Abstract
We previously reported that the local control of pulmonary metastases from colorectal cancer (CRC) following stereotactic body radiotherapy (SBRT) with moderate prescription dose was relatively worse. We investigated the treatment outcomes and toxicities of patients with oligometastases from CRC treated by SBRT using risk-adapted, very high- and convergent-dose regimens. Among patients referred for SBRT from August 2011 to January 2015, those patients were extracted who had liver or pulmonary metastases from CRC, and they were treated with a total dose of 50–60 Gy in five fractions prescribed to the 60% isodose line of the maximum dose covering the surface of the planning target volume. Concurrent administration of chemotherapy was not admitted during SBRT, while neoadjuvant or adjuvant chemotherapy was allowed. A total of 21 patients (12 liver, 9 lung) with 28 oligometastases were evaluated. The median follow-up duration was 27.5 months (range: 6.5–43.3 months). Four patients were treated with SBRT as a series of initial treatments, and 17 patients were treated after recurrent oligometastases. The local control rates at 1 and 2 years from the start of SBRT were 100%. The disease-free and actuarial overall survival rates were 62% and 55%, and 79% and 79%, respectively. No severe toxicities (≥grade 3) occurred during follow-up. The outcomes following high-dose SBRT were excellent. This treatment can provide an alternative to the surgical resection of oligometastases from CRC. Prospective studies are needed to validate the effectiveness of SBRT.
INTRODUCTION
Patients with colorectal cancer (CRC) often have metastases at initial presentation or during follow-up; 20% have metastases at initial presentation, and an additional 25% to 50% develop metastases after treatment for early-stage disease [1, 2]. The major sites of metastases include the liver (60% to 71%) and lung (25% to 40%) [2].
The new cytotoxic and molecular targeting agents have greatly prolonged the progression-free and overall survival (OS) of CRC patients with stage IV disease [3, 4]. The prolonged survival of patients has highlighted the importance of local therapy for CRC patients with limited metastatic disease. Resection combined with chemotherapy has provided decent outcomes for patients despite stage IV disease [5–7].
Stereotactic body radiotherapy (SBRT) is a high-precision conformal external-beam radiation technique that ablates targets at extracranial sites by delivering hypofractionated high-dose radiation while sparing the normal surrounding tissue. SBRT is associated with minimal morbidity and provides high rates of local control for medically inoperable stage I non-small-cell lung cancer (NSCLC) [8]. SBRT is currently considered to be a treatment option for patients with medically inoperable, early-stage NSCLC [9]. Additionally, SBRT has achieved excellent local control and survival for patients with hepatocellular carcinoma [10].
The use of SBRT for patients with oligometastases has been studied retrospectively, and many retrospective study reports have been published. However, the role of SBRT is still under investigation. We have treated patients with pulmonary oligometastases using SBRT with a total dose of 50 Gy in five fractions (50 Gy/5 fr-[80% isodose]). However, the local control (LC) rate was poor for patients with pulmonary oligometastases from CRC, suggesting the need for an increased dose [11]. We performed a study to determine the optimal isodose level for delivering a higher dose to the target while minimizing the dose to the surrounding tissue, and found that a 60% isodose was optimal [12]. Since 2011, we have treated patients with liver and lung tumors using SBRT with risk-adapted, very high- and convergent-dose regimens. In this retrospective study, we evaluated the data of patients with liver or pulmonary oligometastases from CRC who were treated by SBRT.
METHODS AND MATERIALS
Study design
This was a retrospective study of patients with liver or pulmonary oligometastases from CRC, who were treated for the metastases by SBRT between August 2011 and January 2015 at our institution. Data were retrieved from our clinical practice database. Informed consent for SBRT was obtained from all patients, and our institutional review board approved data collection and analysis.
Patients
The study patients satisfied the following criteria: histological diagnosis of primary colorectal adenocarcinoma was verified from prior radical surgery; confined liver or pulmonary metastases were diagnosed by computed tomography (CT) or magnetic resonance imaging (MRI), revealing new nodules or nodules that had grown larger during follow-up; there were one to three metastases; the maximum diameter of tumors was 5–50 mm; no history of other metastases; and Eastern Cooperative Oncology Group performance status (PS) 0–1.
Treatment
We have previously described our methods of SBRT delivery [13, 14]. In short, for treatment planning, the patient was immobilized by a vacuum cushion and abdominal corset, and then underwent long-scan-time CT used for direct visualization of the internal target volume (ITV). No active motion management or respiratory gating was performed for any patient.
The planning target volume (PTV) was determined by adding a margin of 6–8 mm to the ITV. Treatment-planning methods and systems and the calculation algorithm consisted of the following: multi-arc dynamic conformal radiation with eight arcs, FOCUS XiO version 4.2.0–4.3.3 (Computerized Medical Systems, St Louis, MO, USA) and a multigrid superposition algorithm with heterogeneity correction; or volumetric modulated arc therapy with three arcs, Eclipse version 4.2.0–4.3.3 (Varian Medical Systems, Palo Alto, CA, USA) and an Acuros XB algorithm with heterogeneity correction.
SBRT was delivered using risk-adapted total doses in five fractions depending on the location of the metastasis, using either a total dose of 60 Gy or 50 Gy on 5 consecutive days. These prescription doses were set to enclose the PTV surface by the 60% isodose line of the maximum dose. The maximum doses were 100 Gy and 83 Gy in a total dose of 60 Gy and 50 Gy, respectively. For patients with pulmonary metastases, those with peripheral tumors where the PTV did not overlap the chest wall were treated by 60 Gy/5 fr-(60% isodose); those with peripheral tumors where the PTV overlapped the chest wall and those with central tumors where the PTV did not overlap the hilar pulmonary vein or main bronchus were treated by 50 Gy/5 fr-(60% isodose).
For patients with liver metastases, those with liver tumors where the PTV did not overlap the main or right portal vein, or common or right bile duct, were treated by 60 Gy/5 fr-(60% isodose). Other patients were treated by 50 Gy/5 fr-(60% isodose).
The following patients, who were treated by less than these previously described doses, were excluded from the analysis: those patients with centrally located pulmonary metastases where the PTV overlapped the hilar pulmonary vein or main bronchus, or those with normal liver receiving ≥20 Gy exceeding 40% if treated by 50 Gy/5 fr-(60% isodose).
Concurrent administration of chemotherapy was not admitted during SBRT, while neoadjuvant or adjuvant chemotherapy was allowed.
Follow-up and statistical analysis
Follow-up CT scans were performed at 1 and 3 months after SBRT and then at 3-month intervals during the first 2 years for all patients. Subsequent follow-up CT scans were obtained at 4- to 6-month intervals.
Local control (LC) was defined as freedom from local progression according to the response evaluation criteria in solid tumors (RECIST). Toxicity was evaluated using the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Survival curves were constructed using Kaplan–Meier analysis. Data were analyzed using SPSS Statistics 20.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Eligible patients and tumors
A total of 21 patients were eligible for this study. Of these, 12 patients had liver metastases (single nodule in 10 patients, 2 nodules in 1, 3 nodules in 1), and 9 patients had pulmonary metastases (single nodule in 6 patients, 2 nodules in 2, 3 nodules in 1). None of the patients had simultaneous liver and pulmonary metastases. The characteristics of patients and tumors are presented in Table 1. The median follow-up time from SBRT was 27.5 months (range: 6.5–43.3). Four patients were treated with SBRT as a series of initial treatments, and 17 patients were treated after recurrent oligometastases. For the patients with recurrent oligometastases, the median duration between initial treatment and SBRT was 30.1 months (range: 5.3–83.8). Eighteen of 21 patients (86%) received chemotherapy: 15 at initial treatment and 9 at recurrent treatment.
Table 1.
Patient characteristics
| Age (year), median (range) | 72 (38–85) | ||
| Male/female | 15/6 | ||
| Colon/rectum | 16/5 | ||
| Lesion site/numbers | Liver | 1 | 10 |
| 2 | 1 | ||
| 3 | 1 | ||
| Lung | 1 | 6 | |
| 2 | 2 | ||
| 3 | 1 | ||
| Size of maximum diameter (mm), median (range) | 14 (5–50) | ||
| CEA level before SBRT | Normal (≤5 ng/ml) | 14 | |
| Slightly high (5–10 ng/ml) | 4 | ||
| High (≤10 ng/ml) | 3 | ||
| Interval between the initial treatment and SBRT, median (range) (months) | 30.1(5.1–83.8) | ||
| Follow-up duration from SBRT, median (range) (months) | 27.5(6.5–43.3) | ||
| Total dose (BED10 Gy) | 60 Gy/5 fr (132 Gy10) | 15 | |
| 50 Gy/5 fr (100 Gy10) | 13 | ||
| Usage of chemotherapy | Yes/no | 18/3 | |
| As an initial treatment | 15 | ||
| As a treatment for recurrence | 9 | ||
CEA, Carcinoembryonic antigen; SBRT, stereotactic body radiotherapy; BED, biological effective dose.
Local control and overall survival
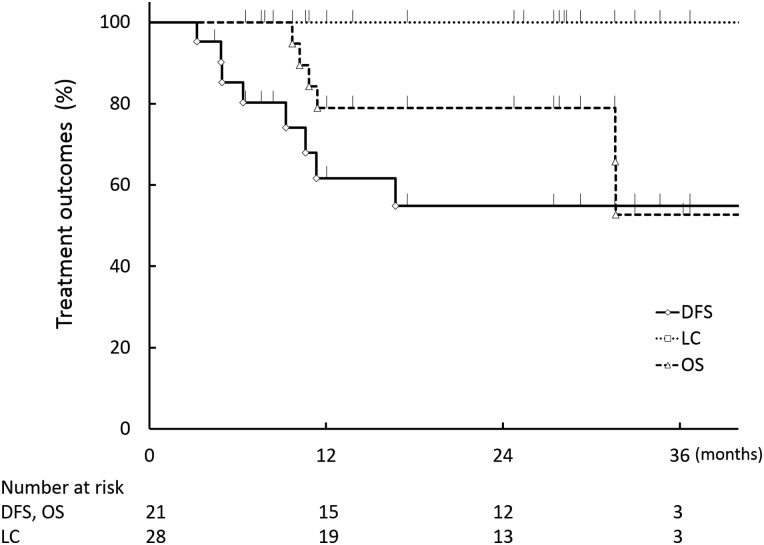
At the time of analysis, 4, 1, 5 and 11 patients were dead from CRC, dead for another reason, alive with disease and alive without disease, respectively. None of the oligometastases treated by SBRT was found to have progressed on CT imaging during the follow-up period. The LC rates at 1 and 2 years from the start of SBRT were 100% (95% confidence interval (CI): 89.3–100) (Fig. 1). The disease-free survival rates at 1 and 2 years from the start of SBRT were 62% (95% CI: 38.1–80.8) and 55% (95% CI: 31.7–76.1), respectively (Fig. 1). Actuarial OS rates at 1 and 2 years were 79% (95% CI: 55.4–91.9) and 79% (95% CI: 55.4–91.9), respectively (Fig. 1).
Fig. 1.
Local control (LC), disease-free survival (DFS) and overall survival (OS).
Toxicities
Among 12 patients with liver oligometastases, 3 patients developed acute grade 1 toxicities, including general fatigue in 3 and fever in 1 patient. Among 9 patients with pulmonary oligometastases, 5 and 1 patients developed acute grade 1 and grade 2 radiation pneumonitis, respectively. One patient developed chronic grade 2 toxicities, including intercostal neuralgia and rib fracture. None of the patients developed grade ≥3 toxicities.
DISCUSSION
The mainstay of treatment for patients with oligometastases confined to the liver or lung is resection combined with chemotherapy, as recommended by the National Comprehensive Cancer Network (NCCN) [15].
For patients with confined liver metastases from CRC, combined therapy has resulted in 5-year overall survival rates of 25% to 50% [5–7]. The cumulative 3-year LC rates following resection of solitary liver metastases were reported to range from 88% to 95% [16, 17]. However, the option of metastasectomy is often limited by an unfavorable anatomical metastatic site, poor function of the remaining hepatic parenchyma and/or poor general patient condition due to advanced age or multiple previous chemotherapy regimens. Actually, hepatic metastases are only resectable in about 20% of patients [18].
For patients with confined pulmonary metastases from CRC, the crude LC rates following resection were reported to be 72% to 80% [19–21]. Synchronous and metachronous pulmonary metastases were resectable in 28% and 42% of surgical patients, respectively [22]. The 5-year overall survival rates of patients with resected metachronous pulmonary metastases were reported to range from 39% to 50% [19, 20].
SBRT is increasingly being considered as a therapeutic option for patients with liver or pulmonary oligometastases. The 2-year LC rates have ranged from 53% to 100% [11, 23–37], and were higher for patients treated with high-dose regimens in two studies [23, 38]. The 2-year OS rates varied from 30% to 86%.
SBRT is less invasive than surgery and has resulted in less deterioration in quality of life (QOL) [39, 40]. Little toxicity was observed; for example, radiation-induced liver disease and gastrointestinal toxicities were not found in several studies [23–27]; and grade ≥3 radiation pneumonitis was observed in 0% to 8% of patients [11, 28–37].
Significant deterioration in health-related QOL has been observed after surgery for patients with stage I NSCLC [41] and liver metastases [42]. By contrast, clinically relevant deterioration has not been observed after SBRT for patients with stage I NSCLC [39] and liver malignancies [40].
Minimally invasive treatments leading to minimal deterioration in QOL are advantageous, especially for patients with oligometastatic disease, since patients with oligometastases from CRC are often treated by chemotherapy and are in poor physical condition. In addition, even with good control of oligometastatic disease, other metastases often recur; and only half of the patients with stage IV CRC survive longer than 5 years. Widder et al. [36] compared the outcomes of patients with pulmonary oligometastases after SBRT with those after pulmonary metastasectomy. Patients were offered pulmonary metastasectomy as the first choice, and SBRT was suggested for patients who were considered to be less suitable surgical candidates. Patients treated by SBRT had more unfavorable prognostic factors. They were significantly older, had a shorter metastasis-free interval and a different distribution of original primary tumors, and therefore they were regarded as having a worse overall prognosis. Despite the selection bias, survival after SBRT was no worse than survival after pulmonary metastasectomy. Prospective comparative studies are therefore required to define the roles of SBRT and pulmonary metastasectomy in oligometastatic disease.
Although the outcomes of SBRT for oligometastases are generally encouraging, many studies have employed a variety of treatment methods, total doses and fractions [11, 23–37]. In addition, many of the retrospective studies reported results on liver and pulmonary oligometastases originating from various primary cancers. If the prescription methods and doses used for SBRT are optimized and standardized, and, subsequently, LC after SBRT is proved to be noninferior to LC after resection, SBRT may be superior to resection because it is minimally invasive and is associated with little deterioration in QOL.
In general, the LC rates of SBRT for oligometastases have been favorable [11, 43]. However, the LC rates of CRC oligometastases were worse than the LC rates of oligometastases from other primary tumors [11, 38, 43, 44]. Before 2010, we treated primary lung cancer and pulmonary oligometastases by SBRT, using 50 Gy/5 fr-(80%-isodose). In our previous analysis, the respective 2- and 3-year LC rates were 73% and 44% for pulmonary oligometastases from CRC, which were significantly worse than the 2- and 3-year LC rates (both 94%) for pulmonary oligometastases from other primaries. Multivariate analysis revealed that the only significant prognostic factor was CRC tumor origin [11]. Thibault et al. [43] reported very similar results using a total dose of 48–60 Gy/4–5 fr. The 2-year LC rate was 76% for oligometastases from CRC, and significantly worse than the 2-year LC rate from other primaries (91%). Singh et al. [44] treated 34 patients, which included 13 colorectal cancer patients, with pulmonary oligometastases, using SBRT with a total dose of 45–60 Gy/5 fr-(80–100% isodose). Five patients recurred and they all had colorectal cancer.
Even when the prescription doses are the same value, the actual dose delivered to the tumor varies markedly, because there are various methods for determining isodose values. Isodose values are given as percentages of the maximum dose within the PTV. In general, as an isodose value decreases, the dose to the central portion of the PTV increases. In order to increase the dose to the target while minimizing the dose volume parameters of the normal adjacent tissue, we previously determined the most suitable isodose value in clinical treatment plans of SBRT. We found that a 60%-isodose plan was the best plan for treating liver and pulmonary oligometastases [12, 45]. The prescription dose is almost the minimum PTV dose, which is equivalent to the 60%-isodose line of the maximum dose. However, the center of the PTV actually receives a much higher dose than the prescribed dose, because the 60%-isodose plan has a very steep dose gradient. For example, 90–100 Gy is delivered to the center of the PTV with a prescription of 60 Gy/5 fr-(60% isodose). Actually, the mean dose to the ITV with a 60%-isodose plan is approximately 155% of the prescription dose [12], which was 93 Gy with the prescription of 60 Gy/5 fr-(60% isodose), and approximately double score in calculating the biological effective dose with α/β of 10 (BED10) (266 vs. 132). Meanwhile, the mean doses and BED10 to the ITV with an 80%-isodose were smaller (approximately 119% and 131%, respectively) than the doses with a 60%-isodose [12]. The increased dose to the central PTV may contribute to increased local control. Indeed, a comparison of the results of this study with those of our previous study revealed apparent differences in the LC rate achieved by our previous prescription of 50 Gy/5 fr-(80%-isodose) and this study's risk-adapted prescription of 50–60 Gy/5 fr-(60%-isodose). The 3-year LC rate was 100% for the study using 50–60 Gy/5 fr-(60%-isodose) and 73% for the study using 50 Gy/5 fr-(80%-isodose) [11]. Therefore, not only the optimal dose/fraction, but also the optimal methods of prescription, or isodose values, should be evaluated.
Another possible reason for the poor LC rates is insufficient clinical target volume (CTV) margin. This hypothesis is supported by Welter et al. [46]. They reported that 8–10 mm for pulmonary metastases (≥2 cm) must be maintained around the lesion to prevent local recurrence. However, as for non-small-cell lung cancer, good LC could be achieved with a 5-mm CTV margin [47] although Giraud et al. [48] also reported that the CTV margin must be increased to 8 mm and 6 mm for adenocarcinoma and squamous cell carcinoma, respectively, to cover 95% of the microscopic extension.
This study has limitations. It was a retrospective, single-institutional case series with a small sample size and a short follow-up period. We are now planning a prospective study using this SBRT regimen for patients with liver and pulmonary oligometastases from CRC.
In conclusion, the LC and OS of patients with liver and pulmonary oligometastases from CRC who underwent SBRT with a risk-adapted dose prescription of maximum doses of 83–100 Gy in five fractions were excellent, even though the patients were not suitable candidates for surgical resection. SBRT is a safe treatment and may be a comparable treatment option for liver and pulmonary oligometastases from CRC. Additional prospective studies are warranted to validate the efficacy of SBRT for patients with liver and pulmonary oligometastases from CRC.
REFERENCES
- 1.Khatri VP, Petrelli NJ, Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit. J Clin Oncol 2005;23 (33):8490–9. [DOI] [PubMed] [Google Scholar]
- 2.Aranda E, Abad A, Carrato A et al. Treatment recommendations for metastatic colorectal cancer. Clin Trans Oncol 2011;13 (3):162–78. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Humblet Y, Siena S et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351 (4):337–45. [DOI] [PubMed] [Google Scholar]
- 4.Giantonio BJ, Levy DE, O'Dwyer P, J et al. A phase II study of high-dose bevacizumab in combination with irinotecan, 5-fluorouracil, leucovorin, as initial therapy for advanced colorectal cancer: results from the Eastern Cooperative Oncology Group study E2200. Ann Oncol 2006;17 (9):1399–403. [DOI] [PubMed] [Google Scholar]
- 5.Rees M, Tekkis PP, Welsh FK et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247 (1):125–35. [DOI] [PubMed] [Google Scholar]
- 6.Pawlik TM, Scoggins CR, Zorzi D et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005;241 (5):715–22, discussion 22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordlinger B, Guiguet M, Vaillant JC et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 1996;77 (7):1254–62. [PubMed] [Google Scholar]
- 8.Timmerman R, Paulus R, Galvin J et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303 (11):1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chi A, Liao Z, Nguyen NP et al. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol 2010;94 (1):1–11. [DOI] [PubMed] [Google Scholar]
- 10.Sanuki N, Takeda A, Oku Y et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol 2014;53 (3):399–404. [DOI] [PubMed] [Google Scholar]
- 11.Takeda A, Kunieda E, Ohashi T et al. Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol 2011;101 (2):255–9. [DOI] [PubMed] [Google Scholar]
- 12.Oku Y, Takeda A, Kunieda E et al. Analysis of suitable prescribed isodose line fitting to planning target volume in stereotactic body radiotherapy using dynamic conformal multiple arc therapy. Pract Radiat Oncol 2012;2 (1):46–53. [DOI] [PubMed] [Google Scholar]
- 13.Takeda A, Kunieda E, Sanuki N et al. Dose distribution analysis in stereotactic body radiotherapy using dynamic conformal multiple arc therapy. Int J Radiat Oncol Biol Phys 2009;74 (2):363–9. [DOI] [PubMed] [Google Scholar]
- 14.Takeda A, Oku Y, Sanuki N et al. Feasibility study of stereotactic body radiotherapy for peripheral lung tumors with a maximum dose of 100 Gy in five fractions and a heterogeneous dose distribution in the planning target volume. J Radiat Res 2014;55 (5):988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benson AB, Grem JL, Saltz L et al. NCCN Clinical Practice Guideline in Oncology Web site. www.nccn.org/professionals/physician_gls/pdf/colon.pdf (September 13 2013, date last accessed). [Google Scholar]
- 16.Aloia TA, Vauthey JN, Loyer EM et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141 (5):460–6; discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 17.Lee WS, Yun SH, Chun HK et al. Clinical outcomes of hepatic resection and radiofrequency ablation in patients with solitary colorectal liver metastasis. J Clin Gastroenterol 2008;42 (8):945–9. [DOI] [PubMed] [Google Scholar]
- 18.Folprecht G, Grothey A, Alberts S et al. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol 2005;16 (8):1311–19. [DOI] [PubMed] [Google Scholar]
- 19.Welter S, Jacobs J, Krbek T et al. Long-term survival after repeated resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg 2007;84 (1):203–10. [DOI] [PubMed] [Google Scholar]
- 20.De Giacomo T, Rendina EA, Venuta F et al. Thoracoscopic resection of solitary lung metastases from colorectal cancer is a viable therapeutic option. Chest 1999;115 (5):1441–3. [DOI] [PubMed] [Google Scholar]
- 21.Welter S, Theegarten D, Trarbach T et al. Safety distance in the resection of colorectal lung metastases: a prospective evaluation of satellite tumor cells with immunohistochemistry. J Thorac Cardiovasc Surg 2011;141 (5):1218–22. [DOI] [PubMed] [Google Scholar]
- 22.Nozawa H, Sunami E, Nakajima J et al. Synchronous and metachronous lung metastases in patients with colorectal cancer: a 20-year monocentric experience. Exp Ther Med 2012;3 (3):449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wulf J, Guckenberger M, Haedinger U et al. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol 2006;45 (7):838–47. [DOI] [PubMed] [Google Scholar]
- 24.Rusthoven KE, Kavanagh BD, Cardenes H et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27 (10):1572–8. [DOI] [PubMed] [Google Scholar]
- 25.van der Pool AE, Mendez Romero A, Wunderink W et al. Stereotactic body radiation therapy for colorectal liver metastases. Br J Surg 2010;97 (3):377–82. [DOI] [PubMed] [Google Scholar]
- 26.Rule W, Timmerman R, Tong L et al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol 2011;18 (4):1081–7. [DOI] [PubMed] [Google Scholar]
- 27.Scorsetti M, Arcangeli S, Tozzi A et al. Is stereotactic body radiation therapy an attractive option for unresectable liver metastases? A preliminary report from a phase 2 trial. Int J Radiat Oncol Biol Phys 2013;86 (2):336–42. [DOI] [PubMed] [Google Scholar]
- 28.Wulf J, Haedinger U, Oppitz U et al. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: a noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys 2004;60 (1):186–96. [DOI] [PubMed] [Google Scholar]
- 29.Okunieff P, Petersen AL, Philip A et al. Stereotactic Body Radiation Therapy (SBRT) for lung metastases. Acta Oncol 2006;45 (7):808–17. [DOI] [PubMed] [Google Scholar]
- 30.Norihisa Y, Nagata Y, Takayama K et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys 2008;72 (2):398–403. [DOI] [PubMed] [Google Scholar]
- 31.Kim MS, Yoo SY, Cho CK et al. Stereotactic body radiation therapy using three fractions for isolated lung recurrence from colorectal cancer. Oncology 2009;76 (3):212–19. [DOI] [PubMed] [Google Scholar]
- 32.Rusthoven KE, Kavanagh BD, Burri SH et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009;27 (10):1579–84. [DOI] [PubMed] [Google Scholar]
- 33.Oh D, Ahn YC, Seo JM et al. Potentially curative stereotactic body radiation therapy (SBRT) for single or oligometastasis to the lung. Acta Oncol 2012;51 (5):596–602. [DOI] [PubMed] [Google Scholar]
- 34.Ricardi U, Filippi AR, Guarneri A et al. Stereotactic body radiation therapy for lung metastases. Lung Cancer 2012;75 (1):77–81. [DOI] [PubMed] [Google Scholar]
- 35.Inoue T, Katoh N, Onimaru R et al. Clinical outcomes of stereotactic body radiotherapy for patients with lung tumors in the state of oligo-recurrence. Pulmonary Med 2012;2012:369820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Widder J, Klinkenberg TJ, Ubbels JF et al. Pulmonary oligometastases: metastasectomy or stereotactic ablative radiotherapy. Radiother Oncol 2013;107 (3):409–13. [DOI] [PubMed] [Google Scholar]
- 37.Inoue T, Oh RJ, Shiomi H et al. Stereotactic body radiotherapy for pulmonary metastases. Prognostic factors and adverse respiratory events. Strahlenther Onkol 2013;189 (4):285–92. [DOI] [PubMed] [Google Scholar]
- 38.Vautravers-Dewas C, Dewas S, Bonodeau F et al. Image-guided robotic stereotactic body radiation therapy for liver metastases: is there a dose response relationship. Int J Radiat Oncol Biol Phys 2011;81 (3):e39–47. [DOI] [PubMed] [Google Scholar]
- 39.Lagerwaard FJ, Aaronson NK, Gundy CM et al. Patient-reported quality of life after stereotactic ablative radiotherapy for early-stage lung cancer. J Thorac Oncol 2012;7 (7):1148–54. [DOI] [PubMed] [Google Scholar]
- 40.Mendez Romero A, Wunderink W, van Os RM et al. Quality of life after stereotactic body radiation therapy for primary and metastatic liver tumors. Int J Radiat Oncol Biol Phys 2008;70 (5):1447–52. [DOI] [PubMed] [Google Scholar]
- 41.Pompili C, Brunelli A, Xiume F et al. Predictors of postoperative decline in quality of life after major lung resections. Eur J Cardiothorac Surg 2011;39 (5):732–7. [DOI] [PubMed] [Google Scholar]
- 42.Langenhoff BS, Krabbe PF, Peerenboom L et al. Quality of life after surgical treatment of colorectal liver metastases. Br J Surg 2006;93 (8):1007–14. [DOI] [PubMed] [Google Scholar]
- 43.Thibault I, Poon I, Yeung L et al. Predictive factors for local control in primary and metastatic lung tumours after four to five fraction stereotactic ablative body radiotherapy: a single institution's comprehensive experience. Clin Oncol (R Coll Radiol) 2014;26 (11):713–19. [DOI] [PubMed] [Google Scholar]
- 44.Singh D, Chen Y, Hare MZ et al. Local control rates with five-fraction stereotactic body radiotherapy for oligometastatic cancer to the lung. J Thorac Dis 2014;6 (4):369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oku Y, Takeda A, Sanuki N et al. Stereotactic ablative body radiation therapy with dynamic conformal multiple arc therapy for liver tumors: optimal isodose line fitting to the planning target volume. Pract Radiat Oncol 2014;4 (1):e7–13. [DOI] [PubMed] [Google Scholar]
- 46.Welter I, Theeqarten D, Trarbach T et al. Safety distance in the resection of colorectal lung metastases: a prospective evaluation of satellite tumor cells with immunohistochemistry. J Thorac Cardiovasc Surg 2011;141 (5):1218–22. [DOI] [PubMed] [Google Scholar]
- 47.Timmerman R, Paulus R, Galvin J et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303 (11):1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giraud P, Antoine M, Larrouy A et al. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int J Radiat Oncol Biol Phys 2000;48 (4):1015–24. [DOI] [PubMed] [Google Scholar]