condition (1 to 4)
| Condition 1 [ILUL] | Condition 2 [IHUH] | Condition 3 [IHUL] | Condition 4 [ILUH]a | |
|---|---|---|---|---|
| IX | ANOVA: P = 8.17 × 10–30* | |||
| P = 2.57 × 10–10** | P = 1.11 × 10–10** | P = 7.48 × 10–3** | P = 4.76 × 10–4** | |
| UY | ANOVA: P = 2.10 × 10–26* | |||
| P = 1.20 × 10–9** | P = 4.56 × 10–6** | P = 2.40 × 10–5** | P = 3.97 × 10–5** | |
| IXUY | ANOVA: P = 1.87 × 10–49* | |||
| P = 0.002** | P = 2.57 × 10–13** | P = 0.390 | P = 1.43 × 10–5** | |
For cases with P ≤ 0.05 for the ANOVA, post-hoc t-tests were performed to further assess the differences between the control and each studied condition (1 to 4).
aReference data obtained in Ref. [34]. *Cases with P ≤ 0.05 were considered statistically significant. **Cases with P ≤ 0.005 (i.e. 0.05/10) were considered statistically significant.
Effects of high and low uranium exposures
In order to study the outcomes of the experimental schemes for [ILUL] (Condition 1) and [IHUL] (Condition 3), it was necessary to study the outcomes of the experimental scheme for [UL] to provide references for comparison (see the section ‘Combined effects of alpha particles and uranium’ below). It was interesting to observe that the normalized net mean number of apoptotic signals for the UL group of embryos was significantly smaller than that of the C group of embryos under both Conditions 1 and 3 (Table 1). Therefore, the LD effect of uranium (i.e. exposures to a low concentration of 10 μg/l) on Zebrafish embryos was similar to the LD effect of alpha particles.
Similarly, in order to study the outcomes of the experimental scheme for [IHUH] (Condition 2), it was necessary to study the outcomes of the experimental scheme for [UH] to provide references for comparison (see the section ‘Combined effects of alpha particles and uranium’ below). By comparing the C and UH groups, the effects of exposing Zebrafish embryos to a high uranium concentration were studied. It was found that the number of apoptotic signals of the UH group of embryos was significantly larger than that of the C group of embryos. A similar result was also obtained in our previous study [34], in which Zebrafish embryos at the same developmental stage were exposed to the same concentration of uranium for 1 h. Therefore, it could be concluded that exposing Zebrafish embryos to a high concentration of uranium (100 μg/l) from 5 to 6 hpf would lead to an increase in the number of apoptotic signals within the embryos.
Combined effects of alpha particles and uranium
Condition 1: [ILUL]
As described above, the normalized net apoptotic signal for these groups of the embryos could be determined by NILUL † = [NILULNet/NC], NIL † = [NILNet/NC] and NUL † = [NULNet/NC]. All the normalized net data for the four sets of experiments were then grouped for further analyses. The combined effects were compared with the effects from individual stressors, as described by Ng et al. [37]. Briefly, two methods were employed to construct the expected mean number of apoptotic signals based on the effects contributed by individual stressors:
adding NIL † to the value for each embryo in the UL group to form the <IL>UL group (Case 1); and
adding NUL † to the value for each embryo in the IL group to form the IL<UL> group (Case 2).
The mean numbers of apoptotic signals obtained for these two groups, i.e. N<I L>UL and NI L<UL>, were equal by definition. To compare whether the <IL>UL or IL<UL> groups were significantly different from the ILUL group, Student's t-tests were performed. Cases with P ≤ 0.05 were considered statistically significant. If P ≤ 0.05 for both Cases 1 and 2, we would conclude either a synergistic effect if (|NILUL†| > |NIL†| + |NUL†|) or an antagonistic effect if (|NILUL†| < |NIL†| + |NUL†|). If P > 0.05 for both cases, but P < 0.1 for at least one of the cases, a weakly synergistic or weakly antagonistic effect would be concluded [37]. All the remaining P values were considered as displaying an additive effect [37].
The results are shown in Table 3. Since P < 0.05 for both Cases 1 and 2, whereas the observed net normalized apoptotic signal (0.15 ± 0.04) was smaller than the expected value (0.58 ± 0.03), these results indicated an antagonistic effect when applying a LD of alpha particles (∼0.44 mGy) and a LD of uranium exposure (10 µg/l) for 1 h on Zebrafish embryos from 5 to 6 hpf.
Table 3.
Expected and observed amounts of apoptosis for combined low-dose alpha-particle irradiation and low-dose uranium exposure
| No. of embryosa | Expected | Observed | P (Case 1b) | P (Case 2c) | Interaction |
|---|---|---|---|---|---|
| 144 | −0.58 ± 0.03 | −0.15 ± 0.04 | 7.06 × 10–11 | 3.16 × 10–11 | Antagonistic |
The expected values (N<I L>UL or NI L<UL>) and observed values (NILUL †) of apoptosis for the combined effects of ∼0.44 mGy alpha-particle irradiation and exposure to 10 µg/l uranium concentration for 1 h on the 24 hpf Zebrafish embryos, indicating whether the interactions were additive, synergistic or antagonistic. aReferring to the total number of embryos in all four sets of samples. bNIL † was added to each embryo in the UL group. cNUL † was added to each embryo in the IL group.
Condition 2: [IHUH]
The net normalized apoptotic signal for the IHUH, IH and UH groups of embryos could be determined by the same method as described in the previous section, where NIHUH† = [NIHUHNet/NC], NIH† = [NIHNet/NC] and NUH† = [NUHNet/NC]. All the normalized net data for the four sets of experiments were then grouped for further analyses. Again, the combined effect was compared with the effects from individual stressors using the same two approaches [37]:
(i) adding NIH† to the value for each embryo in the UH group, to form the <IH>UH group (Case 1); and
(ii) adding NUH† to the value for each embryo in the IH group, to form the IH<UH> group (Case 2).
The mean number of apoptotic signals obtained for these two groups, i.e. N<IH>UH and NIH<UH>, were equal by definition. Student's t-tests were performed to compare whether the <IH>UH or IH<UH> groups were significantly different from the IHUH group. Cases with P ≤ 0.05 were considered statistically significant. If P ≤ 0.05 for both Cases 1 and 2, we would conclude there was either a synergistic effect if (|NIHUH†| > |NIH†| + |NUH†|) or an antagonistic effect if (|NIHUH†| < |NIH†| + |NUH†|). If P > 0.05 for both cases but P < 0.1 for at least one of the cases, a weakly synergistic or weakly antagonistic effect would be concluded [37]. All the remaining P values were considered as displaying an additive effect [37].
The results are shown in Table 4. Since P > 0.05 for both Cases 1 and 2, these results indicated an additive effect when applying a HD of alpha particles (∼4.4 mGy) and a HD of uranium exposure (100 µg/l) for 1 h to Zebrafish embryos from 5 to 6 hpf.
Table 4.
Expected and observed amounts of apoptosis for combined high-dose alpha-particle irradiation and high-dose uranium exposure
| No. of embryosa | Expected | Observed | P (Case 1b) | P (Case 2c) | Interaction |
|---|---|---|---|---|---|
| 144 | 1.11 ± 0.09 | 1.26 ± 0.11 | 0.161 | 0.162 | Additive |
The expected values (N<IH>UH or NIH<UH>) and observed values (NIHUH †) of apoptosis for the combined effects of ∼4.4 mGy alpha-particle irradiation and exposure to 100 µg/l uranium concentration for 1 h on the 24 hpf Zebrafish embryos, indicating whether the interactions were additive, synergistic or antagonistic. aReferring to the total number of embryos in all four sets of samples. bNIH † was added to each embryo in the UH group. cNUH † was added to each embryo in the IH group.
Condition 3: [IHUL]
The normalized net apoptotic signal for the IHUL, IH and UL groups of the embryos could be determined by NIHUL† = [(NIHUL–NC)/NC], NIH† = [(NIH–NC)/NC] and NUL† = [(NUL–NC)/NC]. The experiments were repeated three times, and all the normalized net data were then grouped for analyses. Unlike the previous conditions (Conditions 1 and 2), where the alpha-particle irradiation dose and the uranium exposure corresponded to either (i) both LD effects or (ii) both HD effects, Condition 3 was more complicated.
The results are shown in Table 5. The HD effect for the IH group in terms of the positive normalized net apoptotic signal was in line with that of other studies [37, 40, 41]. Surprisingly, however, when the alpha-particle irradiation was supplemented by a LD exposure to 10 µg/l of DU for 1 h, the normalized net mean number of apoptotic signals in the embryos became –0.013 ± 0.041, which was not significantly different from the number of apoptotic signals in the corresponding control group of embryos. In other words, the number of apoptotic signals appeared to have returned to the background level. Moreover, the normalized net mean number of apoptotic signals for the IHUL group of embryos was significantly lower than that for the IH group of embryos and significantly higher than that for the UL group of embryos. These results indicated that there was an antagonistic effect when applying a HD of alpha particles (∼4.4 mGy) and a LD uranium exposure (10 µg/l) for 1 h to Zebrafish embryos from 5 to 6 hpf.
Table 5.
Apoptotic levels for high-dose alpha particles, low-dose uranium, and a combination of these
| No. of embryos | NIHUL † | NIH † | NUL † |
|---|---|---|---|
| 111a | −0.013 ± 0.041 | 0.356 ± 0.092 | −0.246 ± 0.046 |
| P = 0.0004b* | P = 0.0002c* |
The normalized net mean number of apoptotic signals (N ± SE) obtained from embryos in C, IHUL, IH and UL groups in the four sets of experiments for Condition 3. aReferring to the total number of embryos in all three sets of samples. bP = P value obtained using Student's t-test with the IHUL group of embryos and the IH group of embryos. cP = P value obtained using Student's t-test with the IHUL group of embryos and the UL group of embryos. *Cases with P ≤ 0.05 were considered statistically significant.
Condition 4: [ILUH] (from previous study)
In our previous study [34], we studied the combined effects of a HD DU exposure (100 g/l) and a LD alpha-particle irradiation (0.44 mGy), using experimental procedures similar to those described in the present work. We demonstrated that the LD effect of one stressor, i.e. a low alpha-particle dose, appeared to be nullified by the simultaneous presence of the HD effect brought about by another stressor (DU). Such a result could also be explained using the schematic approach described above. Because the embryos were exposed to a high concentration of DU, it was anticipated that most of the cells damaged by alpha particles were also affected by DU, with the latter delaying some of the cell deaths to and beyond 24 hpf. In other words, the DU-induced delayed cell death had ‘shifted’ the time-point of apoptosis for early apoptotic cells to 24 hpf, thereby increasing the number of apoptotic signals at 24 hpf. Table 6 shows the mean normalized net number of apoptotic signals obtained in our previous study. Incidentally, it was noticed that |NIL †|+ |NUH †| =|–0.163| + |0.240| = 0.403 ∼ |NILUH †|, which supported our conjecture that the combined effects observed at 24 hpf were partly contributed by the apoptotic cells at 24 hpf induced by UH and partly contributed by the early apoptotic cells induced by IL (now with their apoptosis delayed by UH so as to take place at 24 hpf).
Table 6.
Apoptotic levels for low-dose alpha particles, high-dose uranium exposure, and a combination of these
| NILUH † | NIL † | NUH † |
|---|---|---|
| 0.409 ± 0.081 | −0.163 ± 0.047 | 0.240 ± 0.049 |
| P = 1.26×10−7*a | P = 0.0396*b |
Mean normalized net numbers of apoptotic signals (N ± SE) for the C, ILUH, IL and UH groups in three sets of experiments in our previous study [34]. The total number of embryos was 117 in all groups. aP values obtained by comparing IU and I groups using Student's t-test. bP values obtained by comparing IU and U groups using Student's t-test. *Cases with P ≤ 0.05 were considered statistically significant.
DISCUSSION
In the present work, Zebrafish embryos were exposed to a low concentration of DU, i.e. 10 μg/l, or a high concentration of DU, i.e. 100 μg/l, from 5 to 6 hpf. We found that the LD and HD effects obtained for the low and high concentrations of DU, respectively, were opposite in terms of the changes in the number of apoptotic signals within the embryos. These results were similar to those obtained for HD and LD alpha-particle irradiation. Both the results for DU exposure and alpha-particle irradiation were also commensurate with the hormetic response, which was characterized as a biphasic dose–response relationship exhibiting a low-dose stimulation and a high-dose inhibition [14, 15].
Three different experimental schemes, i.e. Condition 1 [ILUL], Condition 2 [IHUH] and Condition 3 [IHUL], were examined in the present work. The results obtained clearly demonstrated that under all experimental conditions, exposures to a low concentration (10 µg/l) of uranium or a low dose of alpha particles alone consistently led to hormesis in Zebrafish embryos, whereas exposures to a high concentration (100 µg/l) of uranium or a high dose of alpha particles alone consistently led to toxic effects in Zebrafish embryos.
The combined effects might not be simply the sum of the effects induced by individual stressors [52, 53]. As described above, additive, synergistic or antagonistic effects were seen to be possible, but most of these combined effects were defined only for HD effects of the individual stressors. For example, Condition 2 [IHUH] considered above corresponded to HD effects for both alpha-particle irradiation (∼4.4 mGy) and DU exposure (100 µg/l for 1 h). Our present results demonstrated an additive effect, because there was no significant difference between the observed net normalized apoptotic signal (1.26 ± 0.11) and the expected value (1.11 ± 0.09).
Condition 1 [ILUL] considered above corresponded to LD effects for both alpha-particle irradiation (∼0.44 mGy) and DU exposure (10 µg/l for 1 h). We note that conclusions about the combined effects should be cautious when at least one of the individual stressors induced LD effects (e.g. below the background level for the selected endpoint) that were opposite to the HD effects (above the background level for the same selected endpoint). In cases where both stressors induced LD effects, a ‘synergistic’ effect should be reflected by a resultant value ‘smaller’ than the values for the individual stressors. In view of this, we focused only on their magnitudes. Our present results for the [ILUL] scheme demonstrated an antagonistic effect because the observed absolute net normalized apoptotic signal (0.15 ± 0.04) was smaller than the absolute expected value (0.58 ± 0.03) (please refer to Table 3).
The combined effect for Condition 3 [IHUL] was even more complicated because the alpha-particle irradiation dose (∼4.4 mGy) inflicted a HD effect on the embryos, whereas exposure to a low concentration of DU (10 µg/l) induced a LD effect. In other words, one net response was positive (alpha particles) whereas the other was negative (DU). Incidentally, when alpha-particle irradiation was supplemented with an exposure to 10 µg/l of DU, the amount of apoptosis in the embryos appeared to have returned to the background level, which was significantly lower than the positive net response for alpha particles and significantly higher than the negative net response for DU. These results showed that both the increase and the decrease in the effects were repressed at the same time, thereby unequivocally leading to the conclusion that the effect was antagonistic. This was in sharp contrast to the case in our previous study [34], where an alpha-particle dose inducing a LD effect together with a DU exposure inducing a HD effect led to an effect even more substantial than the HD effect caused by DU alone. In that case, it was difficult to draw a conclusion about the combined effect because it would be synergistic with reference to the HD effect from DU but it would be antagonistic with reference to the LD effect from alpha particles.
All the above findings regarding the four different schemes were important in that they showed that the combined effects critically depended on the dose responses with which the individual stressors were associated. In summary, for a combination of alpha-particle irradiation and DU exposure, both stressors corresponding to HD effects would lead to an additive effect; DU corresponding to LD effects (with alpha particles corresponding to either LD or HD effects) would lead to an antagonistic effect; and alpha particles corresponding to LD effects together with DU corresponding to HD effects would lead to a new undefined combined effect.
Explanations for the various combined effects observed in the present study requires an understanding of the patterns of and mechanisms behind the cell deaths induced by DU and alpha particles corresponding to both LD and HD effects. A number of different processes, including elimination of naturally aberrant cells by early apoptosis, could explain the radiation-induced LD effect [15]. On the other hand, Miller et al. [33] found DU-induced genomic instabilities, including micronuclei formation and delayed reproductive death, in an in vitro cellular model using HOS cells.
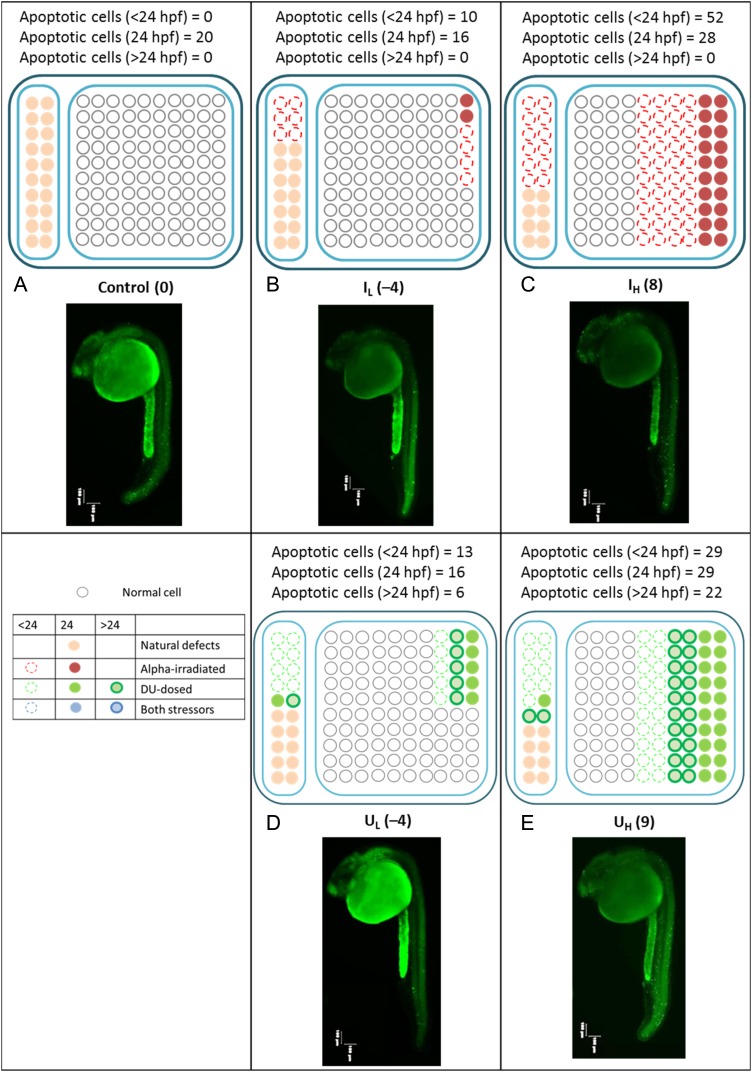
Taking into account the above findings, we attempted to qualitatively explain the combined effects obtained in the present work and in our previous study (e.g. the promotion of early death of cells predisposed to spontaneous transformation by a small alpha-particle dose corresponding to the LD effect, and the delay in cell death resulting from different concentrations of DU exposure). Figure 3 shows simplified schematic diagrams illustrating the patterns of cell deaths induced by the various alpha-particle doses and those induced by different DU concentrations alone. It is emphasized at the outset, here, that these diagrams have only been used to qualitatively illustrate the patterns of cell deaths to clarify this Discussion point for the reader, and should by no means be interpreted quantitatively.
Fig. 3.
Cell deaths induced by alpha particles and depleted uranium. Simplified schematic diagrams showing the patterns of cell deaths induced by different alpha-particle doses alone and by different DU concentrations exposure alone. Numbers in brackets represent the net number of apoptotic cells at 24 hpf (i.e. after subtracting the background number). Left columns: naturally aberrant cells; right columns: healthy cells if no external stressors; red color: cells affected by alpha particles; green color: cells affected by DU; open circles with dotted outlines: cells that undergo apoptosis earlier than 24 hpf; solid circles with colors other than white: cells that undergo apoptosis at 24 hpf; open circles with bold outlines: cells that undergo apoptosis after 24 hpf; white solid circles: normal healthy cells that were not affected by any stressors or those that could repair the damages successfully; pink solid circles: naturally aberrant cells that underwent apoptosis at 24 hpf. Representative images of stained embryos in C, IL, IH, UL and UH groups were also shown. Images of embryos were captured, using a fluorescent microscope with a magnification of ×40. Three images focusing on different sections of the embryos were captured and were then combined into one image.
The cells within the Zebrafish embryos that were not subjected to any stressor could in general be divided into two types, i.e. (i) the naturally aberrant cells; and (ii) healthy cells. When the effects from the different stressors were taken into consideration, the cells could be separated into many different categories according to the stressors or, alternatively, according to the time-points at which they would undergo apoptosis. For simplicity, in Fig. 3, we represented the cells that were affected by different stressors using different colors, i.e. red color for alpha particles and green color for DU, and we represented the time-point for apoptosis using different symbols, i.e. open circles with dotted outlines for apoptosis at earlier than 24 hpf, solid circles for apoptosis at 24 hpf, and open circles with bold outlines for apoptosis after 24 hpf. Furthermore, the naturally aberrant cells and the healthy cells were represented by white and pink solid circles, respectively.
Without any treatment (as in the C group), the naturally aberrant cells underwent apoptosis at 24 hpf. The measurement of this apoptosis revealed the background apoptotic signal (NC) for the corresponding set of experiments. When the embryos received a low alpha-particle dose alone (IL), some healthy cells were damaged and underwent apoptosis before 24 hpf (represented by circles with red dotted outlines) as well as at 24 hpf (represented by red solid circles). Here, we assumed that cell death after 24 hpf due to alpha-particle irradiation was negligible when compared with the apoptosis that had taken place at or before 24 hpf. At the same time, some naturally aberrant cells were triggered to undergo apoptosis before 24 hpf (represented by open circles with red dotted outlines). This early apoptosis decreased the number of apoptotic signals in the embryos at 24 hpf (leading to the LD effect). The same effect was still in action when the embryos were irradiated with a higher alpha-particle dose (IH), but many more naturally aberrant cells and also many more healthy cells were damaged and underwent apoptosis. Because of this, the apoptotic signals observed at 24 hpf were a lot greater in number than those for the low alpha-particle dose, and hence no LD effect was recorded.
Concerning the construction of the responses of the embryos to DU, we understood that DU induced delayed cell deaths when compared with the cell deaths induced by ionizing radiations [33]. For simplicity, we equally divided the cell deaths into three parts, i.e. before 24 hpf, at 24 hpf, and after 24 hpf. We also observed, from Fig. 2, that IL and UL led to similar apoptotic levels, and that IH and UH also led to similar apoptotic levels. These imposed constraints on our construction of the responses to DU. After exposures to a low concentration of DU, some naturally aberrant cells and some healthy cells were damaged. However, as a result of DU-induced delayed cell death, some damaged cells would undergo apoptosis after 24 hpf (represented by open circles with green bold outlines in Fig. 3D). A similar cell death pattern was expected in the case of high DU exposures. However, more damaged cells were expected due to the higher concentration of DU.
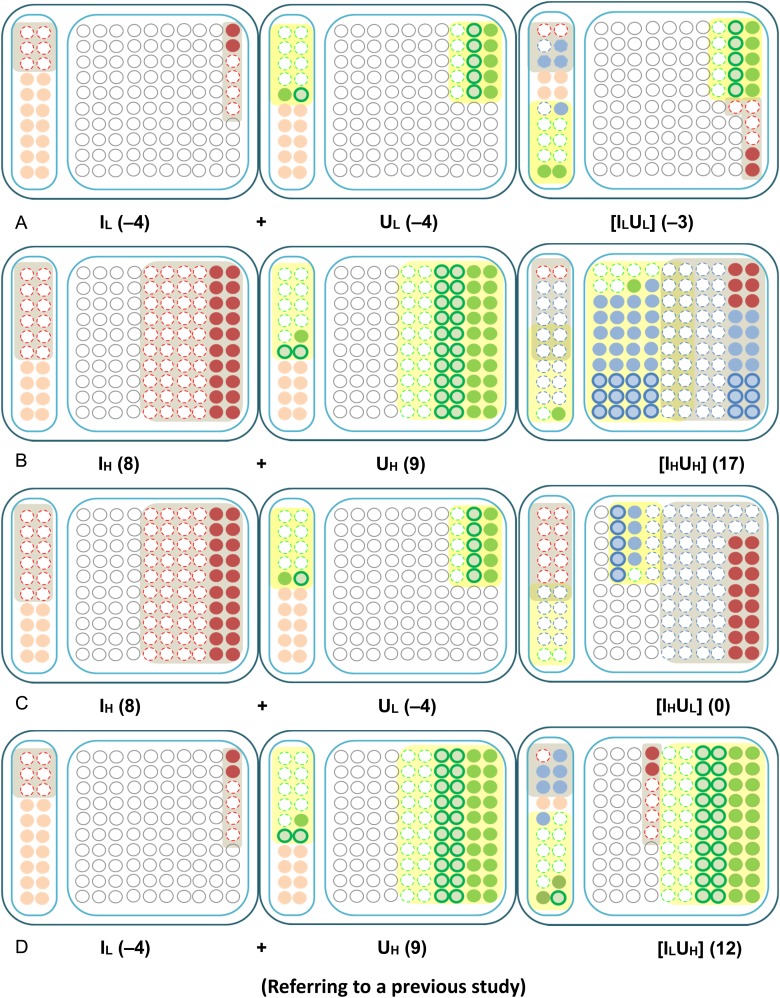
Based on the information in Fig. 3, the combined effects induced when applying different doses of alpha-particle irradiation and different concentrations of DU contamination could then be constructed as shown in Fig. 4. The colors and the symbols were the same as those used in Fig. 3, with the addition of the blue color used to represent cells subjected to both the stressors of alpha particles and DU.
Fig. 4.
Construction of the combined effects from the effects of single stressors. Simplified schematic diagrams showing the construction of combined effects from the effects of single stressors. Numbers in parentheses represent the net number of apoptotic cells at 24 hpf (i.e. after subtracting the background number). Left columns: naturally aberrant cells; right columns: healthy cells if no external stressors; red color: cells affected by alpha particles; green color: cells affected by DU; blue color: cells affected by both alpha particles and DU; open circles with dotted outlines: cells that undergo apoptosis earlier than 24 hpf; solid circles with colors other than white: cells that undergo apoptosis at 24 hpf; open circles with bold outlines: cells that undergo apoptosis after 24 hpf; white solid circles: normal healthy cells that were not affected by any stressors or those that could repair the damages successfully; pink solid circles: naturally aberrant cells that undergo apoptosis at 24 hpf.
Condition 1: [ILUL]
Since the embryos were irradiated with a low alpha-particle dose and also subjected to a low DU concentration in this case, it was reasonable to assume that only a very small number of cells (represented by blue circles in Fig. 4A) would be affected by both stressors at the same time. Thus the delayed cell death upon DU exposure was expected to occur in a small number of cells only; this led to a slight increase in the number of apoptotic signals at 24 hpf. In other words, there was a slight weakening in the LD effect in terms of decreasing the amount of apoptosis at 24 hpf induced by the low alpha-particle dose and the low DU exposure, individually.
Condition 2: [IHUH]
When the embryos were irradiated with a high alpha-particle dose and also subjected to a high DU exposure, most of the damaged cells were expected to be affected by both stressors (represented by blue circles in Fig. 4B). Therefore the delayed cell death due to DU exposure could be offset by the accelerated cell death induced by the significant damages resulting from the high alpha-particle dose and the high DU concentration. As such, the overall effect due to the two stressors revealed through the apoptotic signals observed at 24 hpf appeared to be an additive one (Fig. 4B).
Condition 3: [IHUL]
For Condition 3 (exposure to a high alpha-particle dose and a low DU concentration) (Fig. 4C), it was expected that only a small number of alpha-particle–irradiated cells would be affected by DU and would refrain from undergoing apoptosis at 24 hpf. On the other hand, the delayed cell death due to DU exposure was offset by the additional high alpha-particle dose. Taken together, these effects resulted in a promotion of early cell death, and only a small amount of apoptosis (which was close to the background signal level) could be detected at 24 hpf.
The present work laid the foundation for new study directions concerning combined effects in a realistic environment, e.g. involving stressors that induce LD and HD effects, and involving stressors that promote early cell death or delayed cell death. There have only been a few previous studies on the combined effect of uranium and radiation (e.g. [54]), and in these, the individual stressors induced HD effects or no significant effect on the studied biological endpoint. More studies have been carried out on the combined effect of other heavy metals and radiations. For instance, there have been a number of studies on the combined effect of radiation with cadmium [2, 5, 13, 35–37, 55, 56], with lead [55], and with aluminum [2, 5]. Nevertheless, none of these investigations involved stressors that induced LD effects on the studied endpoints. Apparently, from the results obtained in the present work, the characteristics of different stressors can affect the results obtained using different biological endpoints and different time-points of study. It is expected that the application sequence of the stressors will also affect the results [35–37]. The present work studied the multiple stressor effect on embryos from alpha-particle irradiation followed by uranium exposure. However, different results could be obtained for different sequences of application of stressors. For example, alpha-particle irradiation of Zebrafish embryos followed by cadmium exposure led to induction of an adaptive response being created by the alpha particles against subsequent exposures to cadmium [35], whereas cadmium exposure followed by alpha-particle irradiation led to induction of an adaptive response being created by cadmium against subsequent alpha-particle irradiation [36]. The effects of different sequences of applications of stressors will be investigated in our next study. The present work studied the multiple stressor effect on embryos from treatments with ionizing radiation and heavy metals, with essentially no time-gap. However, different results could be obtained if there was a time-gap between the alpha-particle irradiation and the uranium exposure. For example, an antagonistic effect was reported for a combined treatment of X-rays and the heavy metal cadmium on mice on Day 8 of gestation, which was more significant when the gap between the applications of these two stressors (X-ray first) was 30 min, when compared with time-gaps of 0 or 60 min [57]. On the other hand, the effects on Zebrafish embryos treated with alpha-particle radiation and the heavy metal cadmium simultaneously [37] or with a time-gap of 5 h [35] were studied, and additive or synergistic effects were found for the former, whereas an antagonistic effect was found for the latter (through the induction of an adaptive response being created by the alpha particles against subsequent exposures to cadmium). The effects of time-gaps will be investigated in our next study. More extensive studies on these topics are needed if we are to have a better understanding of the combined effects and achieve more realistic risk assessments.
FUNDING
Funding to pay the Open Access publication charges for this article was provided by the State Key Laboratory in Marine Pollution, City University of Hong Kong, Hong Kong.
REFERENCES
- 1.Salbu B, Rosseland BO, Oughton DH. Multiple stressors; a challenge for the future. J Environ Monit 2005;7:539. [DOI] [PubMed] [Google Scholar]
- 2.Salbu B, Mothersill C, Denbeigh J et al. Environmentally relevant mixed exposures to radiation and heavy metals induce measurable stress responses in Atlantic salmon. Env Sci Technol 2008;42:3441–6. [DOI] [PubMed] [Google Scholar]
- 3.Mothersill C, Salbu B, Heier LS et al. Multiple stressor effects of radiation and metals in salmon (Salmo salar). J Environ Radioact 2007;96:20–31. [DOI] [PubMed] [Google Scholar]
- 4.Mothersill C, Smith RW, Heier LS et al. Radiation-induced bystander effects in the Atlantic salmon (Salmo salar L.) following mixed exposure to copper and aluminum combined with low-dose gamma radiation. Radiat Environ Biophys 2014;53:103–14. [DOI] [PubMed] [Google Scholar]
- 5.Olsvik PA, Heier LS, Rosseland BO et al. Effects of combined γ-irradiation and metal (Al + Cd) exposures in Atlantic salmon (Salmo salar). J Environ Radioact 2010;101:230–6. [DOI] [PubMed] [Google Scholar]
- 6.Vanhoudt N, Vandenhove H, Real A et al. A review of multiple stressor studies that include ionising radiation. Environ Pollut 2012;168:177–92. [DOI] [PubMed] [Google Scholar]
- 7.Heier LS, Teien HC, Oughton D et al. Sublethal effects in Atlantic salmon (Salmo salar) exposed to mixtures of copper, aluminium and gamma radiation. J Environ Radioact 2013;121:33–42. [DOI] [PubMed] [Google Scholar]
- 8.Hallare AV, Schirling M, Luckenbach T et al. Combined effects of temperature and cadmium on developmental parameters and biomarker responses in Zebrafish (Danio rerio) embryos. J Therm Biol 2005;30:7–17. [Google Scholar]
- 9.Stanislav AG, Jin KK, Vladimir GD et al. Cytogenetic effects of combined radioactive (137Cs) and chemical (Cd, Pb, and 2, 4-D herbicide) contamination on spring barley intercalar meristem cells. Mutat Res 2005;586:147–59. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira ALG, Loureiro S, Soares AMVM. Toxicity prediction of binary combinations of cadmium, carbendazim and low dissolved oxygen on Daphnia magna. Aquat Toxicol 2008;89:28–39. [DOI] [PubMed] [Google Scholar]
- 11.Kienle C, Köhler H-R, Filser J et al. Effects of nickel chloride and oxygen depletion on behaviour and vitality of Zebrafish (Danio rerio, Hamilton, 1822) (Pisces, Cypriniformes) embryos and larvae. Environ Pollut 2008;152:612–20. [DOI] [PubMed] [Google Scholar]
- 12.Scheil V, Köhler H-R. Influence of nickel chloride, chlorpyrifos, and imidacloprid in combination with different temperatures on the embryogenesis of the Zebrafish Danio rerio. Arch Environ Contam Toxicol 2009;56:238–43. [DOI] [PubMed] [Google Scholar]
- 13.Danova D, Kafka I, Kalenicova Z et al. The effect of low dose ionizing radiation and cadmium chloride on glucose metabolism in broiler chickens. Acta Vet Brno 2010;79:415–8. [Google Scholar]
- 14.Hoffmann GR. A perspective on the scientific, philosophical, and policy dimensions of hormesis. Dose Response 2009;7:1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calabrese EJ. Hormetic mechanisms. Crit Rev Toxicol 2013;43:580–606. [DOI] [PubMed] [Google Scholar]
- 16.Mathews T, Beaugelin-Seiller K, Garnier-Laplace J et al. A probabilistic assessment of the chemical and radiological risks of chronic exposure to uranium in freshwater ecosystems. Environ Sci Technol 2009;43:6684–90. [DOI] [PubMed] [Google Scholar]
- 17.Priest ND. Toxicity of depleted uranium. Lancet 2001;357:244–6. [DOI] [PubMed] [Google Scholar]
- 18.Magdo HS, Forman J, Graber N et al. Grand rounds: nephrotoxicity in a prepubertal child exposed to uranium from contaminated well water. Environ Health Perspect 2007;115:1237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurttio P, Auvien A, Salonen L et al. Renal effects of uranium in drinking water. Environ Health Perspect 2002;110:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Research Council (NRC). Health Effects of Exposure to Radon. BEIR VI. Washington, Washington DC: National Academies Press, 1999. [PubMed] [Google Scholar]
- 21.Neuberger JS, Gesell TF. Childhood cancers, radon, and gamma radiation. Lancet 2002;360:1437–8. [DOI] [PubMed] [Google Scholar]
- 22.Yu KN, Lau BMF, Nikezic D. Assessment of environmental radon hazard using human respiratory tract models. J Hazard Mater 2006;132:98–110. [DOI] [PubMed] [Google Scholar]
- 23.Stather JW. Dosimetric and epidemiological approaches to assessing radon doses—can the differences be reconciled? Radiat Prot Dosimetry 2004;112:487–92. [DOI] [PubMed] [Google Scholar]
- 24.Collier CG, Strong JC, Humphreys JA et al. Carcinogenicity of radon/radon decay product inhalation in rats – effect of dose, dose rate and unattached fraction. Int J Radiat Biol 2005;81:631–47. [DOI] [PubMed] [Google Scholar]
- 25.Darby S, Hill D, Auvinen A et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case–control studies. BMJ 2005;330:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodge HC. A history of uranium poisoning. In: Hodge HC, Stannard JN, Hursh JB (eds). Handbook of Experimental Pharmacology. New York: Springer, 1973, 5–68. [Google Scholar]
- 27.Leggett RW. The behavior and chemical toxicity of U in the kidney: a reassessment. Health Phys 1989;57:365–83. [DOI] [PubMed] [Google Scholar]
- 28.Morrow P, Gelein R, Beiter H et al. Inhalation and intravenous studies of UF6/UO2F2 in dogs. Health Phys 1982;43:859–73. [DOI] [PubMed] [Google Scholar]
- 29.Taylor DM, Taylor SK. Environmental uranium and human health. Rev Environ Health 1997;12:147–57. [DOI] [PubMed] [Google Scholar]
- 30.Stearns DM, Yazzie M, Bradley AS et al. Uranyl acetate induces hprt mutations and uranium–DNA adducts in Chinese hamster ovary EM9 cells. Mutagenesis 2005;20:417–23. [DOI] [PubMed] [Google Scholar]
- 31.Yazzie M, Gamble SL, Civitello ER et al. Uranyl acetate causes DNA single strand breaks in vitro in the presence of ascorbate (vitamin C). Chem Res Toxicol 2003;16:524–30. [DOI] [PubMed] [Google Scholar]
- 32.Miller AC, Stewart M, Brooks K et al. Depleted uranium-catalyzed oxidative DNA damage: absence of significant alpha particle decay. J Inorg Biochem 2002;91:246–52. [DOI] [PubMed] [Google Scholar]
- 33.Miller AC, Brooks K, Stewart M et al. Genomic instability in human osteoblast cells after exposure to depleted uranium: delayed lethality and micronuclei formation. J Environ Radioact 2003;64:247–59. [DOI] [PubMed] [Google Scholar]
- 34.Ng CYP, Pereira S, Cheng SH et al. Combined effects of depleted uranium and ionising radiation on Zebrafish embryos. Radiat Prot Dosimetry 2015;167:311–5. [DOI] [PubMed] [Google Scholar]
- 35.Yu KN, Tung MMT, Choi VWY et al. Alpha radiation exposure decreases apoptotic cells in Zebrafish embryos subsequently exposed to the chemical stressor, Cd. Environ Sci Pollut Res 2012;19:3831–9. [DOI] [PubMed] [Google Scholar]
- 36.Choi VWY, Ng CYP, Kong MKY et al. Adaptive response to ionizing radiation induced by cadmium in Zebrafish embryos. J Radiol Prot 2013;33:101–12. [DOI] [PubMed] [Google Scholar]
- 37.Ng CYP, Choi VWY, Lam ACL et al. Multiple stressor effect in Zebrafish embryos from simultaneous exposures to ionizing radiation and cadmium. J Radiol Prot 2013;33:113–21. [DOI] [PubMed] [Google Scholar]
- 38.Barbazuk WB, Korf I, Kadavi C et al. The syntenic relationship of the Zebrafish and human genomes. Genome Research 2000;10:1351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyachi Y, Kanao T, Okamoto T. Marked depression of time interval between fertilization period and hatching period following exposure to low dose X-rays in Zebrafish. Environ Res 2003;93:216–9. [DOI] [PubMed] [Google Scholar]
- 40.Yum EHW, Ng CKM, Lin ACC et al. Experimental setup for studying the effects of alpha particles on Zebrafish embryos. Nucl Instrum Meth B 2007;264:171–6. [Google Scholar]
- 41.Yum EHW, Li VWT, Choi VWY et al. Effects of alpha particles on Zebrafish embryos. Appl Radiat Isotop 2010;68:714–7. [DOI] [PubMed] [Google Scholar]
- 42.Chen PK, Cheng SH. Cadmium-induced ectopic apoptosis in Zebrafish embryos. Arch Toxicol 2003;77:69–79. [DOI] [PubMed] [Google Scholar]
- 43.Bladen CL, Lam WK, Dynan WS et al. DNA damage response and Ku80 function in the vertebrate embryo. Nucleic Acids Res 2005;33:3002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geiger G.A., Parker S.E., Beothy A.P et al. Zebrafish as a “Biosensor”? Effects of ionizing radiation and amifostine on embryonic viability and development. Cancer Res 2006;66:8172–81. [DOI] [PubMed] [Google Scholar]
- 45.Choi VWY, Lam RKK, Chong EYW et al. Designing experimental setup and procedures for studying alpha-particle-induced adaptive response in Zebrafish embryos in vivo. Nucl Instrum Meth B 2010;268:651–6. [Google Scholar]
- 46.Tucker B, Lardelli MA. Rapid apoptosis assay measuring relative acridine orange fluorescence in Zebrafish embryos. Zebrafish 2007;4:113–6. [DOI] [PubMed] [Google Scholar]
- 47.Mei J, Zhang QY, Li Z et al. C1q-like inhibits p53-mediated apoptosis and controls normal hematopoiesis during zebrafish embryogenesis. Dev Biol 2008;319:273–84. [DOI] [PubMed] [Google Scholar]
- 48.Yasuda T, Yoshimoto M, Maeda K et al. Rapid and simple method for quantitative evaluation of neurocytotoxic effects of radiation on developing Medaka brain. J Radiat Res 2008;49:533–40. [DOI] [PubMed] [Google Scholar]
- 49.Mishell BB, Shiiqi SM, Henry C et al. Apoptosis: quantitative analysis techniques. In: Mishell BB, Shiigi SM (eds). Selected Methods in Cellular Immunology. Oxford, UK: W.H. Freeman & Co., 1980, 21–2. [Google Scholar]
- 50.Cohen JJ. Apoptosis. Immunol Today 1993;14:126–30. [DOI] [PubMed] [Google Scholar]
- 51.Elmore E. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007;35:495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hertzberg RC. Teuschler LK. Evaluating quantitative formulas for dose–response assessment of chemical mixtures. Environ Health Perspect 2002;110:965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.United States Environmental Protection Agency (USEPA). Framework for cumulative risk assessment. EPA/630/P-02/001F. National Center for Environmental Assessment, USEPA, Washington, Washington DC, 2003. [Google Scholar]
- 54.Vanhoudt N, Vandenhove H, Horemans N et al. The combined effect of uranium and gamma radiation on biological responses and oxidative stress induced in Arabidopsis thaliana. J Environ Radioact 2010;101:923–30. [DOI] [PubMed] [Google Scholar]
- 55.Geras'kin SA, Kim JK, Dikarev VG et al. Cytogenetic effects of combined radioactive (137Cs) and chemical (Cd, Pb, and 2,4-D herbicide) contamination on spring barley intercalar meristem cells. Mutat Res 2005;586:147–59. [DOI] [PubMed] [Google Scholar]
- 56.Hornhardt S, Gomolka M, Walsh L et al. Comparative investigations of sodium arsenite, arsenic trioxide and cadmium sulphate in combination with gamma-radiation on apoptosis, micronuclei induction and DNA damage in a human lymphoblastoid cell line. Mutat Res 2006;600:165–76. [DOI] [PubMed] [Google Scholar]
- 57.Michel C, Fritz-Niggli H. Teratogenic interactions between cadmium and radiation in mice. Experientia 1986;42:80–1. [DOI] [PubMed] [Google Scholar]