Abstract
The subfamily of β2 integrins is implicated in macrophage fusion, a hallmark of chronic inflammation. Among β2 family members, integrin Mac-1 (αMβ2, CD11b/CD18) is abundantly expressed on monocyte/macrophages and mediates critical adhesive reactions of these cells. However, the role of Mac-1 in macrophage fusion leading to the formation of multinucleated giant cells remains unclear. Moreover, the role of integrin αDβ2 (CD11d/CD18), a receptor with recognition specificity overlapping that of Mac-1, is unknown. We found that multinucleated giant cells are formed in the inflamed mouse peritoneum during the resolution phase of inflammation, and their numbers were approximately twofold higher in wild-type mice than in Mac-1−/− mice. Analyses of isolated inflammatory peritoneal macrophages showed that IL-4–induced fusion of Mac-1–deficient cells was strongly reduced compared with wild-type counterparts. The examination of adhesive reactions known to be required for fusion showed that spreading, but not adhesion and migration, was reduced in Mac-1–deficient macrophages. Fusion of αDβ2-deficient macrophages was also significantly decreased, albeit to a smaller degree. Deficiency of intercellular adhesion molecule 1, a counter-receptor for Mac-1 and αDβ2, did not alter the fusion rate. The results indicate that both Mac-1 and αDβ2 support macrophage fusion with Mac-1 playing a dominant role and suggest that Mac-1 may mediate cell-cell interactions with a previously unrecognized counter-receptor(s).
Multinucleated giant cells (MGCs) have long been recognized as a common feature of many granulomatous infections. It is now fully realized that MGCs are also a hallmark of chronic inflammation observed in numerous pathologic disorders, including atherosclerosis, giant cell arteritis, foreign body reactions to implanted biomaterials, Crohn's disease, cancer, amyotrophic lateral sclerosis, and others.1, 2, 3, 4, 5, 6, 7, 8 MGCs are formed from blood monocytes recruited from the circulation to sites of inflammation where they differentiate into macrophages which further undergo fusion as inflammation progresses to the chronic state. Several cytokines, including IL-4, IL-3, and interferon-γ, are shown to induce the formation of MGCs in vitro.4 Furthermore, IL-4 induces fusion in foreign body reactions in vivo.9 Whether macrophage multinucleation is beneficial or detrimental in granulomatous inflammation is still debated4; however, MGCs may be undesirable for implanted biomaterials and probably in many other diseases.
Despite the long history of research on MGCs, the molecular and structural mechanisms of macrophage fusion remain poorly understood. Macrophage fusion inherently involves many adhesive events. Because cell-cell contacts is an obligatory step in fusion, several cell surface receptors, including SIRPα (macrophage fusion receptor), CD47, CD44, E-cadherin, tetraspanins, DC-STAMP, and others were shown to participate in this process.10 Previous in vitro investigations also demonstrated the importance of macrophage attachment to surfaces, with various substrates having different adhesion-, activation-, and fusion-promoting activities.10, 11 Macrophage adhesion appears to be a critical step for macrophage fusion in vivo as well because implanted biomaterials invariably trigger macrophage attachment and their subsequent fusion.5 Finally, macrophages seem to actively migrate to search for fusion partners, implying that adhesion-based migration is part of the process. Supporting evidence for this idea comes from the experiments in mice deficient in the chemokine ligand 2 (monocyte chemoattractant protein 1) which exhibit reduced macrophage fusion during foreign body reaction in vivo and decreased IL-4–induced formation of MGCs in vitro.12 Cell adhesion is mainly mediated by integrins, and previous studies that used function-blocking antibodies reported that β2 integrins play a role in adhesion of macrophages and their subsequent fusion on some, but not all, substrates.13, 14
Monocytes and macrophages express all four members of the β2 integrin subfamily, including αMβ2 (Mac-1, CD11b/CD18), αDβ2 (CD11d/CD18), αXβ2 (CD11c/CD18), and αLβ2 (CD11a/CD18). Among the β2 integrins, integrin Mac-1 is the most abundant and versatile receptor. Ligand engagement by Mac-1 initiates a variety of leukocyte responses, including adhesion, migration, phagocytosis, respiratory burst, degranulation, and others.15, 16 The complexity of Mac-1 functions arises from its ability to recognize a multitude of structurally and functionally dissimilar ligands that include numerous extracellular matrix proteins and those that become extracellular matrix-associated during the inflammatory response and released from damaged cells during tissue injury.17, 18 In addition, Mac-1 can bind counter-receptors expressed on the surface of other cells, including intercellular adhesion molecule 1 (ICAM-1), glycoprotein Ib-IX, and junctional adhesion molecule 3.19, 20, 21 The mechanism that allows Mac-1 to exhibit broad ligand binding specificity is based on the nature of its recognition motif that can be found in many unrelated proteins.18 Integrin αDβ2 is poorly expressed on peripheral blood monocytes but is up-regulated on macrophages at sites of chronic inflammation, for example, in atherosclerotic lesions.22 Integrin αDβ2 shares with Mac-1 many functional properties, including the ability to bind many ligands.23 The role of β2 integrins in macrophage fusion remains controversial. Although previous studies with function-blocking antibodies implicated β2 integrins in macrophage fusion,14 other reports concluded that β2 integrins play a minor role.24 Thus, definitive experimental evidence for the role of β2 integrins and, in particular, of Mac-1 is lacking, and the involvement of αDβ2 in fusion is unclear. Furthermore, it is uncertain exactly which step(s) of the multistage fusion process depend on these integrins.
Here, we examined the role of Mac-1 and αDβ2 in the formation of MGCs with the use of macrophages isolated from mice with deleted Mac-1 and αDβ2 genes. We also determined whether during fusion these receptors engage their ligand, ICAM-1. Our results demonstrate that both β2 integrins participate in fusion, but their contribution appears to correlate with their relative abundance on the surface of macrophages, with Mac-1 being a major player. Furthermore, ICAM-1, a counter-receptor for Mac-1 and αDβ2, is not absolutely required for fusion.
Materials and Methods
Mice
C57BL/6J, Mac-1−/− (B6.129S4-Itgamtm1Myd/J), αDβ2−/− (B6.129S7-Itgadtm1Bll/J), and ICAM-1−/− (B6.129S4-Icam1tm1Jcgr/J) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All procedures were performed in accordance with the animal protocols approved by the Institutional Animal Care and Use Committee at the Arizona State University and the Mayo Clinic Arizona. Mac-1−/−, αDβ2−/−, and ICAM-1−/− mice were housed in irradiated cages, and all mice were maintained under constant temperature (22°C) and humidity, on a 12-hour light/dark cycle in the Animal Facility of the Mayo Clinic Arizona.
Materials, Reagents, and Cells
Permanox dishes and Permanox slide chambers were obtained from Nalge Nunc International (Rochester, NY). Murine IL-4 was purchased from GenScript (Piscataway, NJ). Monoclonal antibody M1/70 directed against the mouse αM subunit was purified from the conditioned medium of hybridoma cells obtained from ATCC (Manassas, VA). Phycoerythrin- and fluorescein isothiocyanate-conjugated M1/70 and monoclonal antibody (mAb) 3.9 (fluorescein isothiocyanate-conjugated) against the αX subunit of integrin αXβ2 (CD11c/CD18) were purchased from eBioscience (San Diego, CA). Polyclonal antibody against the αDI-domain of integrin αDβ2 was described previously.25 Alexa Fluor 546–conjugated phalloidin and Rhodamine phalloidin were purchased from Molecular Probes (Eugene, OR). Opti-MEM I with glutamine and HEPES was purchased from Invitrogen (Gibco, Grand Island, NY). Recombinant human ICAM-1/CD54 Fc chimera was purchased from R&D Systems (Minneapolis, MN). The RAW264.7 murine macrophage cell line was obtained from ATCC. Cells were cultured in Dulbecco's modified Eagle's medium that was supplemented with 10% fetal bovine serum and antibiotics (0.1 mg/mL streptomycin and 0.1 unit/mL penicillin). The αDβ2-expressing HEK293 cells were previously described.23
Isolation of Peritoneal Macrophages
Eight- to 12-week-old male and female mice were used in all experiments with age-matched wild-type (WT) and deficient mice selected for side-by-side comparison. Peritonitis in mice was induced by the intraperitoneal injection of 0.5 mL of a 4% Brewer thioglycollate (TG) solution (Sigma-Aldrich, St. Louis, MO). Cells were collected 3 days after TG injection by peritoneal lavage with 5 mL ice-cold phosphate-buffered saline (PBS) with 5 mmol/L EDTA. The total number of cells in the lavage fluid was counted with a hemacytometer. The percentage of macrophages in the lavage was determined by differential analysis of cytospin preparations dyed with Wright stain. The size of macrophages was assessed by determining their diameter from cytospin photomicrographs. In selected experiments, macrophages were isolated from the peritoneal lavage with the use of the EasySep Mouse selection kit (StemCell Technologies, Vancouver, BC, Canada) with mAb F4/80 conjugated to phycoerythrin.
IL-4–Induced Macrophage Fusion
After isolation, peritoneal cells (2.5 × 105) in 500 μL of Opti-MEM that contained 10% fetal bovine serum were plated as a droplet in 6-cm Permanox dishes and allowed to adhere for 30 minutes at 37°C. RAW264.7 cells were plated at 1.25 × 105/500 μL. Medium (5 mL) was added to the dishes, and cells were incubated for 2 hours at 37°C, in 5% CO2, followed by the addition of 10 ng/mL IL-4. After incubation for 24 to 72 hours, the dishes were washed with PBS, and cells were fixed with 3.7% paraformaldehyde, followed by staining with Wright stain. Photomicrographs of representative fields were obtained with a Leica DM4000B (Leica Microsystems, Buffalo Grove, IL) microscope, and the number of nuclei in MGCs (>2) and mononucleated cells (MNCs) were counted. The extent of MGC formation was evaluated by determining percentage of fusion, which is defined as a fraction of nuclei within MGCs expressed as a percentage of total nuclei counted.26 The number of visible nuclei per MGC was also counted. A total of three to five low-power fields (20×) that contained approximately 100 to 200 cells were analyzed for each experimental condition. The MGC size was determined with ImageJ software (NIH, Bethesda, MD; http://imagej.net). To determine cell spreading, isolated macrophages were seeded on glass coverslips, and fusion was induced as described earlier in this paragraph. Macrophages were fixed with 2% paraformaldehyde, permeabilized with 0.1% Triton X-100, and stained for actin with Rhodamine-phalloidin. Confocal images were obtained with a Leica TCS SP5 AOBS Spectral Confocal System (Exton, PA).
The bifluorescent fusion assay was performed as described previously.13 Briefly, peritoneal macrophages (107/mL) were labeled with either the red fluorescent dye PKH26 (Ex/Em: 551/567) or a green fluorescent dye PKH67 (Ex/Em: 490/502) according to the manufacturer's instructions (Sigma-Aldrich). Cells were washed with PBS and resuspended in Opti-MEM. Equal numbers of PKH26- and PKH67-labeled macrophages were mixed and plated on Permanox dishes, and fusion was induced by IL-4 as described in the previous paragraph. After 48 hours, cells were fixed with 3.7% paraformaldehyde, and images were taken with a Leica DM4000B (Leica Microsystems) microscope. The images shown were captured with 5× objective (1600 × 1200 pixels). Colocalization of PKH26 and PKH67 was quantified with 8-bit images (256 grayscale) with the automated ImageJ colocalization plugin.
Adhesion Assays
For adhesion assays with peritoneal cells, cells were isolated from the peritoneum of WT and Mac-1–deficient 3 three days after TG injection, and 300 μL of 3 × 105 cells in Hanks' balanced salt solution plus 0.1% bovine serum albumin was added to Permanox slide chambers. After 10, 20, and 30 minutes at 37°C, nonadherent cells were removed by washing twice with PBS. Cells were fixed with 3.7% paraformaldehyde and stained with Alexa Fluor 546 phalloidin and DAPI. Images of 10 random fields were taken with the use of a Leica DM4000B microscope with the 20× objective, and the number of adherent cells was counted. Cell spreading was determined as changes in an initial round cell shape. Adhesion assays with αDβ2-expressing HEK293 cells were performed as described.23
Flow Cytometry
Fluorescent-activated cell sorting (FACS) analyses were performed to assess expression of integrins (Mac-1, αDβ2, and αXβ2) on the surface of peritoneal macrophages. Cells (1 × 106) were incubated with 10% normal mouse serum for 20 minutes on ice, washed twice with EDTA/PBS, followed by the addition of fluorescently labeled antibodies directed to the integrin subunits. After 30 minutes on ice, the cells were washed and analyzed with a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ) instrument. The populations of RAW264.7 cells that expressed high and low levels of Mac-1 were sorted with a FACSAria (Becton Dickinson) instrument.
Immunofluorescence
Forty-eight hours after the induction of fusion, RAW264.7 cells were fixed with 3.7% paraformaldehyde, washed three times in PBS, and blocked in a blocking solution (PBS supplemented with 3% normal goat serum and 1% bovine serum albumin) for 45 minutes. Cells were incubated with Alexa Fluor 546–conjugated phalloidin. Cells were mounted in Vectashield medium (Vector Laboratories, Burlingame, CA) that contained DAPI and were visualized with a Leica DM4000B fluorescence microscope. For immunocytochemical procedures required to visualize actin-containing filopodia peritoneal macrophages were incubated until respective time points and were fixed with 2% paraformaldehyde (pH 7.2). The macrophages were permeabilized with 0.1% Triton X-100 made in the aforementioned fixative. The specimens were blocked three times for 15 minutes each in PBS made 1% with bovine serum albumin. Rhodamine phalloidin was applied according to the manufacturer's recommendations, and the specimens were imaged with a Leica SP5 laser scanning confocal microscope. The number of actin-containing protrusions that emanated from the cell periphery was counted from images taken at 20× (three to five images per experiment) from three independent experiments.
Time Lapse Microscopy and Image Processing
Macrophage fusion was visualized with the EVOS Live Cell imaging system (Life Technologies, Grand Island, NY) equipped with the onstage incubator that enabled control of temperature, humidity, and carbon dioxide. Cells were applied on glass coverslips placed in the 36-mm Petri dishes, and fusion was induced by IL-4. The recording continued for 48 hours. Phase contrast images were acquired at 2-minute intervals with the use of a 40× objective. Multifield imaging allowed for simultaneous monitoring of eight areas on the same coverslip. Images acquired at each site were processed with the use of ImageJ software (NIH).
Statistical Analysis
Statistical analysis was performed by using SigmaPlot software version 11.0 (Systat Software, San Jose, CA). Results are presented as the means ± SD from at least three independent experiments. Significance was determined with a Student's t-test. P < 0.05 was considered significant.
Results
Formation of Multinucleated Macrophages in the Mouse Peritoneum during the Resolution Phase of Inflammation
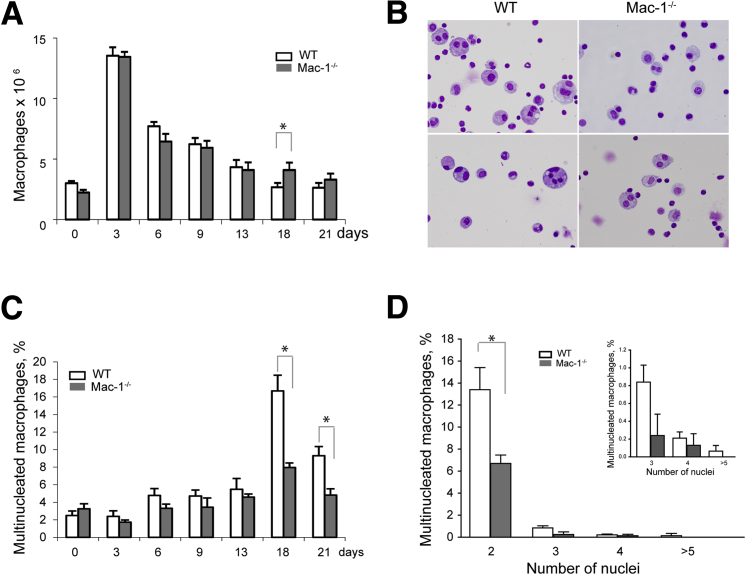
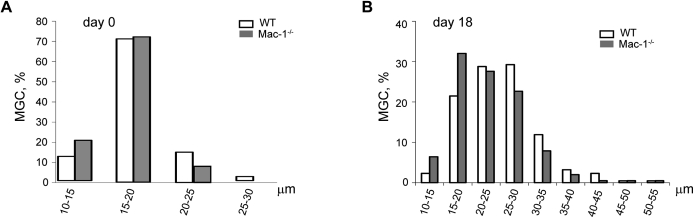
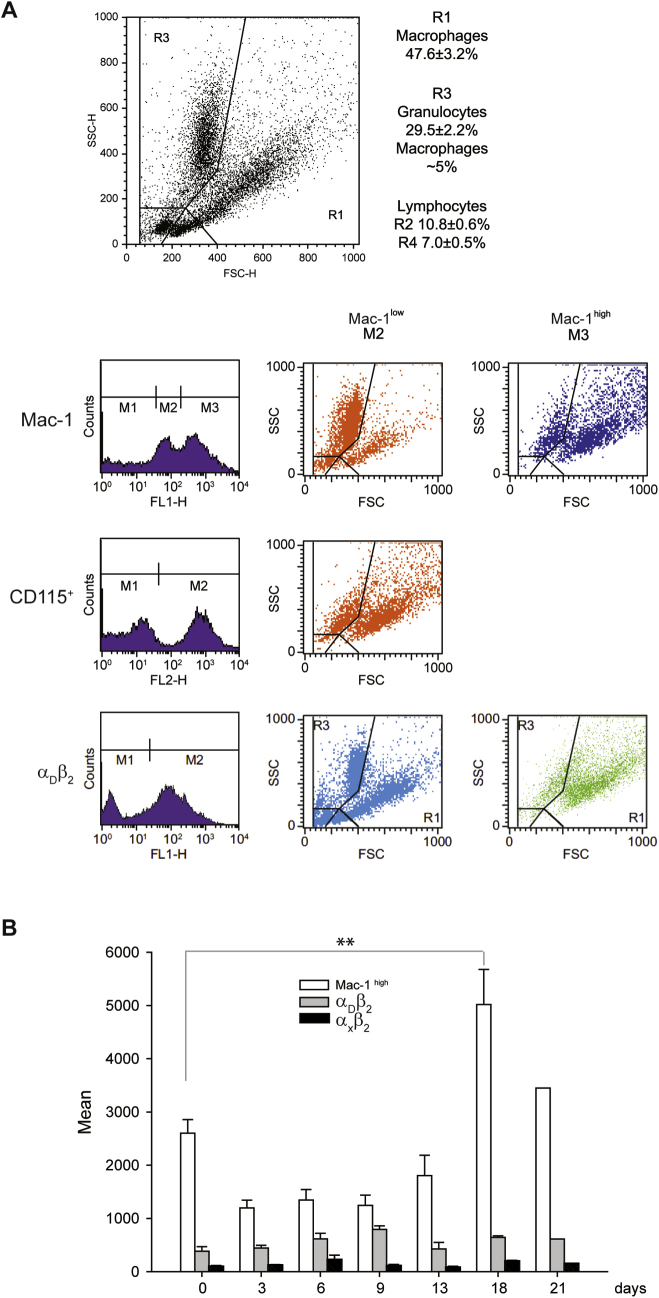
It was reported that most macrophages that were differentiated from peripheral blood monocytes recruited into the inflamed peritoneum do not die locally but leave the inflamed site by lymphatic drainage.27, 28 Although investigating the dynamics of cell populations in the inflamed peritoneum, we have found that macrophages that remain in the peritoneum during the resolution phase of inflammation contain a portion of multinucleated cells. In these studies, we used a mouse model of sterile peritonitis induced with TG, which was shown to be suitable not only for studies of monocyte influx from the circulation (acute phase of inflammation) but also for monitoring the emigration of macrophages from the peritoneum during the resolution phase. In agreement with previous reports,27, 28 the number of cells in the peritoneum was maximal at day 3 after TG injection, and by differential counts, 58% ± 7% of these cells were macrophages. The number of macrophages steadily declined after day 3 (the resolution phase) and by days 18 to 21 the cells stabilized (Figure 1A). The kinetics of macrophage accumulation in and emigration from the peritoneum of the Mac-1−/− mice were not different from those observed in WT mice. The analyses of cell populations in the peritoneum of WT mice at day 18 and 21 showed the presence of macrophages that contained several nuclei (Figure 1B). Although multinucleated macrophages were rarely observed in the noninflamed peritoneum (2.5% ± 0.3% of total resident macrophages), their numbers slightly increased at days 6 to 13 and then sharply rose up to 17% ± 2% at day 18 (Figure 1C). Furthermore, the diameter of resident macrophages (day 0) was slightly smaller than that of macrophages isolated at day 18 (Supplemental Figure S1), which appears to reflect the presence of multinucleated cells. The population of macrophages at day 18 expressed high levels of Mac-1 which was approximately two times higher than on resident macrophages (Supplemental Figure S2). Interestingly, Mac-1 expression over the course of inflammation coincided with the formation of multinucleated macrophages, that is, maximal integrin expression, and number of multinucleated cells were observed at day 18 and 21. Expression of the related integrin αDβ2 peaked at day 9 after TG injection and then slightly decreased (Supplemental Figure S2). Macrophages expressed low levels of αXβ2 (CD11c/CD18) compared with other β2 integrins, and its expression did not significantly change during the progression of inflammation (Supplemental Figure S2).
Figure 1.
Characterization of macrophage populations in the peritoneal lavage of mice at different times after TG injection. A: A peritoneum lavage was isolated 0 to 21 days after TG injection in WT and Mac-1−/− mice, and the number of macrophages was determined from differential counts. B: Representative images of cells from the peritoneal lavage isolated from WT and Mac-1−/− mice at day 18 after TG injection. Cells were stained with Wright stain. C: The number of MGCs in the peritoneum of WT and Mac-1−/− mice at day 18 after TG injection were determined as a percentage of total macrophage number. D: A number of nuclei in multinucleated macrophages were isolated from the peritoneal lavage of WT and Mac-1−/− mice at day 18 after TG injection. Data are expressed as means ± SD. n = 9 to 12 mice per time point determination (3, 6, 9, 13, and 18 days) from 3 to 5 experiments (A); n = 5 resident macrophages (day 0) from 17 mice (A); n = 2 macrophages at day 21 from 3 mice (A); n = 2 to 6 mice per 3 time points (C); n = 4 (D); n ≥ 3 nuclei/cell (inset, D). *P < 0.05. MGC, multinucleated giant cell; TG, thioglycollate, WT, wild-type.
Notably, despite a somewhat higher number of macrophages remaining in the peritoneum of the Mac-1−/− mice by days 18 and 21 compared with WT counterparts (Figure 1A), the number of multinucleated cells was approximately twofold lower (Figure 1C). This was especially clear for binucleate cells, which constituted most multinucleated cells (Figure 1D). The origin of these binucleate cells was not clear because they may arise either by fusion or proliferation of macrophages. Indeed, proliferative self-renewal of peritoneal macrophages was reported.29, 30 Nevertheless, the number of cells with 3, 4, and ≥ 5 nuclei, which most likely arise by fusion, was also less in the population of Mac-1–deficient macrophages (Figure 1D). Furthermore, cells with >5 nuclei, although rare, were observed only in the peritoneum of WT but not Mac-1−/− mice. Although additional work is required to elucidate the role of Mac-1 in the formation of binucleated and multinucleated cells during the resolution of inflammation in peritonitis, the results suggested that Mac-1 may participate in the formation of multinucleated cells.
The investigations of macrophages recovered from the αDβ2−/− mice at day 18 after TG injection showed no changes in the kinetics of macrophage recruitment into and their emigration from the inflamed peritoneum (not shown). Furthermore, similar numbers of multinucleated macrophages were formed in WT and αDβ2−/− mice (18% ± 1% versus 17% ± 2%).
Macrophage Fusion Requires Mac-1
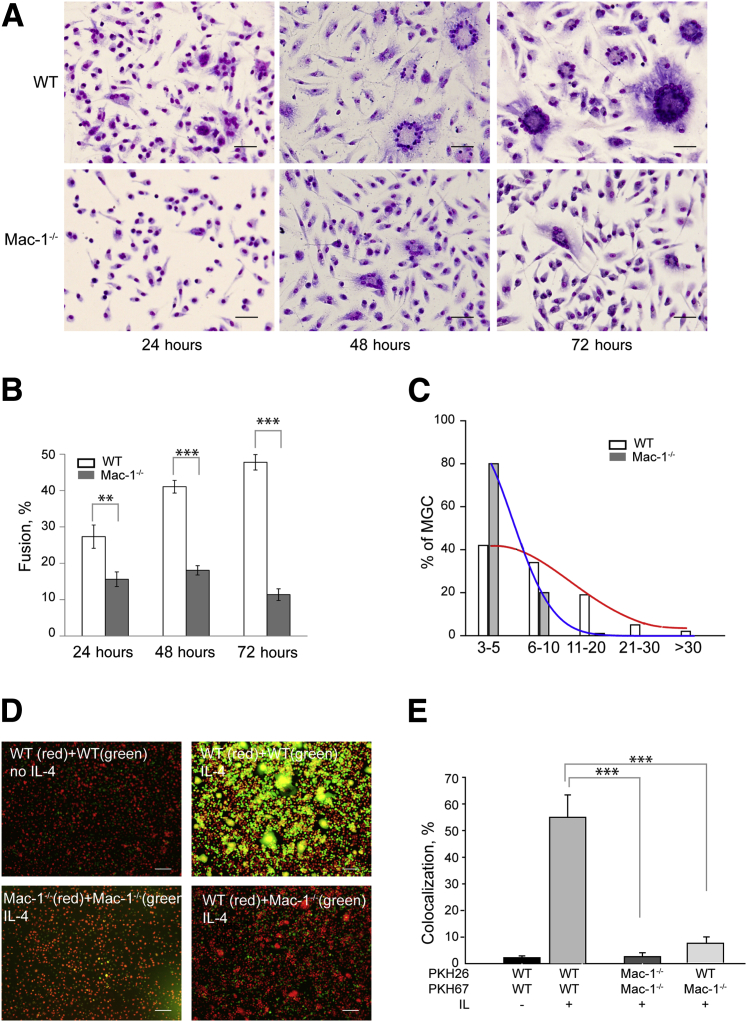
To investigate the role of Mac-1 in macrophage fusion, we examined the capacity of inflammatory peritoneal macrophages isolated from WT and Mac-1−/− mice to fuse in the presence of IL-4. Macrophages were isolated at day 3 after TG injection and were cultured on a permissive fusion substrate, Permanox plastic,13 in the presence of 10 ng/mL IL-4. Fusion was allowed to proceed for 24 to 72 hours, after which time the cells were fixed and treated with Wright stain to quantify the percentage of fusion. The number of MGCs formed from WT macrophages gradually increased to approximately 50% at 72 hours (Figure 2, A and B). In contrast, the fusion rate of macrophages isolated from the Mac-1−/− mice was significantly reduced. By day 3, macrophage fusion of Mac-1–deficient macrophages was 23% ± 2% that of WT macrophages. Moreover, MGCs formed from WT macrophages contained more nuclei than MGCs generated from Mac-1–deficient cells (Figure 2C). MGCs derived from WT macrophages were also somewhat larger than those derived from Mac-1–deficient macrophages (Supplemental Figure S2B). Control macrophage fusion of WT macrophages in the absence of IL-4 by day 3 was 24% ± 2% that of fusion with IL-4.
Figure 2.
Requirement of Mac-1 in mediating macrophage fusion. A: Time course of IL-4–induced fusion of WT and Mac-1–deficient macrophages. Cells were isolated from the peritoneum 3 days after TG injection and plated on Permanox dishes with the use of a procedure described in the Materials and Methods. After 24, 48, and 72 hours, cells were fixed and treated with Wright stain. B: The time-dependent fusion rates were determined for WT and Mac-1–deficient macrophages from Wright stain. C: The number of nuclei in MGCs formed after 72 hours from WT and Mac-1–deficient macrophages. D: Representative images of WT and Mac-1–deficient macrophages labeled with PKH26 (red) or PKH67 (green) and then cultured with 10 ng/mL or without IL-4 for 48 hours. Microscopic images were acquired in the green and red channels and overlaid. E: Colocalization (yellow) of two dyes that indicate the MGC formation was determined using ImageJ program (NIH, Bethesda, MD; http://imagej.net). Data are expressed as means ± SD (B and E) or percentage of a total number of counted MGCs (C). n = 5 to 10 experiments with approximately 400 cells counted in 5 to 10 representative low-power microscopic fields in each experiment (B); n = 25 to 130 MGCs (C); n > 10 experiments each with 5 random fields per slide (E). **P < 0.01, ***P < 0.001. Scale bars: 100 μm (A); 200 μm (D). Original magnification: ×20 or ×40 (B). MGC, multinucleated giant cell; TG, thioglycollate, WT, wild-type.
The involvement of Mac-1 in macrophage fusion was confirmed with a bifluorescent system.13 In this assay, a mixture of two sets of macrophages separately labeled with red (PKH26) and green (PKH67) fluorescent dyes produced hybrid bifluorescent cells, which allowed for the semiquantitative colocalization of two dyes and, hence, the rate of fusion. As determined by this method, IL-4 induced 56% ± 8% fusion of WT macrophages, whereas the fusion rate of Mac-1–deficient macrophages was <5% (Figure 2, D and E). In separate experiments, when PKH26-labeled WT and PKH67-labeled Mac-1–deficient macrophages were mixed and cultured under fusogenic conditions, only PKH26-labeled macrophages were able to form MGCs (red), whereas the colocalization of the two dyes was significantly reduced to 9% ± 1.8% (Figure 2, D and E), suggesting that the presence of Mac-1 is required on both fusing partners.
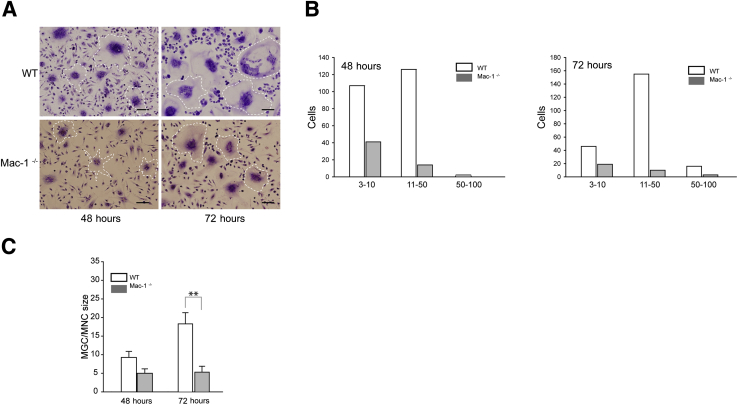
The peritoneal lavage obtained 3 days after TG injection contained several cell types with macrophages and lymphocyte/granulocytes presenting the main populations in both WT and Mac-1−/− mice (Supplemental Figure S2A). Because products secreted by lymphocytes may potentially differently affect fusion of WT and Mac-1–deficient macrophages, we examined the fusogenic capacity of purified cells. WT and Mac-1–deficient macrophages were isolated from the 3-day peritoneal lavage with the use of mAb F4/80 and phycoerythrin selection magnetic beads and used for fusion analyses. The extent of fusion of isolated Mac-1–deficient macrophages was still lower than that of WT cells (Figure 3A). The number of nuclei in WT macrophages after 48 and 72 hours was higher than found in Mac-1–deficient cells (Figure 3B), and the size of MGCs formed from WT cells was proportionally larger than that of Mac-1–deficient cells (Figure 3C).
Figure 3.
Fusion of purified macrophages isolated from WT and Mac-1−/− mice. Macrophages were isolated from the peritoneum lavage with the use of F4/80 mAb and PE selection kit, and fusion was performed. A: Representative images of WT macrophages and Mac-1−/− obtained 48 and 72 hours after the addition of IL-4. Selected MGCs are outlined. B: The number of nuclei in MGCs derived from WT and Mac-1–deficient macrophages was determined from staining with Wright dye. C: The size of MGCs is expressed as a ratio of MGC/MNC, where MNC is the surface occupied by a mononucleated macrophage. Data are expressed as means ± SD. n = 3 experiments each with 5 random fields per slide (B). **P < 0.01. Scale bar = 100 μm. mAb, monoclonal antibody; MGC, multinucleated giant cell; MNC, mononucleated cell; PE, phycoerythrin; WT, wild-type.
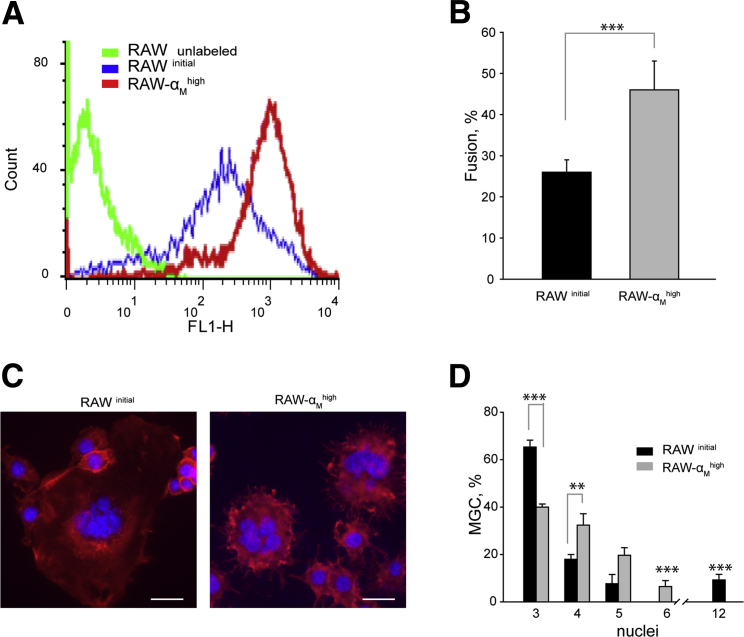
To further explore the importance of Mac-1 in macrophage fusion, we used a murine macrophage cell line RAW264.7. It was reported that these cells undergo efficient fusion in the presence of IL-4.31 The initial population of RAW264.7 cells was sorted to select cells that expressed high levels of Mac-1 (Figure 4A). RAW264.7 cells with high levels of Mac-1 (RAW-αMhigh) fused more frequently than cells in the initial unsorted population (RAWinitial), leading to a greater number of MGCs (Figure 4B). However, MGCs formed from RAW-αMhigh contained fewer nuclei per cell (Figure 4, C and D). Rare cells that contained >10 nuclei were observed only in the unsorted population of RAW264.7 macrophages. In contrast, MGCs generated from RAW-αMhigh contained, on average, three to six nuclei. These data suggest that the increased density of Mac-1 enhanced the macrophage fusion potential so more cells acquired the capacity to fuse. Taken together, these findings indicate that Mac-1 is a key player in the process of macrophage fusion.
Figure 4.
Fusion of RAW264.7 cells that express different levels of Mac-1. A: The initial population of RAW264.7 cells was sorted to select high Mac-1 expressors (RAW264.7-αMhigh). B: RAW264.7initial and RAW264.7-αMhigh were plated on Permanox dishes, and fusion was induced with 10 ng/mL IL-4 for 48 hours. The fusion rate was determined from Wright staining. C: Representative images of MGCs formed from two populations of RAW264.7 cells stained with Alexa Fluor 546 phalloidin for actin and DAPI for nuclei. D: The number of nuclei in MGCs was calculated in samples stained with the Wright dye. Because RAW264.7 cells divide in culture, only cells with >2 nuclei were counted. Data are expressed as means ± SD. n = 3 experiments with 5 random fields analyzed in each experiment (B and D); n = 42 RAW264.7initial and 57 RAW264.7-αMhigh cells. **P < 0.01, ***P < 0.001. Scale bar = 20 μm. MGC, multinucleated giant cell.
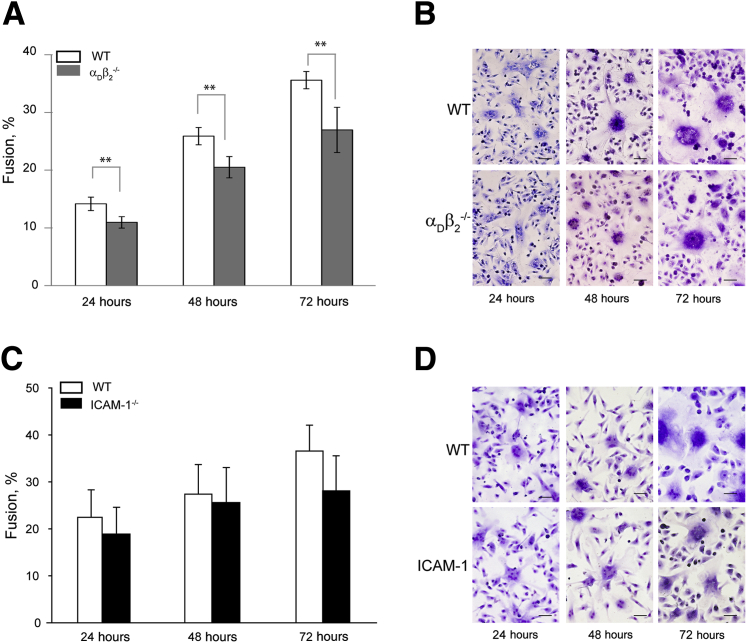
Contribution of αDβ2 and ICAM-1 to Macrophage Fusion
We previously showed that integrin αDβ2 was a receptor with recognition specificity that overlapped that of Mac-1.23 Although no significant decrease in multinucleated macrophages was detected in the peritoneum of the αDβ2−/− mice compared with WT mice during the resolution phase of inflammation, expression of this receptor was elevated by day 9 after TG injection (Supplemental Figure S2). We, therefore, examined the contribution of αDβ2 to macrophage fusion with the use of peritoneal cells isolated from the αDβ2−/− mice. The deficiency of αDβ2 resulted in the decrease of macrophage fusion compared with WT macrophages; however, this reduction was less than that observed for Mac-1–deficient macrophages (75% ± 4% versus 23% ± 2% after 72 hours) (Figure 5, A and B). A limited effect of the αDβ2 deficiency on macrophage fusion may be due to its reduced expression on macrophages.
Figure 5.
Fusion of αDβ2- and ICAM-1–deficient macrophages. A and C: Cells isolated from the peritoneum of αDβ2−/− (A) and ICAM−/− (C) deficient mice 3 days after TG injection were plated on Permanox dishes, and IL-4–induced time-dependent fusion was determined. B and D: Representative images of WT, αDβ2−/−, and ICAM−/− macrophages 24, 48, and 72 hours after the addition of IL-4. Data are expressed as means ± SD. n = 3 separate experiments (A and C). **P < 0.01. Scale bar = 100 μm. ICAM, intercellular adhesion molecule; TG, thioglycollate; WT, wild-type.
Human blood monocyte/macrophages3, 32 and murine peritoneal macrophages (data not shown) express ICAM-1, a counter-receptor for Mac-1. Consistent with its ability to bind the same ligands as Mac-1,23 αDβ2 was also capable of binding ICAM-1. ICAM-1 supported efficient adhesion of αDβ2-expressing cells to ICAM-1, and anti-αDβ2 antibodies efficiently blocked adhesion in a dose-dependent manner (Supplemental Figure S3). It was reported that anti–ICAM-1 antibodies partially inhibited the MGC formation (approximately 40%).32
We directly examined the role of ICAM-1 in macrophage fusion by testing macrophages isolated from the ICAM-1−/− mice. Unexpectedly, no significant difference was found in the fusion rate between ICAM-1–deficient and WT macrophages over a 72-hour period of observation (Figure 5, C and D). Collectively, these results demonstrated that both β2 integrins, Mac-1 and αDβ2, are required for macrophage fusion with Mac-1 playing a dominant role. They also indicated that ICAM-1 is unlikely to be involved in the interaction with these receptors during fusion, suggesting the role of other counter-receptor(s).
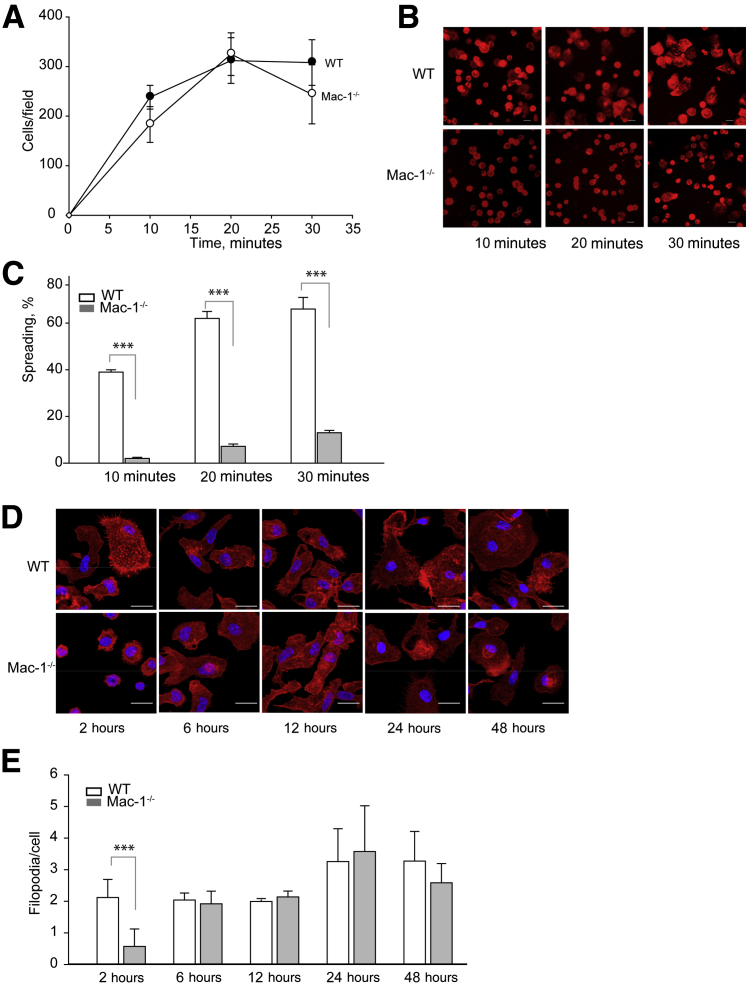
Comparison of Adhesion, Spreading, and Migration of WT and Mac-1–Deficient Macrophages during Fusion
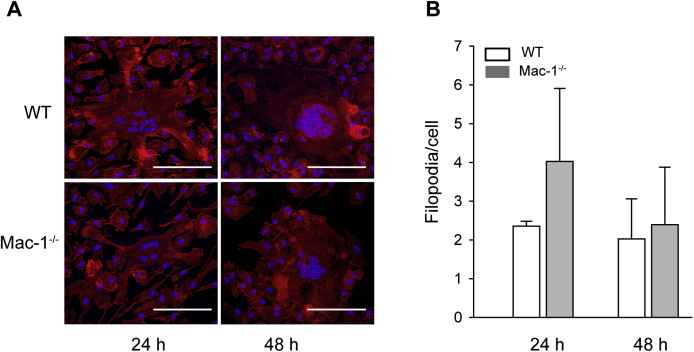
Mac-1 is an adhesion receptor that mediates leukocyte attachment to various extracellular matrix proteins and to the naked plastic.17, 33 Adhesion is a critical step during macrophage fusion,13 and previous studies that used RGD-modified culture plates indicated that β2 integrins play a role in adhesion during fusion.14 However, adhesion of macrophages to the permissive surface Permanox was shown to be integrin β2 independent.13 We confirmed that WT macrophages adhered efficiently to the Permanox dishes. Furthermore, in agreement with earlier results with anti-αM mAb 5C6,13 function-blocking mAb M1/70 directed to αM, although it tended to decrease adhesion, it did not significantly alter it (Supplemental Figure S4). We also found that the inhibitory polyclonal anti-αDβ2 antibody had no effect. Consistent with the lack of a requirement for Mac-1 in macrophage adhesion to Permanox, Mac-1–deficient cells adhered to a similar extent as WT macrophages (Figure 6A). However, in contrast to WT macrophages, spreading of Mac-1–deficient cells was markedly reduced (Figure 6, B and C). To corroborate cell spreading studies with a count of actin-based filopodia, which may serve as a measure of cell spreading, cells were plated on glass coverslips, and fusion was induced with IL-4. The difference in the number of filopodia between WT and Mac-1–deficient mononucleated macrophages were observed 2 hours after cell seeding after which time (determined for 6, 12, 24, and 48 hours) no differences in this morphologic feature were detected (Figure 6, D and E). In addition, the formation of filopodia in MGCs derived from WT and Mac-1–deficient macrophages at 24 and 48 hours was similar (Supplemental Figure S5).
Figure 6.
Adhesion and spreading of WT and Mac-1–deficient macrophages. Peritoneal macrophages isolated from a 3-day inflamed peritoneum of WT and Mac-1−/− mice were plated on Permanox dishes (A–C) or glass coverslips (D and E). A: Macrophages were allowed to adhere for 10, 20, and 30 minutes at 37°C. Nonadherent cells were removed, and adherent cells were labeled with Alexa Fluor 546 phalloidin and DAPI. Ten randomly selected fields were photographed for each condition, and adherent cells were counted with ImageJ software (NIH, Bethesda, MD; http://imagej.net). B: Representative images of spreading of WT and Mac-1–deficient macrophages. C: Percentage of spread cells was determined from the five images of adherent cells. D: Fusion of inflammatory peritoneal macrophages isolated from WT and Mac-1−/− mice plated on glass coverslips for 2 hours was induced with 10 ng/mL IL-4. After various periods of time, cells were fixed with 2% paraformaldehyde and stained with Rhodamine phalloidin. Representative images of cells 2, 6, 12, 24, and 48 hours after plating. E: The total number of actin-based protrusions was counted and divided by the total number of cells in the field. Data are expressed as means ± SD. n = 10 fields with approximately 300 cells per image (A); n > 200 cells in 3 to 5 randomly selected fields of 3 separate experiments (E). ***P < 0.001. Scale bar = 20 μm. Original magnification: ×20 (B and E). WT, wild-type.
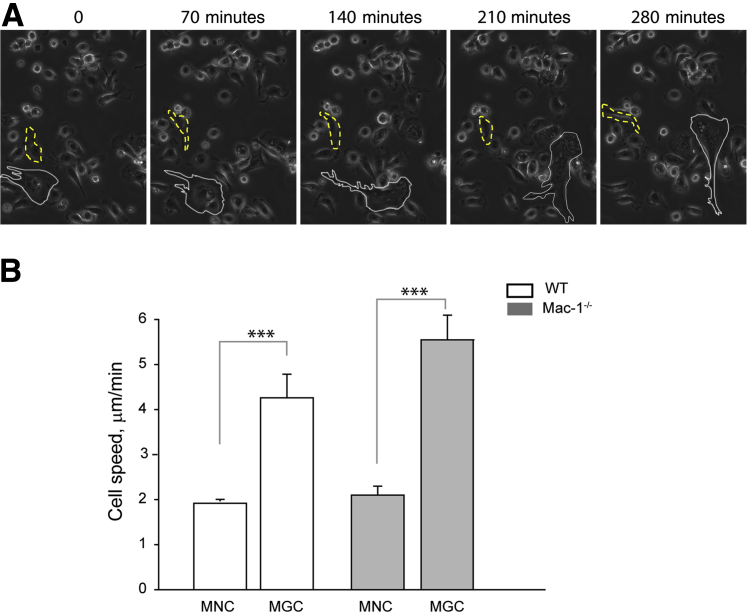
Macrophages move during fusion in vitro,34 and Mac-1, which is known to modulate leukocyte migration,35, 36 may influence the migratory properties of macrophages. To examine whether WT and Mac-1–deficient macrophages have different migratory behavior during IL-4–induced fusion, we determined the rate of cell migration with the use of time-lapse microscopy. Macrophages were plated on glass coverslips, and imaging was initiated after the addition of IL-4 and continued for 48 hours at 37°C in an atmosphere that contained 5% CO2. No difference was found in migration of WT and Mac-1–deficient MNCs (1 ± 0.05 μm/min and 1 ± 0.1 μm/min, respectively) during the first 24 hours. After 24 hours, a population of cells consisted of MNCs and MGCs which allowed us to independently evaluate migration of both cell types. A representative field in Figure 7A and Supplemental Movie S1 shows an MGC and an MNC that were tracked for a period over 4 hours. The difference between MNCs and MGCs was clearly observed, with MGCs generated from both WT and Mac-1–deficient macrophages having an approximately two times higher migration rate (Figure 7B). However, WT and Mac-1–deficient MNCs and MGCs could not be distinguished by their migration rates. These findings indicate that, although Mac-1 is involved in initial macrophage spreading, it is not required for long-term spreading and macrophage migration, suggesting that during fusion Mac-1 may be involved in other as of yet undefined processes.
Figure 7.
Migration of WT and Mac-1–deficient macrophages during fusion. The speed of migration of MGCs and MNCs was determined from time-lapse imaging with the use of the ImageJ TrackMate software (NIH, Bethesda, MD; http://imagej.net). Video recording was initiated after 24 hours of stimulation with IL-4 and continued for an additional 24 hours. A: Representative still frames of WT macrophages show the route traveled by an MGC (white outline) and an MNC (yellow dashed outline). B: Migration rates of WT and Mac-1–deficient cells were determined from the distances traveled by cells. MGCs and MNCs were tracked, and the speed of their migration was calculated. Four different fields were imaged simultaneously for WT and Mac-1–deficient macrophages. Data are expressed as means ± SD. n = 5 individual MGCs and 10 MNCs and representative of 2 experiments. ***P < 0.001. MGC, multinucleated giant cell; MNC, mononucleated cell; WT, wild-type.
Discussion
Integrin Mac-1 is a receptor on the surface of neutrophils and monocyte/macrophages, which supports adhesion of these cells to the extracellular matrix proteins and counter-receptors on various cells, and also regulates many responses, including phagocytosis, migration, aggregation, and degranulation. Here, we showed that Mac-1 is also involved in macrophage fusion because the ability of Mac-1–deficient macrophages to form MGCs was strongly impaired. Furthermore, we found that a related β2 integrin, αDβ2, which shares with Mac-1 its ligand-binding specificity,23 participates in macrophage fusion inasmuch as fusion of αDβ2-deficient macrophages was reduced compared with WT cells, albeit to a less extent than that of Mac-1–deficient cells. Finally, we found that macrophages lacking ICAM-1, a counter-receptor for both Mac-1 and αDβ2, undergo fusion to a similar extent as WT cells. These observations establish a novel function of Mac-1 and αDβ2 in macrophage fusion and implicate an unknown molecule(s) on the surface of fusing macrophages as a counter-receptor for these integrins.
Integrin Mac-1 is a multiligand receptor capable of binding numerous structurally and functionally unrelated proteins. The mechanism that endows Mac-1 with the ability to bind a variety of extremely diverse molecules is the nature of its recognition motif which can be found in many proteins. In particular, the αMI-domain, a ligand-binding region of Mac-1, has affinity for short amino acid sequences that contain a core of basic residues flanked by hydrophobic residues and in which negatively charged residues are strongly disfavored.18, 37 Such ubiquitous sequences are usually present in the interior of folded proteins and become exposed by the conformational alterations on immobilization of proteins on various surfaces. In addition, Mac-1 interacts with several counter-receptors on various cells, including ICAM-1, glycoprotein Ib-IX, and junctional adhesion molecule 3,19, 20, 21 in which Mac-1 recognition sequences apparently conform to the typical Mac-1 binding motif.18 Considering that macrophage fusion inherently involves many adhesive steps, including adhesion to the substrate and cell-cell interactions, the remarkable sticky properties of Mac-1 make it an ideal candidate for mediating this process.
Previous studies with function-blocking antibodies have demonstrated that the initial adhesion to culture plastic of human monocytes, the cells that were used to generate fusing macrophages, depends on β2 integrins.14 Furthermore, on the IL-4 induction of macrophage fusion at days 3 through 7, both anti-β2 and anti-β1 antibodies inhibited adhesion. In addition, the contribution of Mac-1 was noted inasmuch as a mAb to the αM integrin subunit inhibited the late steps of macrophage adhesion.14 However, studies that examined adhesion of TG-elicited macrophages to Permanox plastic found no effect of anti-β2 and anti-αM antibodies.13 These results are consistent with our findings that macrophage adhesion to Permanox was only slightly inhibited by anti–Mac-1 antibodies and that adhesion of Mac-1–deficient macrophages was similar to that of WT cells (Figure 6). Nevertheless, although adhesion of macrophages was not affected by the lack of Mac-1, cell spreading of Mac-1–deficient macrophages determined 30 minutes and 2 hours after cell plating was strongly reduced compared with WT macrophages, and the number of filopodia was less (Figure 6). Therefore, Mac-1–initiated signaling leading to the cytoskeletal rearrangements and formation of actin-based filopodia and lamellipodia may be critical early events during macrophage fusion that are compromised in Mac-1–deficient macrophages, thus affecting their fusogenic capacity. In support of this idea, previous studies have demonstrated that formation of MGCs was preceded by the lamellipodia formation and was attenuated by inhibition of Rac-1, but not Rho activation.31 It is possible that activation of Cdc42, another member of the Rho family of small GTPases which is known to control assembly of filopodia,38 may play a vital role in fusion. Intriguingly, recent studies detected an extensive system of actin-rich Mac-1–bearing ruffles on the surface of Phorbol 12-myristate 13-acetate–activated macrophages and MGCs, with latter cells involved in Mac-1–mediated phagocytosis of large targets.39 Further studies may help to define the relation between Mac-1–mediated adhesion/spreading and formation of actin-based membrane protrusions in macrophage fusion.
Consistent with the role of Mac-1 in macrophage fusion, RAW264.7 cells sorted to express the increased density of integrin fused more frequently in the presence of IL-4 than unsorted cells (Figure 4). The transient up-regulation of Mac-1 that coincided with the formation of multinucleated cells in the peritoneum at days 18 and 21 after TG injection was also observed (Figure 1, C and D). Interestingly, the requirement for Mac-1 under these conditions appears to conflict with the lack of noticeable fusion in the population of resident peritoneal macrophages, which are known to exhibit Mac-1high phenotype.40, 41 Moreover, murine macrophages deficient in the transcription factor GATA binding factor 6, a major regulator of the peritoneal-resident macrophage phenotype, and exhibiting reduced expression of Mac-1 were multinucleate and showed a fusogenic phenotype in the absence of inflammation.42 These observations suggest that studies of macrophage fusion may be markedly influenced by the type of cells, that is, resident versus inflammatory macrophages, the nature of specific inciting stimuli, the density of the receptor, the stage of inflammation, and others. At present, the role of Mac-1 in fusion of different types of macrophages and under various inflammatory conditions is unclear.
Because many studies of macrophage fusion used cell cultures after their fixation, it is poorly appreciated that the process of MGC formation in vitro involves active cell migration34 (Supplemental Movie S1). Cell adhesion and migration are the linked processes, and previous in vitro studies demonstrated that Mac-1 and αDβ2, in cooperation with β1 integrins, are capable of supporting cell migration.25, 35, 36 However, this capacity is realized only when Mac-1 and αDβ2 are expressed on the cell surface at low/moderate densities; the increased density of these integrins inhibits β1-driven cell migration through the formation of excessive adhesive bonds with the substrate.36 Our results of time-lapse microscopy show that MGCs generated either from WT or Mac-1–deficient macrophages actively move with no difference in their migratory rates (Figure 7). Moreover, no difference in migration of WT and Mac-1–deficient mononucleated macrophages was detected, although these cells were less migratory than MGCs. These data are consistent with results demonstrating that other integrins, most probable β1, can support migration of macrophages and suggest that the defect in fusion of Mac-1–deficient macrophages is unlikely associated with their diminished migratory properties.
Macrophage fusion requires bringing two plasma membranes together and may involve the interaction of Mac-1 with its counter-receptor(s) on opposing cells. In this regard, McNally and Anderson14 have demonstrated that anti-β2 antibodies inhibited not only adhesion but also the MGC formation, suggesting that the fusion step is also mediated by β2 integrins. However, whether Mac-1 was involved in this step has not been determined in these studies. One of the Mac-1's counter-receptors, ICAM-1, is expressed on the surface of fusing macrophages32 (data not shown), and previous studies reported that an anti-ICAM antibody inhibited by approximately 40% the formation of MGCs on their induction from human monocytes by interferon-γ.3, 32 Our investigations that used ICAM-1–deficient murine macrophages do not confirm the essential involvement of this molecule (Figure 5B). Thus, although the degree of expression of adhesion receptors, different cytokines used to induce fusion, or the cell type analyzed may account for these discrepancies, the role of ICAM-1 as a chief counter-receptor for Mac-1 and αDβ2 during fusion seems to be unlikely. Instead, other transmembrane proteins, in particular those similar to ICAM-1 belong to the Ig super-family may potentially substitute for ICAM-1. Note that the molecules containing Ig-like domains are widely accepted to participate in fusion. For example, recognition and adhesion between Drosophila myoblasts are mediated by transmembrane proteins that contain Ig-domain.43, 44, 45 Whether such molecules on the surface of fusing macrophages serve as Mac-1 counter-receptors is under investigation.
Our results suggest that IL-4–induced macrophage fusion is most efficient when Mac-1 is present on each fusion partner. This conclusion is supported by the finding that the ability of WT macrophages to fuse with Mac-1–deficient counterparts was strongly impaired and that fusion in the mixture that contained both WT and Mac-1–deficient macrophages occurred mainly between WT cells (Figure 2, D and E). The meaning of this finding is presently unclear, and its complexity is further increased by the lack of information about the molecular and structural mechanisms of macrophage fusion. One interpretation of the reduced ability of Mac-1–deficient macrophages to fuse with each other and with WT cells is that Mac-1 may form a cis complex with the molecule(s) that are involved, directly or indirectly, in fusion. Consistent with this speculation, Mac-1 is known to form lateral complexes in the plasma membrane with several receptors to regulate leukocyte behavior.46, 47 The interaction of Mac-1 with some of the molecules implicated as mediators of fusion (CD47, macrophage fusion receptor, CD44, tetraspanins, matrix metalloprotease 9, and others) may be required to make them competent for fusion by a variety of potential mechanisms, for example, altering their conformation, stabilizing in the plane of the plasma membrane, or cooperating with Mac-1 in intracellular signaling. Alternatively, a lateral complex of Mac-1 with an unknown membrane protein may be necessary for the efficient interaction of Mac-1 with counter-receptors on the opposing cells. At present, whether Mac-1 forms such complexes and, if so, what their function is in fusing macrophages remains to be established.
The identification of Mac-1 as a mediator of macrophage fusion extends the list of its functions in leukocyte biology. Among other receptors that were implicated in macrophage fusion, Mac-1 is unique in that it may, because of its prodigious adhesive and signaling properties, contribute to several functional steps involved in fusion. However, although the contribution of Mac-1 to macrophage fusion is clearly established in the present study, how precisely it acts remains to be defined. The progress in elucidating the function of many fusion mediators identified to date has been impeded by the lack of systematic ultrastructural studies that may reveal intimate processes occurring at the interface between two fusing macrophages. Likewise, modern high-resolution imaging techniques and real-time visualization of live macrophages should provide invaluable insights into the process of their fusion.
Footnotes
Supported by NIH grant HL63199 (T.P.U.).
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.04.001.
Supplemental Data
Supplemental Figure S1.
A and B: The size of resident macrophages (day 0) (A) and macrophages in the peritoneal lavage at day 18 after TG injection (B) were counted. Data are expressed as means ± SD. n = 6 experiments with 100 cells counted for each experimental condition. MGC, multinucleated giant cell; TG, thioglycollate; WT, wild-type.
Supplemental Figure S2.
Expression of β2 integrins on the surface of macrophages. A: FACS analyses of cell populations present in the peritoneal lavage at day 3 after TG injection. The four populations of cells were identified from their appearance on FSC/SSC dot plots (R1 to R4). Macrophages were defined as a population of cells in the R1 region that expressed Mac-1high, CD115+, and αDβ2+ (48% ± 3% of a total cell population in the peritoneum). B: Cells in the R2 region consist of macrophages that expressed Mac-1low/αDβ2+ (approximately 5%) and granulocytes (mainly eosinophils) that expressed Mac-1low/αDβ2+ (approximately 30%). The rest of the cells (approximately 17%) are lymphocytes present in R2 and R4. B: Density of Mac-1, αDβ2, and αXβ2 on macrophages in the R1 region determined at different time points after TG injection. Peritoneal cells isolated from the peritoneum of WT mice were stained with mAb M1/70 (anti–Mac-1), polyclonal anti-αDβ2, and mAb 3.9 (anti-αXβ2) and analyzed by FACS. Data are expressed as means ± SD. n = 3 to 6 experiments. **P < 0.01. FACS, fluorescent-activated cell sorting; FSC, forward scatter; mAb, monoclonal antibody; SSC, side scatter; TG, thioglycollate; WT, wild-type.
Supplemental Figure S3.
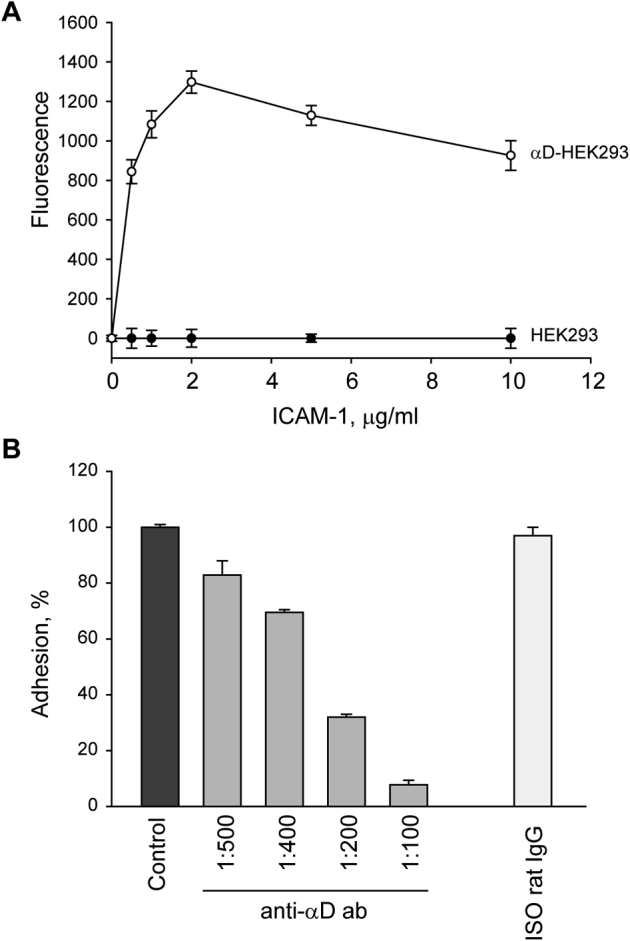
Adhesion of αDβ2-expression cells to ICAM-1. A: Wild-type and αDβ2-expressing HEK293 cells were labeled with calcein, and 5 × 104 cells were added to wells of Immulon 4 microtiter plates coated with different concentrations of ICAM-1 for 30 minutes at 37°C. Nonadherent cells were removed, and fluorescence of adherent cells was determined. B: Calcein-labeled αDβ2-expressing cells were preincubated with different concentration of anti-α αDβ2 polyclonal antibody or isotype control rat IgG for 15 minutes at 22°C and allowed to adhere to wells coated with 2 μg/mL ICAM-1. Data are expressed as percentages of maximal cell adhesion in a control sample without the antibodies and means ± SD. n = 3 independent experiments. ICAM-1, intercellular adhesion molecule 1.
Supplemental Figure S4.
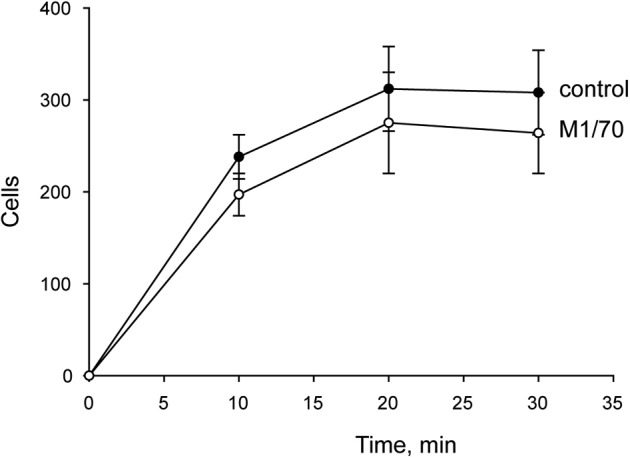
Effect of anti-αM antibody on macrophage adhesion to Permanox. Peritoneal macrophages isolated from a 3-day inflamed peritoneum of wild-type mice were preincubated without or with 20 μg/mL of anti-αM antibody M1/70. Three hundred microliters of 5 × 105cells/mL were added to the Permanox well slides and allowed to adhere for 10, 20, and 30 minutes at 37°C. Nonadherent cells were removed, and adherent cells were labeled with Alexa Fluor 546 phalloidin and DAPI. Randomly selected fields were photographed for each condition, and adherent cells were counted with the use of ImageJ software (NIH, Bethesda, MD; http://imagej.net). Data are expressed as means ± SD. n = 10 fields with approximately 300 cells per image.
Supplemental Figure S5.
Actin-containing protrusions on MGCs formed from WT and Mac-1–deficient macrophages. A: Representative images of MGCs formed from WT and Mac-1–deficient macrophages stained with Rhodamine phalloidin for actin and DAPI for nuclei. B: The number of actin-containing protrusions was calculated from confocal images of MGCs formed from WT and Mac-1–deficient macrophages after 24 and 48 hours of fusion. The total number of actin-based protrusions was counted and divided by the total number of cells in the field. Data are expressed as means ± SD. n = 3 separate experiments. Scale bar = 100 μm. MGC, multinucleated giant cell; WT, wild-type.
The routes of one multinucleated giant cell and one mononucleated cell were tracked for 4 hours to determine the speed of their migration.
References
- 1.Samokhin A.O., Wilson S., Nho B., Lizame M.L., Musenden O.E., Bromme D. Cholate-containing high-fat diet induces the formation of multinucleated giant cells in atherosclerotic plaques of apolipoprotein E-/- mice. Arterioscler Thromb Vasc Biol. 2010;30:1166–1173. doi: 10.1161/ATVBAHA.110.203976. [DOI] [PubMed] [Google Scholar]
- 2.Eberhardt R.T., Dhadly M. Giant cell arteritis: diagnosis, management, and cardiovascular implications. Cardiol Rev. 2007;15:55–61. doi: 10.1097/01.crd.0000218853.05856.b6. [DOI] [PubMed] [Google Scholar]
- 3.Fais S., Burgio V.L., Silvestri M., Capobianchi M.R., Pacchiarotti A., Pallone F. Multinucleated giant cells generation induced by interferon-gamma. Changes in the expression and distribution of the intercellular adhesion molecule-1 during macrophages fusion and multinucleated giant cell formation. Lab Invest. 1994;71:737–744. [PubMed] [Google Scholar]
- 4.Helming L., Gordon S. The molecular basis of macrophage fusion. Immunobiology. 2007;212:785–793. doi: 10.1016/j.imbio.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Anderson J.M., Rodriguez A., Chang D.T. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z.X., Noguchi M., Hiwatashi N., Toyota T. Monocyte aggregation and multinucleated giant-cell formation in vitro in Crohn's disease. The effect of cell adhesion molecules. Scand J Gastroenterol. 1996;31:706–710. doi: 10.3109/00365529609009154. [DOI] [PubMed] [Google Scholar]
- 7.Pawelek J.M., Chakraborty A.K. Fusion of tumour cells with bone marrow-derived cells: a unifying explanation for metastasis. Nat Rev Cancer. 2008;8:377–386. doi: 10.1038/nrc2371. [DOI] [PubMed] [Google Scholar]
- 8.Fendrick S.E., Xue Q.S., Streit W.J. Formation of multinucleated giant cells and microglial degeneration in rats expressing a mutant Cu/Zn superoxide dismutase gene. J Neuroinflammation. 2007;4:9. doi: 10.1186/1742-2094-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kao W.J., McNally A.K., Hiltner A., Anderson J.M. Role for interleukin-4 in foreign-body giant cell formation on a poly(etherurethane urea) in vivo. J Biomed Mater Res. 1995;29:1267–1275. doi: 10.1002/jbm.820291014. [DOI] [PubMed] [Google Scholar]
- 10.Helming L., Gordon S. Molecular mediators of macrophage fusion. Trends Cell Biol. 2009;19:514–522. doi: 10.1016/j.tcb.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Anderson J.M. Multinucleated giant cells. Curr Opin Hematol. 2000;7:40–47. doi: 10.1097/00062752-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Kyriakides T.R., Foster M.J., Keeney G.E., Tsai A., Giachelli C.M., Clark-Lewis I., Rollins B.J., Bornstein P. The CC chemokine ligand, CCL2/MCP1, participates in macrophage fusion and foreign body giant cell formation. Am J Pathol. 2004;165:2157–2166. doi: 10.1016/S0002-9440(10)63265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helming L., Gordon S. Macrophage fusion induced by IL-4 alternative activation is a multistage process involving multiple target molecules. Eur J Immunol. 2007;37:33–42. doi: 10.1002/eji.200636788. [DOI] [PubMed] [Google Scholar]
- 14.McNally A.K., Anderson J.M. Beta1 and beta2 integrins mediate adhesion during macrophage fusion and multinucleated foreign body giant cell formation. Am J Pathol. 2002;160:621–630. doi: 10.1016/s0002-9440(10)64882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coxon A., Rieu P., Barkalow F.J., Askari S., Sharpe A.H., Von Andrian U.H., Arnaout M.A., Mayadas T.N. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 16.Lu H., Smith C.W., Perrard J., Bullard D., Tang L., Entman M.L., Beaudet A.L., Ballantyne C.M. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1 deficient mice. J Clin Invest. 1997;99:1340–1350. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yakubenko V.P., Lishko V.K., Lam S.C., Ugarova T.P. A molecular basis for integrin αMβ2 ligand binding promiscuity. J Biol Chem. 2002;277:48635–48642. doi: 10.1074/jbc.M208877200. [DOI] [PubMed] [Google Scholar]
- 18.Podolnikova N.P., Podolnikov A.V., Haas T.A., Lishko V.K., Ugarova T.P. Ligand recognition specificity of leukocyte integrin alphaMbeta2 (Mac-1, CD11b/CD18) and its functional consequences. Biochemistry. 2015;54:1408–1420. doi: 10.1021/bi5013782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diamond M.S., Staunton D.E., de Fougerolles A.R., Stacker S.A., Garcia-Aguilar J., Hibbs M.L., Springer T.A. ICAM-1 (CD54): a counter-receptor for MAC-1 (CD11b/CD18) J Cell Biol. 1990;111:3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon D.I., Chen Z.P., Xu H., Li C.Q., Dong J.F., McIntire L.V., Ballantyne C.M., Zhang L., Furman M.I., Berndt M.C., López J.A. Platelet glycoprotein Ibα is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Exp Med. 2000;192:193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santoso S., Sachs U.J., Kroll H., Linder M., Ruf A., Preissner K.T., Chavakis T. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med. 2002;196:679–691. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Vieren M., Le Trong H., Wood C.L., Moore P.F., St. John T., Staunton D.E., Gallatin W.M. A novel leukointegrin, αDβ2, binds preferentially to ICAM-3. Immunity. 1995;3:683–690. doi: 10.1016/1074-7613(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 23.Yakubenko V.P., Yadav S.P., Ugarova T.P. Integrin αDβ2, an adhesion receptor up-regulated on macrophage foam cells, exhibits multiligand binding properties. Blood. 2006;107:1643–1650. doi: 10.1182/blood-2005-06-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helming L., Tomasello E., Kyriakides T.R., Martinez F.O., Takai T., Gordon S., Vivier E. Essential role of DAP12 signaling in macrophage programming into a fusion-competent state. Sci Signal. 2008;1:ra11. doi: 10.1126/scisignal.1159665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yakubenko V.P., Belevych N., Mishchuk D., Schurin A., Lam S.C., Ugarova T.P. The role of integrin αDβ2 (CD11d/CD18) in monocyte/macrophage migration. Exp Cell Res. 2008;314:2569–2578. doi: 10.1016/j.yexcr.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNally A.K., Anderson J.M. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am J Pathol. 1995;147:1487–1499. [PMC free article] [PubMed] [Google Scholar]
- 27.Bellingan G.J., Caldwell H., Howie S.E., Dransfield I., Haslet C. In vivo fate of the inflammatory macrophages during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol. 1996;157:2577–2585. [PubMed] [Google Scholar]
- 28.Bellingan G.J., Xu P., Cooksey H., Cauldwell H., Shock A., Bottoms S., Haslett C., Mutsaers S.E., Laurent G.J. Adhesion molecule-dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation. J Exp Med. 2002;196:1515–1521. doi: 10.1084/jem.20011794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies L.C., Rosas M., Smith P.J., Fraser D.J., Jones S.A., Taylor P.R. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur J Immunol. 2011;41:2155–2164. doi: 10.1002/eji.201141817. [DOI] [PubMed] [Google Scholar]
- 30.Davies L.C., Taylor P.R. Tissue-resident macrophages: then and now. Immunology. 2015;144:541–548. doi: 10.1111/imm.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jay S.M., Skokos E.A., Zeng J., Knox K., Kyriakides T.R. Macrophage fusion leading to foreign body giant cell formation persists under phagocytic stimulation by microspheres in vitro and in vivo in mouse models. J Biomed Mater Res A. 2010;93:189–199. doi: 10.1002/jbm.a.32513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Most J., Neumayer H.P., Dierich M.P. Cytokine-induced generation of multinucleated giant cells in vitro requires interferon-gamma and expression of LFA-1. Eur J Immunol. 1990;20:1661–1667. doi: 10.1002/eji.1830200807. [DOI] [PubMed] [Google Scholar]
- 33.Davis G.E. The Mac-1 and p150,95 beta 2 integrins bind denatured proteins to mediate leukocyte cell-substrate adhesion. Exp Cell Res. 1992;200:242–252. doi: 10.1016/0014-4827(92)90170-d. [DOI] [PubMed] [Google Scholar]
- 34.Papadimitriou J.M., Archer M. The morphology of murine foreign body multinucleate giant cells. J Ultrastruct Res. 1974;49:372–386. doi: 10.1016/s0022-5320(74)90051-3. [DOI] [PubMed] [Google Scholar]
- 35.Forsyth C.B., Solovjov D.A., Ugarova T.P., Plow E.F. Integrin αMβ2-mediated cell migration to fibrinogen and its recognition peptides. J Exp Med. 2001;193:1123–1133. doi: 10.1084/jem.193.10.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lishko V.K., Yakubenko V.P., Ugarova T.P. The interplay between integrins αMβ2 and α5β1 during cell migration to fibronectin. Exp Cell Res. 2003;283:116–126. doi: 10.1016/s0014-4827(02)00024-1. [DOI] [PubMed] [Google Scholar]
- 37.Lishko V.K., Podolnikova N.P., Yakubenko V.P., Yakovlev S., Medved L., Yadav S.P., Ugarova T.P. Multiple binding sites in fibrinogen for integrin alpha Mbeta 2 (Mac-1) J Biol Chem. 2004;279:44897–44906. doi: 10.1074/jbc.M408012200. [DOI] [PubMed] [Google Scholar]
- 38.Jaffe A.B., Hall A. Rho GTPases in transformation and metastasis. Adv Cancer Res. 2002;84:57–80. doi: 10.1016/s0065-230x(02)84003-9. [DOI] [PubMed] [Google Scholar]
- 39.Milde R., Ritter J., Tennent G.A., Loesch A., Martinez F.O., Gordon S., Pepys M.B., Verschoor A., Helming L. Multinucleated giant cells are specialized for complement-mediated phagocytosis and large target destruction. Cell Rep. 2015;13:1937–1948. doi: 10.1016/j.celrep.2015.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor P.R., Brown G.D., Geldhof A.B., Martinez-Pomares L., Gordon S. Pattern recognition receptors and differentiation antigens define murine myeloid cell heterogeneity ex vivo. Eur J Immunol. 2003;33:2090–2097. doi: 10.1002/eji.200324003. [DOI] [PubMed] [Google Scholar]
- 41.Dioszeghy V., Rosas M., Maskrey B.H., Colmont C., Topley N., Chaitidis P., Kuhn H., Jones S.A., Taylor P.R., O'Donnell V.B. 12/15-Lipoxygenase regulates the inflammatory response to bacterial products in vivo. J Immunol. 2008;181:6514–6524. doi: 10.4049/jimmunol.181.9.6514. [DOI] [PubMed] [Google Scholar]
- 42.Rosas M., Davies L.C., Giles P.J., Liao C.T., Kharfan B., Stone T.C., O'Donnell V.B., Fraser D.J., Jones S.A., Taylor P.R. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science. 2014;344:645–648. doi: 10.1126/science.1251414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen E.H., Grote E., Mohler W., Vignery A. Cell-cell fusion. FEBS Lett. 2007;581:2181–2193. doi: 10.1016/j.febslet.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 44.Oren-Suissa M., Podbilewicz B. Cell fusion during development. Trends Cell Biol. 2007;17:537–546. doi: 10.1016/j.tcb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Martens S., McMahon H.T. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 46.Xue W., Kindzelskii A., Todd R., III, Petty H. Physical association of complement receptor type 3 and urokinase-type plasminogen activator receptor in neutrophil membranes. J Immunol. 1994;152:4630–4640. [PubMed] [Google Scholar]
- 47.Simon D.I., Rao N.K., Xu H., Wei Y., Majdic O., Ronne E., Kobzik L., Chapman H.A. Mac-1 (CD11b/CD18) and the urokinase receptor (CD87) form a functional unit on monocytic cells. Blood. 1996;88:3185–3194. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The routes of one multinucleated giant cell and one mononucleated cell were tracked for 4 hours to determine the speed of their migration.