Abstract
Both psychological stress (PS) and ionizing radiation (IR) cause varied detrimental effects on humans. There has been no direct evidence so far showing PS alone could cause cancer; however, long-lasting PS may affect our overall health and ability to cope with cancer. Due to their living conditions and occupations, some people may encounter concurrent exposure to both PS and IR to a high extent. In addition to possible health effects resulting directly from exposure to IR on these people, fear of IR exposure is also a cause of PS. The question of whether PS would influence susceptibility to IR, radiocarcinogenesis in particular, is of great concern by both the academic world and the public. Recently, investigations using animal PS models demonstrated that PS could modulate susceptibility to IR, causing increased susceptibility to radiocarcinogenesis in Trp53-heterozygous mice, hematological toxicity in peripheral blood and elevated chromosome aberration (dicentrics) frequency in splenocytes of Trp53–wild-type mice. To actively reduce health risk from exposure to IR, further studies are needed to cumulate more evidence and provide insights into the mechanisms underlying the alterations in susceptibility due to PS modulation. This mini-review gives a general overview of the significance of PS effects on humans and experimental animals, with a special focus on summarizing the latest weight-of-evidence approaches to radiobiological studies on PS-induced alterations in susceptibility in experimental animal models. The susceptibility being investigated is mainly in the context of the impact of the modulatory effect of PS on radiocarcinogenesis; we seek to improve understanding of the combined effects of exposure to both PS and IR in order to facilitate, via active intervention, strategies for radiation risk reduction.
Keywords: psychological stress, ionizing radiation, radiosusceptibility, radiocarcinogenesis, mouse psychological stress model
INTRODUCTION
Both psychological stress (PS) and ionizing radiation (IR) cause varied detrimental effects on humans. The health consequences of PS and the underlying mechanisms of those consequences have been studied for more than 100 years, and there is a pile of extensive review articles in the literature, including those within specialized fields of study [1–12]. It is known that PS can contribute to a number of diseases, including cancer. In intensive lifestyle intervention, stress management focusing on psychological well-being is of the same importance as diet, exercise, and group support. As an important lifestyle component, PS is not merely taken into account for e.g. breast cancer prevention [13], it is treated as one of the causes of disease in lifestyle medicine [14]. Humans, inevitably, are exposed to both PS and IR because they are living in a highly competitive society with diverse stress and on the surface of the Earth, which has a natural background of IR from cosmic, terrestrial and internal sources. Due to people's occupations, activities, lifestyle, living conditions and health conditions, some people may encounter concurrent exposure to PS and IR to a high extent, e.g. nuclear facility workers (cleaning crew and decommissioning workers, in particular), underground miners, uranium mine workers, radiodiagnostic doctors, radiotherapists, patients under radiotherapy, astronauts, space travelers, flight attendants, flight passengers, residents of the high background radiation areas, residents of radiocontaminated areas due to nuclear testing or nuclear plant accidents, etc. [15]. In addition to the possible health effects resulting directly from exposure to IR on such people, fear of health effects such as cancer is also a cause of PS [16–20]. It is believed that fears about and preoccupation with cancer and other health effects attributable to IR would be significant and remain high for years following a radiological attack [21].
Among nuclear and radiation accidents, accidents of nuclear power plants (ANPPs) usually influence millions of people because they are often accompanied by the release of multiple different radioisotopes that result in radioactive contamination of the environment. These released radioisotopes may present a radiation hazard to residents living in radiocontaminated areas because exposure to IR could cause varied deleterious effects, including carcinogenesis. Because IR is neither visible nor able to be sensed, disasters involving IR, in particular, often strike the public with horror of exposure to IR. In fact, exposure to IR due to ANPPs is a significant threat leading to a major health concern. On the other hand, ANPPs also cause PS. Notably, radioactive contamination of the living environment often restricts the outdoor activities of humans, causing further physiological stress and PS [16–23]. Thus ANPPs may pose a long-term threat to health, resulting in adverse health consequences both directly and indirectly.
Although modulation effects from exposure to PS on radiation-induced health consequences and the mechanisms underlying these outcomes still remain largely unknown, recent studies and our latest investigations in mouse models show that chronic restraint–induced PS (CRIPS) can increase the susceptibility of Trp53-heterozygous mice to radiocarcinogenesis, and cause hematological toxicity in peripheral blood and elevated chromosome aberration (dicentrics) frequency in splenocytes of Trp53–wild-type mice. This has had a big impact on the academic world and a sensational effect on the public [24–27]. This mini-review gives an overview of the significance of PS effects on humans and experimental animals, with a special focus on the context of the importance and perspective for study of the modification effect from PS on radiosusceptibility.
It should be noticed that in radiobiology, the IR itself, as an assault to induce varied effects, is also called ‘a stress’ or ‘a stressor’ [1]. To avoid any possible confusion, the term ‘stress’ in this mini-review refers to PS unless otherwise specified.
CONCEPT OF PS AND ITS ANIMAL MODELS
Stress refers to conditions where an environmental demand on an organism exceeds its natural regulatory capacity; it refers, in particular, to situations that include unpredictability and uncontrollability [2]. There are two basic kinds of stress: PS and physical stress. In humans, PS occurs when people confront a situation in which the demands go beyond their coping resources, and it is a feeling of strain and pressure, with emotional and physiological reactions. The response of our body to PS is a highly adaptive phenomenon—when the PS is small, the responses may be desirable, beneficial, and even healthy. However, the response to excessive amounts of PS or chronic PS can become maladaptive, which may be harmful and lead to health consequences—namely, diseases and disorders [3].
Substantial efforts have been made so far in clinical and epidemiological studies about PS; however, the etiology and mechanisms underlying PS-induced health consequences are still poorly understood. This has led to the need to develop clinically relevant animal models, and a number of animal models for studying acute or chronic PS have been developed for research use in the past 50 years. Depending on the objectives and parameters chosen by the experimenter, protocols are available for applying to animals such stressors as neonatal isolation, noise stress, circadian rhythm changes, and predator stress [28, 29]. Psychological stressors have a major physical component and, similarly, some models of physical stress (such as restraint stress and immobilization stress) can induce PS, at least to some extent (e.g. the chronic restraint model has been used to induce PS [24, 27]). As one of the sources of PS, psychosocial stress is also of great concern, and subordinate colony housing has been established as a model in both mice and rats [4, 30–32]. Despite drawbacks, these animal models are invaluable tools for investigation.
HEALTH CONSEQUENCES OF PS IN HUMANS AND IN ANIMAL MODELS
As humans are exposed in daily life to the multitude of stressors that are prevalent in modern society, PS to some extent might be considered normal and even necessary for survival and regular psychological development [5]. On the other hand, PS (excessive amounts of PS and chronic PS, in particular) may cause negative affective states, such as feelings of anxiety and depression, exerting in turn direct effects on biological processes or behavioral patterns, and consequently influencing the immune system, susceptibility to infections, and risk of diseases, including cancer [1, 6–8, 33, 34].
In humans, PS, may contribute to miscellaneous health outcomes that range from highly adaptive to increasing the risk of developing psychopathology, including conditions such as emotional exhaustion, vision disorders, asthma, hypertension, cardiovascular disease, neurological alterations, Alzheimer's disease, immunodeficiency, gastrointestinal alterations, development and maintenance of obesity, metabolic syndrome, diabetes, reproductive disorders, pregnancy complications, infertility, increased amount of mitochondrial DNA (mtDNA), shortened telomeric DNA, and development of cancer [9–12, 35–49]. PS also has an impact on cancer metastasis. Both epidemiological and clinical studies have provided strong evidence for links between PS and cancer progression, but only limited evidence for the role of PS in cancer initiation [50].
In laboratory animal models, studies showed that CRIPS could significantly reduce body weight gain from 1 week after the onset of restraint in rats [51] and from the day following onset in mice [27], and promote immune suppression, inducing lymphocyte reduction [27, 52, 53]. In rats exposed to continuous stress from photoperiod, temperature and noise, a significantly increased incidence of micronuclei in peripheral red blood cells (RBCs) was observed [54]. In mice, it was demonstrated that PS could reduce the RBCs [27], alter the responsiveness to carcinogens, accelerate tumor onset, promote tumor progression and growth, and alter tumor type and location [55–58].
Notably, stress is an inevitable part of human life and it is experienced even before birth. In addition to the health problems caused by acute exposure to PS, experiencing PS repeatedly over a long period of time (i.e. chronic stress) or exposure to PS in the early stages of life may strongly impact on health, including increasing cancer risk [5, 42]. Exposure to chronic stress could result in long-term or permanent changes in the emotional, physiological and behavioral responses that influence susceptibility to and course of disease [6, 59]. Studies show that children who have grown up under PS, namely, in disadvantaged social environments, such as poverty or an unstable family, have an association with adverse health outcomes [60]. Genetics modulates the magnitude of the health consequence, but stress determines the direction [61]. In studies of PS effects following large-scale ANPPs (e.g. the Chernobyl and Fukushima accidents), the sequelae are intense and long lasting, and occur independently of the actual exposure received—and mental health effects were the most significant health consequence [16–20]. It is noted that the evacuee mothers rated their evacuated children's well-being as significantly worse than their own, and the most important risk factors of this health consequence were maternal somatization, and Chernobyl-related stress [62]. Especially in children, there is a wide range of mental and behavioral sequelae due to PS, which can last a long time [60] and cause alterations of mitochondrial DNA copy number and shortening of telomere length [12, 63, 64]. Poor mental health status due to anxiety about IR exposure has been reported, even in the younger generation born in the surrounding area after the Chernobyl accident [65]. In animals, traumatic stress in early life induced altered microRNA expression, and behavioral and metabolic responses in their progeny [66], and CRIPS caused significant apical dendritic atrophy [67, 68]. Prenatal exposure to maternal stress altered physiological and immune functions in the offspring [69–71]. These studies highlight the effects on health of PS that is experienced at a young age [19]. On the other hand, older adults, especially those with multiple co-morbidities, are at risk of increased morbidity after disasters and catastrophic events [72]. However, epidemiological studies on the younger and the older are still rare and the documented works offer limited information. In general, the importance of the PS impact after ANPPs is underrated, and investigations are still far from being complete and comprehensive [73–75].
MECHANISMS INVOLVED IN PS-INDUCED HEALTH CONSEQUENCES
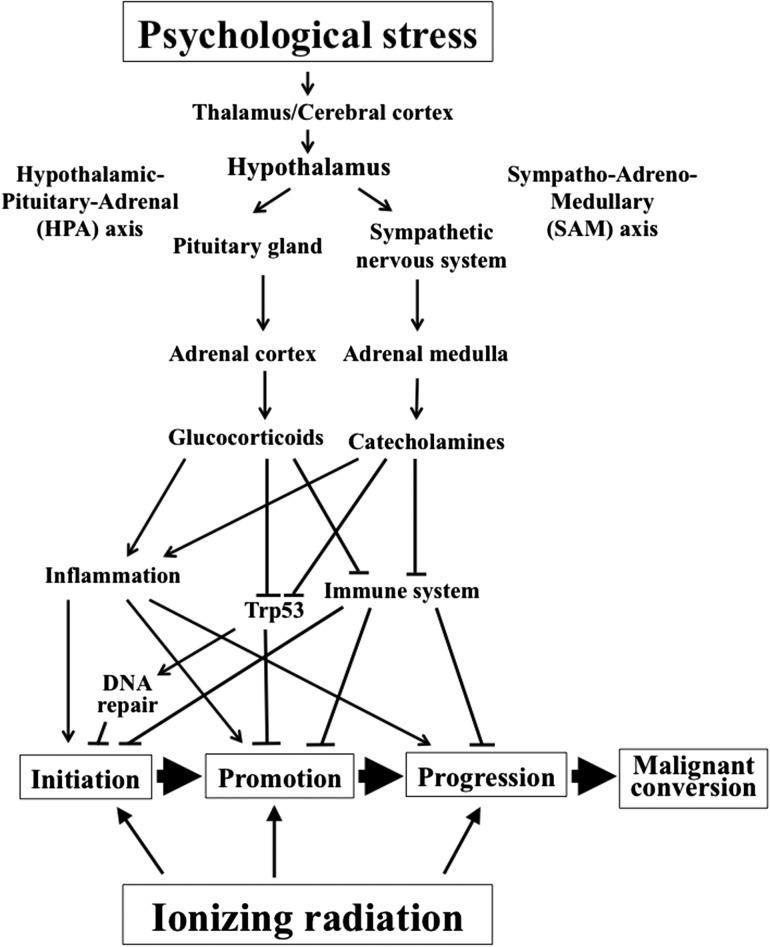
PS, as an important precursor of disease, could reduce quality of life in humans. The biological pathways between PS and the pathophysiological processes underlying disease have attracted substantial scientific attention. Although the mechanisms underlying PS-induced biological and health consequences remain insufficiently understood, based on epidemiological and animal data, a consensus on the physiological reaction to PS has been reached, focusing mainly on the vital roles of two endocrine systems—the hypothalamic–pituitary–adrenal (HPA) axis and the sympatho–adreno–medullary (SAM) axis—with the involvement of the immune system, hematopoietic system, metabolic system, circulatory system, central nervous system (CNS), peripheral nervous system, and behavioral system [2, 5, 9, 11, 12, 24, 29, 38, 76–99] (Fig. 1). For example, PS is thought to influence the pathogenesis of physical disease by causing negative feelings and depression, which in turn promotes biological processes that are associated with disease risk [6]. As shown in the upper part of Fig. 1, (via the HPA axis) PS causes, through regulation of glucocorticoid secretion (cortisol in humans and corticosterone in mice and rats), a variety of physiological changes, such as anti-inflammatory responses; suppression of the immune system; metabolism of carbohydrates, fats and proteins; and gluconeogenesis. Through releasing catecholamines and cooperation with the sympathetic nervous system, SAM regulates the cardiovascular, pulmonary, hepatic, skeletal, muscle, and immune systems. Among the wide variety of physiological changes, the alteration of immune and inflammatory functions may influence infectious, autoimmune, and coronary artery disease and some virally mediated cancers [100]. In addition, immune mechanisms were also involved in the responses of the offspring that were prenatally exposed to maternal stress [69–71]. Examples of PS-induced health consequences will now be described.
PS-induced, chronic, systemic low-grade inflammation seems to be a likely pathway to disease (detrimental health consequences). Transient increases in systemic inflammation have been observed in response to acute PS. There was a potential relationship between inflammatory responses to acute PS and long-term development of disease [101].
For PS-associated increased atherosclerosis, alterations in immune reactions due to activation or depression of HPA regulatory feedback mechanisms influenced both vascular endothelium function and the recruitment of circulating monocytes and their conversion to foam cells, and this involved expression of pro- and anti-inflammatory cytokines by stress hormones, such as catecholamines and glucocorticoids [89].
For PS-associated increased morbidity in humans and animal models, activation by acute stress of the major stress responsive systems, such as the HPA axis and the sympathetic nervous system, played an important role in the regulatory control of the inflammatory cascade. Target tissues for stress system modulation varied in their responses to stress system signaling. Stress-related changes in the sensitivity of target systems toward glucocorticoid regulation were responsible for inflammatory disinhibition and the development of disease related to inflammation [102].
-
Concerning a relationship between PS and cancer, both human and animal studies have shown that PS could impact cancer biology via sympathetic, neuroendocrine and immunologic mechanisms, in part, through regulation of inflammatory mediators.
PS could stimulate neuroendocrine, sympathetic and immune responses, resulting in the activation of the HPA axis, the sympathetic nervous system, and the subsequent regulation of inflammatory responses by immune cells. HPA axis hormones affected the pathogenesis of chronic inflammatory skin diseases and skin tumors, and hyperactive lesional HPA axis hormones could negatively feed back to the central HPA axis and interact with some cytokines and neuropeptides, leading to symptom deterioration [95]. PS-induced immune dysregulation resulted in significant health consequences for immune-related disorders, including tumor growth and metastasis [98]. The HPA axis is also invariably responsive to chronic PS.
Fig. 1.
A schematic of the overall concept proposed relating the modifying effect from psychological stress on radiocarcinogenesis.
Negative mood scores on the questionnaire survey were related to the salivary cortisol level. For example, significantly increased salivary cortisol was observed in students 3 months after the Great East Japan Earthquake [103], and a significantly high level of urinary cortisol was detected 17 months after the accident for residents living within 5 miles of the Three Mile Island accident sites [104]. These facts suggest that cortisol may play a critical role in the chronic pathogenesis of physical disease in people who have experienced catastrophic disasters. In addition, ectopic expression of serum- and glucocorticoid-induced protein kinase 1 (SGK1) in mouse embryonic fibroblasts and HCT116 human colon cancer cells was found to decrease p53 protein accumulation in response to IR. Also, knockdown of SGK1 by siRNA was shown to abolish the blocking effect of cortisol on p53 protein accumulation in response to radiation. In the light of a generally accepted role for Trp53 in the maintenance of genomic integrity, it is plausible that the induction of SGK1 by cortisol mediates reduced function of Trp53, which in turn causes genomic instability, potentially resulting in cancer induction.
Of note, some recent investigations have widened our knowledge of the mechanism by which PS affects health. For example, it appears that PS is conducive to several cell aging processes, leading to accelerated cellular aging—dampening of telomerase, shortening of telomere length, and cell senescence [12, 105]. In humans, increase in glucocorticoid secretion triggered, at least in part, increased mtDNA copy numbers and shortened telomere length, and this was demonstrated in mice by the administration of the stress hormone corticosterone [12]. It was also found that PS could accelerate the erosion of telomeres from very early in life, and possibly even influence the initial setting of telomere length. It was observed that the production of reactive oxygen species (ROS) increased under high levels of PS, being closely associated with oxidative stress [45]. Repeated short-term stress from restraint could synergize ROS signaling through upregulation of NFκB and iNOS expression [106]. Prolonged PS during childhood or adolescence could induce increased oxidative stress in the CNS due to disequilibrium between the oxidant generation and the antioxidant response, resulting in neurobiological modifications; this could enhance the risk of developing psychiatric diseases [5].
A better understanding of the mechanisms underlying PS-induced responses and health consequences would contribute both to new prevention and new treatment strategies. For example, defining the sources of oxidative stress following exposure to early life stress is expected to create new beneficial insights into therapeutic approaches to these mental disorders [5]. Further studies are still required.
EFFECTS FROM CO-EXPOSURE TO PS AND IR, AND THEIR MECHANISMS
The findings to date on the biological effects from concurrent exposure to IR and PS have been obtained mainly from the established experimental PS models in rats and mice. Some pioneering investigations date back several decades, though from a historic point of view, these very early works did not add a critical credit to the quality of the study. As most of the very early investigations were published in non-English journals, and the number of researchers and reports in this field is still low, the progress in this field and the recent significant achievements of the study on the biological effects of concurrent exposure to PS and IR are not widely known to most radiobiologists and psychologists.
Concurrent exposure to PS and IR was studied early, primarily using endpoints in behaviorology, immunology and hematology, rather than cancer biology or carcinogenesis. In a behavioral study, a significantly increased emotional response was observed in rats [107]. Suppression of aggressive behavior was induced by prior exposure to IR in mice in response to PS in the isolation test. Interestingly, the suppression could be induced by only low doses (0.05–0.15 Gy), but not by relatively higher doses (0.25–0.35 Gy), and changes in brain biochemistry (namely, fast turnover of brain serotonin) significantly decreased carnosine content; its synthetase activity in the olfactory bulbs was consistent with the behavioral suppression [108]. In immunological and hematological studies, a significantly decreased number of reticulocytes, neutrophils and thrombocytes in the peripheral blood and an increased number of lymphoid cells in the bone marrow, thymus and spleen were observed in rats [107]. Concurrent exposure to PS from immobilization and IR at a low dose resulted in early adaptation-like changes, such as decrease in body weight, increase in adrenal gland weight, decrease in thymus weight, and slowing down of blood coagulation, while irradiation of immobilized animals prevented stressogenic disturbances, such as depression of anti-aggregation activity of the vascular wall and decrease in the level of leucocytes [109]. Pre-exposure to IR followed by PS reduced the compensatory capability of the blood system. The degree of the disturbances directly depended on the duration of the PS [110]. Exposure to PS after irradiation could complicate the radiation effects, which were especially pronounced under a prolonged and intensive stress. Exposure to PS after low doses of IR showed a reduction in the adaptive and compensator capabilities of the hematopoietic system for exposure to low doses; on the other hand, exposure to PS after a lethal irradiation dose showed inhibition of hematopoiesis recovery, aggravation of the course of acute radiation disease and decrease in the efficiency of radioprotection [111]. Interestingly, exposure to PS prior to IR also showed an inhibitory effect on the development of hyperplasia of the bone marrow [112]. Though these opposite effects were observed, the reasons were unknown. In mice, investigation of the combined effect of PS from immobilization and IR demonstrated that disorders in the blood system were a function of the phase of the general adaptation syndrome (GAS). Acute IR during GAS resistance inhibited the adaptive and compensatory potential of the blood-forming system, and chronic stress by itself increased the rate of spontaneous chromosomal aberrations in the nucleus-containing bone marrow cells [113].
A study indicating the significance of the modulatory effect of immunosuppressed conditions on radiocarcinogenesis was carried out about 20 years ago [114]. Interestingly, the study itself was not designed to specifically test the PS effect, but to demonstrate the existence of preleukemic cells in irradiated mice and to explore the role of an immunosuppressant (dexamethasone) on their promotion to overt leukemia [114]. As mentioned in the previous section, immunosuppression is a very common health consequence resulting from PS, and these findings brought the modulatory effect of PS on radiocarcinogenesis under the spotlight. In brief, Haran-Ghera et al.’s work [114] showed that additional treatment with dexamethasone shortly after exposure of mice to IR (3 Gy) increased the incidence of acute myelomonocytic leukemia from 10–30% to up to 50%. Transplantation of bone marrow cells from irradiated mice into appropriate recipients and treating with dexamethasone could result in acute myeloid leukemia development of donor origin in 70% of the recipients. Moreover, a modulatory (promoting) effect of dexamethasone on radiocarcinogenesis was confirmed, whether administered within several hours or 130 days after IR. It is also worthy of noting that administration of cyclophosphamide shortly after IR could not replace the dexamethasone effect, but it was found to be complementary to the effect of dexamethasone. These results suggest that the underlying mechanisms for radiation-induced acute myeloid leukemia involve a multiphase process, and that preleukemia can be promoted by a stress hormone in the mouse model.
Direct experimental evidence that CRIPS promotes radiocarcinogenesis in vivo has been provided by a recent study by Feng and colleagues [24]. In this work using Trp53-heterozygous mice and a mouse CRIPS model by immobilization, concurrent exposure to both PS and IR (4 Gy) showed significantly reduced IR-induced tumor latency (from ~49 to ~38 weeks of median survival age). The IR-induced tumor spectrum (predominantly lymphomas and sarcomas) was similar regardless of PS, suggesting that the reduced tumor latency was not due to the development of new types of tumor. CRIPS was found to elevate glucocorticoids, induce SGK1, and in turn to increase E3 ubiquitin ligase and MDM2 activity, and to decrease both the Trp53 protein level and Trp53 function in mice, showing promoted growth of human xenograft tumors in a largely Trp53-dependent manner. As regulation of multiple Trp53 stress responses was mediated through MDM2 activation by SGK1 [115, 116], attenuation of Trp53 functions by CRIPS-induced glucocortcoids was believed to be an important part of the mechanism underlying promotion by CRIPS of Trp53-heterozygous mice to radiocarcinogenesis [24].
Feng et al.’s work [24] is a milestone in the field of the study on co-exposure to PS and IR. It has had a big impact on the academic world and a sensational effect on the public—particularly on the residents living in radioactively contaminated areas. However, it should be noticed that Trp53 heterozygous mice were used in this work. Trp53 can prevent radiocarcinogenesis in mice, and IR (4 Gy) can significantly promote tumor development (mainly lymphomas) in Trp53 heterozygous mice, but not in Trp53 wild-type (Trp53wt) mice [117]. Thus, it is important now to investigate the health consequences, especially carcinogenesis, due to concurrent exposure to both IR and PS in Trp53wt animals. Recently, a series of investigations were performed in our laboratory, using the same experimental setup and conditions (CRIPS model, 6 h restraint per day for 28 consecutive days, and IR at 4 Gy on the eighth day), on the biological responses of and subsequent consequences for young mice with normal genotype. Multidisciplinary analyses were carried out on changes in body weight and immune organ weight, alterations in the levels of blood cytokines and stress hormones, changes in the peripheral blood hemogram and anti-oxidative activity of blood cells, chromosome aberrations in splenocytes and in micronuclei in bone marrow erythrocytes, and epigenetic variations (DNA methylation and miRNA expression) and protein expression profiles in the liver. Prior to our ongoing carcinogenesis study, results showed that, concurrent exposure to both CRIPS and IR induced significantly decreased body weight and immune organ weight (spleen and thymus), increased corticosterone and deregulated inflammation-related cytokines in the serum, marked decrease in blood platelet count, and increased chromosomal aberrations (dicentrics) in splenocytes when compared with that in animals exposed to either IR or CRIPS alone [25, 27]. These findings suggest that CRIPS also has a significant impact on radiation-induced detrimental effects in Trp53wt mice.
To date, investigations into concurrent exposure to PS and IR have embraced weights-of-evidence approaches, and in most cases the IR performed was of low LET and at relatively high doses. There is still a lack of information on epidemiological studies, to say nothing of experimental studies using animal models in particular, concerning the issues regarding IR at low doses and IR from high-LET particles. Although the mechanisms remain largely elusive, in addition to the attenuation of Trp53 functions, the altered metabolism and degraded physiological and immune functions observed in studies on PS in combination with pathogen and toxicological assaults may also be critical for additively or synergistically increasing detrimental health consequences, particularly increasing susceptibility to carcinogenesis, as indicated in the study on concurrent exposure to PS and IR [24, 27, 53, 118–124].
DISCUSSION
The mechanisms underlying detrimental radiation effects, for example, radiocarcinogenesis, are likely to be multistep processes, indicating the potential for highly detrimental interactions if two or more consecutive rate-limiting steps are specifically affected by different factors [125], and studies on IR effects should also take the potential for combined effects, namely, synergisms or antagonisms, from other factors into account. To date, combined health effects have only been studied for a few factors, but regarding PS, critical findings are now accumulating. A study on the influence of smoking on radon-induced lung cancer has moved from being an ongoing source of controversy in risk assessment [126] to being an important example of combined exposures leading to a synergically increased health effect risk according to UNSCEAR [127, 128]. Currently, the only attempt to systematically review the combined effects of IR and other factors has been done by UNSCEAR [15, 127]. New findings, especially on such as dietary factors and PS, are widening our knowledge of combined effects, and dietary interventions through calorie restriction before or after IR exposure are associated with a significant reduction in health risk for IR-induced myeloid leukemia and some late-occurring tumors in mice [129, 130]. In view of the multitude of possible interactions among the large number of potentially harmful factors that humans encounter, physiological stress and PS (in particular, excessive amounts of PS and chronic PS) may have a dramatic adverse impact on health for humans living in the modern society. Thus, effects from combined exposures to PS and IR are being revisited and the potential for rapid research progress is considerable.
Regarding the study on combined effects, it should be noticed that, in general, the documented data are still rudimentary, mainly descriptive, and rarely cover exposure ranges large enough to make direct inferences to low-dose exposure situations [125]. Laboratory animal models of PS have provided a novel and powerful tool to probe the mechanisms underlying cancer [98], while the existence of genetic dependence and gender difference should be taken into account [18, 20, 118–121, 124, 131–137]. On the other hand, the development and utilization of new animal stress models should help us to reveal the mechanisms underlying stress-induced health consequences as well as to identify potential clinical interventions based on mechanisms [28].
Psychological intervention, and dietary and pharmacological intervention that are based on PS-reducing mechanisms, are promising means for preventing/reducing PS-attributable health consequences. The interventions are not only symptomatic, but also pathogenetic therapeutics, as demonstrated in humans and experimental animal models [12, 37, 111, 138–143]. Already, both the academic world and the public have recognized the importance of the psychological consequences arising from accident catastrophe and its aftermath, and these consequences challenge our public health activities for the mitigation of IR exposures and risk communication [144]. In response to the challenges, it is urgent that we seek strategies for mitigating the serious consequences [20]. PS-reducing and health interventions should be offered to not only occupational radiation workers, but also the public [145]. It is time to embrace, thoughtfully and authentically, the lifestyle medicine needed to contribute to active reduction of radiation risk. In humans, to normalize PS-resultant altered responses to IR, and to support stress management in lifestyle change is a low-tech and low-cost, simple but simply effective approach, having important implications for the care of high-risk persons and the public as well, with no side effects. This could be done either in combination with other treatments such as pharmacological intervention or as an alternative.
CONCLUSION
This mini-review gives a general overview of the significance of PS effects on humans and experimental animals: PS can cause varied detrimental effects and affect us both emotionally and on a biological level. Although there is no direct evidence showing PS alone can cause cancer, long-lasting PS may affect our overall health and ability to cope with cancer. This paper summarizes the latest weight-of-evidence approaches to radiobiological studies on PS-induced alterations in susceptibility in experimental animal models, mainly in the context of the impact of the modulatory effect of PS on radiocarcinogenesis: it appears that PS could induce increased radiosusceptibility and radiocarcinogenesis in mice. The mechanisms have been attributed to attenuation of Trp53 gene functions, but still remain largely unknown, constraining our capacity to perform a comprehensive epidemiological study in humans. In the meantime, we can ascertain the potential human relevance of the health effects observed in animal models. A better understanding of the mechanisms that govern and regulate PS-induced modulatory effects on responses to IR should help us to understand the etiology and the long-term health consequences in diverse populations and provide intervention/prevention strategies for actively reducing IR risk to humans. It is recommended that experimental mechanistic studies use adequate animal models of different strains in both genders and that investigations include low doses of IR. The present work provides a perspective for understanding the combined effects of exposure to both PS and IR in order to facilitate, via active intervention, strategies for radiation risk reduction. By highlighting the importance of PS modulation of susceptibility to IR, it is becoming increasingly clear that multidimensional approaches to the reduction of PS through active and methodologically adequate interventions promise to be valuable in preventing PS-induced health consequences. Thus, applications of the PS concept, namely, the understanding of PS as a modulatory factor and the use of PS management in reduction of radiation risk are of great importance.
FUNDING
This work was supported in part by MEXT Grant-in-Aid for Scientific Research on Innovative Areas, Grant Number 15H05935 “Living in Space”; National Institute of Radiological Sciences, Japan; and Ministry of the Environment, Japan. This work was conducted as a part of the Study of the Health Effects of Radiation organized by Ministry of the Environment, Japan.
CONFLICT OF INTEREST
The authors have declared that there are no conflicts of interest. The grant providers had no further role in the study design; collection, analysis and interpretation of data; writing of the report; or the decision to submit this paper for publication.
ACKNOWLEDGEMENTS
The authors would like to thank Ms Yasuko Morimoto, Ms Kyoko Sakuma, Ms Taeko Iwai, Mr Sadao Hirobe, Mr Ikutarou Saito, Ms Mikiko Nakajima and Ms Hiromi Arai for their expert technical assistance and administrative support. Critical and constructive comments on the manuscript preparation from Dr Yi Shang are gratefully acknowledged. We greatly appreciate the constructive comments by the anonymous reviewers that strengthened the presentation of this work.
REFERENCES
- 1.Vanhoudt N, Vandenhove H, Real A, et al. . A review of multiple stressor studies that include ionising radiation. Environ Pollut 2012;168:177–92. [DOI] [PubMed] [Google Scholar]
- 2.Koolhaas JM, Bartolomucci A, Buwalda B, et al. . Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev 2011;35:1291–301. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 2008;583:174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartolomucci A, Palanza P, Sacerdote P, et al. . Social factors and individual vulnerability to chronic stress exposure. Neurosci Biobehav Rev 2005;29:67–81. [DOI] [PubMed] [Google Scholar]
- 5.Schiavone S, Colaianna M, Curtis L.. Impact of early life stress on the pathogenesis of mental disorders: relation to brain oxidative stress. Curr Pharm Des 2015;21:1404–12. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S, Kessler RC, Gordon UL.. Strategies for measuring stress in studies of psychiatric and physical disorder In: Cohen S, Kessler RC, Gordon UL (eds). Measuring Stress: A Guide for Health and Social Scientists. New York, NY: Oxford University Press, 1995, 3–26. [Google Scholar]
- 7.Hammen C. Stress and depression. Annu Rev Clin Psychol 2005;1:293–319. [DOI] [PubMed] [Google Scholar]
- 8.Godbout JP, Glaser R.. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol 2006;1:421–7. [DOI] [PubMed] [Google Scholar]
- 9.Claes SJ. CRH, stress, and major depression: a psychobiological interplay. Vitam Horm 2004;69:117–50. [DOI] [PubMed] [Google Scholar]
- 10.Cohen S, Janicki-Deverts D, Miller GE.. Psychological stress and disease. JAMA 2007;298:1685–7. [DOI] [PubMed] [Google Scholar]
- 11.Lucassen PJ, Pruessner J, Sousa N, et al. . Neuropathology of stress. Acta Neuropathol 2014;127:109–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai N, Chang S, Li Y, et al. . Molecular signatures of major depression. Current Biol 2015;25:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kruk J. Lifestyle components and primary breast cancer prevention. Asian Pac J Cancer Prev 2014;15:10543–55. [DOI] [PubMed] [Google Scholar]
- 14.Hyman MA, Ornish D, Roizen M.. Lifestyle medicine: treating the causes of disease. Altern Ther Health Med 2009;15:12–4. [PubMed] [Google Scholar]
- 15.UNSCEAR. II Sources of radiation exposure In: UNSCEAR (eds). Sources and effects of ionizing radiation. UNSCEAR 2000 Report to the general assembly, with scientific annexes. Volume I: Sources. New York, NY: United Nations Publication, 2000, 4–8. [Google Scholar]
- 16.Leon GR. Overview of the psychosocial impact of disasters. Prehosp Disaster Med 2004;19:4–9. [DOI] [PubMed] [Google Scholar]
- 17.Stephan V. Chernobyl: poverty and stress pose ‘bigger threat’ than radiation. Nature 2005;437:181. [DOI] [PubMed] [Google Scholar]
- 18.Bromet EJ, Havenaar JM, Guey LT.. A 25 year retrospective review of the psychological consequences of the Chernobyl accident. Clin Oncol 2011;23:297–305. [DOI] [PubMed] [Google Scholar]
- 19.Boice JD., Jr. Radiation epidemiology: a perspective on Fukushima. J Radiol Prot 2012;32:N33–40. [DOI] [PubMed] [Google Scholar]
- 20.González AJ, Akashi M, Boice J, et al. . Radiological protection issues arising during and after the Fukushima nuclear reactor accident. J Radiol Prot 2013;33:497–571. [DOI] [PubMed] [Google Scholar]
- 21.Tuerk PW, Hall B, Nagae N, et al. . Forty days after the Great East Japan Earthquake: field research investigating community engagement and traumatic stress screening in a post-disaster community mental health training. Int J Psychiatry Med 2013;45:159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kukihara H, Yamawaki N, Uchiyama K, et al. . Trauma, depression, and resilience of earthquake/tsunami/nuclear disaster survivors of Hirono, Fukushima, Japan. Psychiatry Clin Neurosci 2014;68:524–33. [DOI] [PubMed] [Google Scholar]
- 23.Kohzaki M, Ootsuyama A, Moritake T, et al. . What have we learned from a questionnaire survey of citizens and doctors both inside and outside Fukushima?: survey comparison between 2011 and 2013. J Radiol Prot 2015;35:N1–17. [DOI] [PubMed] [Google Scholar]
- 24.Feng Z, Liu L, Zhang C, et al. . Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc Natl Acad Sci U S A 2012;109:7013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsube T, Tanaka K, Wang B, et al. Analysis with FISH method of the modulatory effect from chronic restraint on radiation-induced chromosome aberration in mice. Abstracts of The 57th Annual Meeting of the Japan Radiation Research Society, 2014, 79.
- 26.Wang B, Tanaka K, Katsube T, et al. Effects from total body X-irradiation with or without psychological stress on the hematopoietic system in mice: hematological abnormality in the peripheral blood and residual damage in the bone marrow erythrocytes. Abstracts of The 57th Annual Meeting of the Japan Radiation Research Society, 2014, 108.
- 27.Wang B, Tanaka K, Katsube T, et al. . Chronic restraint-induced stress has little modification effect on radiation hematopoietic toxicity in mice. J Radiat Res 2015;56:760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campos AC, Fogaça MV, Aguiar DC, et al. . Animal models of anxiety disorders and stress. Rev Bras Psiquiatr 2013;35 Suppl 2:S101–11. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Rinwa P, Kaur G, et al. . Stress: neurobiology, consequences and management. J Pharm Bioallied Sci 2013;5:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stefanski V, Engler H.. Effects of acute and chronic social stress on blood cellular immunity in rats. Physiol Behav 1998;64:733–41. [DOI] [PubMed] [Google Scholar]
- 31.Palanza P, Gioiosa L, Parmigiani S.. Social stress in mice: gender differences and effects of estrous cycle and social dominance. Physiol Behav 2001;73:411–20. [DOI] [PubMed] [Google Scholar]
- 32.Nyuyki KD, Beiderbeck DI, Lukas M, et al. . Chronic subordinate colony housing (CSC) as a model of chronic psychosocial stress in male rats. PLoS One 2012;7:e52371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhabhar FS, Miller AH, Stein M, et al. . Diurnal and acute stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain Behav Immun 1994;8:66–79. [DOI] [PubMed] [Google Scholar]
- 34.Zieziulewicz TJ, Mondal TK, Gao D, et al. . Stress-induced effects, which inhibit host defenses, alter leukocyte trafficking. Cell Stress Chaperones 2013;18:279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rozanski A, Blumenthal JA, Kaplan J.. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 1999;99:2192–217. [DOI] [PubMed] [Google Scholar]
- 36.Wischmann TH. Psychogenic infertility – myths and facts. J Assist Reprod Gen 2003;20:485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vizza J, Neatrour DM, Felton PM, et al. . Improvement in psychosocial functioning during an intensive cardiovascular lifestyle modification program. J Cardiopulm Rehabil Prev 2007;27:376–83. [DOI] [PubMed] [Google Scholar]
- 38.Hamer M. Psychosocial stress and cardiovascular disease risk: the role of physical activity. Psychosom Med 2012;74:896–903. [DOI] [PubMed] [Google Scholar]
- 39.Cardwell MS. Stress: pregnancy considerations. Obstet Gynecol Surv 2013;68:119–29. [DOI] [PubMed] [Google Scholar]
- 40.Floras JS. Blood pressure variability: a novel and important risk factor. Can J Cardiol 2013;29:557–63. [DOI] [PubMed] [Google Scholar]
- 41.Huerta-Franco MR, Vargas-Luna M, Tienda P, et al. . Effects of occupational stress on the gastrointestinal tract. World J Gastrointest Pathophysiol 2013;4:108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyland ME, Alkhalaf AM, Whalley B.. Beating and insulting children as a risk for adult cancer, cardiac disease and asthma. J Behav Med 2013;36:632–40. [DOI] [PubMed] [Google Scholar]
- 43.Johansson L, Guo X, Hällström T, et al. . Common psychosocial stressors in middle-aged women related to longstanding distress and increased risk of Alzheimer's disease: a 38-year longitudinal population study. BMJ Open 2013;3:e003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergmann N, Gyntelberg F, Faber J.. The appraisal of chronic stress and the development of the metabolic syndrome: a systematic review of prospective cohort studies. Endocr Connect 2014;3:R55–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue N. Stress and atherosclerotic cardiovascular disease. J Atheroscler Thromb 2014;21:391–401. [DOI] [PubMed] [Google Scholar]
- 46.Seidler A, Thinschmidt M, Deckert S, et al. . The role of psychosocial working conditions on burnout and its core component emotional exhaustion – a systematic review. J Occup Med Toxicol 2014;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toufexis D, Rivarola MA, Lara H, et al. . Stress and the reproductive axis. J Neuroendocrinol 2014;26:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Bonsdorff MB, von Bonsdorff M, Kulmala J, et al. . Job strain in the public sector and hospital in-patient care use in old age: a 28-year prospective follow-up. Age Ageing 2014;43:393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson SM, Sato AF.. Stress and paediatric obesity: what we know and where to go. Stress Health 2014;30:91–102. [DOI] [PubMed] [Google Scholar]
- 50.Moreno-Smith M, Lutgendorf SK, Sood AK.. Impact of stress on cancer metastasis. Future Oncol 2010;6:1863–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiba S, Numakawa T, Ninomiya M, et al. . Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry 2012;39:112–9. [DOI] [PubMed] [Google Scholar]
- 52.Shi Y, Devadas S, Greeneltch KM, et al. . Stressed to death: implication of lymphocyte apoptosis for psychoneuroimmunology. Brain Behav Immun 2003;17 Suppl 1:S18–26. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Foster R, Sun X, et al. . Restraint stress induces lymphocyte reduction through p53 and PI3K/NF-kappaB pathways. J Neuroimmunol 2008;200:71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adam ML, Torres MFP, Franci AC, et al. . On the stress by photoperiod, temperature and noise as possible causes of genomic damaging in an animal model. Stress Health 2011;27:e152–6. [Google Scholar]
- 55.Frick LR, Arcos ML, Rapanelli M, et al. . Chronic restraint stress impairs T-cell immunity and promotes tumor progression in mice. Stress 2009;12:134–43. [DOI] [PubMed] [Google Scholar]
- 56.Flint M, McCarty K, Jenkins F, et al. . Psychological stress accelerates the onset of tumour formation and alters the type and location of tumours in a DMBA mouse carcinogenesis model. Stress Health 2011;27:e129–38. [Google Scholar]
- 57.Hassan S, Karpova Y, Baiz D, et al. . Behavioral stress accelerates prostate cancer development in mice. J Clin Invest 2013;123:874–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin Q, Wang F, Yang R, et al. . Effect of chronic restraint stress on human colorectal carcinoma growth in mice. PLoS One 2013;8:e61435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 1998;338:171–9. [DOI] [PubMed] [Google Scholar]
- 60.Kar N. Psychological impact of disasters on children: review of assessment and interventions. World J Pediatr 2009;5:5–11. [DOI] [PubMed] [Google Scholar]
- 61.Mitchell C, Hobcraft J, McLanahan SS, et al. . Social disadvantage, genetic sensitivity, and children's telomere length. Proc Natl Acad Sci U S A 2014;111:5944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bromet EJ, Goldgabe D, Carlson G, et al. . Children's well-being 11 years after the Chernobyl catastrophe. Arch Gen Psychiatry 2000;57:563–71. [DOI] [PubMed] [Google Scholar]
- 63.Drury SS, Mabile E, Brett ZH, et al. . The association of telomere length with family violence and disruption. Pediatrics 2014;134:e128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tyrka AR, Parade SH, Price LH, et al. . Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biol Psychiatry 2016;15:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Masunaga T, Kozlovsky A, Lyzikov A, et al. . Mental health status among younger generation around Chernobyl. Arch Med Sci 2013;9:1114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gapp K, Jawaid A, Sarkies P, et al. . Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci 2014;17:667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu RJ, Aghajanian GK.. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A 2008;105:359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gądek-Michalska A, Spyrka J, Rachwalska P, et al. . Influence of chronic stress on brain corticosteroid receptors and HPA axis activity. Pharmacol Rep 2013;65:1163–75. [DOI] [PubMed] [Google Scholar]
- 69.Mayer N, Greco C, Bertuzzi M, et al. . Immobilization stress responses in adult rats exposed in utero to immobilization. Stress Health 2011;27:e1–10. [DOI] [PubMed] [Google Scholar]
- 70.Soberanes-Chávez P, López-Rubalcava C, de Gortari P, et al. . Exposure to toluene and stress during pregnancy impairs pups’ growth and dams’ lactation. Neurotoxicol Teratol 2013;40:9–16. [DOI] [PubMed] [Google Scholar]
- 71.Veru F, David P, Laplante DP, et al. . Prenatal maternal stress exposure and immune function in the offspring. Stress 2014;17:133–48. [DOI] [PubMed] [Google Scholar]
- 72.Jenkins JL, Levy M, Rutkow L, et al. . Variables associated with effects on morbidity in older adults following disasters. PLoS Curr 2014;6:ecurrents.dis.0fe970aa16d51cde6a962b7a732e494a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saenko V, Ivanov V, Tsyb A, et al. . The Chernobyl accident and its consequences. Clin Oncol 2011;23:234–43. [DOI] [PubMed] [Google Scholar]
- 74.Fushiki S. Radiation hazards in children – lessons from Chernobyl, Three Mile Island and Fukushima. Brain Dev 2013;35:220–7. [DOI] [PubMed] [Google Scholar]
- 75.Bromet EJ. Emotional consequences of nuclear power plant disasters. Health Phys 2014;106:206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arimura A, Takaki A, Komaki G.. Interactions between cytokines and the hypothalamic–pituitary–adrenal axis during stress. Ann N Y Acad Sci 1994;739:270–81. [DOI] [PubMed] [Google Scholar]
- 77.Song C, Kelly JP, Leonard BE.. The effect of stressful behavioural exposure on endocrine and immune parameters in the rat. Stress Med 1994;10:239–45. [Google Scholar]
- 78.Vogel WH, Jensh R.. Chronic stress and plasma catecholamine and corticosterone levels in male rats. Neurosci Lett 1988;8:183–8. [DOI] [PubMed] [Google Scholar]
- 79.Gispen-de Wied CC, Jansen LM.. The stress-vulnerability hypothesis in psychotic disorders: focus on the stress response systems. Curr Psychiatry Rep 2002;4:166–70. [DOI] [PubMed] [Google Scholar]
- 80.Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry 2002;51:775–87. [DOI] [PubMed] [Google Scholar]
- 81.Segerstrom SC, Gregory EM.. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 2004;130:601–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leonard BE. The HPA and immune axes in stress: the involvement of the serotonergic system. Eur Psychiatry 2005;20 Suppl 3:S302–6. [DOI] [PubMed] [Google Scholar]
- 83.Girdler SS, Klatzkin R.. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol Ther 2007;116:125–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Golden SH. A review of the evidence for a neuroendocrine link between stress, depression and diabetes mellitus. Curr Diabetes Rev 2007;3:252–9. [DOI] [PubMed] [Google Scholar]
- 85.Shelton RC. The molecular neurobiology of depression. Psychiatr Clin North Am 2007;30:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thrall G, Lane D, Carroll D, et al. . A systematic review of the effects of acute psychological stress and physical activity on haemorheology, coagulation, fibrinolysis and platelet reactivity: implications for the pathogenesis of acute coronary syndromes. Thromb Res 2007;120:819–47. [DOI] [PubMed] [Google Scholar]
- 87.Koh KB, Lee Y, Beyn KM, et al. . Counter-stress effects of relaxation on proinflammatory and anti-inflammatory cytokines. Brain Behav Immun 2008;22:1130–7. [DOI] [PubMed] [Google Scholar]
- 88.Vere CC, Streba CT, Streba LM, et al. . Psychosocial stress and liver disease status. World J Gastroenterol 2009;15:2980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gu HF, Tang CK, Yang YZ.. Psychological stress, immune response, and atherosclerosis. Atherosclerosis 2012;223:69–77. [DOI] [PubMed] [Google Scholar]
- 90.Morris MC, Compas BE, Garber J.. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev 2012;32:301–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Silverman MN, Sternberg EM.. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci 2012;1261:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Himmerich H, Fischer J, Bauer K, et al. . Stress-induced cytokine changes in rats. Eur Cytokine Netw 2013;24:97–103. [DOI] [PubMed] [Google Scholar]
- 93.Doom JR, Gunnar MR.. Stress physiology and developmental psychopathology: past, present, and future. Dev Psychopathol 2013;25:1359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Everds NE, Snyder PW, Bailey KL, et al. . Interpreting stress responses during routine toxicity studies: a review of the biology, impact, and assessment. Toxicol Pathol 2013;41:560–614. [DOI] [PubMed] [Google Scholar]
- 95.Kim JE, Cho BK, Cho DH, et al. . Expression of hypothalamic–pituitary–adrenal axis in common skin diseases: evidence of its association with stress-related disease activity. Acta Derm Venereol 2013;93:387–93. [DOI] [PubMed] [Google Scholar]
- 96.Kim JG, Jung HS, Kim K, et al. . Basal blood corticosterone level is correlated with susceptibility to chronic restraint stress in mice. Neurosci Lett 2013;555:137–42. [DOI] [PubMed] [Google Scholar]
- 97.Gądek-Michalska A, Tadeusz J, Rachwalska P, et al. . Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol Rep 2013b;65:1655–62. [DOI] [PubMed] [Google Scholar]
- 98.Powell ND, Tarr AJ, Sheridan JF.. Psychosocial stress and inflammation in cancer. Brain Behav Immun 2013;30:S41–7. [DOI] [PubMed] [Google Scholar]
- 99.Yammine L, Kang DH, Baun MM, et al. . Endothelin-1 and psychosocial risk factors for cardiovascular disease: a systematic review. Psychosom Med 2014;76:109–21. [DOI] [PubMed] [Google Scholar]
- 100.Kiecolt-Glaser JK, McGuire L, Robles TF, et al. . Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Annu Rev Psychol 2002;53:83–107. [DOI] [PubMed] [Google Scholar]
- 101.Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med 2014;76:181–9. [DOI] [PubMed] [Google Scholar]
- 102.Rohleder N. Acute and chronic stress induced changes in sensitivity of peripheral inflammatory pathways to the signals of multiple stress systems – 2011 Curt Richter Award Winner. Psychoneuroendocrinology 2012;37:307–16. [DOI] [PubMed] [Google Scholar]
- 103.Kotozaki Y, Kawashima R.. Effects of the Higashi-Nihon earthquake: posttraumatic stress, psychological changes, and cortisol levels of survivors. PLoS One 2012;7:e34612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schaeffer MA, Baum A.. Adrenal cortical response to stress at Three Mile Island. Psychosom Med 1984;46:227–37. [DOI] [PubMed] [Google Scholar]
- 105.Starkweather AR, Alhaeeri AA, Montpetit A, et al. . An integrative review of factors associated with telomere length and implications for biobehavioral research. Nurs Res 2014;63:36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ali F, Sultana S.. Repeated short-term stress synergizes the ROS signalling through up regulation of NFkB and iNOS expression induced due to combined exposure of trichloroethylene and UVB rays. Mol Cell Biochem 2012;360:133–45. [DOI] [PubMed] [Google Scholar]
- 107.Sudakov KV, Moroz BB, Salieva RM, et al. . The effect of the combined action of chronic gamma irradiation and emotional stress in rats. Fiziol Zh Im I M Sechenova 1995;8:41–9 (in Russian). [PubMed] [Google Scholar]
- 108.Miyachi Y, Yamada T.. Head-portion exposure to low-level X-rays reduces isolation-induced aggression of mouse, and involvement of the olfactory carnosine in modulation of the radiation effects. Behav Brain Res 1996;81:135–40. [DOI] [PubMed] [Google Scholar]
- 109.Zhukova NA, Ziablitskiĭ VM, Mikhal'skaia TIu, et al. . An experimental study of the early changes in the body after simultaneous radiationexposure at a low dose and stress. Radiats Biol Radioecol 1996;36:371–5 (in Russian). [PubMed] [Google Scholar]
- 110.Deshevoĭ I, Moroz BB, Lyrshchikova AV, et al. . Disruptions in the blood system upon exposure to low-dose ionizing radiation depending on duration of emotional stress. Radiats Biol Radioecol 2002;42:384–9 (in Russian). [PubMed] [Google Scholar]
- 111.Moroz BB, Deshevoĭ IuB, Voronina TA, et al. . Effect of mexidol on hemopoietic system in conditions of an emotional stress after exposure to ionizing radiation. Radiats Biol Radioecol 2007;47:163–70 (in Russian). [PubMed] [Google Scholar]
- 112.Moroz BB. Deshevoĭ IuB. Responses of the blood system during the stages of resistance and exhaustion in chronic emotional stress after gamma-irradiation at a dose of 90 rad. Patol Fiziol Eksp Ter 1994;3:17–9 (in Russian). [PubMed] [Google Scholar]
- 113.Muratova MIu, Vorozhtsov SV, Abrosimova AN, et al. . Combined effect of immobilization stress and gamma-irradiation on the blood-forming system in mice. Aviakosm Ekolog Med 2001;35:22–5 (in Russian). [PubMed] [Google Scholar]
- 114.Haran-Ghera N, Peled A, Krautghamer R, et al. . Initiation and promotion in radiation-induced myeloid leukemia. Leukemia 1992;6:689–95. [PubMed] [Google Scholar]
- 115.Webster MK, Goya L, Ge Y, et al. . Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol 1993;13:2031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hu W, Feng Z, Levine AJ.. The regulation of multiple p53 stress responses is mediated through MDM2. Genes Cancer 2012;3:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kemp CJ, Wheldon T, Balmain A.. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet 1994;8:66–9. [DOI] [PubMed] [Google Scholar]
- 118.Matamoros RA, Levine BS.. Stress response and drug metabolism in mice. Fundam Appl Toxicol 1996;30:255–63. [DOI] [PubMed] [Google Scholar]
- 119.Adler NE, Ostrove JM.. Socioeconomic status and health: what we know and what we don't. Ann NY Acad Sci 1999;896:3–15. [DOI] [PubMed] [Google Scholar]
- 120.Friedman EM, Lawrence DA.. Environmental stress mediates changes in neuroimmunological interactions. Toxicol Sci 2002;67:4–10. [DOI] [PubMed] [Google Scholar]
- 121.Cao L, Hudson CA, Lawrence DA.. Immune changes during acute cold/restraint stress-induced inhibition of host resistance to Listeria. Toxicol Sci 2003;74:325–34. [DOI] [PubMed] [Google Scholar]
- 122.Malvandi AM, Haddad F, Moghimi A.. Acute restraint stress increases the frequency of vinblastine-induced micronuclei in mouse bone marrow cells. Stress 2010;13:276–80. [DOI] [PubMed] [Google Scholar]
- 123.Jin J, Wang X, Wang Q, et al. . Chronic psychological stress induces the accumulation of myeloid-derived suppressor cells in mice. PLoS One 2013;8:e74497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Johnson SB, Riley AW, Granger DA, et al. . The science of early life toxic stress for pediatric practice and advocacy. Pediatrics 2013;131:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burkart W. Combined effect of radiation and other agents: is there a synergism trap. J Environ Pathol Toxicol Oncol 2001;20:53–8. [PubMed] [Google Scholar]
- 126.National Research Council (US) Committee on Health Risks of Exposure to Radon (BEIR VI) Appendix C. Tobacco-smoking and its interaction with radon In: National Research Council (US) Committee on Health Risks of Exposure to Radon (BEIR VI) (ed.) Health Effects of Exposure to Radon: BEIR VI. Washington (DC), WA: National Academies Press, 1999;224–53. [PubMed] [Google Scholar]
- 127.UNSCEAR Annex H. Combined effects of radiation and other agents In: UNSCEAR(ed.) Sources and effects of ionizing radiation. UNSCEAR 2000 Report to the general assembly, with scientific annexes. Volume II: Effects. New York, NY: United Nations Publication, 2000;177–295. [Google Scholar]
- 128.Jacob V, Jacob P, Meckbach R, et al. . Lung cancer in Mayak workers: interaction of smoking and plutonium exposure. Radiat Environ Biophys 2005;44:119–29. [DOI] [PubMed] [Google Scholar]
- 129.Yoshida K, Inoue T, Nojima K, et al. . Calorie restriction reduces the incidence of myeloid leukemia induced by a single whole-body radiation in C3H/He mice. Proc Natl Acad Sci U S A 1997;94:2615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shang Y, Kakinuma S, Yamauchi K, et al. . Cancer prevention by adult-onset calorie restriction after infant exposure to ionizing radiation in B6C3F1 male mice. Int J Cancer 2014;135:1038–47. [DOI] [PubMed] [Google Scholar]
- 131.Tannenbaum B, Anisman H.. Impact of chronic intermittent challenges in stressor-susceptible and resilient strains of mice. Biol Psychiatry 2003;53:292–303. [DOI] [PubMed] [Google Scholar]
- 132.Elliott BM, Grunberg NE.. Effects of social and physical enrichment on open field activity differ in male and female Sprague-Dawley rats. Behav Brain Res 2005;165:187–96. [DOI] [PubMed] [Google Scholar]
- 133.Kallai J, Makany T, Karadi K, et al. . Spatial orientation strategies in Morris-type virtual water task for humans. Behav Brain Res 2005;159:187–96. [DOI] [PubMed] [Google Scholar]
- 134.Kajantie E, Phillips DI.. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 2006;31:151–78. [DOI] [PubMed] [Google Scholar]
- 135.van Well S, Kolk AM, Klugkis IG.. Effects of sex, gender role identification, and gender relevance of two types of stressors on cardiovascular and subjective responses: sex and gender match and mismatch effects. Behav Modif 2008;32:427–49. [DOI] [PubMed] [Google Scholar]
- 136.Woolley DG, Vermaercke B, Op de Beeck H, et al. . Sex differences in human virtual water maze performance: novel measures reveal the relative contribution of directional responding and spatial knowledge. Behav Brain Res 2010;208:408–14. [DOI] [PubMed] [Google Scholar]
- 137.Cohen H, Yehuda R.. Gender differences in animal models of posttraumatic stress disorder. Dis Markers 2011;30:141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lengacher CA, Kip KE, Post-White J, et al. . Lymphocyte recovery after breast cancer treatment and mindfulness-based stress reduction (MBSR) therapy. Biol Res Nurs 2013;15:37–47. [DOI] [PubMed] [Google Scholar]
- 139.Carlson LE, Beattie TL, Giese-Davis J, et al. . Mindfulness-based cancer recovery and supportive-expressive therapy maintain telomere length relative to controls in distressed breast cancer survivors. Cancer 2015;121:476–84. [DOI] [PubMed] [Google Scholar]
- 140.Amos T, Stein DJ, Ipser JC.. Pharmacological interventions for preventing post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2014;7:CD006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hruska B, Cullen PK, Delahanty DL.. Pharmacological modulation of acute trauma memories to prevent PTSD: considerations from a developmental perspective. Neurobiol Learn Mem 2014;112:122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maniam J, Morris MJ.. Voluntary exercise and palatable high-fat diet both improve behavioural profile and stress responses in male rats exposed to early life stress: role of hippocampus. Psychoneuroendocrinology 2010;35:1553–64. [DOI] [PubMed] [Google Scholar]
- 143.Moroz BB, Deshevoĭ IuB, Seredenin SB, et al. . Using aphobazol for treatment of the emotional stress after exposure to ionizing radiation. Patol Fiziol Eksp Ter 2005;2:9–13 (in Russian). [PubMed] [Google Scholar]
- 144.Shimura T, Yamaguchi I, Terada H, et al. . Public health activities for mitigation of radiation exposures and risk communication challenges after the Fukushima nuclear accident. J Radiat Res 2015;56:422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shimura T, Yamaguchi I, Terada H, et al. . Radiation occupational health interventions offered to radiation workers in response to the complex catastrophic disaster at the Fukushima Daiichi Nuclear Power Plant. J Radiat Res 2015;56:413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]