Abstract
The aim of this study was to compare three strategies for intensity-modulated radiotherapy (IMRT) for 20 head-and-neck cancer patients. For simultaneous integrated boost (SIB), doses were 66 and 54 Gy in 30 fractions for PTVboost and PTVelective, respectively. Two-phase IMRT delivered 50 Gy in 25 fractions to PTVelective in the First Plan, and 20 Gy in 10 fractions to PTVboost in the Second Plan. Sequential SIB (SEQ-SIB) delivered 55 Gy and 50 Gy in 25 fractions, respectively, to PTVboost and PTVelective using SIB in the First Plan and 11 Gy in 5 fractions to PTVboost in the Second Plan. Conformity indexes (CIs) (mean ± SD) for PTVboost and PTVelective were 1.09 ± 0.05 and 1.34 ± 0.12 for SIB, 1.39 ± 0.14 and 1.80 ± 0.28 for two-phase IMRT, and 1.14 ± 0.07 and 1.60 ± 0.18 for SEQ-SIB, respectively. CI was significantly highest for two-phase IMRT. Maximum doses (Dmax) to the spinal cord were 42.1 ± 1.5 Gy for SIB, 43.9 ± 1.0 Gy for two-phase IMRT and 40.3 ± 1.8 Gy for SEQ-SIB. Brainstem Dmax were 50.1 ± 2.2 Gy for SIB, 50.5 ± 4.6 Gy for two-phase IMRT and 47.4 ± 3.6 Gy for SEQ-SIB. Spinal cord Dmax for the three techniques was significantly different, and brainstem Dmax was significantly lower for SEQ-SIB. The compromised conformity of two-phase IMRT can result in higher doses to organs at risk (OARs). Lower OAR doses in SEQ-SIB made SEQ-SIB an alternative to SIB, which applies unconventional doses per fraction.
Keywords: IMRT, SIB, SEQ-SIB, head and neck cancer, conformity index
INTRODUCTION
Intensity-modulated radiotherapy (IMRT) features highly conformal dose distribution to targets [1]. It was introduced first for head and neck cancer (HNC) to treat nasopharyngeal cancer (NPC), which is characterized by complex shapes as a result of tumor progression and demands a substantial decrease in dosage to the parotids [2]. IMRT has undergone remarkable development and has begun to be used for other HNCs [3].
Two kinds of planning target volume (PTV) are generally used for HNCs: PTVboost is generated by adding a margin to the gross tumor volume (GTV) and PTVelective by including elective volumes. Recently, simultaneous integrated boost technique (SIB), which simultaneously delivers different doses to the two PTVs with a single plan, has become the standard for IMRT [4]. Because SIB uses equal fraction numbers for PTVboost and PTVelective, doses per fraction [fraction sizes (FSs)] for the two PTVs must be different, that is, higher FSs for PTVboost and lower FSs for PTVelective. These FSs are frequently unconventional, and Studer et al. pointed out FS problems involved in SIB for head and neck cancer in their report. They used doses of 2.0 Gy, 2.11 Gy and 2.2 Gy per fraction for PTVboost, and two cases of Grade 4 adverse late effects were observed in the 2.2 Gy group, leading them to conclude that SIB with 2.2 Gy was not recommended for large tumors involving laryngeal structures [5]. Thus, SIB with large FS for PTVboost involves a greater risk of late adverse effects. On the other hand, if the FS for PTVboost is 2 Gy, the FS for PTVelective may be unconventionally low (<1.8 Gy) and thus radiobiologically ineffective, even for controlling subclinical nodal disease [6].
One solution for this FS problem with SIB is two-phase IMRT, which has two sequential plans: the First Plan for the treatment of PTVelective, including PTVboost, and the subsequent Second Plan, exclusively for the treatment of PTVboost. Lee et al. reported on simultaneous modulated accelerated radiotherapy (SMART), which consists of two successive SIB plans [7]. Targets of the First SMART plan were GTV and CTVelective and those of the following Second Plan were GTV and CTVboost. Because we assumed that GTV was an inappropriate target volume, our original modified two-phase IMRT (sequential SIB, SEQ-SIB) has been introduced in our institution. For this approach, the First Plan involves treatment of PTVboost and PTVelective using SIB and the Second Plan treatment of PTVboost only. Because fraction numbers for PTVelective and PTVboost are different for two-phase IMRT and SEQ-SIB, FS can be conventional. To the best of our knowledge, however, few studies have compared the dosimetric parameters (DPs) of these IMRT approaches. We therefore compared the DPs of two-phase IMRT and SEQ-SIB with those of SIB.
MATERIALS AND METHODS
Patient characteristics
The subjects of this study were 20 patients with HNC treated with IMRT at our institution. Ten patients had NPC and the other 10 had oropharyngeal carcinoma (OPC) with histologically diagnosed squamous cell carcinoma. Patient characteristics are presented in Table 1. Bilateral lymphadenopathy was observed in five patients with NPC and in two patients with OPC.
Table 1.
Patient characteristics (n = 20)
| Patients (n = 20) | ||
|---|---|---|
| Age | median | 57 |
| range | 31–89 | |
| Gender | male | 17 |
| female | 3 | |
| Primary site | nasopharynx | 10 |
| oropharynx | 10 | |
| Stage | I | 1 |
| II | 4 | |
| III | 5 | |
| IVA | 8 | |
| IVB | 2 | |
| T-stage | T1 | 3 |
| T2 | 11 | |
| T3 | 2 | |
| T4 | 4 | |
| N-stage | N0 | 3 |
| N1 | 7 | |
| N2 | 9 | |
| N3 | 1 |
Treatment planning
For computed tomography (CT) simulation, patients were immobilized for IMRT. The GE Light speed (GE Medical Systems, Waukesha, WI) was used for CT, and images of 2.5-mm-thick contiguous slices were acquired and transferred to a commercial planning system (Eclipse Version 8.9; Varian Medical Systems, Palo Alto, CA). Target volumes and organs at risk (OARs) (i.e. the spinal cord, brainstem, and bilateral parotid glands) were contoured by radiation oncologists, following recommendations in Reports 50 and 62 of the International Commission on Radiation Units. GTVs consisted of identified masses, and clinical target volumes (CTVs) for boosting were created by adding potential volumes of tumor extensions to GTVs. CTVs for elective treatment included lymph node regions at potential risk of occult metastasis. PTVboost and PTVelective were then created by adding appropriate 3D margins for set-up variations to CTVs for boosting and CTVs for elective treatment, respectively.
Two-phase IMRT and SEQ-SIB were evaluated for comparison with SIB. For two-phase IMRT, a dose was prescribed for PTVelective in the First Plan and the target was confined to PTVboost in the subsequent Second Plan. For SEQ-SIB, PTVelective and PTVboost were simultaneously treated using the SIB technique for the First Plan, and the treatment volume was limited to PTVboost for the Second Plan. The entire SIB plan and the First and Second Plans of two-phase IMRT and SEQ-SIB were created on a pre-treatment simulation CT set. Delivered doses were set at 95% of the volume of the target PTV (D95) for each of the techniques. The dose fractions are listed in Table 2. Biological equivalent doses (BEDs) for the targets were also calculated by following equation:
where n, d, OTT, Tk and DT indicated the number of treatments, fraction size (Gy), overall treatment time (days), kick-off time (days) and doubling time (days), respectively. Tk and DT were set to 28 and 5 days, respectively.
Table 2.
Summary of fractionation schemes of three IMRT techniques
| SIB | Two-phase IMRT | SEQ-SIB | ||
|---|---|---|---|---|
| PTVboost | ||||
| First Plan | 50 Gy/25 fr (2 Gy/fr) | 55 Gy/25 fr (2.2 Gy/fr) | ||
| Second Plan | 20 Gy/10 fr (2 Gy/fr) | 11 Gy/5 fr (2.2 Gy/fr) | ||
| Total | 66 Gy/30 fr (2.2 Gy/fr) | 70 Gy/35 fr (2 Gy/fr) | 66 Gy/30 fr (2.2 Gy/fr) | |
| BED 10 | 74.1 Gy | 74.3 Gy | 74.1 Gy | |
| PTVelective | ||||
| First Plan | 50 Gy/25 fr (2 Gy/fr) | 50 Gy/25 fr (2 Gy/fr) | ||
| Total | 54 Gy/30 fr (1.8 Gy/fr) | 50 Gy/25 fr (2 Gy/fr) | 50 Gy/25 fr (2 Gy/fr) | |
| BED 10 | 57.3 Gy | 60 Gy | 60 Gy |
SIB = simultaneous integrated boost, SEQ-SIB = sequential SIB, BED 10 = biologically equivalent dose (α/β is 10 Gy for early-responding tissue).
The following dose constraints were used for OARs: maximal dose (Dmax) for the spinal cord: 45 Gy or less; Dmax for the brainstem: 54 Gy or less; mean dose (Dmean) for at least one parotid gland: 26 Gy or less. Seven fixed-gantry (210°, 290°, 315°, 45°, 70°, 150°, 180°) angles were designated for SIB and the First Plans of two-phase IMRT and SEQ-SIB. For the Second Plan of two-phase IMRT and SEQ-SIB, gantry angles of some beams were selected so as not to irradiate the contralateral parotid gland. IMRT was planned by means of the analytical anisotropic algorithm with tissue heterogeneity correction using a commercial inverse planning system and performed with a sliding window leaf sequence (MLC type: 120) and 6-MV beams. In the analytical anisotropic algorithm, the calculation grid size was 2.5 mm. CT values of metal-induced artifacts in the oral cavity were replaced with that of soft tissues (40 HU). The following DPs were analyzed: conformity index (CI), V93, D99, D95 and D2 for the PTV, Dmax of the brainstem and spinal cord, and Dmean of the parotid glands. Dx indicates the dose that included x% of the target and Vx indicates the volume as a percentage of the target that received x% of the prescribed dose. CI was determined by dividing the volume receiving the prescribed dose by the target volume. D2 was calculated only for PTVboost. Doses to OARs (Dmax and Dmean) of two-phase IMRT and SEQ-SIB were determined by summing the doses of the First and Second Plans.
Statistical analysis
The paired Student t-test was used to compare DPs. A value of P < 0.05 was defined as having statistical significance.
RESULTS
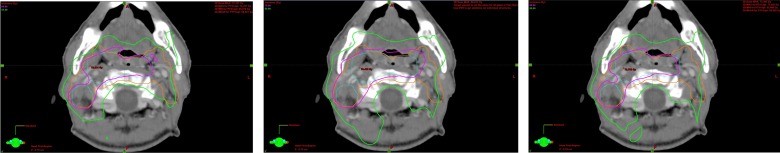
Table 3 shows DPs of the PTV. The CI was significantly lower for PTVboost than for PTVelective using any of the techniques (P < 0.0001). The CIs for both PTVboost and PTVelective were significantly lowest for SIB, followed by SEQ-SIB and two-phase IMRT. The difference in CI for PTVboost was slight between SIB (1.09 ± 0.05) and SEQ-SIB (1.14 ± 0.07), but it was substantially higher for two-phase IMRT (1.39 ± 0.14). CI for PTVelective and PTVboost of the First Plan were 1.12 ± 0.06 and 3.18 ± 0.06 for two-phase IMRT and 1.31 ± 0.12 and 1.17 ± 0.06 for SEQ-SIB. The CI for PTVboost of the Second Plan was 1.12 ± 0.08 for both two-phase IMRT and SEQ-SIB. The exceptionally high CI (3.18 ± 0.06) for PTVboost of the First Plan of two-phase IMRT resulted from the prescription for PTVelective. Figure 1 shows prescribed isodose lines of the total plans of three strategies on the middle plane of the target. The conformity of the isodose lines of PTVboost dose looked similar for SIB and SEQ-SIB, but less so for two-phase IMRT. Conformity of SIB for PTVelective was better than that of the other two techniques.
Table 3.
Dosimetric comparison of CI and PTV for the three IMRT techniques (mean ± SD)
|
P value |
||||||
|---|---|---|---|---|---|---|
| SIB | Two-phase IMRT | SEQ-SIB | SIB vs two-phase IMRT | SIB vs SEQ-SIB | SEQ-SIB vs two-phase IMRT | |
| CI | ||||||
| PTVboost | 1.09 ± 0.05 | 1.39 ± 0.14 | 1.14 ± 0.07 | <0.0001 | 0.02 | <0.0001 |
| PTVelective | 1.34 ± 0.12 | 1.80 ± 0.28 | 1.60 ± 0.18 | <0.0001 | <0.0001 | 0.01 |
| PTVboost | ||||||
| V93 (%) | 99.8 ± 0.2 | 99.8 ± 0.2 | 99.7 ± 0.3 | 0.37 | 0.27 | 0.09 |
| D2 (%) | 108.9 ± 1.5 | 110.4 ± 2.6 | 109.0 ± 1.7 | 0.03 | 0.85 | 0.049 |
| D99 (%) | 96.8 ± 1.1 | 98.4 ± 1.8 | 97.0 ± 1.2 | 0.001 | 0.63 | 0.005 |
| D95 (%) | 100 | 101.9 ± 1.4 | 100.3 ± 0.1 | <0.0001 | <0.0001 | <0.0001 |
| PTVelective | ||||||
| V93 (%) | 99.8 ± 0.2 | 99.9 ± 0.1 | 99.9 ± 0.1 | 0.02 | 0.38 | 0.35 |
| D99 (%) | 97.3 ± 2.2 | 101.9 ± 3.5 | 100.4 ± 2.7 | <0.0001 | 0.0003 | 0.14 |
| D95 (%) | 102.0 ± 1.5 | 106.2 ± 3.7 | 104.6 ± 2.7 | <0.0001 | 0.0007 | 0.14 |
Fig. 1.
Total dose distributions for SIB (left), two-phase IMRT (center) and SEQ-SIB (right). The PTVboost is shown in blue and the PTVelective in orange. The pink line indicates the isodose line prescribed for PTVboost and the green line the isodose line for PTVelective.
V93 of PTVboost and PTVelective were similar and >99.5%. Both D2 and D99 for PTVboost, which reflect the maximum and minimum doses, were respectively slightly and significantly higher for two-phase IMRT than for the other two techniques. D95 for PTVboost was 100% for SIB. Although D95 for PTVboost for both the First and Second Plans of SEQ-SIB was 100%, it slightly exceeded 100% for the total plan. For two-phase IMRT, on the other hand, because the dose for the First Plan was prescribed for PTVelective, D95 for PTVboost in the First Plan exceeded 100% (101.4%) and that in the total plan was significantly higher (101.9%, P < 0.0001) than that of SIB and SEQ-SIB. D99 and D95 for PTVelective of SEQ-SIB and two-phase IMRT were higher than those for SIB.
Table 4 summarizes the OAR doses. Ipsilateral parotid Dmean was similar for SIB and SEQ-SIB, but higher for two-phase IMRT. Contralateral parotid Dmean was lowest for SEQ-SIB and similar for SIB and two-phase IMRT, but the differences were not statistically significant. Spinal cord Dmax was significantly different for the three techniques and was highest for two-phase IMRT and lowest for SEQ-SIB. Brainstem Dmax was significantly lower for SEQ-SIB than SIB (P = 0.007) and two-phase IMRT (P = 0.025). Table 5 shows brainstem and spinal cord Dmax by primary site. Spinal cord Dmax was highest for two-phase IMRT and lowest for SEQ-SIB for both NPC and OPC. Brainstem Dmax was higher for NPC than for OPC for each of the techniques. Brainstem Dmax was significantly different among the three techniques: in NPC, it was highest for two-phase IMRT and lowest for SEQ-SIB, and in OPC, it was significantly lower for SEQ-SIB than for SIB (P = 0.009).
Table 4.
Dosimetric comparison of OARs for the three IMRT techniques (mean ± SD)
|
P value |
||||||
|---|---|---|---|---|---|---|
| SIB | Two-phase IMRT | SEQ-SIB | SIB vs two-phase IMRT | SIB vs SEQ-SIB | SEQ-SIB vs two-phase IMRT | |
| Ipsilateral Parotid Dmean (Gy) | 40.3 ± 12.1 | 44.9 ± 13.3 | 40.4 ± 12.2 | 0.27 | 0.98 | 0.28 |
| Contralateral Parotid Dmean (Gy) | 26.2 ± 4.8 | 26.9 ± 6.3 | 24.8 ± 4.9 | 0.68 | 0.39 | 0.26 |
| Spinal cord Dmax (Gy) | 42.1 ± 1.5 | 43.9 ± 1.0 | 40.3 ± 1.8 | <0.0001 | 0.0007 | <0.0001 |
| Brainstem Dmax (Gy) | 50.1 ± 2.2 | 50.5 ± 4.6 | 47.4 ± 3.6 | 0.753 | 0.007 | 0.025 |
Dmean = mean dose; Dmax = maximum dose.
Table 5.
Dmax for the spinal cord and brainstem by primary site
| Dmax |
P value |
|||||
|---|---|---|---|---|---|---|
| SIB | Two-phase IMRT | SEQ-SIB | SIB vs two-phase IMRT | SIB vs SEQ-SIB | SEQ-SIB vs two-phase IMRT | |
| Spinal cord | ||||||
| Nasopharynx (Gy) | 42.3 ± 1.2 | 43.6 ± 0.8 | 40.0 ± 1.8 | 0.01 | 0.004 | <0.0001 |
| Oropharynx (Gy) | 41.9 ± 1.7 | 44.2 ± 1.2 | 40.6 ± 1.8 | 0.003 | 0.11 | <0.0001 |
| P-value | 0.6 | 0.2 | 0.4 | |||
| Brainstem | ||||||
| Nasopharynx (Gy) | 51.6 ± 1.6 | 53.3 ± 0.9 | 49.9 ± 1.0 | 0.01 | 0.008 | <0.0001 |
| Oropharynx (Gy) | 48.5 ± 1.6 | 47.7 ± 5.1 | 44.9 ± 3.6 | 0.62 | 0.009 | 0.17 |
| P-value | 0.0005 | 0.003 | 0.0005 | |||
DISCUSSION
Our study compared DPs of two-phase IMRT and SEQ-SIB with those of SIB. For SIB, the FS for PTVboost was set at 2.2 Gy on the basis of a report by Chao et al. [8]. This FS was adopted because the volumes of late-responding tissues included in PTVboost were small. The FS for PTVboost for SEQ-SIB in our study was also 2.2 Gy for comparing DPs with SIB. However, Studer et al. reported that two cases developed Grade 4 reactions following the SIB schedule with 2.2 Gy per sessions and concluded that SIB with 2.2 Gy was not recommended [5]. For SEQ-SIB, because of the different number of fractions in PTVboost versus PTVelective, more conventional FSs, such as 2.0 Gy, are available. On the other hand, the large FS (2.2 Gy) was not used for the First Plan of two-phase IMRT because of the large prescribed volume (PTVelective).
Kumar et al. investigated the conformity of SIB for HNC and reported that the range of the ratio of V95 to PTVboost volume was 1.01–1.06 [9]. Mohan et al. performed a phantom study to compare conformity between SIB and two-phase IMRT for HNC [10]. They found that the volume of normal tissues outside the target regions that received the prescribed dose for PTVboost was 34% larger for two-phase IMRT. Our study also demonstrated significantly better conformity of SIB than of two-phase IMRT. For two-phase IMRT, although the CI for PTVboost of the Second Plan (1.12 ± 0.08) was equivalent to that for SIB (1.09 ± 0.05), the prescription for PTVelective of the First Plan compromised conformity of PTVboost in the total plan. On the other hand, the SIB technique adopted in the First Plan of SEQ-SIB achieved conformity to PTVboost higher than two-phase IMRT. Our study also demonstrated that, in the case of PTVelective, CI values were higher not only for two-phase IMRT (1.80 ± 0.28) but also for SEQ-SIB (1.60 ± 0.18). Although CI values of PTVelective were low for the First Plan using these two techniques, they were compromised by additional doses in the Second Plan. The lower dose of the Second Plan of SEQ-SIB than that of two-phase IMRT resulted in a smaller increase in CI values.
Parameters for determining OAR doses were the conformity and prescribed dose for PTV. Emmy et al. compared the parotid dose in HNC treatment using SIB and two-phase techniques [11]. The doses used in their study were 70 Gy for the boost volume in both techniques and 57.75 Gy and 46 Gy for the elective volume in SIB and two-phase IMRT, respectively. The lower dose for the elective volume of two-phase IMRT resulted in a 2.9–3.3 Gy lower mean dose to the parotids away from PTVboost. When PTVboost overlapped with or was close to the parotids, the mean dose was larger for two-phase IMRT. According to the authors, because the higher dose gradient was seen around PTVboost for SIB, SIB showed the superiority. However, the physical mean dose was calculated without adjustment for the dose per fraction, so that when the dose per fraction was taken into consideration, the biological advantage of two-phase IMRT for the parotid, away from PTVboost, became somewhat smaller. Chen et al. in a study of 14 NPC patients also compared the parotid gland dose for two-phase IMRT and SIB [12]. The prescribed doses of the elective and boost volumes for two-phase IMRT and SIB were 54–70.2 Gy and 56.1–69.7 Gy, respectively. Because prescribed doses were similar for the two techniques, the parotid dose depended mainly on conformity. Their study demonstrated that use of two-phase IMRT significantly increased the mean parotid dose by ∼5–7 Gy. They did not assess CI but stated that the accumulation of lower-dose irradiation throughout the second phase might increase the total dose to parotid glands.
In our study, the ipsilateral and contralateral parotid doses were separately assessed. PTVboost was frequently located closer to the ipsilateral than the contralateral parotid. Compared with SIB, two-phase IMRT delivered a higher mean dose to the ipsilateral parotid because of the higher prescribed dose and low conformity of PTVboost. On the other hand, SEQ-SIB showed a slightly poorer conformity, but this did not increase the dose to the ipsilateral parotid. Because contralateral parotid glands are often away from PTVboost and adjacent to or overlapping PTVelective, the prescribed dose for PTVelective and its conformity are major determinants of the contralateral parotid dose. Although two-phase IMRT had lower conformity for PTVelective, its lower dose to PTVelective offset the difference so that the mean dose to the contralateral parotid was similar to that of SIB. For SEQ-SIB, on the other hand, the lower prescribed dose of PTVelective, in spite of its lower conformity, resulted in a lower contralateral parotid dose.
In our study, spinal cord Dmax was higher for two-phase IMRT, because of the higher PTVboost dose and its lower conformity, while SEQ-SIB had significantly lower spinal cord Dmax than SIB in spite of its worse conformity. One possible explanation for this discrepancy is that the same point in the spinal cord received the maximal dose in SIB, whereas the plan change for SEQ-SIB shifted the spinal cord Dmax point. For NPC, the higher PTVboost dose and worse conformity of two-phase IMRT made the brainstem Dmax higher than that for SIB. For OPC, on the other hand, brainstem doses depended on the dose for the PTVelective, which was generally located closer to the brainstem than PTVboost. In two-phase IMRT, lower conformity for PTVelective cancelled the advantage of the lower PTVelective dose. In spite of the lower conformity of SEQ-SIB than SIB, brainstem Dmax of SEQ-SIB was significantly lower than that of SIB, which was attributable to the lower PTVelective dose and the change in the plan (which resulted in a shift in the brainstem Dmax point). We targeted NPC and OPC, for which IMRT is often applied. The equivalence of conformity of SEQ-SIB is suggested in other HNCs, such as hypopharyngeal cancer or oral cancer. However, doses to OARs such as the brainstem and parotid glands need further analysis and evaluation because every HNC has different positional relations between the primary target and the OARs.
There were some shortcomings in our study. Fraction numbers differed between the three techniques. Plan changes between two-phase IMRT and SEQ-SIB resulted in changes in the OAR doses per fraction. Because of these differences, cumulated physical doses to the OARs in the total plans of two-phase IMRT and SEQ-SIB could not be simply compared with OAR doses in SIB. First and Second Plans of two-phase IMRT and SEQ-SIB were planned from a single pre-treatment simulation CT set. However, a patient body changes inevitably during a radiotherapy course, and to compensate for this body change, Second Plans can be created on another simulation CT set acquired during the radiotherapy course. In this manner, dosimetric parameters in our study can be different. In spite of these limitations, our study showed that conformity was significantly different for the three techniques: highest for SIB and lowest for two-phase IMRT. The lower conformity of two-phase IMRT resulted in increased OAR doses. Compared with SIB, SEQ-SIB showed slightly lower conformity, but resulted in OAR doses comparable with those for SIB. SEQ-SIB could thus be a solution for problems of unconventional fraction sizes when SIB is used.
CONFLICT OF INTEREST
No actual or potential conflicts of interest exist.
FUNDING
Funding to pay the Open Access publication charges for this article was provided by Health and Labour Sciences Research Grants for Promotion of Cancer Control Programs [H26-Cancer Policy-General-014].
REFERENCES
- 1.Wu Q, Mohan R, Morris M et al. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas. I: dosimetric results. Int J Radiat Oncol Biol Phys 2003;56:573–85. [DOI] [PubMed] [Google Scholar]
- 2.Stringari L, Benassi M, Arcangeli G et al. A novel dose constraint to reduce xerostomia in head-and-neck cancer patients treated with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2010;77:269–76. [DOI] [PubMed] [Google Scholar]
- 3.Vanetti E, Clivio A, Nicolini G et al. Volumetric modulated arc radiotherapy for carcinomas of the oro-pharynx, hypo-pharynx and larynx: a treatment planning comparison with fixed field IMRT. Radiother Oncol 2009;92:111–7. [DOI] [PubMed] [Google Scholar]
- 4.Wolden SL, Chen WC, Pfister DG et al. Intensity-modulated radiation therapy (IMRT) for nasopharynx cancer: update of the Memorial Sloan–Kettering experience. Int J Radiat Oncol Biol Phys 2006;64:57–62. [DOI] [PubMed] [Google Scholar]
- 5.Studer G, Huguenin PU, Davis JB et al. IMRT using simultaneously integrated boost (SIB) in head and neck cancer patients. Radiat Oncol 2006;1:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Q, Manning M, Schmidt-Ullrich R et al. The potential for sparing of parotids and escalation of biology effective dose with intensity-modulated radiation treatments of head and neck cancers: a treatment design study. Int J Radiat Oncol Biol Phys 2000;46:195–205. [DOI] [PubMed] [Google Scholar]
- 7.Lee SW, Back GM, Yi BY et al. Preliminary results of a phase I/II study of simultaneous modulated accelerated radiotherapy for nondisseminated nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2006;65:152–60. [DOI] [PubMed] [Google Scholar]
- 8.Chao KS, Ozyigit G, Tran BN et al. Patterns of failure in receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2003;55:312–21. [DOI] [PubMed] [Google Scholar]
- 9.Kumar SA, Vivekanandan N, Sriram P et al. A study on conventional IMRT and Rapidarc treatment planning techniques for head and neck cancers. Rep Pract Oncol Radiother 2012;17:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohan R, Wu Q, Manning M et al. Radiobiological consideration in the design of fractionation strategies for intensity-modulated radiation therapy of head and neck cancers. Int J Radiat Oncol Biol Phys 2000;46:619–30. [DOI] [PubMed] [Google Scholar]
- 11.Lamers-Kuijper E, Heemsbergen W, van Mourik A et al. Sequentially delivered boost plans are superior to simultaneously delivered plans in head and neck cancer when the boost volume is located further away from the parotid glands. Radiother Oncol 2011;98:51–6. [DOI] [PubMed] [Google Scholar]
- 12.Chen SW, Yang SN, Liang JA et al. Comparative dosimetric study of two strategies of intensity-modulated radiotherapy in nasopharyngeal cancer. Med Dosim 2005;30:219–27. [DOI] [PubMed] [Google Scholar]