Abstract
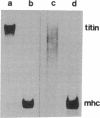
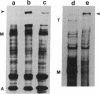
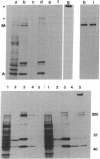
To understand molecular interactions that organize developing myofibrils, we examined the biosynthesis and interaction of titin and myosin heavy chain in cultures of developing muscle. Use of pulse-labeling, immunoprecipitation, and a reversible cross-linking procedure demonstrates that within minutes of synthesis, titin and myosin heavy chain can be chemically cross-linked into very large, detergent-resistant complexes retaining many features of intact myotubes. These complexes, predominantly of titin and myosin, occur very early in myofibrillogenesis as well as later. These data suggest that synthesis and assembly of titin and myosin are temporally and spatially coordinated in nascent myofibrils and support the hypothesis that titin molecules help to organize sarcomere formation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dlugosz A. A., Antin P. B., Nachmias V. T., Holtzer H. The relationship between stress fiber-like structures and nascent myofibrils in cultured cardiac myocytes. J Cell Biol. 1984 Dec;99(6):2268–2278. doi: 10.1083/jcb.99.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton A. B., Isaacs W. B. Titin, a huge, elastic sarcomeric protein with a probable role in morphogenesis. Bioessays. 1991 Apr;13(4):157–161. doi: 10.1002/bies.950130403. [DOI] [PubMed] [Google Scholar]
- Fürst D. O., Osborn M., Nave R., Weber K. The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: a map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J Cell Biol. 1988 May;106(5):1563–1572. doi: 10.1083/jcb.106.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst D. O., Osborn M., Weber K. Myogenesis in the mouse embryo: differential onset of expression of myogenic proteins and the involvement of titin in myofibril assembly. J Cell Biol. 1989 Aug;109(2):517–527. doi: 10.1083/jcb.109.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassner D. Myofibrillar interaction of blot immunoaffinity-purified antibodies against native titin as studied by direct immunofluorescence and immunogold staining. Eur J Cell Biol. 1986 Apr;40(2):176–184. [PubMed] [Google Scholar]
- Hill C. S., Duran S., Lin Z. X., Weber K., Holtzer H. Titin and myosin, but not desmin, are linked during myofibrillogenesis in postmitotic mononucleated myoblasts. J Cell Biol. 1986 Dec;103(6 Pt 1):2185–2196. doi: 10.1083/jcb.103.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs W. B., Cook R. K., Van Atta J. C., Redmond C. M., Fulton A. B. Assembly of vimentin in cultured cells varies with cell type. J Biol Chem. 1989 Oct 25;264(30):17953–17960. [PubMed] [Google Scholar]
- Isaacs W. B., Fulton A. B. Cotranslational assembly of myosin heavy chain in developing cultured skeletal muscle. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6174–6178. doi: 10.1073/pnas.84.17.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs W. B., Kim I. S., Struve A., Fulton A. B. Biosynthesis of titin in cultured skeletal muscle cells. J Cell Biol. 1989 Nov;109(5):2189–2195. doi: 10.1083/jcb.109.5.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Maruyama K., Huang Y. P. Interactions of muscle beta-connectin with myosin, actin, and actomyosin at low ionic strengths. J Biochem. 1984 Aug;96(2):499–506. doi: 10.1093/oxfordjournals.jbchem.a134862. [DOI] [PubMed] [Google Scholar]
- Kimura S., Yoshidomi H., Maruyama K. Proteolytic fragments of connectin cause aggregation of myosin filaments but not of actin filaments. J Biochem. 1984 Dec;96(6):1947–1950. doi: 10.1093/oxfordjournals.jbchem.a135031. [DOI] [PubMed] [Google Scholar]
- Kurzban G. P., Wang K. Giant polypeptides of skeletal muscle titin: sedimentation equilibrium in guanidine hydrochloride. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1155–1161. doi: 10.1016/0006-291x(88)90750-4. [DOI] [PubMed] [Google Scholar]
- Ohtsuki I., Maruyama K., Ebashi S. Regulatory and cytoskeletal proteins of vertebrate skeletal muscle. Adv Protein Chem. 1986;38:1–67. doi: 10.1016/s0065-3233(08)60525-2. [DOI] [PubMed] [Google Scholar]
- Wang S. M., Greaser M. L., Schultz E., Bulinski J. C., Lin J. J., Lessard J. L. Studies on cardiac myofibrillogenesis with antibodies to titin, actin, tropomyosin, and myosin. J Cell Biol. 1988 Sep;107(3):1075–1083. doi: 10.1083/jcb.107.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]