Highlight
Manipulation of AtSLAH1 expression modifies shoot Cl– accumulation and salt tolerance in Arabidopsis, consistent with its proposed role in regulating Cl– transport from root to shoot.
Key words: ABA, Arabidopsis, AtSLAH1, AtSLAH3, chloride, Cl− xylem loading, long-distance transport, nutrition, salinity, slow-type anion channel-associated homologue 1, slow-type anion channel-associated homologue 3.
Abstract
Salinity tolerance is correlated with shoot chloride (Cl–) exclusion in multiple crops, but the molecular mechanisms of long-distance Cl– transport are poorly defined. Here, we characterize the in planta role of AtSLAH1 (a homologue of the slow type anion channel-associated 1 (SLAC1)). This protein, localized to the plasma membrane of root stelar cells, has its expression reduced by salt or ABA, which are key predictions for a protein involved with loading Cl– into the root xylem. Artificial microRNA knockdown mutants of AtSLAH1 had significantly reduced shoot Cl− accumulation when grown under low Cl–, whereas shoot Cl– increased and the shoot nitrate/chloride ratio decreased following AtSLAH1 constitutive or stelar-specific overexpression when grown in high Cl–. In both sets of overexpression lines a significant reduction in shoot biomass over the null segregants was observed under high Cl– supply, but not low Cl– supply. Further in planta data showed AtSLAH3 overexpression increased the shoot nitrate/chloride ratio, consistent with AtSLAH3 favouring nitrate transport. Heterologous expression of AtSLAH1 in Xenopus laevis oocytes led to no detectible transport, suggesting the need for post-translational modifications for AtSLAH1 to be active. Our in planta data are consistent with AtSLAH1 having a role in controlling root-to-shoot Cl– transport.
Introduction
Chloride (Cl–) is classified as a micronutrient, but it is often present in plant tissues at concentrations typical of a macronutrient (i.e. 2–20 rather than 0.1–200 μg g–1 DW) (Marschner, 1995; Xu et al., 2000; Broadley et al., 2012; Franco-Navarro et al., 2015). Cl– has vital roles in regulating numerous physiological processes including turgor, enzyme activity, photosynthesis and membrane potential (Rognes, 1980; White and Broadley, 2001; Teakle and Tyerman, 2010). Although the pathways for Cl– entry and movement within the plant have been characterized biochemically, their molecular determinants are poorly defined (Teakle and Tyerman, 2010; Henderson et al., 2014).
High concentrations of sodium chloride (NaCl) in soils reduces crop yield (Rengasamy, 2010, Roy et al., 2014), which can impose significant economic costs to farmers (Munns and Gilliham, 2015). Na+ transport and its impact on plant growth have been relatively well documented at both a physiological and a molecular level in a variety of plant species (Blumwald et al., 2000; Zhu, 2003; Horie and Schroeder, 2004; Davenport et al., 2005; Garthwaite et al., 2005; Chinnusamy et al., 2006; Apse and Blumwald, 2007; Horie et al., 2008; Müller et al., 2014; Roy et al., 2014; Maathuis et al., 2014; Flowers et al., 2015). However, in other economically important crop plants like soybean, grapevine, citrus and lotus, leaf Cl– accumulation (not Na+) is correlated with decreased plant growth and photosynthesis when plants are under salt stress (Storey and Walker, 1999; Walker et al., 2002; Tregeagle et al., 2006; Teakle et al., 2007; Teakle and Tyerman, 2010; Gong et al., 2011). So, although Cl– is a micronutrient it can also accumulate to concentrations that inhibit plant growth when plants encounter salinity. The cause of both Na+- and Cl–-induced reductions in photosynthesis and growth, and the cause of salt-induced cell death are yet to be definitively determined and are a priority area for research (Munns and Gilliham, 2015). Some studies have investigated the relative impact of Na+ and Cl– on barley and wheat (e.g. Tavakkoli et al., 2011; Genc et al., 2015). Growth and photosynthesis of several cultivars of barley appeared to be more sensitive to the addition of Cl– than of Na+ (Tavakkoli et al., 2011). These findings highlight the importance of investigating the regulation of both Na+ and Cl– transport for improving plant salt tolerance, even in species that are classically considered to be more Na+-sensitive than Cl–-sensitive under saline conditions.
Identification of genes that underpin root-to-shoot Cl– transport, and the related signalling pathways, should provide information that could be used to reduce Cl– sensitivity in commercial crops. A key pathway in controlling Cl– accumulation in the shoot is its loading from xylem parenchyma cells into the transpiration stream (Teakle and Tyerman, 2010). Recently, a nitrate (NO3 –) transporter 1/peptide transporter family member (NPF2.4) was identified as the first protein to be directly involved in loading Cl– into the root xylem (Li et al., 2016). However, Cl– accumulation in the shoot is predicted to be a multigenic trait in a number of plant groups and species including soybean, maize, grapevine, citrus and legumes (Abel, 1969; Storey and Walker, 1999; Moya et al., 2003; Sibole et al., 2003; Gilliham and Tester, 2005; Gong et al., 2011; Henderson et al., 2014; Fort et al., 2015). Knockouts of Atnpf2.4 had a 20% reduction in shoot Cl– (Li et al., 2016), providing further evidence of the multigenic nature of shoot Cl– accumulation. Therefore, other anion transport proteins are likely to be involved in root-to-shoot Cl– transport (e.g. Henderson et al., 2014), but these remain to be identified and functionally characterized at a molecular level.
Three anion conductances have been identified using electrophysiology in barley root xylem parenchyma protoplasts, namely an inwardly rectifying anion channel (X-IRAC), a quickly activating anion conductance (X-QUAC) and a slowly activating anion conductance (X-SLAC) (Köhler and Raschke, 2000). Similar results were found in maize root stelar cells (Gilliham and Tester, 2005) and Arabidopsis root pericycle cells (Kiegle et al., 2000). X-QUAC is the most prevalent conductance observed in xylem parenchyma cells and is likely to load the majority of Cl– (and NO3 –) ions into the xylem under non-saline conditions (Köhler et al., 2002; Gilliham and Tester, 2005) as the estimated flux through this conductance could easily account for the Cl– release from the xylem vessels measured using a 36Cl– tracer (Pitman, 1982; Köhler and Raschke, 2000).
The hormone abscisic acid (ABA) regulates solute transport from root to shoot (Cram and Pitman, 1972). Excised barley roots treated with ABA for 2h accumulated significantly more Cl– than untreated roots (Cram and Pitman, 1972). Cl– efflux to the xylem was also reduced following the ABA treatment, but Cl– influx into the root was unaffected (Cram and Pitman, 1972). These results indicate that ABA down-regulates xylem loading of Cl– in roots but not root Cl– influx. Furthermore, the anion conductances in maize, barley and Arabidopsis stele, as well as the potassium conductance through the stelar K+ outwardly rectifying channel (SKOR), are also down-regulated by ABA (Cram and Pitman, 1972; Gilliham and Tester, 2005). In Arabidopsis, AtSKOR was transcriptionally down-regulated by ABA (Gaymard et al., 1998). Therefore, it may be possible to identify candidate genes for Cl– loading into the root xylem by characterizing those genes encoding putative anion transporters that are expressed in the stele and are down-regulated (either transcriptionally or post-translationally) by ABA.
Early electrophysiological studies on stomatal guard cells revealed the slowly activated anion conductance (SLAC) (Linder and Raschke, 1992). More recently, the gene encoding the protein responsible for this conductance, SLAC1, was identified (Negi et al., 2008; Vahisalu et al., 2008). SLAC1 is a plasma membrane (PM) localized protein, highly permeable to malate and chloride (Negi et al., 2008; Chen et al., 2010), and slac1 mutants have increased Cl– in guard cells (Negi et al., 2008; Vahisalu et al., 2008). Four homologues of SLAC1, the slow-type anion channel-associated homologues 1 to 4 (SLAH1 to 4), have been identified that also localize to the PM and are predicted to be involved in anion transport (Negi et al., 2008; Vahisalu et al., 2008). Expression of SLAH1 or SLAH3 in slac1 knockout mutants could complement both the defective closure response of slac1 stomata to high CO2 and its ionic profile when constitutively overexpressed (Negi et al., 2008). SLAH3 is expressed in guard cells and roots, and is preferentially selective for NO3 – over Cl–; it has a role in NO3 – alleviation of ammonium toxicity (Geiger et al., 2009, 2011; Demir et al., 2013; Zheng et al., 2015). SLAH2 is also expressed in roots (Maierhofer et al., 2014), and the protein is predominantly permeable to NO3 – (with a NO3 –/Cl– permeability ratio of 82) (Maierhofer et al., 2014). Both SLAH2 and SLAH3 have been predicted to have roles in loading NO3 – into the root xylem (Maierhofer et al., 2014; Zheng et al., 2015). SLAH1 is also expressed in the root; however, the role of AtSLAH1 is currently unknown. As SLAH1 is expressed in the root stele and can complement the stomatal phenotype of the Atslac1 mutant when ectopically expressed (Negi et al., 2008; Vahisalu et al., 2008; Zheng et al., 2015), we examined whether AtSLAH1 has a role in loading Cl– into the root xylem.
Materials and methods
Plant materials and growth conditions
All chemicals were obtained from Sigma-Aldrich unless stated. Arabidopsis thaliana ecotype (Col-0) seeds were purchased from the European Arabidopsis Stock Centre (Nottingham, UK). Plants were grown within temperature controlled growth rooms. Arabidopsis plants grown in soil were kept in long day conditions (16h day/8h night), while those in hydroponics were kept in short day conditions (10h day/14h night). In both long day and short day conditions, the temperature was maintained at 21–23 °C, the humidity was maintained between 60–75%, and the irradiance during the light period was 150 μmol m–2 s–1. Plants were grown in hydroponics following protocols described in Conn et al. (2013) and in soil following methods described in Møller et al. (2009).
Generation of AtSLAH1 artificial microRNA lines
The AtSLAH1 T-DNA knockout mutant (FLAG_329G06) was ordered through the Versailles Arabidopsis Stock Centre; however, the expression of AtSLAH1 (At1G62280) was detectable in all homozygous mutant lines (Supplementary Fig. S1 at JXB online). To elucidate the function of AtSLAH1 in planta, artificial microRNAs (amiRNAs) were designed to knockdown AtSLAH1 expression. To produce AtSLAH1 knockdown mutants, specific amiRNAs were designed against the AtSLAH1 mRNA sequence using Micro RNA Designer (http://wmd3.weigelworld.org/cgi-bin/webapp.cgi) following the protocol of Schwab et al. (2006). Two 21bp target sequences (TAAAACGCTATTTGGTTCCGT and TTATGTCTAGTGTCGAGACTG) were identified from the AtSLAH1 coding sequence and two independent amiRNA constructs were generated with a set of primers (Supplementary Table S1) to incorporate the 21bp amiRNA sequence into the MIR319a vector (Schwab et al., 2006). Both full-length SLAH1-amiRNA products were cloned using high-fidelity Phusion® polymerase (New England Biolabs, USA) into a Gateway® enabled pCR8 entry vector (Invitrogen, CA, USA) and transferred into the pMDC32 expression vector (Curtis and Grossniklaus, 2003) through an LR reaction (Invitrogen). The constructs were transformed into Arabidopsis using Agrobacterium-mediated floral dip transformation (Clough and Bent, 1998). Hygromycin B (20 μg ml–1) was used to select the transformants following the protocol described in Harrison et al. (2006).
Generation of cell type-specific overexpression lines
An Arabidopsis enhancer trap line (E2568; Møller et al., 2009) was used to generate plants with AtSLAH1 root stelar-cell-specific overexpression. AtSLAH1 full length cDNA was cloned using high-fidelity Phusion® polymerase (New England Biolabs, USA) from Arabidopsis root cDNA (following RNA extraction and cDNA synthesis following Henderson et al. (2015)) into a Gateway-enabled pCR8 entry vector (Life Technologies, CA, USA). AtSLAH1 was then transferred into a pTOOL5 destination vector (pMDC132+UAS+NOS) (Plett, 2008) containing the GAL4-inducible promoter UAS, which drives target gene expression specifically in root stelar cells in line E2568. The construct was transformed into Arabidopsis line E2586 using Agrobacterium-mediated transformation (Clough and Bent, 1998). The seeds from transformed plants were harvested and germinated in soil. When the seedling had two to four true leaves, 20mg l–1 BASTA (Bayer, Germany) was sprayed on the seedlings to select for plants with the transgenic insertion.
Generation of constitutive overexpression lines
Full-length AtSLAH1 and AtSLAH3 coding sequences were amplified (the primers used are listed in Supplementary Table S1) from Arabidopsis root cDNA, using Phusion® polymerase, cloned into the Gateway-enabled pCR8 entry vector (Life Technologies) and transferred into the pMDC32 expression vector (Curtis and Grossniklaus, 2003) through an LR reaction (Life Technologies). The construct was transformed into Arabidopsis (Col-0) using Agrobacterium-mediated transformation (Clough and Bent, 1998). Hygromycin B (20 μg ml–1) was used to select lines containing the transgene insertion following the protocol described in Harrison et al. (2006).
Salinity and ABA treatment
Both salinity and ABA treatment were performed in hydroponics (Conn et al., 2013). For the 7-day salinity treatment, NaCl was added to basal nutrient solution (BNS) (Conn et al., 2013) to make a final concentration of 50, 75, and 100mM. Additional CaCl2 was added to each solution to achieve a constant Ca2+ activity of 1.3mM following the addition of high concentrations of monovalent cations, which act to reduce the activity of other cations and induce calcium deficiency (as detailed in Conn et al. (2013)). For ABA treatment, a stock solution of 100mM (±)-cis-trans-abscisic acid was made in absolute ethanol. When applying 20 µM ABA, this resulted in a final ethanol concentration of 0.01% (v/v) when added into the growth solution.
Expression analysis
Gene expression analysis by qRT-PCR was performed following the method described in Burton et al. (2008). The primers for examining AtSLAH1 expression were 5′TCTTCATGTCCCTGGTCTG3′ (forward) and 5′ATTGCTGTTTGCTGCTGTC3′ (reverse) and for AtSLAH3 were 5′ATCTCTCGGTCGTTGGGAACTTTG3′ (forward) and 5′CTCGTTGGTCGGTAGCCTTTGG3′ (reverse). The selected Arabidopsis housekeeping genes (AtGAPDH (At3G26650), AtActin2 (At3G18780), AtTubulin (At1G50010) and AtCyclophilin (At2G36130)) and data normalization followed the methods described in Jha et al. (2010). For relative gene expression, AtActin2 was used as a control gene, and the relative expression level of target genes was detected using the same primer pairs as listed above using a QuantStudio 12K Flex Real-Time PCR system (Life Technologies).
Phenotyping transgenic plants
For determining the shoot NO3 − concentration, a method that uses salicylic acid to form a chromophore with NO3 − under alkaline conditions (pH>12) was used (Cataldo et al., 1975). In brief, 3–5mg of Arabidopsis dried tissue was extracted in 0.5ml deionized water, with 0.05ml of the extraction incubated with 0.2ml of 5% (w/v) salicylic acid–H2SO4 for 20min at room temperature; 0.05ml of this mixture was transferred into a fresh tube and 0.95ml of 2M NaOH was added. A 0.2ml aliquot was transferred to a well of a transparent 96-well plate and the absorbance at OD410nm determined. A serial dilution of known concentrations of potassium nitrate (KNO3) was used for the standard, which ranged from 0 to 50mM.
For determining the shoot Cl– concentration, 20–30mg of freeze-dried Arabidopsis tissue was extracted in 500 μl 1% nitric acid at 80 °C overnight. A chloride analyser (Model 926S, Sherwood Scientific, Cambridge, UK) was used to examine the Cl– concentration by following the manufacturer’s instructions.
Expression and electrophysiological characterization of AtSLAH1 in X. laevis oocytes
The AtSLAH1, AtSnRK2.2, and AtSnRk2.3 coding sequences (primers are listed in Supplementary Table S1) were cloned using high-fidelity Phusion® polymerase (New England Biolabs, USA) from Arabidopsis root cDNA into a Gateway-enabled pCR8 entry vector (Life Technologies) before being transferred into the pGEM-HE (DEST) vector, and cRNA was synthesized using the mMESSAGE mMACHINE® kit (Ambion, Australia) as previously described (Preuss et al, 2011). Healthy stage IV–VI defoliculated oocytes were obtained through surgery and enzymatic digestion of ovaries from toads kept in an Xenopus colony at the Waite Campus (University of Adelaide). The cRNA (46 nl/23ng per oocyte) was injected using a micro injector (Drummond Nanoject II injector, USA) with a glass microcapillary pipette following the manufacturer’s procedures. The same volume of nuclease-free water was injected into control oocytes. Injected oocytes were incubated at 18 °C for 2 days in an ND96 solution (96mM NaCl, 2mM KCl, 1mM MgCl2, 5mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1.8mM CaCl2, pH 7.4 with 1M Tris) combined with horse serum (50 ml l–1), tetracycline (50 μg ml–1) and penicillin (50 μg ml–1). After 2 days, AtSLAH1 cRNA-injected oocytes were voltage clamped from +40 to –120 mV in 20 mV decrements for 3s perfusing in the following bath solutions (basal: 2mM calcium gluconate, 5mM HEPES and 0.1mM LaCl3) plus 1 or 20mM CsNO3/CsCl at pH 7.5. Two-electrode voltage clamping (TEVC) was performed on oocytes as previously described in Roy et al. (2008) using an OC-725C amplifier (Warner Instruments Corp., USA), signals were digitized with a Digidata 1440A (Molecular Devices, USA), and then the data were recorded and analysed using pCLAMP 10.2 (Molecular Devices, USA).
Results
AtSLAH1 expression is down-regulated by both salt and ABA
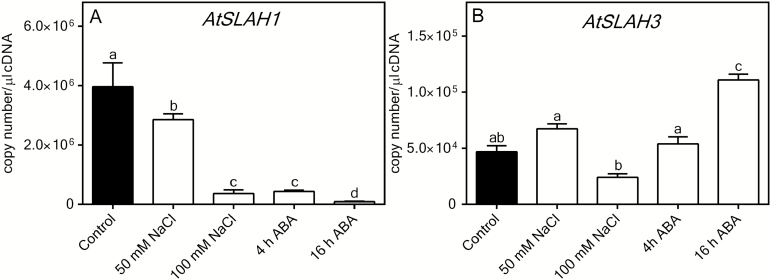
A qRT-PCR was performed on Arabidopsis root cDNA to determine whether AtSLAH1 transcript abundance altered following salt or ABA treatment. The expression level of AtSLAH1 was significantly reduced by 91% after 7 days of 100mM NaCl treatment, and by 97% after 16h of 20 µM ABA treatment when compared with the control (Fig. 1A). In contrast, the close homologue AtSLAH3, which shares the same cell location in xylem parenchyma and pericycle cells of the root stele and is also PM localized (Negi et al., 2008), was not down-regulated by ABA or salt treatment (Fig. 1B). All these data are consistent with previous observations (Kilian et al., 2007; Brady et al., 2007; Gifford et al., 2008; Supplementary Fig. S2).
Fig. 1.
Expression level of AtSLAH1 (A) and AtSLAH3 (B) treated with control (2mM NaCl), 50mM and 100mM NaCl for 7 days, or 20 μM ± cis-trans-ABA for 4 or 16h. Arabidopsis (Col-0) were grown in hydroponics for 5 weeks and exposed to NaCl treatment for 7 days. The ABA was applied 4 or 16h before harvest. Transcripts were detected in the whole root cDNA. Results are presented as means+SEM, n=5. The expression levels were normalized to four control genes (AtGAPDH, AtActin2, AtTubulin and AtCyclophilin). Statistical significance was determined by one-way analysis of variance (ANOVA) and Tukey’s test (P≤0.05); a, b and c represent data groups that are statistically different from each other.
AtSLAH1 amiRNA knockdown lines have low Cl– accumulation in the shoot under low Cl– supply
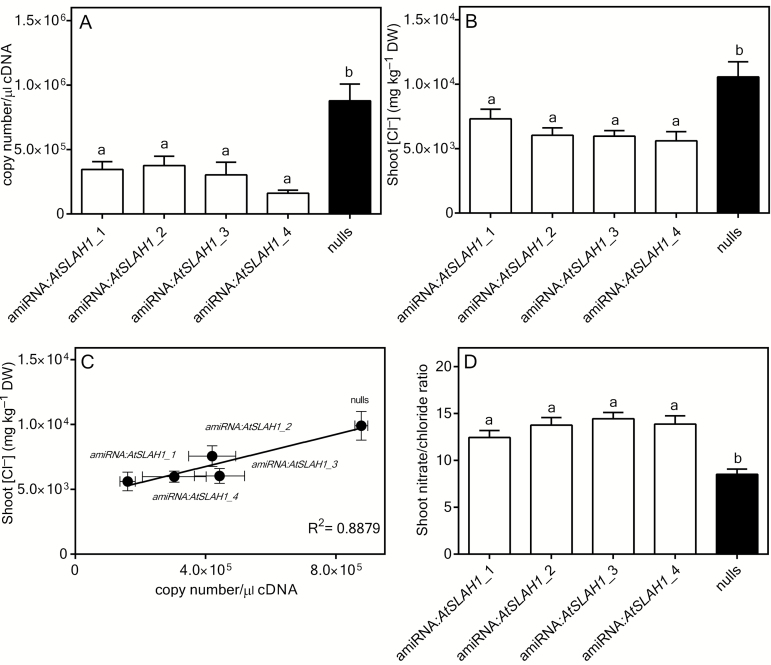
To investigate whether AtSLAH1 was involved in root-to-shoot anion transport, different Arabidopsis lines with an increase or decrease in AtSLAH1 expression were generated. Atslah1 knockout lines (FLAG_329G06) were ordered from the Versailles Arabidopsis Stock Centre. Homozygous lines were successfully identified (Supplementary Fig. S1); however, RT-PCR performed using AtSLAH1-specific primers (Supplementary Table S1) found that the expression of AtSLAH1 was not abolished in these mutant lines (Supplementary Fig. S1C). Therefore, four independent amiRNA:AtSLAH1 mutant lines were generated, which were named amiRNA:AtSLAH1_1 (two inserts), amiRNA:AtSLAH1_2 (two inserts), amiRNA:AtSLAH1_3 (three inserts) and amiRNA:AtSLAH1_4 (two inserts). Under low salt conditions (2mM NaCl), qRT-PCR showed that the transcript abundance of AtSLAH1 in the root of all independent amiRNA lines was less than half of that found in the null segregants (P≤0.005) (Fig. 2A). In all amiRNA:AtSLAH1 lines, the shoot Cl– concentration was significantly lower than that of the null segregants under low salt conditions, being reduced by 30–47% (P≤0.005) (Fig. 2B). The expression level of AtSLAH1 was plotted against shoot Cl– concentration for each plant and a highly significant positive relationship was observed with an R 2 of 0.89. The shoot NO3 − concentration under low Cl− supply was also determined and no difference was found between the mutants and the null segregants (Supplementary Fig. S3A), but the reduction in shoot Cl– led to a significantly greater shoot NO3 –/Cl– ratio in all mutants when compared with the null segregants (Fig. 2D). No differences were found in shoot biomass in any of the amiRNA:AtSLAH1 lines under low Cl– conditions (Supplementary Fig. S3B). The shoot Na+ and K+ concentrations were determined in these plants and no differences were found between all mutants and null segregants (Supplementary Fig. S3C, D). The experiment was repeated using the same set of seeds, under the same treatments, and found to have similar results where shoot Cl– accumulation was decreased in amiRNA:SLAH1 lines (Supplementary Fig. S3E). Under high Cl– supply, amiRNA lines had significantly reduced AtSLAH1 transcript abundance compared with the null segregants in the same conditions (Supplementary Fig. S4A); however, the AtSLAH1 expression in the null segregants was reduced by a quarter compared with low Cl– conditions (Fig. 1A and Supplementary Fig. S4A). At the same time there was no difference in shoot Cl– concentration between the knockdown plants and controls under high Cl– supply (Supplementary Fig. S4B). This was presumably due to the native downregulation of AtSLAH1 expression by high salt (Figs 1A and 2A and Supplementary Fig. S4A).
Fig. 2.
Under low Cl– conditions, amiRNA:AtSLAH1 mutants had significantly reduced expression levels of AtSLAH1 and reduced shoot Cl– compared with null segregants. Plants were grown hydroponically for 6 weeks in BNS containing 2mM NaCl (low Cl– conditions). (A) AtSLAH1 expression in roots of all amiRNA-AtSLAH1 mutants (amiRNA:AtSLAH1_1, 2, 3 and 4) and null segregants (nulls). (B) Shoot Cl– accumulation of amiRNA-AtSLAH1 mutants and nulls under low Cl– conditions. (C) Correlation between transcript level of AtSLAH1 and shoot Cl– concentration. (D) The shoot NO3 –/Cl– ratio in all amiRNA:AtSLAH1 mutant and null segregant lines grown under low Cl– conditions. Results are mean+SEM (n>8), except (C), which is ±SEM. Statistical differences determined by one-way ANOVA and Tukey’s test (P≤0.005); a and b represent statistically significant differences between data groups.
Plants constitutively overexpressing AtSLAH1 accumulate high Cl– in the shoot under high Cl– supply
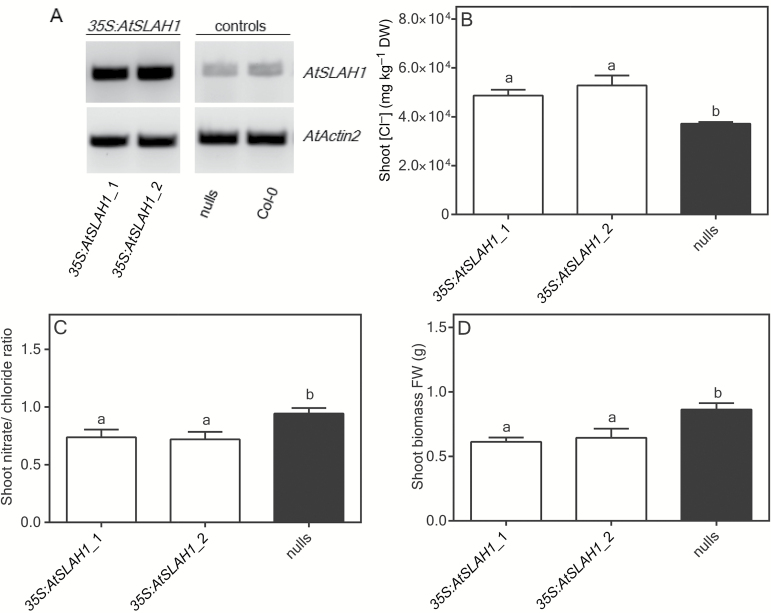
Plants with constitutive overexpression of AtSLAH1 and their null segregants were selected by determining the presence or absence of the AtSLAH1 transgene by PCR of genomic DNA. Relative expression of total AtSLAH1 (consisting of both the native and the transgenic AtSLAH1) was then determined in root tissue by semi-quantitative RT-PCR. AtSLAH1 was found to be highly expressed in both 35S:AtSLAH1 lines generated, whereas the null segregants had less abundant expression (Fig. 3A). When 75mM NaCl was applied to 35S:AtSLAH1_1, 35S:AtSLAH1_2 and null segregant lines for 7 days, significantly higher shoot Cl– concentration accumulated in the overexpression lines when compared with the null segregants (P≤0.05), with no difference found between the two independent overexpression lines in shoot Cl– concentration (Fig. 3B). The shoot NO3 – in both overexpression lines displayed no differences compared with null segregants (Supplementary Fig. S5A). Therefore, the increase in shoot Cl– accumulation resulted in a decrease in shoot NO3 –/Cl– ratio in both overexpression lines under high Cl– conditions (P≤0.05) (Fig. 3C). Both 35S:AtSLAH1 overexpression lines had significantly less whole shoot biomass when compared with the null segregants (P≤0.005) (Fig. 3D).
Fig. 3.
Under high Cl– conditions, 35S:AtSLAH1 overexpression lines accumulated higher shoot Cl– and showed reduced NO3 –/Cl– ratio compared with null segregants (nulls). Plants were grown hydroponically in BNS until 6 weeks old and then exposed to BNS containing 75mM NaCl (high Cl– conditions) for 7 days. (A) Semi-quantitative RT-PCR of 35S:AtSLAH1 overexpression lines and nulls. (B) Shoot Cl– concentration under high Cl– conditions. (C) Shoot NO3 –/Cl– ratio. (D) Whole shoot biomass (fresh weight) as measured after high Cl– treatment. Results are mean+SEM (n>6). Statistical differences determined by one-way ANOVA and Tukey’s test (P≤0.05); a and b represent statistically significant differences between data groups.
Under low Cl– supply, the shoot Cl– and NO3 – concentration of both 35S:AtSLAH1 overexpression lines was not significantly different from each other or the null segregants (Supplementary Fig. S5B, C), and the shoot biomass between all the genotypes was not significantly different (Supplementary Fig. S5D).
Stelar-specific overexpression of AtSLAH1 is correlated with increased shoot Cl– accumulation under high Cl– supply
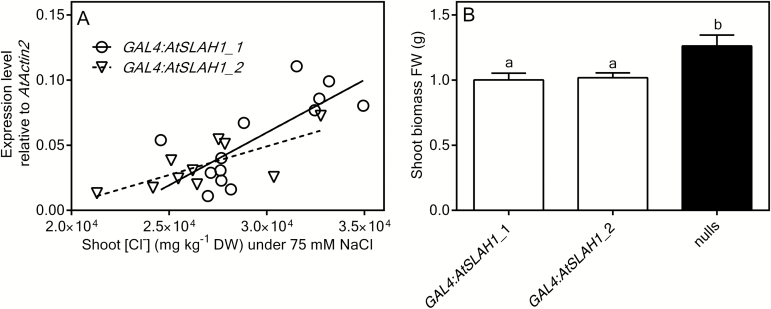
To further study the function of AtSLAH1 and avoid potential problems caused by non-targeted over expression in all cell types, root stelar cell-type specific over expression lines were generated following the method outlined by Møller et al. 2009. Two independent lines, named GAL4:AtSLAH1_1 and GAL4:AtSLAH1_2 were grown in hydroponics for 6 weeks before being supplied with 2 or 75mM NaCl for a further 7 days. Under high Cl– conditions, both cell-specific overexpression lines had greater accumulation of Cl– within the shoot; AtSLAH1 expression and shoot Cl– accumulation were again positively correlated (R 2=0.5, P≤0.01) (Fig. 4A). As with the constitutively overexpressing AtSLAH1 plants, high salt treatment led to greater shoot Cl– accumulation and no alteration in shoot concentration of Na+, K+ or NO3 – in the cell-specific overexpression lines compared to the null segregant lines (Supplementary Fig. S6A, B, C). Furthermore, the shoot biomass of the cell-specific overexpression lines was reduced under high salt treatment compared with the null segregants (Fig. 4B).
Fig. 4.
Stelar cell type-specific overexpression of AtSLAH1 in E2586 significantly increased shoot Cl– accumulation and reduced shoot biomass under high Cl– conditions. (A) Correlation between shoot Cl– accumulation and relative expression of AtSLAH1 in GAL4:AtSLAH1 overexpression lines under high Cl– (75mM NaCl) supply. Open circles and solid line: GAL4:AtSLAH1_1; open triangle and dash line: GAL4:AtSLAH1_2. Plants were grown hydroponically in BNS for 6 weeks and then exposed to BNS containing 75mM NaCl (high Cl– conditions) for 7 days. Correlation between shoot Cl– concentration and the abundance of AtSLAH1 in GAL4:AtSLAH1_1 (R 2=0.5805, P ≤ 0.005, significant deviation from zero), GAL4:AtSLAH1_2 (R 2=0.5395, P ≤ 0.005, significant deviation from zero). (B) Whole shoot biomass (fresh weight) as measured after high Cl– treatment. Results are mean+SEM (n>6). Statistical differences determined by one-way ANOVA and Tukey’s test (P≤0.05); a and b represent statistically significant differences between data groups.
Under low Cl– conditions, no significant differences in shoot Cl– or NO3 – accumulation were identified between stelar-specific AtSLAH1 overexpression lines and the null segregants (Supplementary Fig. S7A, B), but significantly less shoot Cl– was accumulated in these plants in low Cl– than when in high Cl– treatment (Fig. 4A). Under low Cl– conditions, the shoot biomass of both stelar-specific AtSLAH1 overexpression lines was not significantly different from the null segregants (Supplementary Fig. S7C).
AtSLAH3 overexpression increases the shoot NO3 –/Cl– ratio under high and low Cl–
To compare the effects of AtSLAH1 misexpression with that of a close homologue known to have a preference for NO3 – transport, we examined the phenotype of plants constitutively overexpressing AtSLAH3. In contrast to the greater Cl– accumulation we observed in shoots of AtSLAH1-overexpressing plants, we observed a lower accumulation of shoot Cl– under both high and low Cl– supply in AtSLAH3-overexpressing plants (Supplementary Fig. S8A, B). The mean value for NO3 – concentration of the shoot was higher in the overexpression lines (Supplementary Fig. S8C, D), but not significantly compared with the null segregants under any condition tested, but coupled to the Cl– data led to a significantly greater NO3 –/Cl– ratio in all conditions tested (Supplementary Fig. 8E, F).
AtSLAH1 is likely to require additional co-factors to be active in X. laevis oocytes
AtSLAH1 was expressed in X. laevis oocytes in an attempt to examine whether it could directly catalyse the transport Cl– (Supplementary Materials and methods). No functional activity could be detected when AtSLAH1 was expressed by itself (Supplementary Fig. S9). The AtSLAH1 homologue, AtSLAC1, was also found to be electrically silent in oocytes when expressed by itself, but when expressed with sucrose non-fermenting-1-related protein kinase 2.6 (SnRK2.6) AtSLAC1 carried currents (Geiger et al., 2009). To investigate whether a similar regulatory process was also required to trigger anion transport by AtSLAH1 in heterologous systems, AtSnRk2.2 and AtSnRk2.3 (root localized homologues of AtSnRk2.6 that have their expression regulated by ABA; Yoshida et al., 2006; Fujii and Zhu, 2009; Nakashima et al., 2009) were co-injected with AtSLAH1 in X. laevis oocytes (Supplementary Fig. S9F–H) but this resulted in no consistent activation of current, suggesting additional cofactors that regulate SLAH1 function still need to be identified.
Discussion
AtSLAH1 meets the predicted characteristics for a gene controlling Cl– loading into the root xylem
Previous studies showed that AtSLAH1 belongs to the AtSLAC1 family; SLAC1 and SLAH3 underpin components of the slow type (S-type) anion conductance involved in anion efflux across the PM of stomatal guard cells in response to CO2 and O3 (Negi et al., 2008; Vahisalu et al., 2008; Geiger et al., 2011; Demir et al., 2013). The guard cell PM S-type anion conductance was found to be permeable to malate, Cl– and NO3 – and its activation triggered by ABA (Schroeder and Hagiwara, 1989; Hedrich, 2012). AtSLAH3 and another family member, AtSLAH2, have been predicted to load NO3 – into the root stele (Maierhofer et al., 2014; Zheng et al., 2015). Whilst AtSLAH1 is not usually expressed in guard cells it could complement the wild-type guard cell function of the slac1 knockout when ectopically expressed, indicating it may encode or regulate a functional channel (Negi et al., 2008; Vahisalu et al., 2008); however, its true physiological functions are yet to be deciphered. Here, we confirmed that AtSLAH1 was highly expressed in the Arabidopsis root and its expression was down-regulated strongly by ABA and NaCl treatment (Fig. 1). Previously AtSLAH1 was shown to be expressed in the root stele and pericycle of Arabidopsis roots and present on the PM (Brady et al., 2007; Gifford et al., 2008; Negi et al., 2008; Supplementary Fig. S2). As the stelar-localized PM conductances capable of loading anions into the root xylem (and consequently the shoot) have been observed to be down-regulated by ABA (Gilliham and Tester, 2005) this suggests that AtSLAH1 could be involved in significant Cl– loading of the root xylem. In contrast, we found that AtSLAH3 transcript abundance was not reduced by salt or down-regulated by ABA (Fig. 1B). This coupled to the higher NO3 –/Cl– ratio of AtSLAH3-overexpressing plants (Supplementary Fig. S8E and F) suggests it does not, by itself, contribute to a significant proportion of Cl– accumulation in the shoot.
AtSLAH1 regulates Arabidopsis shoot Cl– accumulation
As the ‘Atslah1’ T-DNA insertion mutant from the European Arabidopsis Stock Centre retained expression of SLAH1, we generated amiRNA lines that had reduced expression of AtSLAH1 (Fig. 2A). Under low Cl– supply (2mM NaCl), all AtSLAH1 amiRNA lines had lower accumulation of Cl– but not of NO3 – in the shoot (Fig. 2A, B and Supplementary Fig. S3A). There was also a strong positive correlation between AtSLAH1 expression levels and shoot Cl– (Fig. 2C), which suggests that AtSLAH1 might play an important role in regulating Cl– transport from root to shoot by affecting net loading of xylem vessels in the root. Whilst the shoot NO3 – concentration did not significantly alter when compared with the null segregants (Supplementary Fig. S3A), reduced AtSLAH1 expression did lead to an increased shoot NO3 –/Cl– ratio due to a lower amount of Cl– in the shoot (Fig. 2D).
Shoot Cl– concentration was also examined in all amiRNA:AtSLAH1 mutants exposed to high salt stress. No shoot Cl– concentration differences were found between mutants and the null segregants under these conditions (Supplementary Fig. S4B). AtSLAH1 expression is naturally decreased under high concentrations of NaCl (Fig. 1A). Therefore it is reasonable to suggest that the unchanged shoot Cl– concentration in these plants was probably due to the endogenous down-regulation of AtSLAH1 caused by high salinity. Therefore, the results of AtSLAH1 overexpression lines might be expected to be more instructive for determining AtSLAH1 function under high salt conditions.
In 35S:AtSLAH1 overexpression lines we observed significantly increased shoot Cl– accumulation compared with null segregants when grown under high Cl– (75mM NaCl) (Fig. 3B); this is again consistent with AtSLAH1 being involved in xylem Cl– loading. No difference in shoot NO3 –, K+ or Na+ accumulation was observed between 35S:AtSLAH1 overexpression lines and null segregants under high Cl– supply (Supplementary Fig. S5A, E, F) indicating that the role of AtSLAH1 is specific for Cl–. This translated into a reduced NO3 –/Cl– ratio in these lines compared with nulls (Fig. 3C). In overexpression lines, there was a concomitant decrease in shoot biomass compared with null segregant lines (Fig. 3C), suggesting that the level of Cl– accumulated, and the reduction in NO3 –/Cl– ratio over this time period is suboptimal for growth. In many studies, the shoot K+/Na+ ratio is widely used to evaluate the plant’s salt tolerance: a higher K+/Na+ ratio value normally indicates a better salinity tolerance (Tester and Davenport, 2003). Due to the antagonism between Cl– and NO3 – transport and the key roles of NO3 – in plant metabolism, it is reasonable to suggest that mechanisms that maintain high NO3 –/Cl– ratios might also be beneficial for improving salt tolerance.
These effects on shoot Cl– accumulation, shoot NO3 –/Cl– ratio and shoot biomass were replicated in AtSLAH1 root stelar cell-specific overexpression lines in high Cl– conditions (in the cell types in which AtSLAH1 is ordinarily expressed (Brady et al., 2007; Gifford et al., 2008; Negi et al., 2008) (Fig. 4). This indicates that constitutive overexpression of AtSLAH1 did not result in significant pleiotropic responses, which may be to do with the need for an unknown interacting partner in its native cell type for AtSLAH1 to be functional. What is important to note is that in AtSLAH1 constitutively overexpressing plants and in the root stelar specific AtSLAH1 overexpression lines in low Cl– growth conditions, there was no growth phenotype compared with the null segregants (Figs 3 and 4). This linked to the fact that other ion contents (K+, Na+ or NO3 –) were not altered in any conditions (Supplementary Figs S5 and S6), demonstrates that the growth of AtSLAH1-overexpressing plants was not altered by overexpression of the AtSLAH1 protein per se. Rather the inhibition of growth seen for AtSLAH1-overexpressing plants in high Cl– was specifically due to the additional accumulation of Cl– in the shoot (Figs 3 and 4).
Chloride accumulation in the shoot is a multigenic trait (White, 2001). We have shown that AtSLAH1 is likely to make up a component of this, as it has a significant effect on shoot Cl– accumulation (~20–40% in various conditions), which suggests that other Arabidopsis anion transport proteins might be involved in root to shoot Cl– transport. Recently, NFP2.4, a transport protein localized to the PM of stelar cells, was found to be important for Cl– but not for NO3 – accumulation in shoots (Li et al., 2016). AtCCC has also been shown to have an impact on shoot Cl– accumulation (Colmenero-Flores et al., 2007; Henderson et al., 2015). However, AtCCC is predominantly localized to the Golgi and trans-Golgi network and so is unlikely to have a direct role in net loading of Cl– into the xylem (Henderson et al., 2015). Other candidates include transporters designated as NO3 – permeable, but which may also transport some Cl–, such as NRT1.5/NPF7.3, NRT1.8/NPF7.2 and SLAH3 (Lin et al., 2008; Li et al., 2010). A transcriptional comparison between the roots of good and poor Cl–-excluding grapevine rootstocks suggested further candidate genes for this multigenic trait including aluminium-acitivated malate transporters (ALMT), chloride channels (CLC) and their putative activating kinases (Henderson et al., 2014). However, the true involvement of these candidate proteins requires that their substrates are resolved by functional assays. Therefore, the observed phenotypes in any single gene mutant are likely to be complicated by other functional proteins involved in Cl– accumulation in the shoot.
AtSLAH1 is likely to require unknown interacting proteins to function
No anion-mediated currents were identified when AtSLAH1 cRNA was injected into X. laevis oocytes (Supplementary Fig. S9C, D). AtSLAC1, AtSLAH2 and AtSLAH3 are known to require protein kinases to be functional in oocytes (Geiger et al., 2009; Vahisalu et al., 2010; Brandt et al., 2012; Demir et al., 2013; Gutermuth et al., 2013; Maierhofer et al., 2014). For instance, when SLAC1 was expressed in X. laevis oocytes no clear anion currents were generated (Vahisalu et al., 2008); however, co-expression with the protein kinase SnRk2.6 in oocytes was found to activate SLAC1 by phosphorylation of multiple serines in the SLAC1 hydrophilic N-terminal sequence (Geiger et al., 2009; Lee et al., 2009; Vahisalu et al., 2010). This evidence suggests that a similar regulatory component is important for activating the S-type anion channels and may be required for SLAH1 activity. As SnRK2.6 has low expression in roots we concentrated on the root expressed members of the ABA-activated SNF1-related protein kinases 2, SnRK2.2 and SnRK2.3, which are both involved in ABA signalling pathways in roots (Yoshida et al., 2006; Fujii and Zhu, 2009; Nakashima et al., 2009). To identify whether a phosphorylation process initiated by a protein kinase was required to activate SLAH1 in oocytes, SnRK2.2/2.3 was co-injected with AtSLAH1 into oocytes (Supplementary Fig. S9G–J). However, no activity was observed. Therefore, it is likely that AtSLAH1 requires additional factors for it to be active, or that it is itself a regulator of transport through its interaction with another transport protein.
Conclusions
Manipulating AtSLAH1 expression level in Arabidopsis resulted in significant alterations in shoot Cl– concentrations suggesting AtSLAH1 is involved in Cl– xylem loading in roots and the regulation of Cl– accumulation in the shoot in response to salt stress. In contrast, overexpression of AtSLAH3 resulted in relatively greater NO3 – content of the shoot under low and high Cl– treatments. In the present work, heterologous expression studies were unable to distinguish whether AtSLAH1 acts directly as a transport protein or transport regulator. As AtSLAH1 expression decreases under salinity and ABA, but AtSLAH3 is still expressed, the relative capacity for roots to deliver NO3 – to the shoot is increased under saline conditions but the capacity for Cl– loading is reduced. This will serve to maximize important NO3 – delivery to the shoot despite the increased competition from Cl– during salinity stress. Therefore, it appears likely that AtSLAH1 and AtSLAH3 act in tandem to regulate NO3 – and Cl– loading to the shoot and are both targets for manipulation in crops to improve salinity tolerance.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. AtSLAH1 is still expressed in the slah1 homozygous T-DNA insertion line FLAG_336C06.
Figure S2. The transcript level changes of AtSLAH1 and AtSLAH3 upon NaCl or ABA treatment.
Figure S3. Under low Cl– conditions, the shoot NO3 –, Na+, K+ concentrations and biomass were detected in all amiRNA:AtSLAH1 mutants and null segregants (nulls).
Figure S4. Transcript abundance of AtSLAH1 amiRNA containing lines (T2) and shoot Cl– concentration under high Cl– stress.
Figure S5. The shoot NO3 –, Cl– concentrations and shoot biomass were detected under low and high Cl– supply in both 35S:AtSLAH1_1 and 35S:AtSLAH1_2, and null segregants (nulls).
Figure S6. Under high Cl– conditions, the shoot Na+, K+ and NO3 – concentrations were detected in all GAL4:AtSLAH1 overexpression lines and null segregants (nulls).
Figure S7. Under low Cl– conditions, shoot Cl–, NO3 – concentrations and whole shoot biomass were detected in all GAL4:AtSLAH1 overexpression lines and null segregant.
Figure S8. The shoot NO3 – and Cl– concentrations were detected under low and high Cl– supply in both 35S:AtSLAH3_1 and 35S:AtSLAH3_2, and null segregant (nulls) lines.
Figure S9. Electrophysiological characterization of AtSLAH1 in X. laevis oocytes.
Table S1. Primers used for generating amiRNA:AtSLAH1 constructs, for screening homozygous Atslah1 T-DNA mutant lines and for cloning AtSLAH1/AtSLAH3 from Arabidopsis.
Acknowledgements
The authors thank Dr Darren Plett for donating the destination vector (pTOOL5) in this study; Yuan Li and Hui Zhou for performing the qRT-PCRs (Australian Centre for Plant Function Genomics, Adelaide, Australia). The work was supported by: the Grains Research and Development Corporation (UA000145 to S.J.R., M.G.); the Australian Research Council (ARC) through Centre of Excellence (CE14010008) and Future Fellowship (FT130100709) funding to M.G.; and, the University of Adelaide Graduate Research Scholarship and the Australian Centre for Plant Functional Genomics student scholarship to J.Q.
References
- Abel GH. 1969. Inheritance of capacity for chloride inclusion and chloride exclusion by soybeans. Crop Science 9, 697–698. [Google Scholar]
- Apse MP, Blumwald E. 2007. Na+ transport in plants. FEBS Letters 581, 2247–2254. [DOI] [PubMed] [Google Scholar]
- Blumwald E, Aharon GS, Apse MP. 2000. Sodium transport in plant cells. Biochimica et Biophysica Acta 1465, 140–151. [DOI] [PubMed] [Google Scholar]
- Brady SM, Orlando DA, Lee J-Y, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, Benfey PN. 2007. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318, 801–806. [DOI] [PubMed] [Google Scholar]
- Brandt B, Brodsky DE, Xue S, Negi J, Iba K, Kangasjarvi J, Ghassemian M, Stephan AB, Hu H, Schroeder JI. 2012. Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proceedings of the National Academy of Sciences of the United States of America 109, 10593–10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley M, Brown P, Cakmak I, Rengel Z, Zhao F. 2012. Function of nutrients: micronutrients. In Marschner P, ed. Mineral nutrition of higher plants. London: Acadmic Press, 191–248. [Google Scholar]
- Burton RA, Jobling SA, Harvey AJ, Shirley NJ, Mather DE, Bacic A, Fincher GB. 2008. The genetics and transcriptional profiles of the cellulose synthase-like HvCslF gene family in barley. Plant Physiology 146, 1821–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo DA, Haroon M, Schrader LE, Youngs VL. 1975. Rapid colorimetric determination of nitrate in plant-tissue by nitration of salicylic-acid. Communications in Soil Science and Plant Analysis 6, 71–80. [Google Scholar]
- Chen Y, Hu L, Punta M, Bruni R, Hillerich B, Kloss B, Rost B, Love J, Siegelbaum SA, Hendrickson WA. 2010. Homologue structure of the SLAC1 anion channel for closing stomata in leaves. Nature 467, 1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Zhu J, Zhu J-K. 2006. Salt stress signaling and mechanisms of plant salt tolerance. Genetic Engineering 27, 141–177. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Colmenero-Flores JM, Martinez G, Gamba G, Vazquez N, Iglesias DJ, Brumos J, Talon M. 2007. Identification and functional characterization of cation-chloride cotransporters in plants. The Plant Journal 50, 278–292. [DOI] [PubMed] [Google Scholar]
- Conn SJ, Hocking B, Dayod M, et al. 2013. Protocol: optimising hydroponic growth systems for nutritional and physiological analysis of Arabidopsis thaliana and other plants. Plant Methods 9, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram WJ, Pitman MG. 1972. Action of abscisic acid on ion uptake and water flow in plant roots. Australian Journal of Biological Sciences 25, 1125–1132. [Google Scholar]
- Curtis MD, Grossniklaus U. 2003. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology 133, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport R, James RA, Zakrisson-Plogander A, Tester M, Munns R. 2005. Control of sodium transport in durum wheat. Plant Physiology 137, 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir F, Horntrich C, Blachutzik JO, Scherzer S, Reinders Y, Kierszniowska S, Schulze WX, Harms GS, Hedrich R, Geiger D, Kreuzer I. 2013. Arabidopsis nanodomain-delimited ABA signaling pathway regulates the anion channel SLAH3. Proceedings of the National Academy of Sciences of the United States of America 110, 8296–8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers TJ, Munns R, Colmer TD. 2015. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Annals of Botany 115, 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort KP, Heinitz CC, Walker MA. 2015. Chloride exclusion patterns in six grapevine populations. Australian Journal of Grape and Wine Research 21, 147–155. [Google Scholar]
- Franco-Navarro JD, Brumós J, Rosales MA, Cubero-Font P, Talón M, Colmenero-Flores JM. 2015. Chloride regulates leaf cell size and water relations in tobacco plants. Journal of Experimental Botany 67, 873–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Zhu JK. 2009. Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proceedings of the National Academy of Sciences of the United States of America 106, 8380–8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite AJ, von Bothmer R, Colmer TD. 2005. Salt tolerance in wild Hordeum species is associated with restricted entry of Na+ and Cl– into the shoots. Journal of Experimental Botany 56, 2365–2378. [DOI] [PubMed] [Google Scholar]
- Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferriere N, Thibaud JB, Sentenac H. 1998. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94, 647–655. [DOI] [PubMed] [Google Scholar]
- Geiger D, Maierhofer T, Al-Rasheid KAS, et al. 2011. Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Science Signaling 4, ra32. [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, et al. 2009. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proceedings of the National Academy of Sciences of the United States of America 106, 21425–21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc Y, Oldach K, Taylor J, Lyons GH. 2015. Uncoupling of sodium and chloride to assist breeding for salinity tolerance in crops. New Phytologist 210, 145–156. [DOI] [PubMed] [Google Scholar]
- Gifford ML, Dean A, Gutierrez RA, Coruzzi GM, Birnbaum KD. 2008. Cell-specific nitrogen responses mediate developmental plasticity. Proceedings of the National Academy of Sciences of the United States of America 105, 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliham M, Tester M. 2005. The regulation of anion loading to the maize root xylem. Plant Physiology 137, 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Blackmore D, Clingeleffer P, Sykes S, Jha D, Tester M, Walker R. 2011. Contrast in chloride exclusion between two grapevine genotypes and its variation in their hybrid progeny. Journal of Experimental Botany 62, 989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutermuth T, Lassig R, Portes MT, Maierhofer T, Romeis T, Borst JW, Hedrich R, Feijo JA, Konrad KR. 2013. Pollen tube growth regulation by free anions depends on the interaction between the anion channel SLAH3 and calcium-dependent protein kinases CPK2 and CPK20. The Plant Cell 25, 4525–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SJ, Mott EK, Parsley K, Aspinall S, Gray JC, Cottage A. 2006. A rapid and robust method of identifying transformed Arabidopsis thaliana seedlings following floral dip transformation. Plant Methods 2, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R. 2012. Ion channels in plants. Physiological Reviews 92, 1777–1811. [DOI] [PubMed] [Google Scholar]
- Henderson SW, Baumann U, Blackmore DH, Walker AR, Walker RR, Gilliham M. 2014. Shoot chloride exclusion and salt tolerance in grapevine is associated with differential ion transporter expression in roots. BMC Plant Biology 14, 273–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SW, Wege S, Qiu J, Blackmore DH, Walker AR, Tyerman SD, Walker RR, Gilliham M. 2015. Grapevine and Arabidopsis cation-chloride cotransporters localize to the Golgi and trans-Golgi network and indirectly influence long-distance ion transport and plant salt tolerance. Plant Physiology 169, 2215–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Schroeder JI. 2004. Sodium transporters in plants. Diverse genes and physiological functions. Plant Physiology 136, 2457–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Sugawara M, Okunou K, Nakayama H, Schroeder JI, Shinmyo A, Yoshida K. 2008. Functions of HKT transporters in sodium transport in roots and in protecting leaves from salinity stress. Plant Biotechnology 25, 233–239. [Google Scholar]
- Jha D, Shirley N, Tester M, Roy SJ. 2010. Variation in salinity tolerance and shoot sodium accumulation in Arabidopsis ecotypes linked to differences in the natural expression levels of transporters involved in sodium transport. Plant Cell and Environment 33, 793–804. [DOI] [PubMed] [Google Scholar]
- Kiegle E, Gilliham M, Haseloff J, Tester M. 2000. Hyperpolarisation-activated calcium currents found only in cells from the elongation zone of Arabidopsis thaliana roots. The Plant Journal 21, 225–229. [DOI] [PubMed] [Google Scholar]
- Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, D’Angelo C, Bornberg-Bauer E, Kudla J, Harter K. 2007. The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. The Plant Journal 50, 347–363. [DOI] [PubMed] [Google Scholar]
- Köhler B, Raschke K. 2000. The delivery of salts to the xylem. Three types of anion conductance in the plasmalemma of the xylem parenchyma of roots of barley. Plant Physiology 122, 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler B, Wegner LH, Osipov V, Raschke K. 2002. Loading of nitrate into the xylem: apoplastic nitrate controls the voltage dependence of X-QUAC, the main anion conductance in xylem-parenchyma cells of barley roots. The Plant Journal 30, 133–142. [DOI] [PubMed] [Google Scholar]
- Lee LY, Lan W, Buchanan BB, Luan S. 2009. A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proceedings of the National Academy of Sciences of the United States of America 106, 21419–21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Byrt C, Qiu J, Baumann U, Hrmova M, Evrard A, Johnson AAT, Birnbaum KD, Mayo GM, Jha D, Henderson SW, Tester M, Gilliham M, Roy SJ. 2016. Identification of a stelar-localized transport protein that facilitates root-to-shoot transfer of chloride in Arabidopsis. Plant Physiology 170, 1014–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Fu YL, Pike SM, et al. 2010. The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. The Plant Cell 22, 1633–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SH, Kuo HF, Canivenc G, et al. 2008. Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. The Plant Cell 20, 2514–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder B, Raschke K. 1992. A slow anion channel in guard-cells, activating at large hyperpolarization, may be principal for stomatal closing. FEBS Letters 313, 27–30. [DOI] [PubMed] [Google Scholar]
- Maathuis FJ, Ahmad I, Patishtan J. 2014. Regulation of Na+ fluxes in plants. Frontiers in Plant Science 5, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maierhofer T, Diekmann M, Offenborn JN, et al. 2014. Site- and kinase-specific phosphorylation-mediated activation of SLAC1, a guard cell anion channel stimulated by abscisic acid. Science Signaling 7, ra86. [DOI] [PubMed] [Google Scholar]
- Marschner H. 1995. Mineral nutrition of higher plants, 2nd edn Houston: Gulf Professional Publishing. [Google Scholar]
- Møller IS, Gilliham M, Jha D, Mayo GM, Roy SJ, Coates JC, Haseloff J, Tester M. 2009. Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. The Plant Cell 21, 2163–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya JL, Gomez-Cadenas A, Primo-Millo E, Talon M. 2003. Chloride absorption in salt-sensitive Carrizo citrange and salt-tolerant Cleopatra mandarin citrus rootstocks is linked to water use. Journal of Experimental Botany 54, 825–833. [DOI] [PubMed] [Google Scholar]
- Müller M, Kunz HH, Schroeder JI, Kemp G, Young HS, Neuhaus HE. 2014. Decreased capacity for sodium export out of Arabidopsis chloroplasts impairs salt tolerance, photosynthesis and plant performance. The Plant Journal 78, 646–658. [DOI] [PubMed] [Google Scholar]
- Munns R, Gilliham M. 2015. Salinity tolerance of crops – what is the cost? New Phytologist 208, 668–673. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Fujita Y, Kanamori N, et al. 2009. Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant and Cell Physiology 50, 1345–1363. [DOI] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K. 2008. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452, 483–486. [DOI] [PubMed] [Google Scholar]
- Pitman MG. 1982. Transport across plant roots. Quarterly Reviews of Biophysics 15, 481–554. [DOI] [PubMed] [Google Scholar]
- Plett DC. 2008. Spatial and temporal alterations of gene expression in rice. Ph.D. Thesis, University of Adelaide. [Google Scholar]
- Preuss CP, Huang CY, Tyerman SD. 2011. Proton-coupled high-affinity phosphate transport revealed from heterologous characterization in Xenopus of barley-root plasma membrane transporter, HvPHT1;1. Plant Cell and Environment 34, 681–689. [DOI] [PubMed] [Google Scholar]
- Rengasamy P. 2010. Soil processes affecting crop production in salt-affected soils. Functional Plant Biology 37, 613–620. [Google Scholar]
- Rognes SE. 1980. Anion regulation of lupin asparagine synthetase-chloride activation the glutamine-utilizing reactions. Phytochemistry 19, 2287–2293. [Google Scholar]
- Roy SJ, Gilliham M, Berger B, et al. 2008. Investigating glutamate receptor-like gene co-expression in Arabidopsis thaliana . Plant Cell and Environment 31, 861–871. [DOI] [PubMed] [Google Scholar]
- Roy SJ, Negrao S, Tester M. 2014. Salt resistant crop plants. Current Opinion in Biotechnology 26, 115–124. [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S. 1989. Cytosolic calcium regulates ion channels in the plasma-membrane of Vicia faba guard-cells. Nature 338, 427–430. [Google Scholar]
- Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D. 2006. Highly specific gene silencing by artificial microRNAs in Arabidopsis. The Plant Cell 18, 1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibole JV, Cabot C, Poschenrieder C, Barcelo J. 2003. Efficient leaf ion partitioning, an overriding condition for abscisic acid-controlled stomatal and leaf growth responses to NaCl salinization in two legumes. Journal of Experimental Botany 54, 2111–2119. [DOI] [PubMed] [Google Scholar]
- Storey R, Walker RR. 1999. Citrus and salinity. Scientia Horticulturae 78, 39–81. [Google Scholar]
- Tavakkoli E, Fatehi F, Coventry S, Rengasamy P, McDonald GK. 2011. Additive effects of Na+ and Cl– ions on barley growth under salinity stress. Journal of Experimental Botany 62, 2189–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teakle NL, Flowers TJ, Real D, Colmer TD. 2007. Lotus tenuis tolerates the interactive effects of salinity and waterlogging by ‘excluding’ Na+ and Cl– from the xylem. Journal of Experimental Botany 58, 2169–2180. [DOI] [PubMed] [Google Scholar]
- Teakle NL, Tyerman SD. 2010. Mechanisms of Cl– transport contributing to salt tolerance. Plant Cell and Environment 33, 566–589. [DOI] [PubMed] [Google Scholar]
- Tester M, Davenport R. 2003. Na+ tolerance and Na+ transport in higher plants. Annals of Botany 91, 503–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregeagle JM, Tisdall JM, Blackmore DH, Walker RR. 2006. A diminished capacity for chloride exclusion by grapevine rootstocks following long-term saline irrigation in an inland versus a coastal region of Australia. Australian Journal of Grape and Wine Research 12, 178–191. [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, et al. 2008. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452, 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Puzorjova I, Brosche M, et al. 2010. Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. The Plant Journal 62, 442–453. [DOI] [PubMed] [Google Scholar]
- Walker RR, Blackmore DH, Clingeleffer PR, Correll RL. 2002. Rootstock effects on salt tolerance of irrigated field-grown grapevines (Vitis vinifera L. cv. Sultana). 1. Yield and vigour inter-relationships. Australian Journal of Grape and Wine Research 8, 3–14. [Google Scholar]
- White PJ. 2001. The pathways of calcium movement to the xylem. Journal of Experimental Botany 52, 891–899. [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR. 2001. Chloride in soils and its uptake and movement within the plant: A review. Annals of Botany 88, 967–988. [Google Scholar]
- Xu GH, Magen H, Tarchitzky J, Kafkafi U. 2000. Advances in chloride nutrition of plants. Advances in Agronomy 68, 97–150. [Google Scholar]
- Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K. 2006. The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. Journal of Biological Chemistry 281, 5310–5318. [DOI] [PubMed] [Google Scholar]
- Zheng X, He K, Kleist T, Chen F, Luan S. 2015. Anion channel SLAH3 functions in nitrate-dependent alleviation of ammonium toxicity in Arabidopsis. Plant Cell and Environment 38, 474–486. [DOI] [PubMed] [Google Scholar]
- Zhu JK. 2003. Regulation of ion homeostasis under salt stress. Current Opinion in Plant Biology 6, 441–445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.