Abstract
The in vivo low-dose responses of zebrafish (Danio rerio) embryos to 150 kV X-rays with different levels of hardness were examined through the number of apoptotic events revealed at 24 h post fertilization by vital dye acridine orange staining. Our results suggested that a triphasic dose response was likely a common phenomenon in living organisms irradiated by X-rays, which comprised an ultra-low-dose inhibition, low-dose stimulation and high-dose inhibition. Our results also suggested that the hormetic zone (or the stimulation zone) was shifted towards lower doses with application of filters. The non-detection of a triphasic dose response in previous experiments could likely be attributed to the use of hard X-rays, which shifted the hormetic zone into an unmonitored ultra-low-dose region. In such cases where the subhormetic zone was missed, a biphasic dose response would be reported instead.
INTRODUCTION
Low-dose exposures to ionizing radiation have attracted much attention from scientists as these are relevant to environmental exposures. For radiation protection practices, the linear no-threshold (LNT) hypothesis has been widely accepted, which assumes that the radiation risk is linearly proportional to the dose and that there is not a threshold dose below which no radiation risk exists. However, accumulating evidence has shown that the LNT hypothesis does not hold in a low-dose regime, e.g. the reduced mutations and cancers induced by low-dose radiation shown in in vitro and in vivo studies [1–7]. In particular, the well-known hormetic responses typified by a biphasic dose response (BDR) demonstrating low-dose stimulation and high-dose inhibition (with respect to the zero-dose background value) do not fit the LNT hypothesis [8–10]. The dose ranges (or zones) with below-background and above-background responses are commonly referred to as the hormetic and toxic zones, respectively.
A very interesting but much less studied phenomenon related to the BDR is the ‘triphasic dose response’ (TDR) discovered by Hooker et al. in the low-dose region [11]. By studying the chromosomal inversion frequency in the spleen tissue of pKZ1 mice, the authors found an extra zone with doses below those for the hormetic zone, in which the responses were above the background. In other words, the TDR comprised ultra-low-dose inhibition, low-dose stimulation and high-dose inhibition [11]. Apparently, TDR also did not fit the LNT hypothesis. The zone exhibiting the ultra-low-dose inhibition was referred to as the ‘subhormetic zone’ [11]. By using X-rays generated from a machine operated at 250 kV with a filter of 0.6 mm tin + 2.5 mm copper + 1 mm aluminum, the dose ranges for the subhormetic, hormetic and toxic zones were found to be 5–10 μGy, 1–10 mGy and >100 mGy, respectively [11].
Surprisingly, TDR was not extensively reported. To the best of our knowledge, only our group have further studied and demonstrated TDR in zebrafish (Danio rerio) embryos induced by microbeam 3.37-MeV protons from the SPICE (Single-Particle Irradiation System to Cell) facility at the National Institute of Radiological Sciences (NIRS), Japan [12]. Dechorionated zebrafish embryos were irradiated at 0.75 h post fertilization (hpf) (i.e. at the two-cell stage) by microbeam protons, and the levels of apoptosis in the embryos at 25 hpf were quantified through terminal dUTP transferase-mediated nick end-labeling (TUNEL) assay. When both cells of the two-cell stage embryos were irradiated, TDR was displayed with subhormetic, hormetic and toxic zones at doses <30 mGy, 30–60 mGy and >90 mGy.
Many in vivo studies on the dose response in the low-dose regime have been performed using X-rays, so it is surprising that TDR has not been consistently reported. As such, it is reasonable to suggest that there are factors other than the X-ray dose that can affect the observation of TDR, among which the hardness of X-ray photons is a plausible candidate. The hardness of an X-ray beam describes its penetrating power, which increases with the average energy of the X-ray photons in the beam. When a filter is applied, lower-energy photons are preferentially attenuated and the average energy of X-ray photons in the filtered X-ray beam becomes higher, so the X-ray beam has been hardened upon filtration.
In fact, as early as 1925, Arntzen and Krebs had already explored the biological effect of X-rays with different hardness on Pisum sativum (Victoria-peas). A stimulatory effect was demonstrated when filters were used but there was no such effect without the filters [13]. More recently, Dong et al. also revealed that, for the same X-ray dose, the more energetic X-ray photons significantly increased the apoptotic events in early Xenopus laevis embryos [14]. These studies hinted that the biological effect could not be solely determined by the X-ray dose, but would also depend on the hardness of the X-ray photons.
As such, the present paper aims to explore whether the observation of TDR depends on the hardness of the X-rays through the use of X-ray photons generated by the same X-ray irradiator operated at the same voltage but with different filters. Zebrafish (D. rerio) embryos were used as the vertebrate model for studying the in vivo biological effects. Zebrafish has become a popular model in many fields of research studies, such as developmental biology, physiology, toxicology and environmental research, as well as cancer research [15, 16]. Our group has been actively using zebrafish embryos to study the effect of low-dose radiation, including the hormetic effect, bystander effect and adaptive response [17–23]. Approximately 70% of human genes have at least one obvious zebrafish ortholog, as revealed by whole zebrafish genome sequencing technique [24]. Human and zebrafish genomes share considerable homology, including conservation of most DNA repair-related genes [24, 25].
MATERIALS AND METHODS
Ethics statement
The animal studies were approved by the Department of Health, Government of the Hong Kong Special Administrative Region, with the ref. no. Ref: (13–7) in DH/HA&P/8/2/5 Pt.1, and were performed in accordance with the guidelines.
Zebrafish maintenance
Approximately 35 adult zebrafish of both genders were kept in a 45-l fish tank. The water temperature was maintained at 28°C using thermostats. The fish were adapted to a 14/10 h light/dark cycle to maintain a good production of embryos. The fish were fed four times daily with fish food (TetraMin, Melle, Germany) and brine shrimp (Brine Shrimp Direct, Ogden, UT, USA).
To ensure synchronization of the embryonic stages, the embryos were collected using specially designed plastic collectors [26] within 30 min from the start of the light-induced spawning. The collected embryos were incubated in an incubator with the temperature set at 28.5°C to allow continuous development of the embryos until 4 h post fertilization (hpf). Healthy developing embryos were chosen and then manually dechorionated using a pair of sharp forceps (Dumont, Hatfield, PA, USA) under the stereomicroscope (Nikon, Chiyoda-ku, Tokyo, Japan). During dechorionation, the embryos were placed into a Petri dish lined with an agarose (Invitrogen, Life Technologies Corporation, Carlsbad, CA, USA) gel layer and filled with E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, 0.1% methylene blue).
X-ray irradiation
In this study, an X-ray generator, X-RAD 320 irradiator (Precision X-Ray INC., North Branford, CT, USA) was used. The X-RAD 320 irradiator has been widely used to study in vitro and in vivo biological effects induced by X-rays [27–29]. In the present study, the voltage was set at 150 kV and the source–surface distance was 70 mm for the experiments. Three different X-ray hardness conditions (f) were examined; namely, (a) no filter used (referred to as F0), (b) application of the filter (F1) made of 2 mm thick aluminum (Al) and (c) application of the filter (F2) made of 1.5 mm Al + 0.25 mm Copper (Cu) + 0.75 mm Tin (Sn). X-rays generated without any filters (F0 case) contained a larger amount of soft X-rays. F1 was an Al filter that could preferentially remove the lower-energy X-ray photons generated from the tungsten target. F2 was a thoraeus filter designed to harden and smooth the spectrum of higher-energy kilovoltage beams generated from a tungsten target. The hardness values of the X-ray beam under these conditions were F0 < F1 < F2.
The hardness of the X-ray beam could also be characterized by the average energy, as well as the first and second half-value layers; namely, HVL1 and HVL2, of Cu. Here, HVL1 was the thickness of Cu, which reduced the filtered X-ray exposure by half, while HVL2 was the thickness of Cu, to further reduce the filtered X-ray exposure by half. The HVL1 and HVL2 values together with the mean X-ray energies for the 150-kVp X-rays from the RAD 320 X-ray irradiator for the different filter conditions were:
F0: HVL1 (Cu) = 0.0389 mm; HVL2 (Cu) = 0.101 mm; mean energy = 47.4 keV;
F1: HVL1 (Cu) = 0.143 mm; HVL2 (Cu) = 0.410 mm; mean energy = 57.3 keV;
F2: HVL1 (Cu) = 1.72 mm; HVL2 (Cu) = 2.02 mm; mean energy = 102 keV.
Since different filters were employed, in order to maintain similar dose rates in all experiments, different currents were set. For the F0 case, the current was set as 1 mA and the dose rate was ∼33 mGy/min. For the F1 case, the current was set as 2 mA and the dose rate was ∼46 mGy/min. For the F2 case, the current was set as 12.5 mA and the dose rate was ∼ 32 mGy/min. The dose rates were monitored using a PTW UNIDOSE Universal Dosemeter (SN006861, PTW, Freiburg, Germany).
For each X-ray hardness condition (f), six different doses (d) were studied. For each experiment, dechorionated zebrafish embryos at 5 hpf were divided into 7 groups (with 10 embryos each), which were referred to as the (a) control group, (b) 5 mGy group, (c) 10 mGy group, (d) 15 mGy group, (e) 25 mGy group, (f) 50 mGy group and (g) 100 mGy. Throughout the experiments, the embryos were kept at room temperature. After irradiation, the embryos were incubated at 28.5°C until 24 hpf for the analysis.
Quantification of apoptosis by vital dye acridine orange (AO) staining
In the present study, the number of apoptotic events on the embryos at 24 hpf was employed as the biological endpoint, which has been commonly adopted for studying radiation-induced effects on zebrafish embryos [16]. Briefly, the embryos were transferred into a culture medium with 2 μg/ml of the vital dye acridine orange (AO) (Sigma, St. Louis, MO, USA) to stain for 45 min. During staining, in order to minimize fading of the AO color, the embryos were kept in a dark environment. Then embryos were washed twice thoroughly using deionized water to remove excessive AO. After anesthetizing the embryos using 0.0016 M tricaine (Sigma, St. Louis, MO, USA), three images with focuses on different sections of each anesthetized embryo were captured using SpotBasic (SPOT 4.7, Diagnostic Instruments Inc., Sterling Heights, MI, USA). The apoptotic events appeared as bright green dots under a fluorescent microscope with a magnification of 40×. Images captured for different sections of an embryo were combined into a single image for determination of the total number of apoptotic events.
Statistical analysis
For each X-ray hardness condition (f) and for each X-ray dose (d), a total of three sets of experiments were carried out on different days (k). Furthermore, a control was also prepared on each day of the experiments. The number of apoptotic events in an embryo irradiated on day k with a dose d under the X-ray hardness condition f was denoted as (NI)kfd, while the number of apoptotic events on a control embryo on day k was denoted as (NC)k. In the present study, normalized data were used to account for the different (NC)k values on different days (k) [30–33]. On a particular day k, the background number Bk of apoptotic events was taken as the average number of apoptotic events on control embryos, i.e. Bk = <(NC)k>. For each filter f and for each X-ray dose d, the normalized mean number of apoptotic events for an irradiated sample was then given by (N')fd = <((NI)kfd –Bkf)/Bkf>, where the data from all days k have been included to calculate the mean value. All data were expressed as the normalized mean apoptotic events ((N')fd) ± standard error of the mean (SEM). The statistical significance for differences between the control group and a specific treatment group was obtained using the t-test. Cases with P < 0.05 were considered to correspond to statistically significant differences between the compared groups.
RESULTS
Dose response for F0
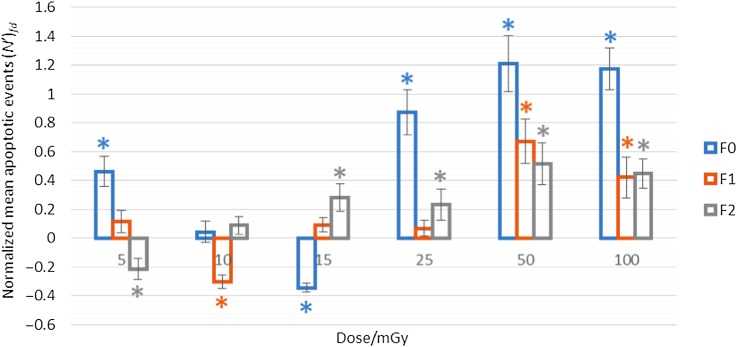
Table 1 shows the normalized mean number of apoptotic events (N')fd obtained in zebrafish embryos at different doses (d) for F0. An inhibition effect (defined as a positive (N')fd value) occurred at 5 mGy, which was statistically significant when compared to the control group. However, the effect became insignificant at the X-ray dose of 10 mGy. Notably, a stimulation effect (defined as a negative (N')fd value) occurred at 15 mGy, which was statistically significant. This stimulation effect was also referred to as the hormetic effect in the present paper. At 25 mGy and beyond, the inhibition effects reappeared again, which were statistically significant. In other words, the TDR was present for the F0 case.
Table 1.
Normalized mean number of apoptotic events (N')fd (± SEM) obtained in zebrafish embryos at different doses (d) for F0, F1 and F2. n was the number of zebrafish embryos in a particular sample. *Cases with P < 0.05 are asterisked and regarded as corresponding to statistically significant differences
| d | 5 mGy | 10 mGy | 15 mGy | 25 mGy | 50 mGy | 100 mGy | |
|---|---|---|---|---|---|---|---|
| F0 | (N')fd | 0.46 ± 0.11 (n = 23) | 0.04 ± 0.07 (n = 25) | −0.34 ± 0.03 (n = 23) | 0.87 ± 0.16 (n = 24) | 1.21 ± 0.19 (n = 22) | 1.17 ± 0.14 (n = 23) |
| p | 2.68 × 10–4* | 0.32 | 1.14 × 10–5* | 8.82 × 10–6* | 1.82 × 10–6* | 1.99 × 10–8* | |
| F1 | (N')fd | 0.12 ± 0.08 (n = 23) | −0.30 ± 0.05 (n = 26) | 0.09 ± 0.05 (n = 21) | 0.07 ± 0.05 (n = 26) | 0.67 ± 0.15 (n = 28) | 0.42 ± 0.14 (n = 23) |
| p | 0.13 | 1.09 × 10–4* | 0.13 | 0.21 | 1.66 × 10–4* | 7.00 × 10–3* | |
| F2 | (N')fd | −0.21 ± 0.08 (n = 25) | 0.09 ± 0.06 (n = 28) | 0.28 ± 0.10 (n = 28) | 0.23 ± 0.11 (n = 28) | 0.52 ± 0.15 (n = 27) | 0.45 ± 0.10 (n = 28) |
| p | 0.017* | 0.17 | 0.011* | 0.032* | 1.52 × 10–3* | 4.36 × 10–4* |
Dose response for F1
Table 1 also shows the normalized mean number of apoptotic events (N')fd obtained in zebrafish embryos at different doses (d) for F1. An inhibition effect occurred at 5 mGy but without statistical significance. Notably, a stimulation effect occurred at 10 mGy, which was statistically significant. At 15 mGy and beyond, the inhibition effects reappeared again, which were statistically insignificant at 15 and 25 mGy, and statistically significant at 50 and 100 mGy. In other words, a response similar to the TDR was observed for the F1 case, but the apoptotic level in the first zone was not significantly different from the background level.
Dose response for F2
Table 1 also shows the normalized mean number of apoptotic events (N')fd obtained in zebrafish embryos at different doses (d) for F2. A stimulation effect occurred at 5 mGy, which was statistically significant. At 10 mGy and beyond, the inhibition effects were present, which were statistically insignificant at 10 mGy, and statistically significant at 15 mGy and beyond. In contrast to the cases for F0 and F1, TDR was not observed, and only a BDR was observed.
The raw data have been presented in Tables S1 to S4 as Supplementary Information. Figure 1 summarizes the dose responses in the zebrafish embryos induced by 150 kV X-rays under the filtration conditions of F0, F1 and F2. Representative images of stained embryos for F0, F1 and F2 are shown in Figure 2. Apparently, the hormetic zone was shifted towards lower doses with the application of filters. Moreover, the normalized mean number of apoptotic events (N')fd, which surrogated the biological effects, confirmed that the amount of apoptotic events did not solely depend on the absorbed X-ray dose, but also on the hardness of the X-ray beam.
Fig. 1.
Normalized mean apoptotic events (N')fd induced by 150 kV X-rays under the different filtration conditions of F0, F1 and F2, with respect to different X-ray doses. For each filtration condition, three sets of experiments were performed and the normalized data were pooled together. Cases with P < 0.05 are asterisked and regarded as corresponding to statistically significant differences.
Fig. 2.
Representative images of stained embryos for (a) F0 (NC)k, (b) F0 (NI)kf50, (c) F1 (NC)k, (d) F1 (NI)kf50, (e) F2 (NC)k and (f) F2 (NI)kf50. Images of embryos were captured using a fluorescent microscope with 40× magnification.
Although the number of apoptotic events appeared to be larger at 50 mGy than 100 mGy under all filtration conditions, not all the differences were statistically significant. The P values obtained using the two-tailed Student's t-test for the differences under F0, F1 and F2 conditions were 0.88, 0.26 and 0.71, respectively. The insignificant difference between the number of apoptotic signals for 50 and 100 mGy was likely due to the insufficient separation of the doses. We had previously studied the dose response for a much larger separation of the dose above the hormetic region (up to 3 Gy) and indeed noted that the number of apoptotic signals (revealed using TUNEL assay) increased with the X-ray dose up to 3 Gy [34].
DISCUSSION
The low-dose responses in zebrafish (D. rerio) embryos induced by 150 kV X-rays with different hardness values were examined. Our results suggested that TDR was common, which comprised an ultra-low-dose inhibition, low-dose stimulation and high-dose inhibition, as originally discovered by Hooker et al. [11] using X-rays and subsequently confirmed by our group using microbeam protons [12]. Our results also suggested that the hormetic zone (or the stimulation zone) was shifted towards lower doses with the application of filters. The subhormetic inhibition effect only began to appear at the dose of 5 mGy, although not significantly with the application of filter F1, while the effect did not even start to appear at the dose of 5 mGy with the application of filter F2. To be compatible with the view that the hormetic zone was shifted towards lower doses with the application of filters, it was expected that statistically significant inhibition effects might occur at doses <5 mGy. This conjecture also agreed with the results of Hooker et al., which revealed the dose ranges for the subhormetic, hormetic and toxic zones as 5–10 μGy, 1–10 mGy and >100 mGy, respectively [11]. In particular, the dose range (1–10 mGy) of their hormetic zone was commensurate with the dose ranges of the hormetic zones identified in the present work; namely, 5–15 mGy and <10 mGy under the F1 and F2 conditions, respectively. As such, the subhormetic zone predicted at doses <5 mGy from the data in the present paper agreed with that found as 5–10 μGy by Hooker et al. [11]. The X-ray photons employed by Hooker et al. [11] had a HVL (Cu) of 3 mm, which was larger than the HVL1 (Cu) of 1.72 mm for the hardest X-ray beams under the F2 condition in the present paper. The shifting of the hormetic zone (or the stimulation zone) towards smaller doses for harder X-rays proposed in the present paper was strongly supported by the results of Hooker et al. [11].
On the other hand, TDR was also revealed by our group through TUNEL assay of zebrafish embryos irradiated with microbeam protons at the two-cell stage (0.75 hpf). The subhormetic, hormetic and toxic zones were identified at doses <30 mGy, 30–60 mGy and >90 mGy, respectively [12]. However, unlike the case for X-ray irradiation, deposition of proton energy is highly non-uniform. It is well established that energy deposition is most significant towards the end of the proton range. The range of 3.37-MeV protons in water was about 180 μm. Since the thickness of a cell in the two-cell stage zebrafish embryo was larger than 250 μm, and since the protons came from the bottom of the cells, it is likely that maximum energy deposition (at the Bragg peak) occurred above the cell nucleus, and the dose received by the nucleus could be smaller than that in the case of uniform energy deposition. As such, for the same X-ray and proton doses, energy deposition from protons in the cell nuclei in a two-cell stage zebrafish embryo could be smaller. If the mid-value of the hormetic zone was scaled down to 10 mGy, the hormetic zone would correspond to doses <6.7 mGy, which, again, was compatible with the current data.
The current results thus show that TDR is likely a common phenomenon in living organisms irradiated by X-rays. The non-detection of TDR in previous experiments can likely be attributed to the use of hard X-rays (e.g. using filters), which shifted the hormetic zone into an unmonitored ultra-low-dose regime (e.g. <5 mGy as demonstrated in the present paper, or even into the μGy range as revealed by Hooker et al., 2004). In such cases where the subhormetic zone was missed, BDR would be reported. However, if the hormetic zone was also missed, i.e. if the responses at doses less than ∼20 mGy were not monitored, even the BDR would not be noticed. The TDR, or the BDR, did not fit the LNT hypothesis. However, these low-dose responses are very important for the purpose of radiological protection, since these low doses are relevant to realistic environmental exposures.
Our current results also confirm that apoptotic events do not solely depend on the absorbed X-ray dose, but also on the hardness of the X-ray beam. Arntzen and Krebs have demonstrated that there was a stimulatory effect on P. sativum (Victoria-peas) when an X-ray filter was used, while the absence of the filter led to no stimulatory effect [13]. Apparently, the dose used in that study and in the peas system would correspond to the dose of ∼5 mGy in our zebrafish (D. rerio) embryo system. In other words, the dose employed by the authors fell into the hormetic zone when an X-ray filter was used and the subhormetic zone when no X-ray filter was used. At this dosage, from our results as shown in Fig. 1, the use of softer X-rays would decrease the stimulatory effect and increase the inhibitory effect. This agrees with the observation of Arntzen and Krebs that the inhibitory effect on peas increased when a thinner filter was used, which increased the proportion of the softer X-rays [13].
There are some overlaps between hormesis in the ‘triphasic dose responses’ reported in this paper, and Increased Radio-Resistance (IRR) and Hyper-Radio-Sensitivity (HRS). IRR and HRS were previously defined with reference to the common linear-quadratic (LQ) survival curves for cell populations exposed to ionizing radiations (e.g. [35]). HRS referred to sub-LQ survival values, while IRR referred to the abrupt return of response to the LQ values. As described in the Introduction, hormetic responses are biphasic dose responses demonstrating low-dose stimulation and high-dose inhibition [8–10]. Bonner [35] noted that interaction of HRS and IRR, among other phenomena, could lead to hormesis. The author also remarked that identifying the cellular and molecular mechanisms underlying HRS and IRR could aid better understanding of hormetic effects.
More recently, Dong et al. also studied how the energy of X-ray photons and the exposure time affected the apoptotic events in early X. laevis embryos [14]. The authors concluded that for the same absorbed dose, the response was enhanced when higher-energy X-rays were employed. It was noticed that the doses involved in that study were >10 Gy, i.e. at least two orders of magnitude larger than the doses involved in the present study. For such high doses, low-dose responses such as BDR or TDR would not be anticipated. However, one interesting observation was that in the dose range from 25 to 100 mGy (i.e. above the hormetic zone), the dose response caused by softer X-rays (without filters) was much larger than those caused by harder X-rays (with filters). The reasons behind the transition to the enhanced response for higher-energy X-rays in the high-dose regime are still not understood. It would be pertinent to explore the dose regime in which the transition would take place, and to study the mechanisms underlying such a transition in the future.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Radiation Research online.
FUNDING
Funding to pay the Open Access publication charges for this article was provided by the State Key Laboratory in Marine Pollution, City University of Hong Kong, Hong Kong.
Supplementary Material
ACKNOWLEDGMENTS
The present work was supported by a research grant from the State Key Laboratory in Marine Pollution, City University of Hong Kong.
REFERENCES
- 1.Hosoi Y, Sakamoto K. Suppressive effect of low dose total body irradiation on lung metastasis: dose dependency and effective period. Radiother Oncol 1993;26:177–9. [DOI] [PubMed] [Google Scholar]
- 2.Ishii K, Hosoi Y, Yamada S et al. Decreased incidence of thymic lymphoma in AKR mice as a result of chronic, fractionated low-dose total-body X irradiation. Radiat Res 1996;146:582–5. [PubMed] [Google Scholar]
- 3.Mitchel RE, Jackson JS, McCann RA et al. The adaptive response modifies latency for radiation-induced myeloid leukemia in CBA/H mice. Radiat Res 1999;152:273–9. [PubMed] [Google Scholar]
- 4.Redpath JL, Lu Q, Lao X et al. Low doses of diagnostic energy X-rays protect against neoplastic transformation in vitro. Int J Radiat Biol 2003;79:235–40. [DOI] [PubMed] [Google Scholar]
- 5.Elmore E, Lao XY, Ko M et al. Neoplastic transformation in vitro induced by low doses of 232 MeV protons. Int J Radiat Biol 2005;81:291–7. [DOI] [PubMed] [Google Scholar]
- 6.Moskalev AA, Plyusnina EN, Shaposhnikov MV. Radiation hormesis and radioadaptive response in Drosophila melanogaster flies with different genetic backgrounds: the role of cellular stress-resistance mechanisms. Biogerontology 2011;12:253–63. [DOI] [PubMed] [Google Scholar]
- 7.Phan N, De Lisio M, Parise G et al. Biological effects and adaptive response from single and repeated computed tomography scans in reticulocytes and bone marrow of C57BL/6 mice. Radiat Res 2012;177:164–75. [DOI] [PubMed] [Google Scholar]
- 8.Calabrese EJ, Baldwin LA. Defining hormesis. Hum Exp Toxicol 2002;21:91–7. [DOI] [PubMed] [Google Scholar]
- 9.Calabrese EJ. Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem 2008;27:1451–74. [DOI] [PubMed] [Google Scholar]
- 10.Calabrese EJ, Linda AB. Toxicology rethinks its central belief. Nature 2003;421:691–2. [DOI] [PubMed] [Google Scholar]
- 11.Hooker AM, Bhat M, Day TK et al. The linear no threshold model does not hold for low-dose ionizing radiation. Radiat Res 2004;162:447–52. [DOI] [PubMed] [Google Scholar]
- 12.Choi VWY, Yum EHW, Konishi T et al. Triphasic low-dose response in zebrafish embryos irradiated by microbeam protons. J Radiat Res 2012;53:475–81. [PubMed] [Google Scholar]
- 13.Arntzen L, Krebs C. Investigation into the biological effect of filtered and unfiltered X rays, as measured on peas. Acta Radiologica 1925;4:5–31. [Google Scholar]
- 14.Dong JJ, Mury SP, Drahos KE et al. Shorter exposures to harder X-rays trigger early apoptotic events in Xenopus laevis embryos. PLoS ONE 2010;5:e8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genetic 2007;8:353–67. [DOI] [PubMed] [Google Scholar]
- 16.Choi VWY, Yu KN. Embryos of the zebrafish Danio rerio in studies of non-targeted effects of ionizing radiation. Cancer Lett 2015;356:91–104. [DOI] [PubMed] [Google Scholar]
- 17.Yum EHW, Cheng SH, Yu KN. Zebrafish embryos for studying radiation response in vivo. J Radiat Res 2009;50:Supplement A, A93. [Google Scholar]
- 18.Yum EHW, Choi VWY, Nikezic D et al. Alpha-particle-induced bystander effects between zebrafish embryos in vivo. Radiat Meas 2009;44:1077–80. [Google Scholar]
- 19.Choi VWY, Cheng SH, Yu KN. Radioadaptive response induced by alpha-particle-induced stress communicated in vivo between zebrafish embryos. Environ. Sci Technol 2010;44:8829–34. [DOI] [PubMed] [Google Scholar]
- 20.Choi VWY, Wong MYP, Cheng SH et al. Dosimetric study of radioadaptive response of zebrafish embryos using PADC-film substrates. Radiat Meas 2011;46:1795–8. [Google Scholar]
- 21.Choi VWY, Ng CYP, Cheng SH et al. α-Particle irradiated zebrafish embryos rescued by bystander unirradiated zebrafish embryos. Environ Sci Technol 2012;46:226–31. [DOI] [PubMed] [Google Scholar]
- 22.Kong EY, Choi VWY, Cheng SH et al. Some properties of the signals involved in unirradiated zebrafish embryos rescuing α-particle irradiated zebrafish embryos. Int J Radiat Biol 2014;90:1133–42. [DOI] [PubMed] [Google Scholar]
- 23.Ng CYP, Kong EY, Kobayashi A et al. Neutron induced bystander effect among zebrafish embryos. Radiat Phys Chem 2015;117:153–9. [Google Scholar]
- 24.Howe K, Clark MD, Torroja CF et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013;496:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbazuk WB, Korf I, Kadavi C et al. The syntenic relationship of the zebrafish and human genomes. Genome Res 2000;10:1351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi VWY, Lam RKK, Chong EYW et al. Designing experimental setup and procedures for studying alpha-particle-induced adaptive response in zebrafish embryos in vivo. Nucl Instr Meth B 2010;268:651–6. [Google Scholar]
- 27.Bao S, Wu Q, Mclendon RE et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006;444:756–60. [DOI] [PubMed] [Google Scholar]
- 28.Mok MTS, Henderson BR. The in vivo dynamic interplay of MDC1 and 53BP1 at DNA damage-induced nuclear foci. Int J Biochem Cell Biol 2012;44:1398–409. [DOI] [PubMed] [Google Scholar]
- 29.Yoo H, Kang JW, Lee DW et al. Pyruvate metabolism: a therapeutic opportunity in radiation-induced skin injury. Biochem Biophys Res Commun 2015;460:504–10. [DOI] [PubMed] [Google Scholar]
- 30.Choi VWY, Ng CYP, Kong MKY et al. Adaptive response to ionizing radiation induced by cadmium in zebrafish embryos. J Radiol Prot 2013;33:101–12. [DOI] [PubMed] [Google Scholar]
- 31.Choi VWY, Ng CYP, Kobayashi A et al. Bystander effect between zebrafish embryos in vivo induced by high-dose X-rays. Environ Sci Technol 2013;47:6368–76. [DOI] [PubMed] [Google Scholar]
- 32.Ng CYP, Choi VWY, Lam ACL et al. Multiple stressor effect in zebrafish embryos from simultaneous exposures to ionizing radiation and cadmium. J Radiol Prot 2013;33:113–21. [DOI] [PubMed] [Google Scholar]
- 33.Ng CYP, Pereira S, Cheng SH et al. Combined effects of depleted uranium and ionising radiation on zebrafish embryos. Radiat Prot Dosim 2015;167:311–15. [DOI] [PubMed] [Google Scholar]
- 34.Choi VWY, Konish T, Oikawa M et al. Adaptive response in zebrafish embryos induced using microbeam protons as priming dose and x-ray photons as challenging dose. J Radiat Res 2010;51:657–64. [DOI] [PubMed] [Google Scholar]
- 35.Bonner WM. Phenomena leading to cell survival values which deviate from linear-quadratic models. Mutation Research 2004;568:33–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.