Highlight
Plant auxin efflux and influx proteins redirect the plant hormone auxin towards the feeding site upon root-knot nematode infection in Arabidopsis thaliana roots.
Key words: Arabidopsis, AUX1, auxin, galls, LAX3, PIN, root-knot nematode.
Abstract
Plant-parasitic root-knot nematodes induce the formation of giant cells within the plant root, and it has been recognized that auxin accumulates in these feeding sites. Here, we studied the role of the auxin transport system governed by AUX1/LAX3 influx proteins and different PIN efflux proteins during feeding site development in Arabidopsis thaliana roots. Data generated via promoter–reporter line and protein localization analyses evoke a model in which auxin is being imported at the basipetal side of the feeding site by the concerted action of the influx proteins AUX1 and LAX3, and the efflux protein PIN3. Mutants in auxin influx proteins AUX1 and LAX3 bear significantly fewer and smaller galls, revealing that auxin import into the feeding sites is needed for their development and expansion. The feeding site development in auxin export (PIN) mutants was only slightly hampered. Expression of some PINs appears to be suppressed in galls, probably to prevent auxin drainage. Nevertheless, a functional PIN4 gene seems to be a prerequisite for proper nematode development and gall expansion, most likely by removing excessive auxin to stabilize the hormone level in the feeding site. Our data also indicate a role of local auxin peaks in nematode attraction towards the root.
Introduction
Plant roots are constantly challenged by pathogens and parasites present in the rhizosphere. Among them, plant-parasitic nematodes (PPN) inflict considerable damage to a wide range of plant species (Sasser and Freckman, 1986). Because of their economic importance, the best-studied nematodes are the cyst nematodes (CN; Heterodera and Globodera spp.) and root-knot nematodes (RKN; Meloidogyne spp.), which are both biotrophs with sedentary lifestyles.
Second-stage juvenile (J2) nematodes penetrate the plant root at the elongation zone and move towards the root stele, where they manipulate pathways implicated in root development to induce feeding sites called syncytia (for CN) or giant cells (GC; for RKN). GCs induced by RKNs are most commonly derived from parenchymatic cells within the stele that surround the nematode head during parasitism. GC formation starts with the induction of binucleate cells (de Almeida Engler et al., 1999, 2011) followed by repeated rounds of nuclear division, DNA amplification, and cell growth in the absence of cytokinesis. This process, which occurs in five to seven cells around the nematode head, causes them to become multinucleate and hypertrophied, reaching up to 100 times the size of normal root vascular parenchyma cells. Hyperplasia of surrounding cells results in the formation of typical root-knots or galls.
Plant hormones are known to control the regulation of plant growth and development, with transport-dependent auxin gradients triggering the formation of plant organs (Benkova et al., 2003). Auxin plays a major role in plant root development, where it is mainly responsible for cell division, and establishing and maintaining root primordia (De Smet et al., 2010). This hormone is transported from the aerial producing sites towards the root tip through basipetal transport involving influx and efflux transporter proteins. Members of the AUXIN RESISTANT 1 (AUX1) and LIKE AUX1 (LAX) transmembrane protein family govern auxin influx, while the PIN family proteins are responsible for auxin efflux. The spatial and subcellular localization of these proteins drives the auxin flow from source to sink tissues, including plant roots (Wisniewska et al., 2006). Generally, regions with increased auxin levels correlate with the initiation sites of organ primordia in both plant root and shoot tissues (Tanaka et al., 2006).
Interestingly, auxin also plays an important role during the initiation and early development of syncytia induced by CN (Grunewald et al., 2009). An auxin-insensitive tomato (Solanum lycopersicum) mutant, diageotropica (dgt), was found to be almost completely resistant to these nematodes and the Arabidopsis axr2/iaa7 mutant, defective in auxin signalling, had a significantly reduced development of CN (Goverse et al., 2000). Chemical inhibition of auxin transport resulted in a reduction of CN development (Goverse et al., 2000) and experiments using the auxin-responsive DR5 reporter revealed that auxin accumulates within young syncytia (Karczmarek et al., 2004). CN manipulate the auxin distribution route (Grunewald et al., 2009), a process which involves enhanced expression of the auxin influx protein AUX1 in the primary syncytial cell (Mazarei et al., 2003) and downregulation of the efflux protein PIN1 (Grunewald et al., 2009). Knowing that nematode infection of Arabidopsis thaliana pin1 mutants results in a reduced number of cysts and pin3 and pin4 mutants support only smaller cysts (Goverse et al., 2000; Grunewald et al., 2009), these auxin export proteins must play important roles in CN parasitism. The Hs19C07 effector of the beet CN H. schachtii was shown to interact with the auxin influx protein LAX3 in Arabidopsis roots (Lee et al., 2011). LAX3 is transcriptionally active within developing syncytia and in cells that are to be incorporated in the syncytium. Although the single lax3 and aux1 mutant showed no defects in nematode development, the aux1 lax3 double mutant and the aux1 lax1 lax2 lax3 quadruple mutant had significant decreases in female CN numbers at both 14 and 30 days after inoculation (DAI) (Lee et al., 2011).
In contrast to CN, the role of auxin transport on RKN-induced GC formation and development is poorly understood. There is accumulating evidence that auxin also plays a role in the compatible interaction between plants and RKN, but the underlying mechanisms are unknown. For example, application of a synthetic auxin (1-naphthaleneacetic acid) was shown to increase the susceptibility for M. javanica in otherwise resistant peach plants (Prunus persica; Kochba and Samish, 1971). Likewise, application of the natural auxin indole acetic acid (IAA) to tomato roots resulted in a concentration-dependent weight increase of M. javanica-induced galls (Glazer et al., 1986). Similar to its resistance to CN, the auxin-insensitive tomato mutant dgt does not support RKN development due to an arrest in early feeding site formation (Richardson and Price, 1984). Hutangura et al. (1999) observed a strong expression of an auxin-reporter fusion (GH3pro:GUS) in M. javanica-induced GCs on white clover (Trifolium repens) at early (48–72h) time points post inoculation, whereas the signal decreased at later time points (96–120h). Similarly, Karczmarek et al. (2004) observed a specific and strong activation of the auxin-responsive DR5pro:GUS within the initial feeding cells induced by RKN in Arabidopsis, with the signal most prominent from 18h until 5 DAI, whereas later on the signal decreased. At later time points (7 to 14 DAI), Absmanner et al. (2013) reported DR5pro:GUS expression in neighbouring cells but not in the GCs in Arabidopsis. Generally, auxin seems to be early and locally accumulating within RKN-induced feeding sites, as in CN-induced syncytia, and thus might also have an important role during gall development. Although strong activity of the AUX1 promoter within GCs (3 to 14 DAI) has been reported (Mazarei et al., 2003), the role of additional players in auxin transport coordination during gall development is currently unknown.
This gap in current understanding prompted us to study the role of auxin transport governed by AUX1/LAX and PIN proteins during GC development upon RKN infection in Arabidopsis roots. GUS and GFP Arabidopsis reporter lines were used to investigate the redistribution of these proteins during feeding cell development and mutant lines were used to test the importance of the proteins for the establishment of a feeding site. From these data, a model for the redirection of auxin during GC formation is proposed, which was compared to results of former studies regarding the role of polar auxin transport during syncytium development.
Materials and methods
Sterilization and sowing of A. thaliana seeds for in vitro infection experiments
Seeds of A. thaliana wild type (ecotype Columbia 0) and En-2 different mutant and transgenic lines were cold-stratified at 4°C for 4 days to synchronize germination. Vapour sterilization of the seeds (50ml H2O, 40ml NaOCl, 4.4ml HCl 25%) was performed for 5h followed by further surface sterilization with 70% C2H6O (ethanol) for 2min and 5% NaOCl for 5min. Seeds were thoroughly rinsed in sterile water.
Approximately 80 seeds were plated for germination on 9cm diameter Petri dishes with Murashige and Skoog medium (MS with vitamins 4.7g/L, 2% sucrose, 0.8% Daichin agar, pH 5.7) and 0.15% plant agar. To allow root development on the surface of the growth medium for ease of transplanting, the Petri dishes were placed vertically in the plant growth chamber at 24°C under a 12h light/12h dark regime. After 5 days, the seedlings were transferred using sterile toothpicks to six-well tissue culture plates (Falcon) containing 4ml of MS medium. Each treatment was replicated 10 times. Growth conditions were maintained at 24°C on a 12h light/12h dark cycle for a period of 7 days to allow sufficient root growth.
Nematode culture and sterilization
Hatched J2s were collected from tomato roots (pieces of 2–3cm) in a mistifier chamber and subsequently purified with 35% sucrose solution. The juveniles were surface sterilized with HgCl2 solution (0.002% Triton X-100 w/v, 0.004% NaN3 w/v, 0.004% HgCl2 w/v) and rinsed three times in sterile tap water. Prior to inoculation the juveniles were transferred to 0.7% Gelrite solution to allow even distribution of juvenile nematodes and facilitate their movement through the medium.
Nematode infection assay
Twelve-day-old seedlings of A. thaliana were each inoculated with approximately 300 pre-parasitic juveniles (J2) of M. incognita. The J2 were equally distributed at the base of the root system using a repetitive pipette (Eppendorf Multipette® Plus). The plants were then transferred to a plant growth chamber operating at 18°C under a 24h dark regime, conditions that favoured nematode infection. We analysed the nematode susceptibility of the plants by counting the number of parasitic J2s, galls, females, and egg masses in roots collected at 3, 7, 35, and 42 DAI. Clean roots were soaked in 10ml of 2.5% NaOCl for 5min to bleach them. To remove residual NaOCl, the roots were rinsed and soaked in tap water. Thereafter, the roots were transferred into acid fuchsin (1:30 acid fuchsin to distilled water) and boiled in a microwave for 30s. After cooling, the excess liquid was drained and the roots were washed with running tap water. Roots were de-stained by boiling in 70% acidified glycerol. Using a binocular microscope, observations and recordings were made of J2s, galls, females, and egg masses. For each line, the number of galls and nematodes in the root system was counted on at least 10 individual plants per experiment. The whole infection experiment was twice independently repeated, giving similar results. In one of these experiments with 10 plants, the developmental stage (in a total of ~400 nematodes per line) and the gall size (in a total of ~250 galls per line) was additionally registered. Data were statistically analysed using SPSS Statistics 20 (IBM®), applying a Student’s t-test for pairwise comparisons, or ANOVA and Tukey’s test for multiple comparisons of group means.
Analysis of promoter GUS fusion lines
Different transgenic Arabidopsis plants (Col-0) with the promoters of the auxin efflux genes fused to GUS (PIN1pro:GUS, PIN2pro:GUS, PIN3pro:GUS, PIN4pro:GUS, PIN7pro:GUS) as well as of the auxin influx genes AUX1pro:GUS and LAX3pro:GUS were grown and after 2 weeks infected with M. incognita. GUS staining on non-sectioned galls was done at 3 DAI using the protocol described in Karczmarek et al. (2004).
GUS staining on sections of galls formed in promoter fusion lines was done at different time points after nematode inoculation (3, 7, and 14 DAI) as described by de Almeida Engler et al. (1999). To avoid diffusion of the GUS precipitate, galls were fixed in 2.0% glutaraldehyde overnight and were then embedded in Technovit 7100, sectioned (3 µm), and microscopically analysed by dark-field optics.
In vivo confocal microscopy
Observation of PIN1pro:GUS-GFP, PIN7pro:GUS-GFP, PIN1pro:PIN1-GFP, PIN2pro:PIN2-GFP, PIN4pro:PIN4-GFP, and PIN7pro:PIN7-GFP expression was performed in nematode-infected Arabidopsis transgenic seedlings. Galls at various time points after infection (7–14 DAI) were dissected from roots and mounted in 5% agar. Thick vibroslices ranging from 50 to 150 μm were made using a Microm HM650V Vibratome (Walldorf). Slices were mounted on microscope slides, a cover slip placed in position, and the slice immediately observed using an inverted confocal microscope (model LSM510 META; Zeiss). Samples were excited with a 488nm argon laser and the GFP-specific fluorescence emission was captured using the lambda spectral mode with a 499–550nm detection bandwidth range. Image analysis and Z-stack projections were done with the LSM 510 software (Zeiss).
Results
Spatial distribution and transcriptional regulation of PIN/AUX1/LAX in M. incognita-induced feeding sites in Arabidopsis
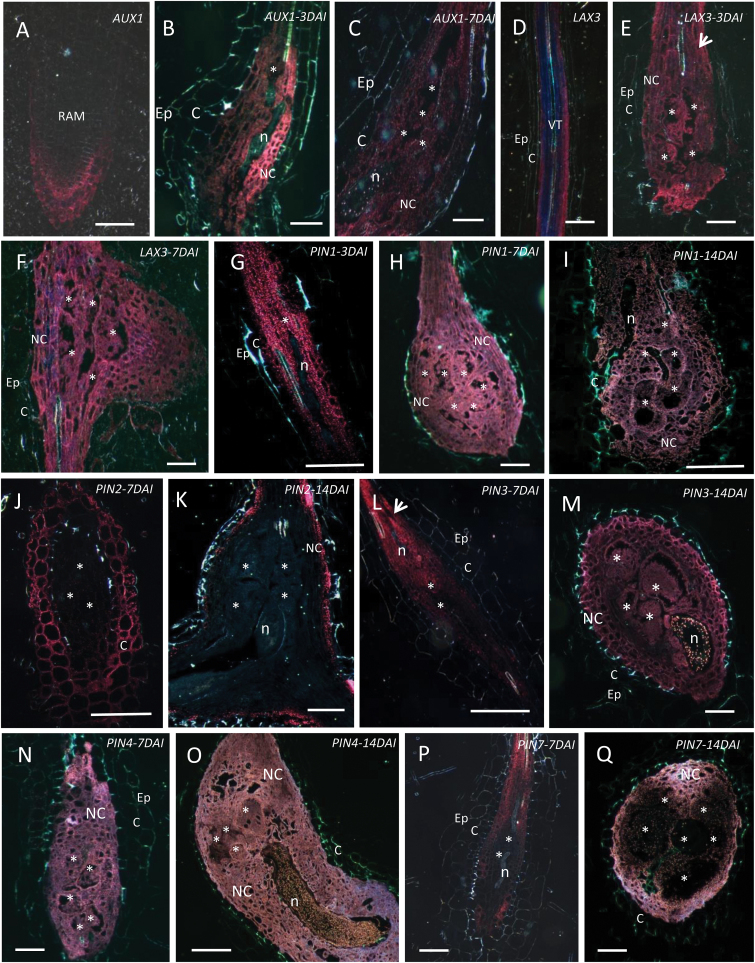
It is well known that the activity of different PIN/AUX1/LAX proteins is required for asymmetric auxin distribution in developmental processes, such as leaf, flower, and lateral root initiation (Tanaka et al., 2006). Therefore, PIN, AUX1, or LAX promoter:GUS lines were investigated for their expression during RKN feeding site initiation and development. Previous comparisons with in situ hybridization demonstrated that these transgenic lines display similar root expression patterns as the endogenous genes (Friml et al., 2002a ; Friml et al., 2002b ; Friml et al., 2003; Abas et al., 2006). PIN, AUX1, or LAX3 promoter::GUS-lines were inoculated with the RKN M. incognita and promoter activity was investigated at different time points after inoculation. Whole GUS-stained roots and galls are depicted in Fig. 1. For a detailed visualization of tissue and cellular expression, sections are illustrated in Fig. 2. GUS staining of uninfected roots confirmed AUX1pro:GUS expression in root tips, mainly at the columella root cap (Figs 1A and 2A). Expression was induced in young galls 3 DAI (Figs 1B and 2B) and 7 DAI (Fig. 2C) and was weak or absent in root tissues surrounding the gall, suggesting an auxin influx during early stages of gall development. In contrast, in uninfected roots LAX3pro:GUS expression was absent at the root tip but strong in the root stele (Figs 1C and 2D). During RKN infection, LAX3 promoter activity was high within young galls (3 DAI and 7 DAI; Figs 1D and 2E, F), suggesting an auxin influx in galls. In addition, both AUX1pro:GUS and LAX3pro:GUS plants showed slightly stronger staining in cells located at the basipetal side of the developing gall (Fig. 1B, D). The locally induced expression of AUX1 and LAX3 reveals that RKN might modulate acropetal auxin transport, most likely to direct enhanced auxin import into the developing feeding site.
Fig. 1.
Promoter activity of Arabidopsis auxin transporter genes in uninfected roots (UR) and in young M. incognita-induced galls at 3 DAI. (A, B) AUX1pro:GUS UR and 3 DAI, (C, D) LAX3pro:GUS UR and 3 DAI, (E, F) PIN1pro:GUS UR and 3 DAI, (G, H) PIN2pro:GUS UR and 3 DAI, (I, J) PIN3pro:GUS UR and 3 DAI, (K, L) PIN4pro:GUS UR and 3 DAI, (M, N) PIN7pro:GUS UR and 3 DAI. Arrows point to the basipetal part of the gall where AUX1, LAX3, and PIN3 expression is activated. G, gall; n, nematode. Bars = 100 µm.
Fig. 2.
Promoter activity of Arabidopsis auxin transporter genes in uninfected roots (UR) and in M. incognita-induced galls at 3–14 DAI. (A) AUX1pro:GUS in UR and (B, C) galls at 3 and 7 DAI, (D) LAX3pro:GUS in UR and (E, F) galls at 3 and 7 DAI, (G, I) PIN1pro:GUS in galls at 3, 7, and 14 DAI, (J, K) PIN2pro:GUS at 7 and 14 DAI, (L, M) PIN3pro:GUS at 7 and 14 DAI, (N, O) PIN4pro:GUS at 7 and 14 DAI, (P, Q) PIN7 pro:GUS at 7 and 14 DAI. Arrows point to the basipetal part of the gall where LAX3 and PIN3 expression is activated. GUS staining is visualized in red. C, cortex; Ep, epidermis; n, nematode; NC, neighbouring cells; RAM, root apical meristem; asterisk, giant cell. Bars = 50 µm.
PIN1pro:GUS was detected in the stele of uninfected roots (Fig. 1E), as well as in young (3 and 7 DAI) and maturing (14 DAI) galls (Figs 1F and 2G, H, I). The expression pattern of the gene encoding the PIN1 auxin efflux carrier, a protein which is responsible for acropetal auxin transport through the root stele towards the root tip (Feraru and Friml, 2008), did not seem to change strongly upon RKN infection in Arabidopsis.
PIN2pro:GUS, responsible for basipetal auxin transport (Feraru and Friml, 2008), was expressed in the root cortex and epidermis of uninfected roots, and strongly expressed in the root elongation zone (Fig. 1G). PIN2pro:GUS expression was not observed in GCs or neighbouring cells (Fig. 1H), although cortex cells showed GUS staining at different time points after infection (7 and 14 DAI; Fig. 2J, K). This observation could be due to maintenance of the basipetal auxin transport driven by PIN2 in the root cortex, or might indicate that these cortex cells are exporting auxin towards the GCs through the neighbouring cells.
In uninfected roots, PIN3pro:GUS showed expression in the root stele and at the root tip (Fig. 1I). At early time points after infection (3 and 7 DAI), high PIN3 promoter activity was detected in the neighbouring cells at the basipetal side of the gall, but not strongly inside the GCs (Figs 1J and 2L). However, at later time points after infection (14 DAI), PIN3pro:GUS expression was clearly observed within GCs and all neighbouring cells (Fig. 2M).
PIN4pro:GUS was strongly expressed in the root apical meristem in uninfected roots (Fig. 1K). Whereas PIN4 promoter activity was weak in the root stele (Fig. 1K), its activity was significantly enhanced within young and mature GCs and neighbouring cells (7 and 14 DAI; Figs 1L and 2N, O).
Similar to PIN1, PIN7pro:GUS was not expressed in the root tip and GUS staining was observed in the vascular cylinder of uninfected roots (Fig. 1M). PIN7 expression was suppressed in GCs at all investigated time points (Figs 1N and 2P, Q) but its expression was detected in neighbouring cells (Fig. 2P, Q).
Promoter activity as observed by GUS analyses was confirmed by GFP localization studies performed for one promoter that was found to be active (PIN1pro:GUS-GFP) and one that was found to be inactive in GCs (PIN7pro:GUS-GFP), and was evaluated in fresh gall slices (Supplementary Fig. S1). Results confirmed the lack of PIN7 promoter activity at all investigated time points in galls. PIN1 promoter activity was observed inside GCs until at least 7 DAI, but decreased at later time points. Promoter activity of both PIN genes was observed in the root vascular tissue of uninfected roots (Supplementary Fig. S1). Based on these observations as well as Figs 1E, F and 2G–I, we conclude that, for PIN1, the basal expression profile is slightly enhanced in young galls (until 7 DAI), whereas PIN7 expression is specifically suppressed inside the GCs.
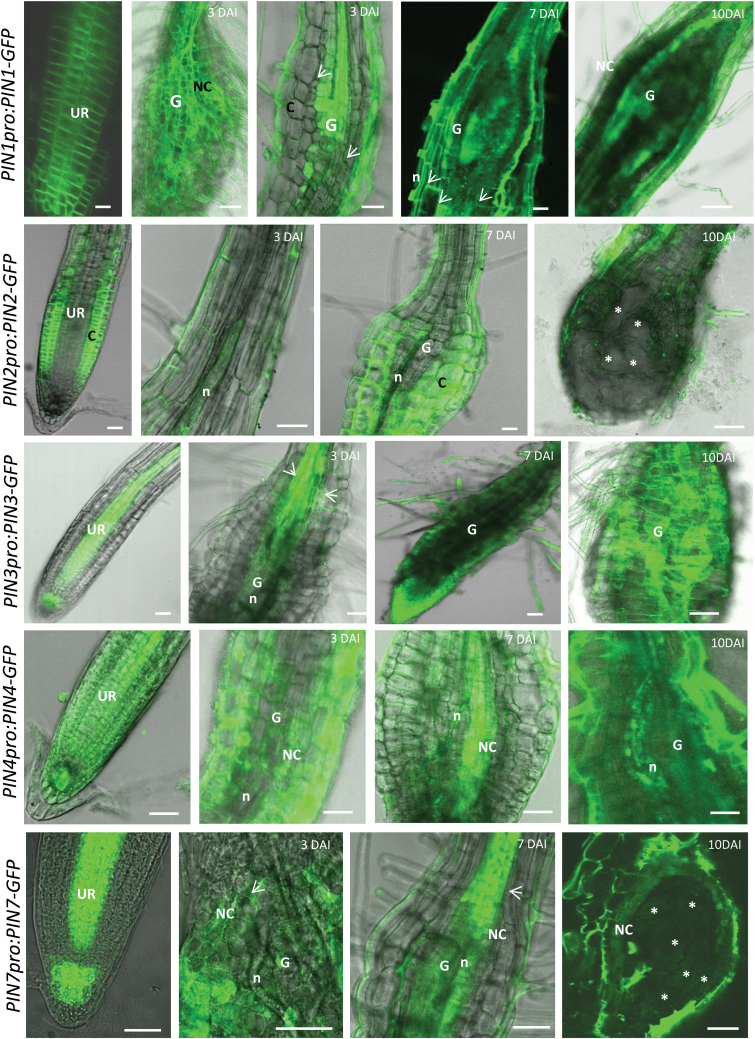
Localization of PIN proteins in M. incognita-induced feeding sites in Arabidopsis
Protein localization was analysed using protein–GFP fusion lines of PIN1pro:PIN1-GFP, PIN2pro:PIN2-GFP, PIN3pro:PIN3-GFP, PIN4pro:PIN4-GFP, and PIN7pro:PIN7-GFP in uninfected roots and in galls at different developmental stages (Fig. 3). PIN1-GFP signal was more intense at the acropetal side of the root cells in uninfected roots and this localization was not changed in cells surrounding the GCs (cortex and epidermal cells). This typical pattern was less clear in GCs due to the high concentration of the PIN1 protein in mainly young GCs (3–7 DAI) (Fig. 3). PIN2 did not accumulate during nematode migration within the root nor at later time points after infection. PIN2 protein was seen in the cortex cells around the GCs, with the same pattern as in uninfected roots (Fig. 3). PIN3 showed localization on the basipetal side of the young gall tissue, as also seen by promoter activity (Figs 1 and 2). Its acropetal localization in cells was not disturbed by the feeding site development. PIN4 was detected around the infecting nematode (3 DAI), with strong expression in GCs and neighbouring cells mainly at 7 DAI, slightly decreasing at 10 DAI. In uninfected roots, PIN7 was typically located in the vascular tissue and in the columella root cap cells (Ferari and Friml, 2008; Fig. 3). In galls, PIN7 was detected in the neighbouring cells, but this protein was absent in GCs (Fig. 3), confirming promoter activity results (Fig. 2 and Supplementary Fig. S1).
Fig. 3.
PIN1pro:PIN1-GFP, PIN2pro:PIN2-GFP, PIN3pro:PIN3-GFP, PIN4pro:PIN4-GFP, and PIN7pro:PIN7-GFP analysis in uninfected roots and in M. incognita-induced galls at 3, 7, and 10 DAI in Arabidopsis. Arrows point to the accumulation of PIN1-GFP, PIN3-GFP, and PIN7-GFP at the acropetal side of cells. C, cortex; G, gall; n, nematode; NC, neighbouring cells; UR, uninfected root; asterisk, giant cell. Bars = 25 µm.
Disruption of auxin transport affects nematode penetration, feeding site initiation, and development
To further investigate the importance of the different auxin influx and efflux proteins for nematode penetration, feeding site initiation, and gall and nematode development, we investigated pin1, pin2, pin3, pin4, pin7, aux1, and lax3 mutants and the aux1 lax3 double mutant. All mutants were infected with RKN M. incognita. Preliminary infection experiments on the pin7 mutant showed no significant differences in gall number (data not shown) and this mutant was therefore not further studied. For all other mutants, the infection success was monitored at different time points after infection: (1) at 3 and 7 DAI to evaluate nematode penetration and feeding site initiation; and (2) at 35 and 42 DAI to monitor gall and nematode development.
The role of auxin influx/efflux in penetration and feeding site initiation
Acid fuchsin staining of infected plants grown in vitro allowed us to monitor nematode penetration within Arabidopsis roots. In wild-type plants at 3 DAI, J2 nematodes had penetrated the plant roots at the elongation zone, and some had already started to initiate a feeding site. By 7 DAI, most nematodes had initiated their feeding sites. In roots of susceptible plants, the number of nematodes was expected to be equal at both time points or slightly higher at 7 DAI (since infection is not synchronized). Because the pin1mutant has a different genetic background, it was analysed separately.
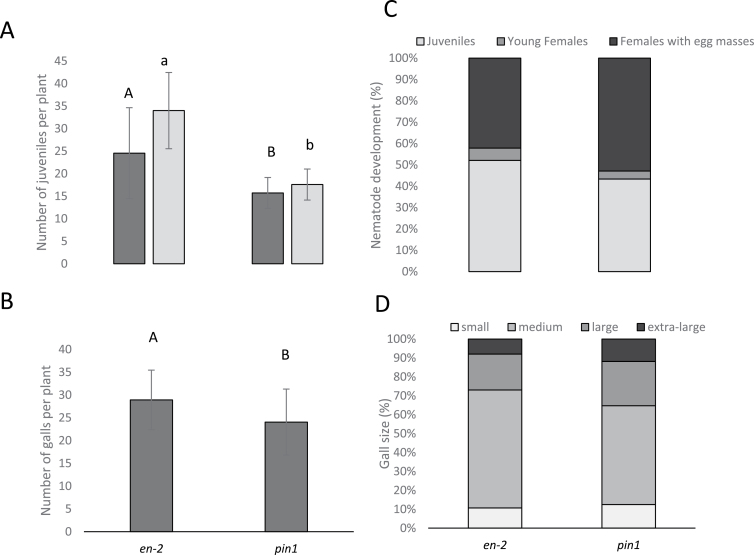
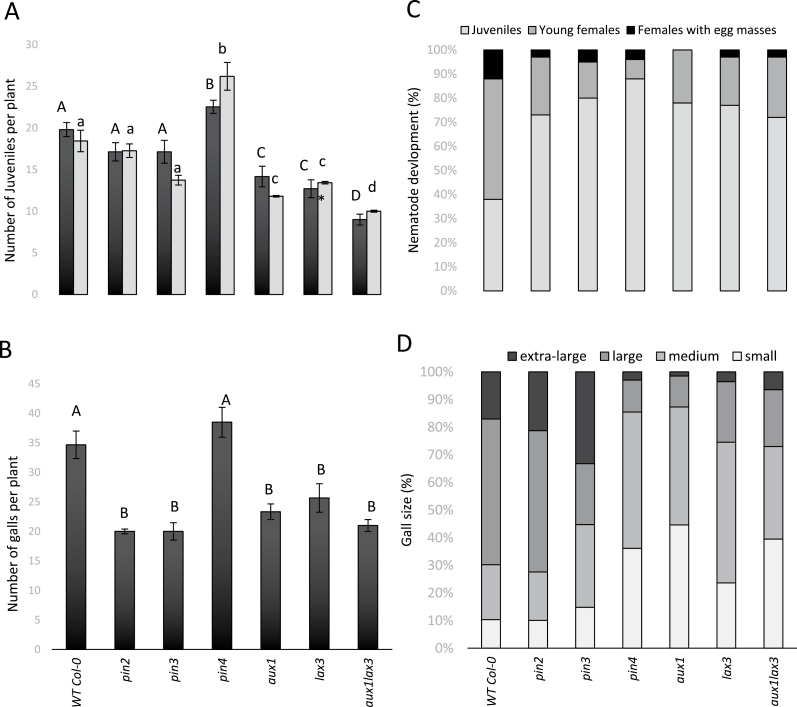
Our infection experiments on the mutant lines (Figs 4 and 5) showed that, compared to the wild-type line En-2, the pin1 mutant contained significantly fewer nematodes inside the roots at both 3 and 7 DAI (Fig. 4A). The pin2 and pin3 mutants (Fig. 5A), by contrast, contained a similar number of nematodes within the roots at 3 and 7 DAI as the wild type. The aux1, lax3 and aux1lax3 mutants were found to have a 25–50% reduction in juvenile nematodes penetrating the root (analysed at 3 DAI) and during feeding site establishment (analysed at 7 DAI) compared to wild type. For the pin3 and aux1 mutant, the number of juveniles was slightly lower at 7 DAI than at 3 DAI, suggesting that feeding site initiation was hampered. Strikingly, the pin4 mutant showed significantly enhanced nematode penetration and establishment compared to the wild-type plants (Fig. 5A). Taken together, these data suggest a possible increased attractiveness of the pin4 mutant, whereas the pin1, aux1, lax3, and aux1 lax3 mutant plants were significantly less penetrated by the nematodes.
Fig. 4.
Analyses of pin1 Arabidopsis mutant and its corresponding wild type En-2, infected by the RKN M. incognita. (A) Number of J2 per plant, counted at 3 (dark grey) and 7 DAI (light grey). Different letters indicate statistically significant differences based on a Student’s t-test (P < 0.05), using upper case for 3 DAI and lower case for 7 DAI. (B) Number of galls at 35 DAI. Different letters indicate statistically significant differences based on a Student’s t-test (P < 0.05). (C) Developmental stages of the observed nematodes within the galls at 42 DAI, shown as percentages. (D) Classification of gall sizes at 35 DAI, shown as percentages. Extra-large (> 2.5mm), large galls (1.5–2.5mm), medium galls (1–1.5mm), and small galls (<1mm). In A and B, bars represent the average ± standard deviation of at least 10 individual plants. The whole infection experiment was twice independently repeated, giving similar results. In C and D, bars represent the percentage of each developmental stage (of ~400 nematodes per line) or gall size (of ~250 galls per line) counted on all 10 individual plants in one infection experiment.
Fig. 5.
Analyses of aux1, lax3, aux1 lax3, pin2, pin3, and pin4 Arabidopsis mutants infected by the RKN M. incognita. (A) Number of J2 per plant, counted at 3 and 7 DAI. Different letters indicate statistically significant differences based on ANOVA and Tukey’s test (P < 0.05), using upper case for 3 DAI and lower case for 7 DAI. (B) Number of galls at 35 DAI. Different letters indicate statistically significant differences (P < 0.05). (C) Developmental stages of the observed nematodes within the galls 42 DAI, shown as percentages. (D) Classification of gall sizes at 35 DAI, shown as percentages. Extra-large (> 2.5mm), large galls (1.5–2.5mm), medium galls (1–1.5mm), and small galls (<1mm). In A and B, bars represent the average ± standard deviation of at least 10 individual plants. The whole infection experiment was twice independently repeated, giving similar results. In C and D, bars represent the percentage of each developmental stage (of ~400 nematodes per line) or gall size (of ~250 galls per line) counted on all 10 individual plants in one infection experiment.
The role of auxin influx/efflux on gall and nematode development
In wild-type plants, gall expansion is typically observed until around 20 DAI (Vieira et al., 2012). After this, nematodes will develop further inside the gall and will lay egg masses on the surface of this root swelling. Gall number and size were evaluated in all mutants (Figs 4B, D and 5B, D), while nematode development within the galls was investigated 42 DAI (Figs 4C and 5C).
Mutants in auxin influx proteins (aux1, lax3, and aux1 lax3) showed low gall number in comparison with wild-type plants (Fig. 5B). This decrease in gall number correlated well with the reduced nematode penetration and feeding site establishment in the roots of these auxin influx mutants (Fig. 5A). Female development within these feeding sites was also hampered (Fig. 5C), and galls in aux1, lax3, and aux1 lax3 mutant lines were smaller than in the wild-type Col-0 (Fig. 5D). Remarkably, in the aux1 mutant line, none of the females produced egg masses at 42 DAI, while the lax3 mutant and the double mutant allowed egg mass production. Although there was a synergistic effect of aux1 and lax3 on nematode penetration (Fig. 5A), aux1 seems to have the strongest effect on nematode development (Fig. 5C).
Compared to its wild-type en-2, the pin1 mutant held a significantly lower number of galls (Fig. 4B), which correlated with the reduced number of nematodes inside the pin1 roots (Fig. 4A). However, nematode development and gall size on the pin1 mutant line was similar to the wild-type En-2 (Fig. 4C, D).
In the pin2 and the pin3 mutants, the number of galls and nematode development were significantly reduced (Fig. 5B, C), although gall sizes were apparently similar to wild-type Col-0 (Fig. 5D). pin4 mutants held the same number of galls as wild-type Col-0, but we observed a significant inhibition of nematode maturation and gall expansion (Fig. 5B–D). This shows that, although the lack of PIN4 resulted in enhanced nematode penetration and feeding site initiation (Fig. 5A), galls did not expand well (Fig. 5D) and nematode development was strongly hampered by the lack of PIN4 (Fig. 5C).
Discussion
Herein, we have investigated how influx and efflux proteins are redirecting auxin within the plant root during RKN infection in Arabidopsis. In the data interpretation and discussion, two distinct infection phases were considered: (1) nematode penetration and establishment in the host root (initiation of the feeding site); and (2) feeding site and nematode development. Because our observations strongly suggest that auxin transport plays different roles during those two phases, our results will here be discussed per infection phase.
The role of auxin during RKN penetration and establishment
The here-reported data on infections of mutant lines validate previous indications that auxin is an important molecule in root attractiveness for nematodes (Curtis et al., 2007). Our results show that pin4 mutants, reported to accumulate higher auxin levels in the root tip (Friml et al., 2002a ), are more susceptible to RKN penetration, as seen by the high number of juveniles within the plant host (3–7 DAI). A second argument for the role of auxin in root attractiveness is that the investigated mutants in auxin influx proteins (AUX1, LAX3) and in the polar auxin transporter PIN1 are less susceptible to RKN penetration. These proteins are necessary to direct auxin transport from the source (shoot apical meristem) towards the sink tissues, such as roots where nematodes invade and establish their feeding sites. In aux1 and pin1 mutants, the IAA levels within the apical root regions were consistently lower than those found in comparable regions of the wild-type root (Marchant et al., 2002; Zhang et al., 2014). These observations suggest that local auxin maxima at the root tip direct nematode penetration in host roots. It has also been previously suggested that auxin induces changes in the surface cuticle and behaviour of Meloidogyne spp., illustrated by the increased stylet thrusting and higher motility (Curtis et al., 2007). High auxin concentrations have also been shown to attract Aphelenchoides besseyi nematodes (Feng et al., 2014). An alternative explanation for the observed increased attractiveness of the studied mutant is a potential auxin-induced change in root exudates, which are involved in host location. For example, elevated levels of ethylene were shown to be correlated with decreased host attraction by RKN (Fudali et al., 2013). We cannot, therefore, exclude the possibility that a local increase in auxin upon nematode infection results in disturbances in ethylene production, which ultimately affects host attractiveness. Possible crosstalk between auxin and ethylene in host location is further supported by the Arabidopsis auxin transport mutant pin2, which has also been described as the ethylene mutant eir1 (Luschnig et al., 1998). However, no change in host penetration was observed for the pin2 mutant in the current study.
The role of auxin during feeding site and nematode development
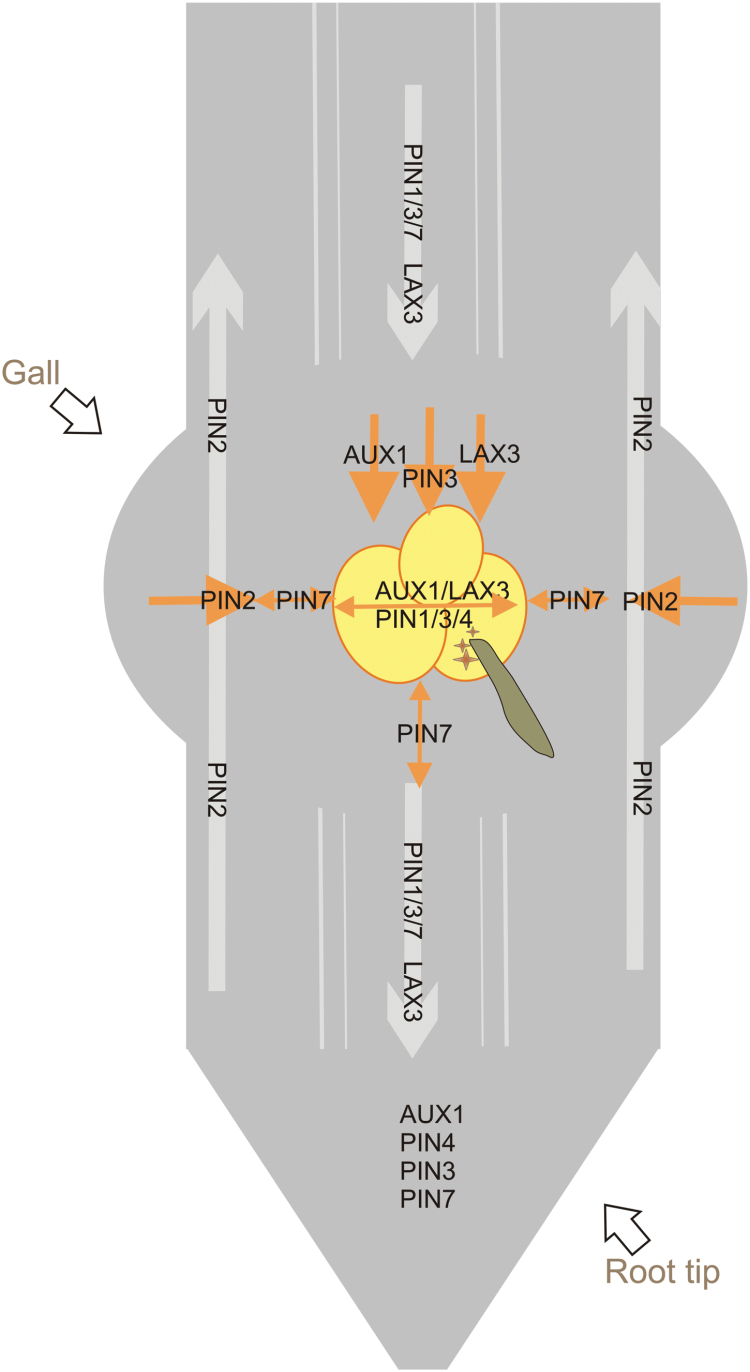
Previous research using auxin-responsive promoters showed that auxin accumulates in young GCs, but that this process is transient and the auxin response shifts to neighbouring cells 2–5 DAI (Hutangura et al., 1999; Karczmarek et al., 2004). Auxin inside GCs might be partially derived from M. incognita secretions, which have been shown to contain auxin conjugates, albeit in very low quantities (De Meutter et al., 2005). In addition, and probably more important for the reported accumulation of auxin in the GCs, our results provide evidence for a redirected flow of endogenous auxin within the plant during RKN feeding site development. Based on our data, the model depicted in Fig. 6 illustrates this auxin redirection in the RKN-induced feeding sites up to 7 DAI. The normal auxin transport from the shoot apex towards the root tip is shown with grey arrows. The redirected flow during feeding site development is visualized in orange.
Fig. 6.
Model of AUX1/LAX3/PIN-mediated auxin transport during nematode feeding site development, based on the 3 and 7 DAI data provided in this manuscript. The giant cells are shown as yellow circles, and the nematode is the green-brown worm. Grey arrows show the direction of the auxin flow in uninfected Arabidopsis roots, and the auxin import/export proteins that are mainly responsible for this flow (based on Feraru and Friml, 2008). Orange arrows show the redirected auxin flow in nematode feeding sites, based on the data provided in this study. n, nematode; brown stars, nematode-secreted auxin. This figure is available in colour at JXB online.
GUS analyses showed that at the basipetal region of the gall and in the GCs, AUX1 and LAX3 expression were notably induced at 3 and 7 DAI, indicating that AUX1 and LAX3 actively import auxin into the young GCs and their neighbouring cells, which make up the nematode feeding site. LAX3pro:GUS expression is also reportedly induced within young syncytia (Lee et al., 2011). For AUX1, promoter activity in whole galls and syncytia was comparable to that reported by Mazarei et al. (2003), although those authors did not section the feeding sites to visualize expression at the cellular level. Herein, we present the cellular expression pattern of AUX1 in sectioned galls, revealing GUS expression in GCs as well as in neighbouring cells. Taken together with the observation that the aux1 and the lax3 mutants as well as the aux1 lax3 double mutant contained significantly fewer nematodes, fewer galls, and slower nematode development, we propose that auxin import through AUX1 and LAX3 is required for GC initiation, gall expansion, and, thus, nematode development. In comparison, LAX3 is transcriptionally active within developing syncytia and in cells to be incorporated in the syncytium. While the single lax3 and aux1 mutants showed no defects in CN development, the aux1 lax3 double mutant and the aux1 lax1 lax2 lax3 quadruple mutant had significant decreases in female CN numbers (Lee et al., 2011).
The similar expression pattern of AUX1, LAX3, and PIN3 strongly suggests that the PIN3 protein facilitates the export of auxin from neighbouring cells at the basipetal side towards the developing GCs. The presence of plasmodesmata between GCs and neighbouring cells (Hofmann et al., 2010) might further facilitate auxin transport. Interestingly, there are some recent insights that show the need for a sequential regulation of LAX3 and PIN3 expression during lateral root emergence. During that process, LAX3-dependent auxin accumulation induces cell wall–modifying enzymes that loosen the cell wall and allow the newly formed lateral root to emerge through the existing root tissues (Swarup et al., 2008; Péret et al., 2013). Mathematical modelling and experimental validation in Arabidopsis roots showed that the interplay between PIN3 and LAX3 can create sharp intercellular gradients in LAX3 expression. The authors suggested that, by expressing PIN3, cells can communicate effectively with their neighbours, thereby allowing them to coordinate which of the cells is going to express LAX3 (Péret et al., 2013). Therefore, considering the similar spatiotemporal expression pattern of AUX1, LAX3, and PIN3, a similar pathway could be expected in galls. During CN infection, PIN3 gene expression was observed within young syncytia (2 and 5 DAI) (Grunewald et al., 2009). The PIN3-GFP fusion protein revealed a change in the polar localization of PIN3 during syncytium development (Grunewald et al., 2009), whereas at later stages (>5 DAI) PIN3 was more highly expressed in cells neighbouring the developing syncytium. This local expression has been assumed to be important for incorporating neighbouring cells into the growing syncytium (Grunewald et al., 2009). Differently for galls, PIN3pro:PIN3-GFP analysis showed similar PIN3 localization, at the acropetal side of cells, in both uninfected roots and galls/GCs. This might be explained by the fact that GCs do not fuse with neighbouring cells in galls. Intracellular PIN1 and PIN7 localization was also not changed by gall formation.
During gall initiation and development, GUS analyses and protein localization analyses showed that the activity of PIN2 and PIN7 genes as well as protein expression was remarkably absent in the GCs. These genes are also not active in young syncytia (Grunewald et al., 2009). The lack of expression of these two auxin export proteins in GCs probably prevents auxin drainage, hence leading to enhanced auxin levels within GCs. In addition, PIN2 expression in the cortex and PIN7 expression in neighbouring cells might be involved in the export of auxin from these cells towards GCs, allowing their proper development and, consequently, nematode maturation. For pin2, this hypothesis is supported by functional analysis using the pin2 mutant lines. Whereas this mutant is equally as susceptible as the wild type to CNs (Grunewald et al., 2009), RKN development was delayed in the pin2 mutant line, resulting in fewer mature females and egg masses than the wild type. Infection experiments on a pin7 mutant showed no significant differences in gall number (data not shown) and this mutant was therefore not further studied. PIN7 expression is clearly suppressed in GCs (Figs 1–4), and this might explain why a complete knock-out does not have an effect on gall formation.
Despite the fact that PIN1 is expressed in GCs, and a reduced number of nematodes are present inside the mutant roots, only a slight difference in gall number was observed in pin1 mutant compared to wild-type roots. Gall size and nematode development were not influenced by the pin1 mutation. This indicates that PIN1 is not needed for gall and nematode development, but seems to be necessary for acropetal auxin transport towards the root tip, where its accumulation could affect nematode attraction. In contrast, PIN1 expression is downregulated in young syncytia, and pin1 mutants support significantly fewer and smaller cysts (Grunewald et al., 2009). PIN1 downregulation most likely is correlated with decreased auxin export from CN-induced syncytia (Goverse et al., 2000; Grunewald et al., 2009). In contrast to syncytia (Grunewald et al., 2009), PIN1 expression does not seem to be important for GC development.
Interestingly, the PIN4-promoter is active in RKN-induced galls, whereas the PIN4 protein is normally mainly expressed at the root quiescent centre, where this protein is known to be regulating auxin homeostasis and patterning through sink-mediated auxin distribution at the root tip (Friml et al., 2002a ). Although a lack of PIN4 leads to enhanced nematode penetration and feeding site initiation, PIN4 expression is needed for proper gall expansion and consequently nematode development, as seen by the lower number of mature females (with egg masses) in the pin4 mutant compared to wild-type galls. Having determined the PIN4 gene expression and protein levels within GCs and neighbouring cells, this protein might be considered as an important regulator of auxin homeostasis within developing GCs. Similarly, in CN-induced syncytia, the PIN4-promoter was shown to be induced at early time points (2 and 5 DAI) during development and cysts were smaller in the pin4 mutants compared to the wild type (Grunewald et al., 2009).
Even though similarities were observed (e.g. the importance of PIN4 expression in the feeding sites), results obtained in this RKN study show some differences from the data obtained during CN infection (e.g. the importance of PIN1 for syncytia versus PIN2/3 for GCs development) of the same Arabidopsis transgenic/mutant lines (Goverse et al., 2000; Grunewald et al., 2009). Based on the observed differences, we hypothesize that, due to the evolutionary divergence between RKN and CN (Holterman et al., 2009), both sedentary types of nematodes have evolved different strategies to manipulate auxin transport, which also correlates with a different ontogeny, architecture, and cell wall modification pattern in both types of feeding sites. Our data support the idea that a different set of effectors unique for either CN or RKN are most probably involved in the establishment of the nematode feeding site. For example, as far as we know, no ortholog of the H. schachtii effector Hs19C07, interacting with LAX3 (Lee et al., 2011), has been found in the RKN secretome. Thus, which mechanisms RKN are using to commandeer auxin distribution in the plant root remain to be investigated. However, as no RKN effector with similar action to Hs19C07 has been found yet, it is equally possible that auxin transport rearrangements arise in response to RKN infection, and are not actively manipulated by the nematode.
Our data conclusively support a model (Fig. 6) in which RKN infection of plant roots affects the auxin distribution patterns during feeding site development. Auxin import at the basipetal side of the gall seems to be induced by the concerted action of AUX1, LAX3, and PIN3. This phenomenon would ultimately lead to auxin accumulation within the GCs induced by M. incognita. Local auxin maxima correlate with the initiation sites of organ primordia in both plant roots and shoots (Tanaka et al., 2006) and most likely feeding sites. During plant root development, auxin is responsible for cell division and for the establishment and maintenance of root primordia (De Smet et al., 2010). Moreover, auxin is known to facilitate radial expansion in the root elongation zone (Strader et al., 2010), where RKN infect host roots and initiate their feeding sites, supporting the role for auxin in GC expansion.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Promoter activity of Arabidopsis PIN1pro:GUS-GFP and PIN7pro:GUS-GFP in uninfected roots and in Meloidogyne incognita-induced galls at 7–14 DAI.
Acknowledgments
This work benefited from interactions funded through COST Actions 872 and FA1208. Part of the work was executed during a COST-supported Short Term Scientific Mission (COST-STSM-872–05044). This research is supported by the Dutch Technology Foundation STW, which is part of the Netherlands Organisation for Scientific Research (NWO), and which is partly funded by the Ministry of Economic Affairs. TK is supported by an FWO postdoctoral fellowship.
References
- Abas L, Benjamins R, Malenica N, Paciorek T, Wirniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C. 2006. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nature Cell Biology 8, 249–256. [DOI] [PubMed] [Google Scholar]
- Absmanner B, Stadler R, Hammes UZ. 2013. Phloem development in nematode-induced feeding sites: the implications of auxin and cytokinin. Frontiers in Plant Science 4, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J. 2003. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602. [DOI] [PubMed] [Google Scholar]
- Curtis RHC. 2007. Do phytohormones influence nematode invasion and feeding site establishment? Nematology 9, 155–160. [Google Scholar]
- De Smet I, Lau S, Voss U, et al. 2010. Bimodular auxin response controls organogenesis in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 107, 2705–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Engler J, De Vleesschauwer V, Burssens S, Celenza JL, Inze D, Van Montagu M, Engler G, Gheysen G. 1999. Molecular markers and cell cycle inhibitors show the importance of cell cycle progression in nematode-induced galls and syncytia. The Plant Cell 11, 793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Engler J, Engler G, Gheysen G. 2011. Unravelling the plant cell cycle in nematode induced feeding sites. In: Jones J, Gheysen G, Fenoll C, eds. Genomics and molecular genetics of plant-nematode interactions. Dordrecht: Springer, 349–368. [Google Scholar]
- De Meutter J, Tygat T, Prinsen E, Gheysen G, Van Onckelen H, Gheysen G. 2005. Production of auxin by the plant-parasitic nematodes Heterodera schachtii and Meloidogyne incognita . Communication in Agricultural and Applied Biological Sciences 70, 51–60. [PubMed] [Google Scholar]
- Feng H, Shao Y, Wei LH, Gao CY, Zhou YJ. 2014. The white-tip nematode, Aphelenchoides besseyi, exhibits an auxin-orientated behaviour affecting its migration and propagation. Nematology 16, 837–845. [Google Scholar]
- Feraru E, Friml J. 2008. PIN polar targeting. Plant Physiology 147, 1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Benkova E, Blilou I, et al. 2002. a. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108, 661–673. [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jurgens G. 2003. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426, 147–153. [DOI] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K. 2002. b. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415, 806–809. [DOI] [PubMed] [Google Scholar]
- Fudali SL, Wang CL, Williamson VM. 2013. Ethylene signaling pathway modulates attractiveness of host roots to the root-knot nematode Meloidogyne hapla . Molecular Plant-Microbe Interactions 26, 75–86. [DOI] [PubMed] [Google Scholar]
- Glazer I, Epstein E, Orion D, Apelbaum A. 1986. Interactions between auxin and ethylene in root-knot nematode (Meloidogyne javanica) infected tomato roots. Physiological and Molecular Plant Pathology 28, 171–179. [Google Scholar]
- Goverse A, Overmars H, Engelbertink J, Schots A, Bakker J, Helder J. 2000. Both induction and morphogenesis of cyst nematode feeding cells are mediated by auxin. Molecular Plant-Microbe Interactions 13, 1121–1129. [DOI] [PubMed] [Google Scholar]
- Grunewald W, Cannoot B, Friml J, Gheysen G. 2009. Parasitic nematodes modulate PIN-mediated auxin transport to facilitate infection. PloS Pathogens 5, e1000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann J, Youssef-Banora M, de Almeida-Engler J, Grundler FMW. 2010. The role of callose deposition along plasmodesmata in nematode feeding sites. Molecular Plant-Microbe Interactions 23, 549–557. [DOI] [PubMed] [Google Scholar]
- Holterman M, Karssen G, van den Elsen S, van Megen H, Bakker J, Helder J. 2009. Small subunit rDNA-based phylogeny of the Tylenchida sheds light on relationships among some high-impact plant-parasitic nematodes and the evolution of plant feeding. Phytopathology 99, 227–235. [DOI] [PubMed] [Google Scholar]
- Hutangura P, Mathesius U, Jones MGK, Rolfe BG. 1999. Auxin induction is a trigger for root gall formation caused by root-knot nematodes in white clover and is associated with the activation of the flavonoid pathway. Australian Journal of Plant Physiology 26, 221–231. [Google Scholar]
- Karczmarek A, Overmars H, Helder J, Goverse A. 2004. Feeding cell development by cyst and root-knot nematodes involves a similar early, local and transient activation of a specific auxin-inducible promoter element. Molecular Plant Pathology 5, 343–346. [DOI] [PubMed] [Google Scholar]
- Kochba J, Samish RM. 1971. Effect of kinetin and 1-naphthylacetic acid on root-knot nematodes in resistant and susceptible peach rootstocks. Journal of the American Society for Horticultural Science 96, 458–461. [Google Scholar]
- Lee C, Chronis D, Kenning C, Péret B, Hewezi T, Davis EL, Baum TJ, Hussey R, Bennett M, Mitchum MG. 2011. The novel cyst nematode effector protein 19C07 interacts with the Arabidopsis auxin influx transporter LAX3 to control feeding site development. Plant Physiology 155, 866–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. 1998. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana . Genes & Development 12, 2175–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, Eklof J, Casero PJ, Bennett M, Sandberg G. 2002. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14, 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarei M, Lennon KA, Puthoff DP, Rodermel SR, Baum TJ. 2003. Expression of an Arabidopsis phosphoglycerate mutase homologue is localized to apical meristems, regulated by hormones, and induced by sedentary plant-parasitic nematodes. Plant Molecular Biology 53, 513–530. [DOI] [PubMed] [Google Scholar]
- Péret B, Middleton AM, French AP, et al. 2013. Sequential induction of auxin efflux and influx carriers regulates lateral root emergence. Molecular Systems Biology 9, 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson L, Price NS.1984. Observations on the biology of Meloidogyne incognita and the Diageotropica tomato mutant. Revue Nematologique 7, 97–99. [Google Scholar]
- Sasser JN, Freckman DW. 1986. A world perspective on nematology - the role of the society. In: Veech JA, Dickson DW, eds. Vistas on nematology. Hyattsville, MD, USA: Society of Nematologists, 7–14. [Google Scholar]
- Strader LC, Chen GL, Bartel B. 2010. Ethylene directs auxin to control root cell expansion. The Plant Journal 64, 874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Kargul J, Marchant A, et al. 2004. Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. The Plant Cell 16, 3069–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Dhonukshe P, Brewer PB, Friml J. 2006. Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cellular and Molecular Life Sciences 63, 2738–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira P, Banora MY, Castagnone-Sereno P, Rosso MN, Engler G, Engler JD. 2012. An immunocytochemical procedure for protein localization in various nematode life stages combined with plant tissues using methylacrylate-embedded specimens. Phytopathology 102, 990–996. [DOI] [PubMed] [Google Scholar]
- Wisniewska J, Xu J, Seifertova D, Brewer PB, Ruzicka K, Blilou I, Rouquie D, Benkova E, Scheres B, Friml J. 2006. Polar PIN localization directs auxin flow in plants. Science 312, 883. [DOI] [PubMed] [Google Scholar]
- Zhang KX, Xu HH, Gong W, Jin Y, Shi YY, Yuan TT, Li J, Lu YT. 2014. Proper PIN1 distribution is needed for root negative phototropism in Arabidopsis. PloS One 9, e85720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.