Highlight
This work provides the mechanistic explanation for differential salt stress sensitivity amongst Brassica species and links it with regulation of root plasma membrane potential and the cytosolic K/Na ratio
Key words: H+-ATPase, ion homeostasis, membrane potential, potassium retention, ROS detoxification, sodium exclusion, tissue tolerance.
Abstract
Brassica species are known to possess significant inter and intraspecies variability in salinity stress tolerance, but the cell-specific mechanisms conferring this difference remain elusive. In this work, the role and relative contribution of several key plasma membrane transporters to salinity stress tolerance were evaluated in three Brassica species (B. napus, B. juncea, and B. oleracea) using a range of electrophysiological assays. Initial root growth assay and viability staining revealed that B. napus was most tolerant amongst the three species, followed by B. juncea and B. oleracea. At the mechanistic level, this difference was conferred by at least three complementary physiological mechanisms: (i) higher Na+ extrusion ability from roots resulting from increased expression and activity of plasma membrane SOS1-like Na+/H+ exchangers; (ii) better root K+ retention ability resulting from stress-inducible activation of H+-ATPase and ability to maintain more negative membrane potential under saline conditions; and (iii) reduced sensitivity of B. napus root K+-permeable channels to reactive oxygen species (ROS). The last two mechanisms played the dominant role and conferred most of the differential salt sensitivity between species. Brassica napus plants were also more efficient in preventing the stress-induced increase in GORK transcript levels and up-regulation of expression of AKT1, HAK5, and HKT1 transporter genes. Taken together, our data provide the mechanistic explanation for differential salt stress sensitivity amongst these species and shed light on transcriptional and post-translational regulation of key ion transport systems involved in the maintenance of the root plasma membrane potential and cytosolic K/Na ratio as a key attribute for salt tolerance in Brassica species.
Introduction
In today’s context of global climate change, salinization of arable land is a major threat to the agricultural production system. Although improving salt tolerance in major cultivated crops is of paramount importance to global food security in the 21st century, the process is significantly handicapped by the physiological and genetic complexity of the salinity tolerance trait and, as such, requires understanding of the orchestrated regulation of major subtraits at the tissue-specific level (Zhu, 2001).
Oilseeds and vegetables are two very important components of human food and are dietary necessities. The family Brassicaceae includes a number of cultivated crop species that have a considerable degree of variation in their salt tolerance (Purty et al., 2008; Kumar et al., 2009; Chakraborty et al., 2016a ). A considerable inter- and intraspecific variation in the overall growth, electrolyte leakage, proline accumulation, and maintenance of the K+/Na+ ratio was observed for different Brassica species (Ashraf and McNeilly, 2004; Purty et al., 2008; Chakraborty et al., 2012). At the whole-plant level, the mechanism of salt tolerance varied considerably among three Brassica species (B. napus, B. juncea, and B. oleracea) tested with 150mM NaCl stress (Chakraborty et al., 2016b ). It was shown that the overall superior salinity stress tolerance in B. napus was achieved by the higher osmo-tolerance matched by the moderate tissue tolerance and superior K+ retention ability in the leaf mesophyll. At the same time, while showing relatively modest overall tolerance, B. oleracea was far more superior in terms of shoot tissue tolerance. However, to the best of our knowledge, no specific details on the molecular/cellular mechanisms conferring this intra- and interspecific variability in salinity tolerance are available in the literature, and no significant quantitative trait locus (QTL) related to salinity tolerance has been identified in Brassica to date (Nayidu et al., 2013).
For the majority of glycophytes, salinity tolerance is achieved through more than one strategy operating either simultaneously or in isolation, depending upon the duration and intensity of the stress (Munns, 2002). Such strategies include improved osmotic adjustment, exclusion of Na+ from uptake, intracellular Na+ sequestration, K+ retention in the cytosol, control of xylem ion loading, and oxidative stress tolerance (Ashraf et al., 2008; Adem et al., 2014; Bose et al., 2014a ). Which of these make the greatest contribution to salinity stress tolerance in Brassica?
Many glycophytes including cultivated crop species employ Na+ exclusion and/or partitioning strategies to achieve salt tolerance. Induced expression of the plasma membrane Na+/H+ antiporter (encoded by the SOS1 gene in Arabidopsis) results in improved salt tolerance in several species (Zhu, 2001). In contrast, the Arabidopsis salt overly sensitive1 (sos1) mutant, not having the capacity to exclude Na+ from its uptake, was found to accumulate 5-fold more Na+ in the shoot tissue and showed a highly sensitive phenotype (Nublat et al., 2001). Unlike SOS1 which pumps cytosolic Na+ into the apoplastic space, the tonoplast-based NHX1 Na+/H+ antiporter removes cytotoxic Na+ by pumping it into the vacuole for sequestration (Fan et al., 2015). At the same time, no significant difference in unidirectional (channel-mediated) Na+ uptake was reported between genotypes contrasting in salinity stress tolerance in various crop species [e.g. wheat (Davenport et al., 2005) and barley (Chen et al., 2007)]. Is this also the case for Brassica?
Another important aspect of achieving tissue tolerance is through maintenance of K+ homeostasis (Anschutz et al., 2014; Shabala et al., 2016a ). By screening nearly 70 barley varieties contrasting in salinity stress tolerance, Chen et al. (2007) showed that >60% of genetic variability in salinity stress tolerance in barley was related to the roots’ ability to retain K+ in the mature root zone upon acute NaCl treatment. Similar reports were later published for wheat (Cuin et al., 2008), lucerne (Smethurst et al., 2008), and poplar (Sun et al., 2009), and strong evidence for the inheritance of this trait was provided (Chen et al., 2008; Cuin et al., 2011). At the same time, NaCl-induced K+ efflux from wheat roots was shown to be an order of magnitude smaller compared with barley, for the same experimental conditions (for comparison, see Chen et al., 2007; Cuin et al., 2009). Does K+ retention in roots play a major role in salinity stress tolerance in Brassica? If the answer is yes, how is this K+ retention achieved? Does this occur at the transcriptional or post-translational level? Several pathways were reported to mediate NaCl-induced K+ efflux from plant tissues. These are highly tissue specific and include (Wu et al., 2015; Shabala et al., 2016a ): (i) depolarization-activated outward-rectifying K+-selective channels; (ii) weakly voltage-dependent non-selective cation channels (NSCCs); and (iii) reactive oxygen species (ROS)-activated K+-permeable channels. Which of these pathways (if any) operates in Brassica roots?
Previous reports also suggested that cytosolic Ca2+ signalling is an essential component of plant adaptation to saline conditions (Dodd et al., 2010). At least several concurrent signalling loops have to be considered. One of them is related to the salt overly sensitive (SOS) pathway. The activity of SOS1 is regulated by the CBL–CIPK complex that is achieved by phosphorylating its C-terminus (Quintero et al., 2011). To enable this activation, a salt stress-induced Ca2+ signal is first detected by SOS3 (CBL4), a myristoylated calcium-binding protein; SOS3 is then activated by recruiting SOS2 (CIPK24), a serine/threonine protein kinase (reviewed in Shabala et al., 2015). Elevated apoplastic Ca2+ levels are also essential to NSCC-mediated Na+ uptake (Demidchik and Tester, 2002) and prevent K+ leakage from the cell (Shabala et al., 2006). Finally, salinity stress results in a substantial build-up of the cytotoxic hydroxyl radical and induces K+ efflux and Ca2+ influx in both root and shoot tissues (Demidchik et al., 2003, 2010), with NADPH oxidase being one of the major sources of such ROS production and accumulation in the apoplast (Bose et al., 2014b ; Demidchik, 2015; Shabala et al., 2015). Also, both constitutive and ROS-induced ion fluxes could vary significantly between different parts of the root (i.e. the mature and elongation zone) (Demidchik et al., 2007). In this context, it was shown that the Ca2+ transport protein Annexin 1 in Arabidopsis thaliana (AtANN1) mediates root responses to extracellular H2O2 (Laohavisit et al., 2012), and so are cyclic nucleotide-gated Ca2+-permeable channels (Ordonez et al., 2014). What role do Ca2+ transport systems play in Brassica root responses to salinity?
The present study attempted to address the above questions by conducting a detailed electrophysiological investigation of mechanisms underlying interspecific variability in salinity stress tolerance in Brassica, at the tissue level. The non-invasive micro-electrode ion flux estimation (MIFE) technique was employed to study the kinetics of stress-induced net fluxes of Na+, K+, Ca2+, and H+ from various root zones and then link these changes with changes in the transcriptional profile of several key candidate genes. The molecular identity of the transporters involved was further confirmed in a series of pharmacological experiments.
Materials and methods
Plant material and experimental condition
The Brassica seeds of three different species, namely B. napus, B. juncea, and B. oleracea, were obtained from commercial suppliers (Zepson Seeds, Richmond and Hollander Imports, Hobart, Australia). Seeds were surface sterilized with 1% (v/v) HClO for 10min, thoroughly rinsed with distilled water, and then grown using the paper roll method (Pandolfi et al., 2010) in non-buffered basic salt medium (BSM) solution (0.5mM KCl+0.2mM NaCl+0.1mM CaCl2, pH 5.7) in the dark for 4–5 d at room temperature (24±1°C). Plants were used for electrophysiological measurements when their root length was between 50mm and 70mm.
For long-term salinity treatment experiments [root growth assay, viability staining, membrane potential (MP), steady-state Na+ flux measurement, and gene expression studies], the seeds of all three Brassica species were surfaced sterilized and placed in paper rolls, and allowed to germinate and grow for 3 d in dark as described above. Then the uniformly grown seedlings were transferred to a 1.5 litre plastic container of aerated BSM added with the appropriate strength of NaCl as per the treatment requirement. The seedlings were suspended on a plastic grid so that their roots were completely immersed in the BSM. Plants were then grown under 16/8h light/dark cycle at 24 °C air temperature with an irradiance of 150 µmol m−2 s−1 for another 2–4 d.
Root growth assay
Root length was measured from control and 150mM NaCl-treated plants, which were subjected to at least 48h of salt treatment. The reduction in the root length of salt-treated seedlings was expressed as a percentage of the control by comparing them with plants of the same age grown in BSM without salt.
Viability staining
For viability staining, the Brassica seeds were grown in BSM without (control) and with NaCl added (treatment) as described above and the roots were collected after 2 d of 150mM NaCl treatment. The viability of the Brassica roots was assessed by using the fluorescein diacetate (FDA)–propidium iodide (PI) double staining method as described in Koyama et al. (1995). FDA (Cat. No. F7378; Sigma, St. Louis, MO, USA) is a non-polar ester permeable through the intact plasma membrane, and shows green colour under a fluorescent microscope in viable cells after hydrolysis by the internal esterases, while PI (Cat. No. P4864; Sigma-Aldrich) is impermeable to the intact plasma membrane and enters only cells with a damaged plasma membrane with large pores (i.e. dead or dying cells), which is indicated by red colour under a fluorescent light upon PI–nuclear DNA conjugate formation.
Control and 150mM NaCl-treated seedlings were stained in the darkness with freshly prepared FDA solution (5 µg ml–1) for 3min, followed by PI solution (3 µg ml–1) for 10min. The double-stained roots were observed under a florescence microscope (Leica MZ12; Leica Microsystems, Wetzlar, Germany) illuminated by an ultra-high-pressure mercury lamp (Leica HBO Hg 100W; Leica Microsystems) and fitted with a Leica I3-wavelength filter cube (Leica Microsystems). The excitation and emission wavelengths were 488/505–530nm and 543/585nm for FDA and PI, respectively. Photographs were taken with a Leica (DFC295; Leica Microsystems) camera fitted on the microscope using image acquisition and processing software LAS V3.8 (Leica Microsystems). During image acquisition, all the automatic exposure features of the LAS V3.8 were disabled, and exposure time (0.62s), gain (2.3×), saturation (0.35), and gamma (2.37) were set to constant values for all the measurements. To determine the extent of cell death, the red and green channels of the fluorescent images were separated and the fluorescent intensity of the respective images was quantified using Fiji (ImageJ) software.
Ion flux measurements
Net fluxes of H+, K+, Ca2+, and Na+ were measured by a non-invasive ion flux measurement technique using vibrating ion-selective microelectrodes (the MIFE technique; University of Tasmania) as described previously (Cuin et al., 2011; Wu et al., 2015). Briefly, microelectrodes were prepared from borosilicate glass capillaries by pulling capillaries followed by drying and silanization with tributylchlorosilane. The electrode tips were broken to achieve external tip diameters of 2–3 µm before they were backfilled with the corresponding back-filling solutions, followed by front-filling with appropriate ion-selective cocktails (see Supplementary Table S1 at JXB online).
Electrodes were mounted on a 3D-micromanipulator (MMT-5, Narishige) and calibrated in an appropriate set of standards encompassing measured ranges of particular ions using a three-point calibration. For MIFE measurements, the plant sample with intact roots (already adapted to BSM for 40min) was immobilized in a measuring chamber, mounted on a microscope stage, and electrode tips positioned 40 µm away from the root surface. Net fluxes were measured from the elongation (800–1000 µm from the root tip) and mature (20mm from the root tip) root zones. The electrochemical potential difference between the two positions was recorded for each electrode by the MIFE CHART software (Shabala et al., 1997) and converted to ion concentration difference using the calibrated Nernst slope of the electrode. Net ion fluxes were calculated using the MIFEFLUX software for cylindrical diffusion geometry (Newman, 2001). All treatments [either 150mM NaCl for salinity stress; or 0.3mM CuCl2+1mM Na-ascorbate (referred to as Cu/A) for oxidative stress] were applied after recording steady fluxes of the respective ions for at least 5min. For the steady-state Na+ flux measurements, both the control and roots treated with 150mM NaCl for 48h were immobilized as described above and net fluxes were recorded for at least 5min from the mature and elongation zones.
Pharmacology
In pharmacological experiments, plant roots were pre-treated for 30–60min prior to application of NaCl or Cu/A stresses with one of the following: 1mM sodium orthovanadate (vanadate; a potent blocker of the H+-ATPase pump; Sigma, Cat. S6508); 1mM amiloride (a potent blocker of Na+/H+ antiport; Sigma Cat. A4562); or 1 µM Eosin Yellow (EY; a known blocker of the Ca2+-ATPase pump; Sigma Cat. 119830).
Membrane potential measurement
Both control and salt-treated seedlings were immobilized in a measuring chamber as described earlier (Bose et al., 2014a ) and were kept for pre-conditioning in BSM (with/without NaCl) for 50–60min. A conventional microelectrode (GC 150F-10, Harvard Apparatus Ltd, Kent, UK) with a tip diameter of ~0.5 µm was filled with 0.5M KCl and connected to a MIFE electrometer (Shabala et al., 1997) via an Ag–AgCl half-cell. The mounted electrode was then impaled in the external cortex cells of intact roots using a manually operated hydraulic micromanipulator (MHW-4-1; Narishige, Tokyo, Japan). The steady-state MP measurements were conducted from at least six individual roots (from both the mature and elongation zones as described above) with no more than four impalements per root undertaken. Each measurement was averaged for at least a 30s interval (Bose et al., 2014a ).
Gene expression study
For gene expression studies, Brassica seedlings grown as described earlier were subjected to 150mM NaCl stress for a period for 1h and 48h in order to simulate the short- and long-term salt stress condition, which finally correlated with ion flux measurement and other root growth studies. Total RNAs from root tissues of control and salt-treated plants were isolated using the RNASure Plant Kit (Genetix Brand, Cat NP-84905) according to the manufacturer’s protocol, with minor modifications. Briefly, root tissues (~100mg) were homogenized in liquid nitrogen to a fine powder and suspended in lysis buffer, before they were subjected to on-column DNase digestion to eliminate any genomic DNA contamination. Finally, the RNA pellet was re-suspended in 20 µl of RNase-free water and stored at −20 °C for further use. The integrity of the RNA was confirmed in the gel, and the absorbance of the isolated RNA was recorded using a Nanodrop Spectrophotometer (ND 1000). Approximately 2 µg of total RNA was used for cDNA synthesis using a First strand cDNA synthesis kit (Thermo Scientific), and the synthesized cDNA was confirmed by PCR using all cDNA samples (~100ng) as template with an 18S rRNA primer. To test gene-specific primers (see Supplementary Table S2 for sequence details), the above experiment was carried out for each set of primers and a single amplicon PCR product was confirmed in agarose gel.
Changes in expression of the above genes (transcript levels) were studied by real-time quantitative PCR (StepOnePlus™ Real-Time PCR System from Applied Biosystems using a QuantiFast SYBR Green PCR kit, Qiagen,USA). The reaction mixture included ~100ng of cDNA, 0.16 µM of primers, and 12.5 µl of QuantiFast SYBR Green PCR mix. The reaction volume was maintained at 20 µl by sterile nuclease-free water. Reactions were run under the following conditions: 95 °C for 5min for one cycle; 95 °C for 10s and 60 °C for 30s for 40 cycles. At the end of the PCR cycles, the products were put through a melt curve analysis to determine the specificity of amplification. The fold changes in transcript in salt-treated roots were compared with those of control plants in terms of fold change, analysed by the comparative 2–ΔΔCt method (Schmittgen and Livak, 2008). The Br-18s rRNA gene was used as the internal control to normalize the PCRs (Sarkar et al., 2014).
Results
Differential salt sensitivity was observed in three Brassica species under both short- and long-term salinity stress
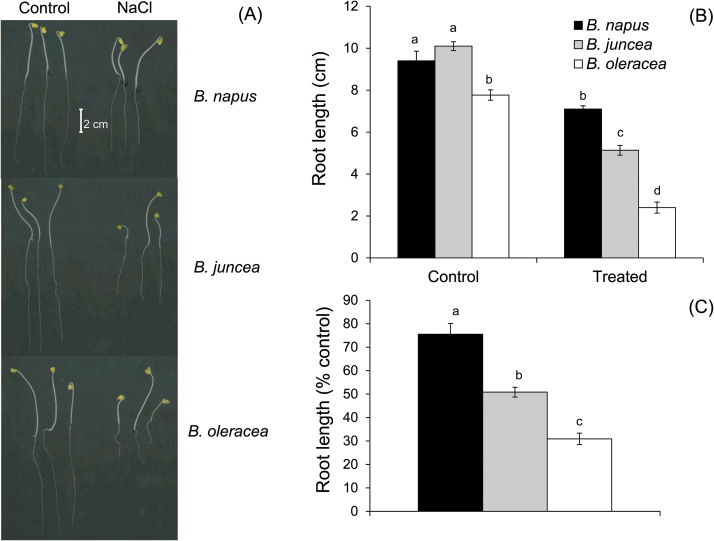
The three Brassica species (B. napus, B. juncea, and B. oleracea) do vary significantly in terms of tolerance to salinity stress when studied in long-term experiments under glasshouse or field conditions (Ashraf and McNeilly, 2004; Chakraborty et al., 2016b ). This differential salt sensitivity is phenotypically detected even under a much shorter duration of stress. Significant differences in the root growth rate were found in 5-day-old seedlings grown under control and saline (150mM NaCl) conditions (Fig. 1). The visible difference in the root growth (Fig. 1A) of salt-treated plants suggested the order of salt tolerance as B. napus> B. juncea>B. oleracea, at the seedling stage. Under control conditions, the root growth varied between 8cm and 10cm in 5-day-old seedlings of the three Brassica species tested. Treatment with 150mM NaCl restricted root growth to a mere ~30% and ~50% in the case of B. oleracea and B. juncea, respectively, while there was nearly 75% retention of root length in B. napus compared with control plants (Figs 1B, C).
Fig. 1.
Effect of salinity stress on root growth of three Brassica species (5-day-old seedling) grown in non-buffered basic salt medium (BSM) solution (0.5mM KCl+0.2mM NaCl+0.1mM CaCl2, pH 5.7) and exposed to 150mM NaCl stress for 72h. Values are the mean ±SE (n=5 individual plants).
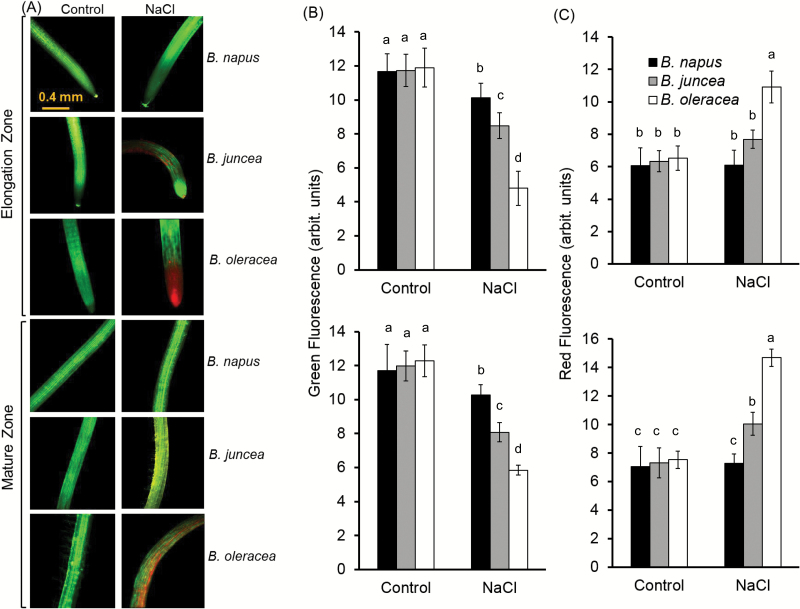
The three Brassica species showed distinctive variability in the tissue-specific sensitivity to salt stress, as revealed by viability staining experiments (Fig. 2A). After 48h of 150mM NaCl treatment, the root tip of B. napus (most tolerant) was almost as healthy as non-treated root, while both B. juncea (>25% mortality) and B. oleracea (>50% mortality) showed a considerably higher level of mortality around the root tip (Fig. 2B, C). Unlike B. napus and B. juncea, B. oleracea, showed symptoms of dying from the root tip spreading towards the mature zone upon long-term salt exposure. Similar to the elongation zone, the mature zone of all the three species showed loss of viability to different extents, with B. napus being the least damaged (~10%), while B. juncea (~30%) and B. oleracea (~50%) exhibited comparatively much higher loss of viability (Fig. 2B, C).
Fig. 2.
Viability staining of the elongation and mature root zones of three Brassica species exposed to 150mM NaCl stress for 48h. (A) One (of five) typical image is shown for each treatment/species. (B, C) Intensity of the green (B) and red (C) fluorescent signal. Values are the mean ±SE (n=15–20).
Mature and elongation zones behaved differently in terms of Na+ exclusion and sensitivity towards salt stress
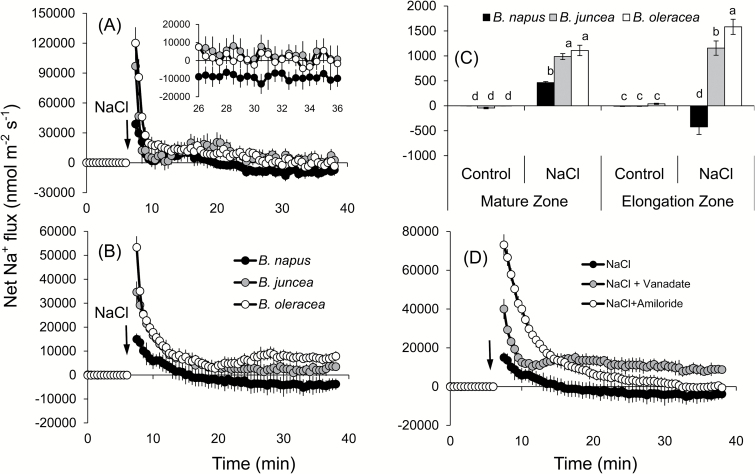
Addition of 150mM NaCl to the root medium resulted in a massive Na+ uptake in plant roots (Fig. 3), in both the elongation (Fig. 3A) and mature (Fig. 3B) zones, although with rather different magnitude (~2-fold higher in the root apex). This uptake gradually slowed down, and net Na+ efflux was measured from B. napus roots at ~15min after onset of 150mM NaCl stress in both zones. In the other two species, however, net Na+ flux significantly decreased (to nearly zero) but never turned into net efflux (Fig. 3A, B). Also different were the peak magnitudes of net Na+ uptake immediately after NaCl application. In the mature zone (the major bulk of the root), the most tolerant B. napus had a peak value of 15 000±2200 nmol m−2 s−1, while it was much higher in B. juncea (>2-fold) and B. oleracea (>3.5-fold). Also, B. napus was able to extrude Na+ in the mature zone (Fig. 3B), while both B. juncea and B. oleracea showed net Na+ uptake of ~3500 nmol m−2 s−1 and ~8000 nmol m−2 s−1, respectively, 30min after the salt treatment. The above findings reported for transient Na+ fluxes were further confirmed in experiments involving long-term salinity treatments. Root exposure to 150mM NaCl for 48h showed that B. napus had 2-fold less steady-state Na+ uptake in the mature zone compared with the two other Brassica species (Fig. 3C) and was the only species that showed a statistically significant net Na+ efflux of ~425 nmol m−2 s−1 in the elongation zone (Fig. 3C), while the other two species showed only net Na+ uptake (Fig. 3C).
Fig. 3.
Transient net Na+ flux kinetics measured in three Brassica species from elongation (A) and mature (B) root zones in response to 150mM NaCl stress. The inset in (A) resolves a genotypic difference in the net Na+ efflux at the end of the transient recording. (C) Steady-state Na+ efflux from roots (both mature and elongation zones) of three Brassica species exposed to 150mM NaCl stress for 48h. (D) Transient net Na+ flux kinetics in response to 150mM NaCl treatment measured from the mature root zone of B. napus pre-treated for 1h in solutions containing a specific metabolic inhibitor or a channel blocker. Values are the mean ±SE (n=6–8). The sign convention for all MIFE measurements is ‘efflux negative’.
A series of pharmacological experiments was conducted to reveal the identity of the above strong Na+ exclusion mechanism in B. napus. Pre-treatment of roots with sodium orthovanadate (a known blocker of H+-ATPase) and amiloride (an inhibitor of the Na+/H+ plasma membrane exchanger; Cuin et al., 2011) for 1h completely changed the pattern of Na+ fluxes in B. napus roots (Fig. 3D). Both chemicals shifted net Na+ fluxes towards more positive values by increasing the peak magnitude of net Na+ uptake by >2.5-fold and ~5-fold for vanadate and amiloride treatment, respectively. Also, no net Na+ efflux was measured in either treatment at the end of the transient recordings (Fig. 3D). While control plants showed a net Na+ efflux of −3800±2700 nmol m−2 s−1 after 30min of NaCl treatment, amiloride-treated roots had a net Na+ uptake of 1500±1250 nmol m−2 s−1. These results strongly suggest that the Na+ exclusion mechanism in B. napus is mediated by the plasma membrane-localized Na+/H+ antiporter fuelled by H+-ATPase.
K+ flux kinetics in mature and elongation zones upon salinity treatment
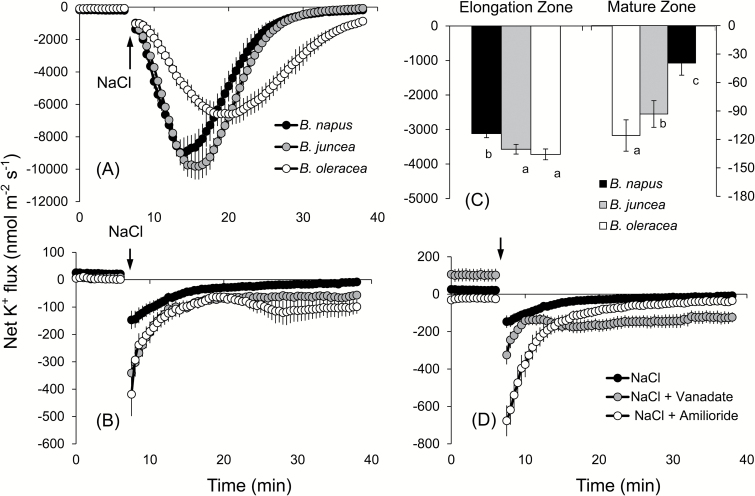
The strong efflux of K+ was observed upon addition of NaCl to the bath medium in both the elongation and mature zones of all the three species, although the pattern differed significantly between the two root zones (Fig. 4A, B). In the elongation zone, the peak K+ efflux was highest in B. juncea followed by B. napus and B. oleracea. The salt-induced K+ leakage was slowly restored to the pre-stress values in B. napus and B. juncea 25min after the stress application, while K+ efflux in B. oleracea persisted throughout the duration of the flux measurements (up to 40min in Fig. 4A; indicated as negative flux values). We also observed a delay in the timing of peak K+ efflux which occurred 5±1min (B. napus), 7±0.5min (B. juncea), and 14±2min (B. oleracea) after application of the stress (Fig. 4A). The overall amount of K+ leaked over the 30min interval since the stress (calculated as the area under the curve in the respective graphs) was the smallest in the most tolerant B. napus (Fig. 4C, left panel; significant at P<0.05). In the mature zone, the highest K+ efflux (peak value) was observed immediately after the imposition of a salt stress. This flux was slowly restored to pre-stress values in B. napus (Fig. 4B) while it remained highly negative (net K+ efflux) in the other two varieties (significant at P<0.05). The magnitude of the peak K+ efflux also varied between the three species, with minimum values observed in B. napus followed by B. juncea and B. oleracea. Similar to the elongation zone, the overall salt-induced K+ leakage was the highest in the most salt-sensitive species B. oleracea and the least leakage was observed in the most salt-tolerant B. napus (Fig. 4C, right panel). Pre-treatment of B. napus (the most salt tolerant among the varieties tested) with vanadate resulted in a higher peak K+ efflux and the loss of the ability to restore K+ values to the pre-stress level (Fig. 4D), suggesting involvement of H+-ATPase. Pre-treatment of roots with 1mM amiloride led to a >4-fold increase in the peak K+ efflux and remained higher (more negative) than the respective value without the inhibitor 30min after the treatment (−80±15 nmol m−2 s−1 and 0±8 nmol m−2 s−1, respectively). Taken together, the observed results suggest involvement of the voltage-gated K+ channels in K+ retention in Brassica roots under salt stress.
Fig. 4.
Transient net K+ flux kinetics measured in three Brassica species from the elongation (A) and mature (B) root zones in response to 150mM NaCl stress. (C) Average K+ flux from roots over the 30min period after Na+ addition to three Brassica species (both mature and elongation zones). (D) Transient net K+ flux kinetics in response to 150mM NaCl treatment measured from the mature root zone of B. napus pre-treated for 1h in solutions containing specific metabolic inhibitors. Values are the mean ±SE (n=6–8).
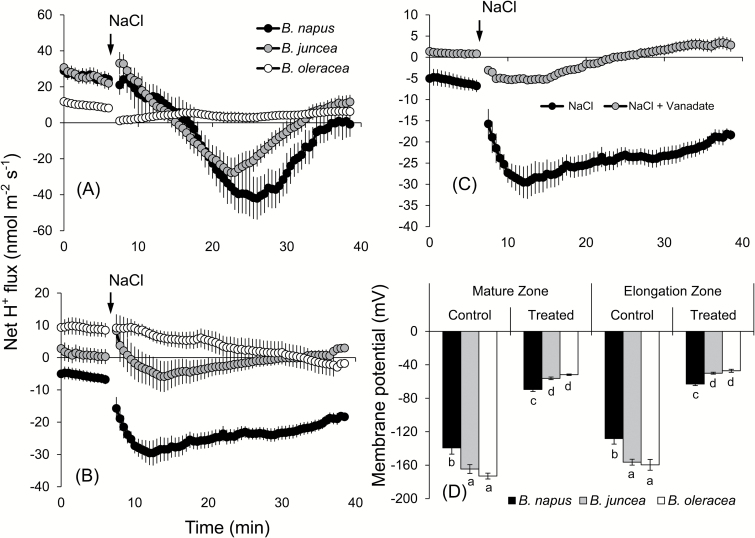
Brassica napus spends more energy through active H+ pumping for maintaining the membrane potential under salinity stress
The onset of salinity stress led to a significant shift in H+ fluxes towards more negative values in all species and both root zones assessed. The magnitude of this shift, however, showed a clear species and tissue dependence (Fig. 5). In the elongation zone, all three species had net initial uptake (zero to 5min; Fig. 5A). Addition of NaCl led to a shift towards H+ efflux. This shift was strongest in B. napus followed by B. juncea and then by B. oleracea (Fig. 5A). A similar pattern was also observed in the mature zone (Fig. 5B), although here the steady-state initial H+ flux values were more negative in all species (net efflux of −6±0.9 nmol m−2 s−1 in B. napus and 0.2±1.1 nmol m−2 s−1 in B. juncea, compared with ~22±1.8 nmol m−2 s−1 in both species in the elongation zone. The peak H+ flux values in the mature zone were −30±4, −6±4, and −2.5±2 nmol m−2 s−1 for B. napus, B. juncea, and B. oleracea, respectively (Fig. 5B), and −42±12, −28±8, and 0±2 nmol m−2 s−1 in the elongation zone (Fig. 5A).
Fig. 5.
Transient net H+ flux kinetics measured in three Brassica species from elongation (A) and mature (B) root zones in response to 150mM NaCl stress. (C) Transient net H+ flux kinetics in response to 150mM NaCl treatment measured from the mature root zone of B. napus pre-treated for 1h with 1mM sodium orthovanadate. Values are the mean ±SE (n=6–8). (D) Membrane potential (mV) of epidermal root cells of three Brassica species measured from roots exposed to 150mM NaCl stress for 48h. Mean ±SE (n=20–25).
The NaCl activation of H+ pumping was largely prevented by vanadate which reduced H+ efflux peak values from −30±4 nmol m−2 s−1 to −5±1 nmol m−2 s−1 upon vanadate pre-treatment (demonstrated as an example for B. napus; Fig. 5C). Also affected was the steady-state initial H+ flux (negative in the control; slightly positive after vanadate pre-treatment). Taken together, these results point towards the involvement of H+-ATPase. Given the fact that the plasma membrane H+-ATPase is a critical player in determining the MP of plant cells (Palmgren and Niessen, 2011), MP values were tested in three varieties under control and salt treatment (48h NaCl) conditions. The largest decrease in MP values was observed in B. oleracea in both the mature (from −170±4 mV in control to −50±0.8 mV after salt treatment) and elongation (from −160±6.5 to −47±1.8 mV) zones, respectively (Fig. 5D). Brassica. napus not only showed the least reduction in MP under long-term salt stress, but also was able to maintain the highest MP under 150mM salt treatment in both the mature (−70±2.5 mV) and elongation (−63±1.9 mV) zones.
ROS-induced K+ leakage is related to the active efflux of Ca2+ through Ca2+-ATPase
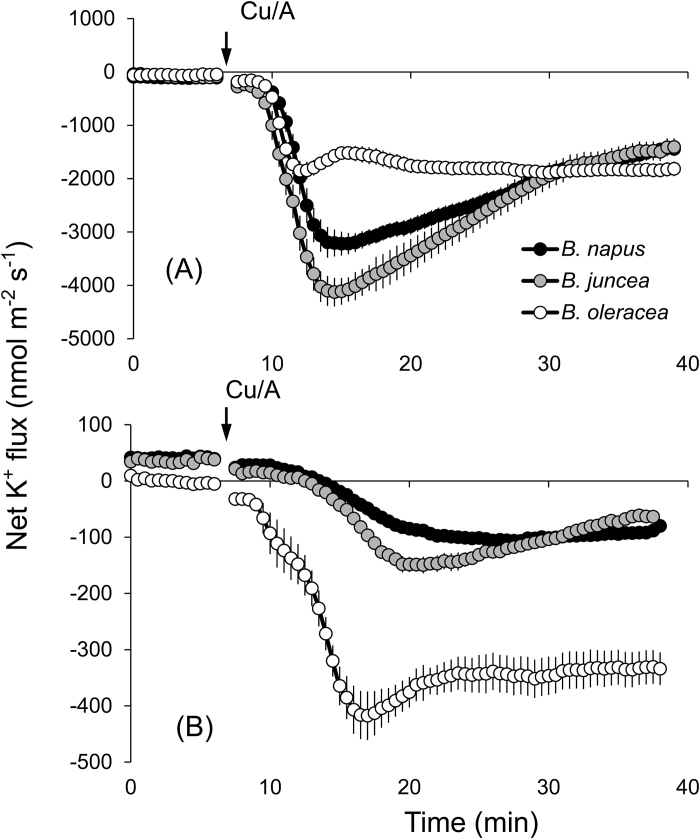
Production of ROS under salinity stress is inevitable, and thus a plant’s ability to withstand ROS stress is often associated with the overall salt tolerance (Bose et al., 2014b ). To assess the sensitivity of the three Brassica species to oxidative stress, the seedlings were subjected to hydroxyl radical generation by Cu/A treatment (Demidchik et al. 2003, 2010). Addition of Cu/A to the BSM solution resulted in a profuse K+ efflux, in both the mature and elongation zones (Fig. 6). The magnitude of this efflux differed significantly between the two zones, with almost a 10-fold higher efflux observed from the elongation zone. Here, the peak values of K+ efflux occurred 7–8min after the treatment (Fig. 6A). In the mature zone, the extent of Cu/A activation of the K+ efflux was inversely correlated with the overall salinity stress tolerance (B. oleracea>B. juncea>B. napus) (Fig. 6B).
Fig. 6.
Transient net K+ flux kinetics measured in three Brassica species from the elongation (A) and mature (B) root zones in response to oxidative (1mM Cu/A) stress. Values are the mean ±SE (n=4–6).
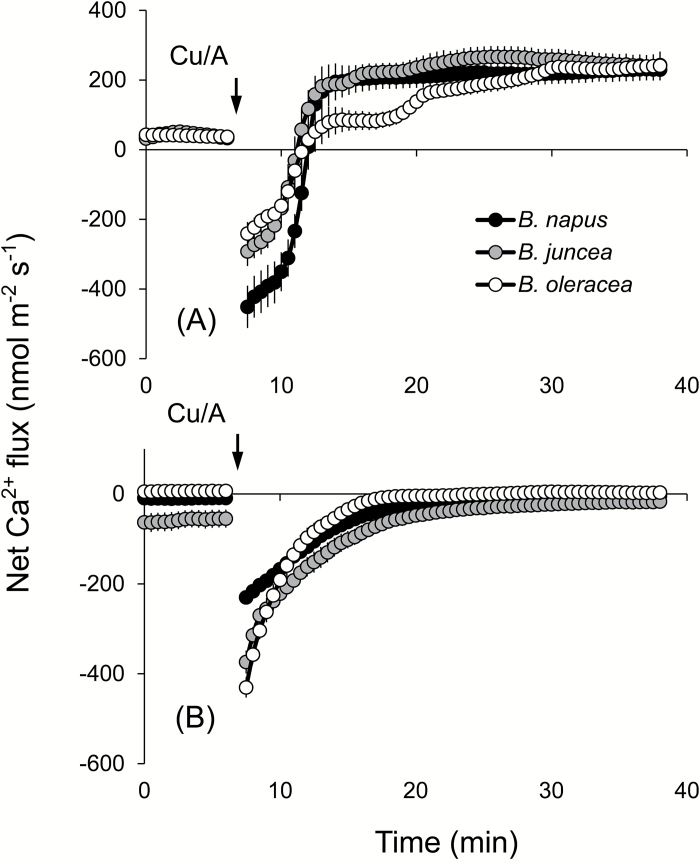
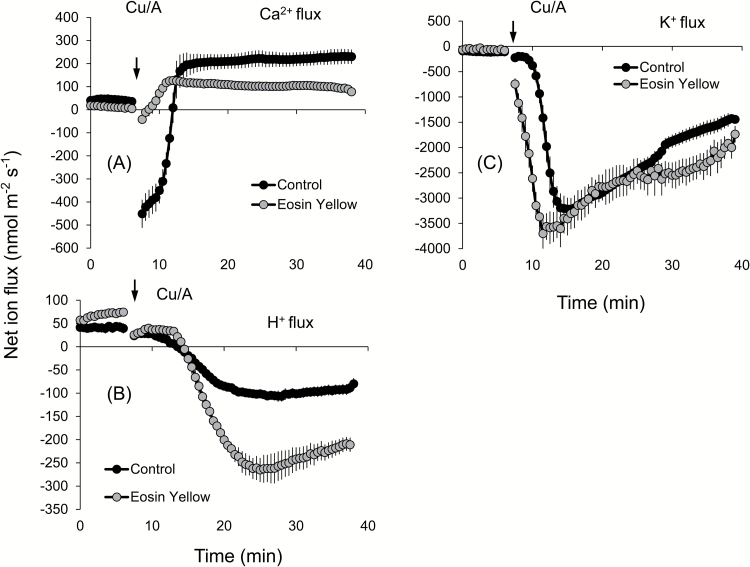
ROS stress is known to affect Ca2+ homeostasis in plants (Demidchik et al., 2010; Zepeda-Jazo et al., 2011), with possible implications for salinity stress signalling. Therefore, we measured the kinetics of net Ca2+ fluxes in response to Cu/A treatment. Of all ROS produced under saline conditions, the hydroxyl radical is the most aggressive and cannot be controlled by means of enzymatic antioxidants (for a review, see Bose et al., 2014b ). In addition, due to the signalling role of H2O2 in plant adaptive responses (Gilroy et al., 2014; Niu and Liao, 2016; Shabala et al., 2016b ), it is often rather difficult to separate ‘detrimental’ and ‘beneficial’ effects of H2O2 on the activity of ion transporters. Taken together, these two facts determined our use of the hydroxyl radical as a ROS agent in this work. Application of the Cu/A hydroxyl radical-generating mix to Brassica roots stimulated net Ca2+ uptake in the elongation zone (Fig. 7A) but resulted in a gradually decaying net Ca2+ efflux from the mature zone (Fig. 7B). No clear differences between species have emerged. Interestingly, immediately upon Cu/A application, a brief transient Ca2+ efflux was also measured in all three Brassica species for several minutes, before turning into net Ca2+ uptake (Fig. 7A). Due to the ~100-fold higher Ca2+ concentration gradient across the plasma membrane, the passive Ca2+ efflux is thermodynamically impossible and could either originate from the cell wall (as a result of the Donnan exchange; Kinraide, 1998; Shabala and Newman, 2000) or be mediated by some active plasma membrane Ca2+ efflux system. To differentiate between these two possible sources, we pre-treated roots with Eosin Yellow (EY), a known inhibitor of Ca2+-ATPase (Romani et al., 2004; Beffagna et al., 2005). Pre-treatment of B. napus roots with EY completely blocked the initial Ca2+ efflux, suggesting involvement of Ca-ATPase in active Ca2+ pumping out from the cells (Fig. 7C). EY was also found to shorten the timing of the response to Cu/A, with the peak K+ efflux in the presence of EY occurring 3min prior to the peak values without EY in the elongation zone (Fig. 8A), and exacerbated the extent of Cu/A-induced activation of net K+ efflux from the mature zone (Fig. 8B).
Fig. 7.
Transient net Ca2+ flux kinetics measured in three Brassica species from the elongation (A) and mature (B) root zones in response to oxidative (1mM Cu/A) stress. Values are the mean ±SE (n=4–6).
Fig. 8.
Transient net Ca2+ (A), H+ (B), and K+ (C) flux kinetics measured in response to oxidative (1mM Cu/A) stress from the elongation root zone of B. napus pre-treated for 1h in solutions containing 1 µM Eosin Yellow, a known blocker of the Ca2+-ATPase pump. Values are the mean ±SE (n=4–6).
Selective induction of key ion transporters/pumps in B. napus explains its higher salinity tolerance among Brassica species
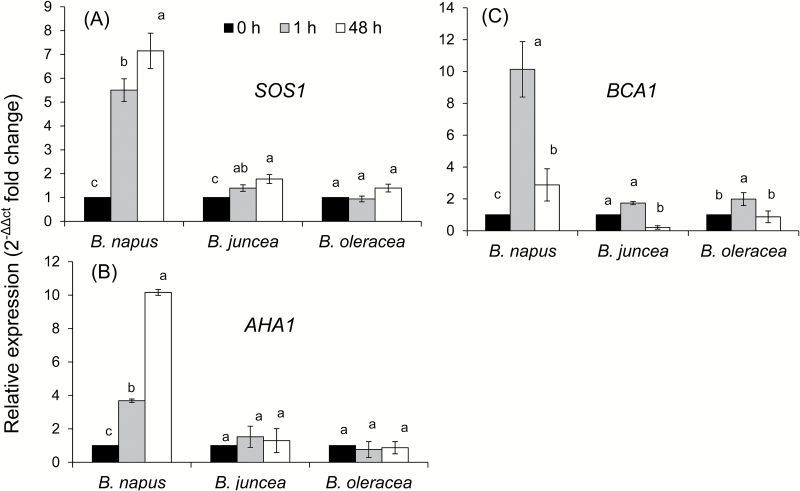
Quantitative real-time PCR analyses were carried out to assess the relative abundance of the transcript level in control (0h) and after short-term (1h) and long-term (48h) salt stress in the three Brassica species. As postulated from the ion flux data and pharmacological tests, we found a significant induction of the plasma membrane Na+/H+-antiporter (SOS1) transcript in B. napus, after both 1h and 48h of 150mM salt treatment. The relative induction of SOS1 transcript was ~5.5- and ~7-fold after 1h and 48h from the onset of salt stress, respectively (Fig. 9A), while it was induced only marginally in B. juncea (1.4- and 1.8-fold induction 1h and 48h after salt treatment, respectively). In the case of B. oleracea, the transcript level was not increased 1h after the treatment, while it was increased slightly (~1.4-fold) 48h afterwards. Upon exposure to salt stress, the transcript level of the plasma membrane H + -ATPase (AHA1) was also induced sharply only in B. napus (3.7- and 10-fold induction 1h and 48h after onset of the stress, respectively) (Fig. 9B). Neither B. juncea nor B. oleracea showed any significant (P<0.05) up-regulation of the AHA1 transcript. Unlike SOS1 and AHA1 genes, the expression of the Ca-ATPase gene did not show a continuous increasing pattern with the increase of the salt treatment period (Fig. 9C). The highest induction of the Ca-ATPase transcript was observed in B. napus, 1h after the salt treatment (~10-fold), which decreased after 48h salt treatment (compared with 1h treatment) while still remaining ~3-fold higher than the control value. The minimal induction of Ca-ATPase transcript was observed in B. juncea, that was not statistically significant.
Fig. 9.
Quantitative real-time PCR analysis of the time-dependent expression pattern of SOS1 (A), AHA1 (B), and BCA1 (C) genes in roots of three Brassica species grown in non-buffered basic salt medium (BSM) solution (0.5mM KCl+0.2mM NaCl+0.1mM CaCl2, pH 5.7) and exposed to 150mM NaCl stress for 0, 1, and 48h. The total RNA was extracted collectively from at least five individual plants for each treatment combination. Values are the mean ±SE (n=6 independent real-time PCRs performed). The relative fold-change values are given as the mean ±SE in each case.
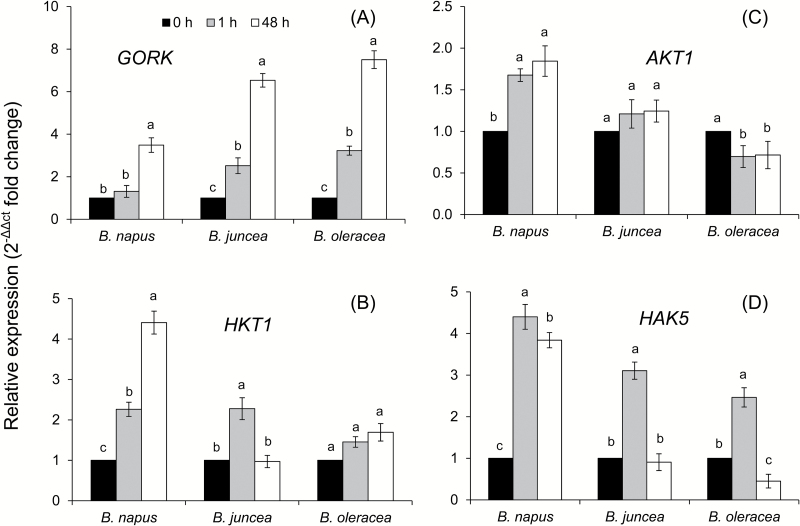
The expression patterns of other key cellular ion transporters mediating intracellular K+ and Na+ homeostasis were also investigated (Fig. 10). Our first candidate was the GORK channel that mediates salt stress-induced K+ efflux from the cell in many plant species (Shabala and Cuin, 2008; Shabala and Pottosin, 2014). Salinity stress induced GORK transcript levels in all three Brassica species, in a time-dependent manner (Fig. 10A). After 1h of salt stress, there was no induction of GORK observed in B. napus, while there was a >2.5-fold induction in both B. juncea and B. oleracea (Fig. 10A). Although 48h of NaCl stress led to significant induction in GORK transcript abundance even in B. napus, the magnitude of induction was ~2-fold lower compared with the other two species. The quantitative PCR data on the relative transcript abundance of HKT1, AKT1, and HAK5 showed a comparatively higher level of induction in B. napus for all three genes (Fig. 10B–D). In fact, the AKT1 transcript did not show any significant change in either B. juncea or B. oleracea upon exposure to salt stress, while HKT1 showed induction upon 1h of salt exposure in B. juncea, but no significant induction in B. oleracea. Strong up-regulation (although with differential magnitude) of the HAK5 transcript was observed in all three Brassica species with short-term salt stress (1h), but it was either decreased to the initial level or even down-regulated with a longer duration (48h) of stress, except in B. napus, which was able to maintain ~3.8-fold up-regulation of the HAK5 transcript level even after 48h of stress.
Fig. 10.
Quantitative real-time PCR analysis of the time-dependent expression pattern of GORK (A), HKT1 (B), AKT1 (C), and HAK5 (D) genes in roots of three Brassica species grown in non-buffered basic salt medium (BSM) solution (0.5mM KCl+0.2mM NaCl+0.1mM CaCl2, pH 5.7) and exposed to 150mM NaCl stress for 0, 1, and 48h. The total RNA was extracted collectively from at least five individual plants for each treatment combination. Values are the mean ± SE (n=6–9 independent real-time PCRs performed). The relative fold-change values are given as the mean ±SE in each case.
Discussion
Similar to other cultivated glycophyte species, Brassica are also sensitive to salinity stress and possess a certain degree of variability in salinity tolerance at the species level (Ashraf et al., 2001; Kumar et al., 2009). Genetically, such variability in salt tolerance in Brassica is thought to be associated with the ploidy level of the species (Ashraf and McNeilly, 2004). In the present study, we found distinctive differences in salt tolerance of the three Brassica species studied. The root growth assay suggested the order of salt tolerance as B. napus>B. juncea>B. oleracea (Fig. 1); this was further confirmed by the viability staining data (Fig. 2). While these findings are consistent with some previous observations (Ashraf et al., 2001; Purty et al., 2008), no explanation for this intraspecies differential sensitivity to salt was given until now, at the mechanistic level. Here we provide strong evidence that higher salt tolerance in B. napus is conferred by at least three complementary physiological mechanisms: (i) higher Na+ extrusion ability from roots resulting from increased expression and activity of plasma membrane SOS1-like Na+/H+ exchangers; (ii) better root K+ retention ability resulting from stress-inducible activation of H+-ATPase and the ability to maintain a more negative MP under saline conditions; and (iii) reduced sensitivity of B. napus root K+-permeable channels to ROS. Of these, the two latter mechanisms played the dominant role in conferring salinity stress tolerance in Brassica. The supporting arguments are given below.
SOS1-mediated Na+ extrusion may be essential for higher salt tolerance in B. napus
A massive influx of Na+ was observed in all three Brassica species immediately after imposition of salinity stress. Given the strong cytotoxicity of Na+ (Munns and Gilliham, 2015), plant performance under saline conditions will be critically dependent on the ability of Brassica species to remove excessive Na+ from the cytosol, either to the external media or by sequestering it into the vacuole. In contrast to mammalian systems, higher plants do not harbour Na+ ATPase pumps (Maathuis, 2014), and the above exclusion is believed to be mediated by Na+/H+ exchangers [either SOS1 at the plasma membrane (Shi et al., 2000) or NHX at the tonoplast membrane (Blumwald et al., 2000)]. Here we provide strong evidence that superior salinity stress tolerance in B. napus is conferred by higher SOS1 activity. Indeed, B. napus was the only species showing active Na+ exclusion in both the mature and elongation root zones (Fig. 3A, B). This Na+ efflux was found to be sensitive to both vanadate (a known inhibitor of H+ -ATPase that provides a driving force for Na+/H+ antiporter operation) and amiloride [a known blocker of Na+/H+ exchangers in both mammalian (Grinstein et al. 1988) and plant (Cuin et al., 2011) systems] (Fig. 3D), strongly suggesting the involvement of SOS1. This is further confirmed by measuring stress-induced changes in SOS1 transcript levels (Fig. 9) that were several fold greater in B. napus compared with the two other Brassica species. Also strongly induced was AHA1 expression (a 10-fold increase in transcript level), conferring the possibility to fuel the SOS1 operation. Thus, it is plausible to suggest that B. napus possesses a very efficient salt-sensing system that is able to translate changes in the rhizosphere conditions into both transcriptional (Fig. 9) and functional (Fig. 3) changes in the SOS1 activity. Specific details of the mechanisms behind thhe observed activation remain obscure. Zhu (2003) has argued that SOS1 protein has a long tail that resides in the cytoplasm and, by analogy with bacterial and yeast systems, may potentially operate as an Na+ sensor per se. However, no direct experimental support for this hypothesis has been provided so far, and it was argued (Shabala et al., 2015) that SOS1 activity is regulated by a SOS3/SOS2 complex, which activates its C-terminus from the cytosolic site and thus relies on changes in cytosolic free Ca2+ (not external Na+) concentrations. Given the fact that changes in SOS1 activity strongly correlated with changes in net K+ and H+ fluxes, involvement of the H+-ATPase/GORK tandem system as a potential Na+ sensor (Shabala et al., 2015) may be plausible. This issue is discussed in more detail in the following sections. It should be also noted that net Na+ efflux was not observed in either the elongation or the mature zone of the other two Brassica species (Fig. 3C), and thus cannot be responsible for the reported higher tolerance of B. juncea as compared with B. oleracea (Figs 1, 2). Thus, it is suggested that Na+ exclusion from uptake plays an important but not a crucial role as a determinant of genetic variability in salinity stress tolerance in Brassica.
Maintenance of K+ homeostasis is critical for salt tolerance in B. napus
Maintenance of K+ homeostasis is essential for enzyme activities, ionic and pH homeostasis, and charge balance (Dreyer and Uozumi, 2011), and cytosolic K+ is considered to be the common denominator of plant adaptive responses to a broad range of environmental stresses (Shabala and Pottosin, 2014; Shabala et al., 2016a ). Changes in the cytosolic K+ are also suggested to be an important signal that may determine the cell’s fate and also switch its operation from the metabolic to defence mode (Demidchik et al., 2014). Both X-ray crystallographic and ion-selective microelectrode-based measurements of the cytosolic K+ level were shown to be decreased considerably under salinity stress (Flowers and Hajibagheri, 2001; Cuin et al., 2003), and a strong correlation between the root’s K+ retention ability and plant salinity stress tolerance was reported for several species including wheat (Cuin et al., 2008, 2012), barley (Chen et al., 2005, 2007; Wu et al., 2015), poplar (Sun et al., 2009), and lucerne (Smethurst et al., 2008). Here we extend this list and show that the above mechanism is also applicable to explain the intraspecific variability in salinity tolerance in Brassica. Indeed, the average total K+ leak was found to be in the order B. napus<B. juncea<B. oleracea, which was in an agreement with the overall ability of these species to tolerate external salt stress (Fig. 4). A strong correlation (see Supplementary Table S3) between K+ retention and net Na+ uptake observed in the Brassica species (especially in the mature root zone); sensitivity of NaCl-induced K+ efflux to vanadate and amiloride (Fig. 4D); and a positive correlation between root K+ retention ability and plasma membrane potential under saline conditions (Fig. 5D) collectively point towards involvement of GORK channels as a major pathway for the salt stress-induced K+ efflux from Brassica roots. GORK (outward-rectifying potassium selective) channels are activated by membrane depolarization (Very et al., 2014), and their gating is strongly dependent upon the extracellular K+ concentration (Anschutz et al., 2014). GORK channels are ubiquitously expressed in Arabidopsis tissues and represent an essential element in volume control of various cell types such as guard cells, pollen tube, or root hairs (Becker et al., 2003; Hosy et al., 2003). They are also known to be ROS sensitive and determine cell fate by mediating induction of programmed cell death under saline conditions (Demidchik et al., 2010). These findings reported for Arabidopsis are in a very good agreement with our data reporting much smaller activation of K+ efflux by the hydroxyl radical-generating Cu/A mix in B. napus roots (Fig. 6B) compared with the two other species. Thus, it may be concluded that the intraspecific variability in salinity stress tolerance among Brassica species is critically dependent on the functional expression and/or regulation of GORK channels under saline conditions.
Interestingly, GORK transcript levels were up-regulated by 4- to 7-fold, in a clear time-dependent manner (Fig 10A). This is counterintuitive and contradicts the above notion of the essentiality of K+ retention in salinity stress tolerance. The most likely explanation is the comment made above that plants may use K+ efflux as a switch from metabolic to defence mode (Demidchik et al., 2014), and increased expression of GORK channels may assist plants in achieving this goal. As such a switch has a caveat of losing too much K+, a very fine balance between the amount of K+ lost in this signalling process and the amount of K+ retained in the root for turgor and/or metabolic purposes is needed. Brassica napus plants seem to be very efficient in maintaining such a balance. Not only is the reported increase in GORK transcript 2-fold lower in B. napus compared with the other two species, but B. napus plants showed much higher transcript levels of both low (AKT1) and high (HAK5) affinity transport systems involved in K+ acquisition (hence, restoring cytosolic K+ levels once the signalling is over). Also 2 - to 3-fold higher were the HKT1 transcript levels in B. napus 48h after stress onset (Fig. 10B). Traditionally, the role of HKT1 is attributed to retrieval of Na+ from the xylem (Munns and Tester, 2008). This suggest a better Na+ retention in the root of B. napus and a potential for its use for osmotic (turgor maintenance) purposes, to compensate for K+ lost.
Maintenance of a higher membrane potential under salinity stress is achieved by an active H+ pumping by B. napus
An active H+ pumping is the key to maintaining the negative MP required for optimal plant growth (Sze et al., 1999). Under saline conditions, NaCl induces activation of both plasma membrane and vacuolar H+-ATPases that are required to restore the otherwise depolarized MP in both halophytes and glycophytes (Yang et al., 2007; Bose et al., 2015). Here we show that the differential sensitivity of Brassica species to salinity stress is determined by the extent of NaCl-induced activation of H+-ATPase, at both the transcriptional (Fig. 9) and post-translational (Fig. 5) level.
A significant correlation between membrane depolarization and K+ leakage from the root tissue was established in earlier reports (Chen et al., 2007; Bose et al., 2014a ) and attributed to the depolarization-activated outward-rectifying K+ channels (Shabala and Pottosin, 2014). In agreement with this, a dramatic K+ efflux was observed in all Brassica species tested (Fig. 4) that correlated with the extent of MP depolarization (Fig. 5). The NaCl-induced H+ efflux was almost instantaneous (Fig. 5B) and strongly blocked by vanadate, a known inhibitor of H+-ATPase (Fig. 5C). The causal link between the cytosolic K+ concentration and H+-ATPase activity was suggested by Buch-Pedersen et al. (2006) who showed that the plant plasma membrane H+-ATPase is regulated by potassium bound to the proton pump at a site involving Asp617 in the cytoplasmic phosphorylation domain, suggesting a role for K+ as an intrinsic uncoupler. Thus, it was suggested that GORK channels may be located in close proximity to H+-ATPase, forming a ‘microdomain’ in the lipid raft (Shabala et al., 2015), and that a reduction in the cytosolic K+ concentration in the H+-ATPase microdomain may affect operation of the H+ pump as described above. This GORK/H+-ATPase ‘tandem’ may then operate as a salt sensor and explain the differential sensitivity between Brassica species.
Differential ROS sensitivity in salt stress signalling in Brassica
Plants growing in a saline environment are facing cytotoxic ROS produced as a secondary effect of salinity stress. Demidchik et al. (2010) reported a 2.5- to 3-fold increase in hydroxyl radicals in Arabidopsis roots exposed to 100mM NaCl stress. Also the amount of H2O2 accumulated in the roots showed a significant increase under salt stress (Xie et al., 2011). This increased load of ROS under saline conditions was shown to activate GORK channels, which conduct large outwardly rectifying K+ currents and thus increase K+ leakage from the tissue (Demidchik et al., 2010). In full agreement with this, we report a high degree of K+ leakage in the mature zone of all three Brassica as soon as they are exposed to oxidative stress (Fig. 6B). The most salt-sensitive species, B. oleracea, not only showed the highest K+ efflux but the rate of induction of K+ leakage was also much faster in this species (judged by the timing of peak K+ efflux). Overall, K+ retention capacity in the mature root zone under oxidative stress (imposed by the hydroxyl radical-generating Cu/A mix; Demidchik et al., 2003) was B. napus>B. juncea>B. oleracea as the order of tolerance, which is similar to the respective order for root growth and root viability under salt stress (Figs 1, 2). Interestingly, the pattern of K+ efflux was not similar in the elongation zone (Fig. 6A). In fact, it was almost the other way around, with B. juncea and B. napus showing higher K+ efflux than B. oleracea. Again, it may be tempting to suggest, therefore, that ROS-induced K+ leak from the root apex may perform a ‘positive’ (signalling) role, either switching cell metabolism from the metabolic into defence mode of operation (Demidchik et al., 2014), thus enabling a rapid plant adaptation to altered conditions in the rhizosphere, or initiating a long-distance (root to shoot) signalling cascade (Shabala et al., 2016b ).
Oxidative stress is known also to affect Ca2+ homeostasis (Demidchik et al., 2003) through a large number of Ca2+ transport systems (Demidchik et al., 2010; Zepeda-Jazo et al., 2011). Accordingly, ROS-induced net Ca2+ fluxes were also measured and assessed in this work. A rapid initial efflux of Ca2+ was observed from both the mature and elongation zones of Brassica roots immediately upon exposure to the oxidative stress (Fig. 7A, B). In the elongation zone, the highest Ca2+ efflux was observed in B. napus, which was converted into steady uptake of Ca2+ 7–12min after the stress application. For the other two species, the trend was also similar, with a slight difference between the periods of efflux in these species. As passive (channel-mediated) Ca2+ efflux is thermodynamically not possible, the reported data suggest a rapid activation by ROS of some active Ca2+ efflux systems, which is more pronounced in the salt-tolerant B. napus species. Cellular membranes harbour a large number of Ca2+ efflux systems (Ca2+-ATPase family) and Ca2+ exchangers (CAX family) (McAinsh and Pittman, 2009; Bose et al., 2011), and activation of plasma membrane-based Ca2+-ATPase pumps by hydroxyl radicals was demonstrated in pharmacological and patch–clamp experiments in pea root cells (Zepeda-Jazo et al., 2011). Accordingly, we have hypothesized that a similar scenario may be applicable to Brassica roots. Pharmacological tests revealed that the ROS-induced Ca2+ efflux was sensitive to EY, a known blocker of Ca2+-ATPase. The activation of Ca2+-ATPase also happened to be the highest in the elongation zone of B. napus and the lowest in the mature zone of B. napus, suggesting tissue-specific Ca2+ signalling in this species. The observed efflux is transient and is gradually transformed into net Ca2+ uptake that most probably results from the hydroxyl radical-induced activation of Ca2+-permeable channels in root plasma membranes, as reported in direct patch–clamp experiments on other species (Demidchik et al., 2003; Foreman et al., 2003; Demidchik and Maathuis, 2007; Zepeda-Jazo et al., 2011). Taken together, a concurrent (or very closely timed) activation of both Ca2+ efflux and Ca2+ influx systems may shape the cytosolic Ca2+ signature, conferring specificity of the stress signal (Knight and Knight, 2001; Demidchik et al., 2002; Ludwig et al., 2004; Demidchik and Maathuis, 2007; Dodd et al., 2010). Consistent with this suggestion were changes in BCA1 transcript levels that peaked at 1h and declined afterwards (once the signalling was over). The reported induction in BCA1 transcript was significantly higher in B. napus (Fig. 9C), consistent with the reported strongest net Ca2+ efflux from its roots Fig. 7A).
Conclusions
To summarize, the superior salinity stress tolerance of B. napus was conferred by its better ability to exclude Na+ and retain K+, thus providing an optimal cytosolic K/Na ratio. This ability was determined by the efficient transcriptional and post-translational regulation of key transport systems, namely the plasma membrane H+-ATPase pump, the SOS1 Na+/H+ exchanger, P-type Ca2+-ATPase, and low- and high-affinity K+ uptake systems. Specific details of their regulation and salt stress perception and signalling pathways remain to be revealed in follow-up studies.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Propidium iodide-stained B. oleracea root apex showing dead cells after 48h of 150mM NaCl stress.
Table S1. List of liquid ionophores and respective back-filling solution used for micro-electrode preparation.
Table S2. Nucleotide sequences of different primers used in the study along with amplicon length.
Table S3. Correlation matrix (two-tailed Pearson’s correlation) for different physiological and ion uptake parameters with transcript abundance of key transporters/proteins
Acknowledgements
This work has been supported by an Endeavour Research Fellowship, Australia Awards, Australian Government Department of Education to KC, and Australian Research Council and Grain Research and Development Corporation grants to SS.
References
- Adem GD, Roy SJ, Zhou M, Bowman JP, Shabala S. 2014. Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biology 14, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anschütz U, Becker D, Shabala S. 2014. Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. Journal of Plant Physiology 171, 670–687. [DOI] [PubMed] [Google Scholar]
- Ashraf M, Athar HR, Harris PJC, Kwon TR. 2008. Some prospective strategies for improving crop salt tolerance. Advances in Agronomy 97, 45–110. [Google Scholar]
- Ashraf M, McNeilly T. 2004. Salinity tolerance in Brassica oilseeds. Critical Reviews in Plant Science 23, 157–174. [Google Scholar]
- Ashraf M, Nazir N, McNeilly T. 2001. Comparative salt tolerance of amphidiploid and diploid Brassica species. Plant Science 160, 683–689. [DOI] [PubMed] [Google Scholar]
- Becker D, Hoth S, Ache P, Wenkel S, Roelfsema MR, Meyerhoff O, Hartung W, Hedrich R. 2003. Regulation of the ABA-sensitive Arabidopsis potassium channel gene GORK in response to water stress. FEBS Letters 554, 119–126. [DOI] [PubMed] [Google Scholar]
- Beffagna N, Buffoli B, Busi C. 2005. Modulation of reactive oxygen species production during osmotic stress in Arabidopsis thaliana cultured cells: involvement of the plasma membrane Ca2+-ATPase and H+-ATPase. Plant and Cell Physiology 46, 1326–1339. [DOI] [PubMed] [Google Scholar]
- Blumwald E, Aharon GS, Apse MP. 2000. Sodium transport in plant cells. Biochimica et Biophysica Acta 1465, 140–151. [DOI] [PubMed] [Google Scholar]
- Bose J, Pottosin II, Shabala SS, Palmgren MG, Shabala S. 2011. Calcium efflux systems in stress signaling and adaptation in plants. Frontiers in Plant Science 2, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J, Rodrigo-Moreno A, Lai D, Xie Y, Shen W, Shabala S. 2015. Rapid regulation of the plasma membrane H+-ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa . Annals of Botany 115, 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J, Rodrigo-Moreno A, Shabala S. 2014. b ROS homeostasis in halophytes in the context of salinity stress tolerance. Journal of Experimental Botany 65, 1241–1257. [DOI] [PubMed] [Google Scholar]
- Bose J, Shabala L, Pottosin I, Zeng F, Velarde-Buendía A, Massart A, Poschenrieder C, Hariadi Y, Shabala S. 2014. a Kinetics of xylem loading, membrane potential maintenance, and sensitivity of K+-permeable channels to reactive oxygen species: physiological traits that differentiate salinity tolerance between pea and barley. Plant, Cell and Environment 37, 589–600. [DOI] [PubMed] [Google Scholar]
- Buch-Pedersen MJ, Rudashevskaya EL, Berner TS, Venema K, Palmgren MG. 2006. Potassium as an intrinsic uncoupler of the plasma membrane H+-ATPase. Journal of Biological Chemistry 281, 38285–38292. [DOI] [PubMed] [Google Scholar]
- Chakraborty K, Bose J, Shabala L, Eyles A, Shabala S. 2016. b Evaluating relative contribution of osmo- and tissue-tolerance mechanisms towards salinity stress tolerance in three Brassica species. Physiologia Plantarum. doi:10.1111/ppl.12447. [DOI] [PubMed] [Google Scholar]
- Chakraborty K, Sairam RK, Bhaduri D. 2016. a Effects of different levels of soil salinity on yield attributes, accumulation of nitrogen, and micronutrients in Brassica spp. Journal of Plant Nutrition 39, 1026–1037. [Google Scholar]
- Chakraborty K, Sairam RK, Bhattacharya RC. 2012. Salinity induced expression of pyrrolline-5-carboxylate synthetase determine salinity tolerance in Brassica spp. Acta Physiologiae Plantarum 34, 1935–1941. [Google Scholar]
- Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S. 2005. Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant, Cell and Environment 28, 1230–1246. [Google Scholar]
- Chen Z, Pottosin II, Cuin TA, et al. 2007. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiology 145, 1714–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Shabala S, Mendham N, Newman I, Zhang G, Zhou M. 2008. Combining ability of salinity tolerance on the basis of NaCl-induced K flux from roots of barley. Crop Science 48, 1382–1388. [Google Scholar]
- Cuin TA, Betts SA, Chalmandrier R, Shabala S. 2008. A root’s ability to retain K+ correlates with salt tolerance in wheat. Journal of Experimental Botany 59, 2697–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuin TA, Bose J, Stefano G, Jha D, Tester M, Mancuso S, Shabala S. 2011. Assessing the role of root plasma membrane and tonoplast Na+/H+ exchangers in salinity tolerance in wheat: in planta quantification methods. Plant, Cell and Environment 34, 947–961. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Miller AJ, Laurie SA, Leigh RA. 2003. Potassium activities in cell compartments of salt-grown barley leaves. Journal of Experimental Botany 54, 657–661. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Tian Y, Betts SA, Chalmandrier R, Shabala S. 2009. Ionic relations and osmotic adjustment in durum and bread wheat under saline conditions. Functional Plant Biology 36, 1110–1119. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Zhou M, Parsons D, Shabala S. 2012. Genetic behaviour of physiological traits conferring cytosolic K+⁄ Na+ homeostasis in wheat. Plant Biology 14, 438–446. [DOI] [PubMed] [Google Scholar]
- Davenport R, James RA, Zakrisson-Plogander A, Tester M, Munns R. 2005. Control of sodium transport in durum wheat. Plant Physiology 137, 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V. 2015. Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environmental and Experimental Botany 109, 212–228. [Google Scholar]
- Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, Sokolik A, Yurin V. 2010. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. Journal of Cell Science 123, 1468–1479. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Davenport RJ, Tester M. 2002. Nonselective cation channels in plants. Annual Review of Plant Biology 53, 67–107. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Maathuis FJM. 2007. Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytologist 175, 387–404. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM. 2003. Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. Journal of Cell Science 116, 81–88. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Davies JM. 2007. Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. The Plant Journal 49, 377–386. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Straltsova D, Medvedev SS, Pozhvanov GA, Sokolik A, Yurin V. 2014. Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. Journal of Experimental Botany 65, 1259–1270. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Tester M. 2002. Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiology 128, 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D. 2010. The language of calcium signaling. Annual Review of Plant Biology 61, 593–620. [DOI] [PubMed] [Google Scholar]
- Dreyer I, Uozumi N. 2011. Potassium channels in plant cells. FEBS Journal 278, 4293–4303. [DOI] [PubMed] [Google Scholar]
- Fan W, Deng G, Wang H, Zhang H, Zhang P. 2015. Elevated compartmentalization of Na+ into vacuoles improves salt and cold stress tolerance in sweet potato (Ipomoea batatas). Physiologia Plantarum 154, 560–571. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Hajibagheri MA. 2001. Salinity tolerance in Hordeum vulgare: ion concentrations in root cells of cultivars differing in salt tolerance. Plant and Soil 231, 1–9. [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, et al. 2003. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422, 442–446. [DOI] [PubMed] [Google Scholar]
- Gilroy S, Suzuki N, Miller G, Choi WG, Toyota M, Devireddy AR, Mittler R. 2014. A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends in Plant Science 19, 623–630. [DOI] [PubMed] [Google Scholar]
- Grinstein S, Smith JD, Onizuka R, Cheung RK, Gelfand EW, Benedict S. 1988. Activation of Na+/H+ exchange and the expression of cellular proto-oncogenes in mitogen- and phorbol ester-treated lymphocyte. Journal of Biological Chemistry 263, 8658–8665. [PubMed] [Google Scholar]
- Hosy E, Vavasseur A, Mouline K, et al. 2003. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proceedings of the National Academy of Sciences, USA 100, 5549–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB. 1998. Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiology 118, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Knight MR. 2001. Abiotic stress signalling pathways: specificity and cross-talk. Trends in Plant Science 6, 262–267. [DOI] [PubMed] [Google Scholar]
- Koyama H, Toda T, Yokota S, Dawair Z, Hara T. 1995. Effects of aluminium and pH on root growth and cell viability in Arabidopsis thaliana strain Landsberg in hydroponic culture. Plant and Cell Physiology 36, 201–205. [Google Scholar]
- Kumar G, Purty RS, Sharma MP, Singla-Pareek SL, Pareek A. 2009. Physiological responses among Brassica species under salinity stress show strong correlation with transcript abundance for SOS pathway-related genes. Journal of Plant Physiology 166, 507−520. [DOI] [PubMed] [Google Scholar]
- Laohavisit A, Shang Z, Rubio L, et al. 2012. Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca2+- and K+-permeable conductance in root cells. The Plant Cell 24, 1522–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig AA, Romeis T, Jones JD. 2004. CDPK-mediated signalling pathways: specificity and cross-talk. Journal of Experimental Botany 55, 181–188. [DOI] [PubMed] [Google Scholar]
- Maathuis FJ. 2014. Sodium in plants: perception, signalling, and regulation of sodium fluxes. Journal of Experimental Botany 65, 849–858. [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Pittman JK. 2009. Shaping the calcium signature. New Phytologist 181, 275–294. [DOI] [PubMed] [Google Scholar]
- Munns R. 2002. Comparative physiology of salt and water stress. Plant, Cell and Environment 25, 239–250. [DOI] [PubMed] [Google Scholar]
- Munns R, Gilliham M. 2015. Salinity tolerance of crops—what is the cost? New Phytologist 208, 668–673. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681. [DOI] [PubMed] [Google Scholar]
- Nayidu N, Bollina V, Kagale S. 2013. Oilseed crop productivity under salt stress. In: Ahmad P, Azouz MM, Prasad MNV, eds. Ecophysiology and responses of plants under salt stress. Heidelberg: Springer, 249–265. [Google Scholar]
- Newman IA. 2001. Ion transport in plants: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant, Cell and Environment 24, 1–14. [DOI] [PubMed] [Google Scholar]
- Niu LJ, Liao WB. 2016. Hydrogen peroxide signaling in plant development and abiotic responses: crosstalk with nitric oxide and calcium. Frontiers in Plant Science 7, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nublat A, Desplans J, Casse F, Berthomieu P. 2001. Sas1, an Arabidopsis mutant over-accumulating sodium in the shoot, shows deficiency in the control of the root radial transport of sodium. The Plant Cell 13, 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordoñez NM, Marondedze C, Thomas L, Pasqualini S, Shabala L, Shabala S, Gehring C. 2014. Cyclic mononucleotides modulate potassium and calcium flux responses to H2O2 in Arabidopsis roots. FEBS Letters 588, 1008–1015. [DOI] [PubMed] [Google Scholar]
- Palmgren MG, Nissen P. 2011. P-Type ATPases. Annual Review of Biophysics 40, 243–266. [DOI] [PubMed] [Google Scholar]
- Pandolfi C, Pottosin I, Cuin T, Mancuso S, Shabala S. 2010. Specificity of polyamine effects on NaCl-induced ion flux kinetics and salt stress amelioration in plants. Plant and Cell Physiology 51, 422–434. [DOI] [PubMed] [Google Scholar]
- Purty RS, Kumar G, Singla-Pareek SL, Pareek A. 2008. Towards salinity tolerance in Brassica: an overview. Physiology and Molecular Biology of Plants 14, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero FJ, Martinez-Atienza J, Villalta I, et al. 2011. Activation of the plasma membrane Na/H antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proceedings of the National Academy of Sciences, USA 108, 2611–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani G, Bonza MC, Filippini I, Cerana M, Beffagna N, De Michelis MI. 2004. Involvement of the plasma membrane Ca2+-ATPase in the short-term response of Arabidopsis thaliana cultured cells to oligogalacturonides. Plant Biology 6, 192–200. [DOI] [PubMed] [Google Scholar]
- Sarkar T, Thankappan R, Kumar A, Mishra GP, Dobaria JR. 2014. Heterologous expression of the AtDREB1A gene in transgenic peanut-conferred tolerance to drought and salinity stresses. PLoS One 9, e110507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nature Protocols 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Shabala S, Bose J, Fuglsang AT, Pottosin I. 2016. a On a quest for stress tolerance genes: membrane transporters in sensing and adapting to hostile soils. Journal of Experimental Botany 67, 1015–1031. [DOI] [PubMed] [Google Scholar]
- Shabala S, Cuin TA. 2008. Potassium transport and plant salt tolerance. Physiologia Plantarum 133, 651–669. [DOI] [PubMed] [Google Scholar]
- Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Miller AJ, Davies JM, Newman IA. 2006. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiology 141, 1653–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Newman I. 2000. Salinity effects on the activity of plasma membrane H+ and Ca2+ transporters in bean leaf mesophyll: masking role of the cell wall. Annals of Botany 85, 681–686. [Google Scholar]
- Shabala S, Newman IA, Morris J. 1997. Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiology 113, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Pottosin I. 2014. Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiologia Plantarum 151, 257–279. [DOI] [PubMed] [Google Scholar]
- Shabala S, White RG, Djordjevic MA, Ruan YL, Mathesius U. 2016. b Root to shoot signalling: integration of diverse molecules, pathways and functions. Functional Plant Biology 43, 87–104. [DOI] [PubMed] [Google Scholar]
- Shabala S, Wu H, Bose J. 2015. Salt stress sensing and early signalling events in plant roots: current knowledge and hypothesis. Plant Science 241, 109–119. [DOI] [PubMed] [Google Scholar]
- Shi H, Quintero FJ, Pardo JM, Zhu JK. 2002. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. The Plant Cell 14, 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smethurst CF, Rix K, Garnett T, Auricht G, Bayart A, Lane P, Wilson SJ, Shabala S. 2008. Multiple traits associated with salt tolerance in lucerne: revealing the underlying cellular mechanisms. Functional Plant Biology 35, 640–650. [DOI] [PubMed] [Google Scholar]
- Sun J, Chen S, Dai S, et al. 2009. NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiology 149, 1141–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H, Li X, Palmgren MG. 1999. Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. The Plant Cell 11, 677–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry AA, Nieves-Cordones M, Daly M, Khan I, Fizames C, Sentenac H. 2014. Molecular biology of K+ transport across the plant cell membrane: what do we learn from comparison between plant species? Journal of Plant Physiology 171, 748–769. [DOI] [PubMed] [Google Scholar]
- Wu H, Zhu M, Shabala L, Zhou M, Shabala S. 2015. K+ retention in leaf mesophyll, an overlooked component of salinity tolerance mechanism: a case study for barley. Journal of Integrative Plant Biology 57, 171–185. [DOI] [PubMed] [Google Scholar]
- Xie YJ, Xu S, Han B, Wu MZ, Yuan XX, Han Y, Gu Q, Xu DK, Yang Q, Shen WB. 2011. Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role of RbohD-derived reactive oxygen species synthesis. The Plant Journal 66, 280–292. [DOI] [PubMed] [Google Scholar]
- Yang Y, Xu S, An L, Chen N. 2007. NADPH oxidase-dependent hydrogen peroxide production, induced by salinity stress, may be involved in the regulation of total calcium in roots of wheat. Journal of Plant Physiology 164, 1429–1435. [DOI] [PubMed] [Google Scholar]
- Zepeda-Jazo I, Velarde-Buendía AM, Enríquez-Figueroa R, Bose J, Shabala S, Muñiz-Murguía J, Pottosin II. 2011. Polyamines interact with hydroxyl radicals in activating Ca2+ and K+ transport across the root epidermal plasma membranes. Plant Physiology 157, 2167–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. 2001. Plant salt tolerance. Trends in Plant Science 6, 66–71. [DOI] [PubMed] [Google Scholar]
- Zhu JK. 2003. Regulation of ion homeostasis under salt stress. Current Opinion in Plant Biology 6, 441–445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.