Highlight
BjMYB1, an R2R3-MYB protein from Brassica juncea, binds to W-box-like elements rather than AC elements to mediate plant defence against fungus.
Key words: BjMYB1, Botrytis cinerea, fungus, MYB transcription factor, pathogen defence, W-box-like element.
Abstract
We previously identified the W-box-like-4 (Wbl-4) element (GTAGTGACTCAT), one of six Wbl elements in the BjC-P promoter of the unusual chitinase gene BjCHI1 from Brassica juncea, as the core element responsive to fungal infection. Here, we report the isolation and characterization of the cognate transcription factor interacting with the Wbl-4 element. Using Wbl-4 as a target, we performed yeast one-hybrid screening of a B. juncea cDNA library and isolated an R2R3-MYB transcription factor designated as BjMYB1. BjMYB1 was localized in the nucleus of plant cells. EMSA assays confirmed that BjMYB1 binds to the Wbl-4 element. Transiently expressed BjMYB1 up-regulated the activity of the BjC-P promoter through its binding to the Wbl-4 element in tobacco (Nicotiana benthamiana) leaves. In B. juncea, BjMYB1 displayed a similar induced expression pattern as that of BjCHI1 upon infection by the fungus Botrytis cinerea. Moreover, heterogeneous overexpression of BjMYB1 significantly elevated the resistance of transgenic Arabidopsis thaliana to the fungus B. cinerea. These results suggest that BjMYB1 is potentially involved in host defence against fungal attack through activating the expression of BjCHI1 by binding to the Wbl-4 element in the BjC-P promoter. This finding demonstrates a novel DNA target of plant MYB transcription factors.
Introduction
Plants are sessile organisms and therefore vulnerable to various pathogens in their habitat. Among the pathogens encountered, fungi pose a widespread threat to the conservation of plant species and food security of human beings (Fisher et al., 2012). It has been estimated that fungal diseases of the five major food crops—rice (Oryza sativa), wheat (Triticum aestivum), corn (Zea mays), soybean (Glycine max), and potato (Solanum tuberosum)—cause a yield loss of 125 million tons yearly (Fisher et al., 2012). For example, Botrytis cinerea, the second most important plant fungal pathogen, infects more than 200 plant species and causes massive economic losses in agriculture (Dean et al., 2012; Lu et al., 2013; Weiberg et al., 2013).
Over the course of evolution, plants have evolved sophisticated mechanisms to defend against pathogen attack (Jones and Dangl, 2006; Lu et al., 2013). In responding to pathogen infection, plant genes for defence are activated, and transcription factors (TFs) play important roles (Windram et al., 2012; Liu et al., 2013). The major TFs that activate plant defence genes against B. cinerea are the WRKY and AP2/ERF families (Berrocal-Lobo et al., 2002; Chen and Chen, 2002; Dong et al., 2003; Lai et al., 2008; Hu et al., 2012). A number of investigations have revealed that the highly conserved WRKY domains in most WRKY TFs specifically bind to the W-box elements characterized by the nucleotide motif (T/C)TGAC(C/T) (Eulgem et al., 2000; Eulgem and Somssich, 2007; Rushton et al., 2010; Chi et al., 2013), although some recent reports have shown that WRKY proteins also display binding affinity to various W-box-like (Wbl) elements in which only a central GAC-core motif is required for binding (Ciolkowski et al., 2008; Yamasaki et al., 2012; Brand et al., 2013).
MYB proteins comprise the largest family of TFs in plants and play regulatory roles in plant development, secondary metabolism, hormone signalling, disease resistance, and abiotic stress tolerance (Katiyar et al., 2012). MYB TFs have been classified into four subfamilies (R1-MYB, R2R3-MYB, 3R-MYB, and 4R-MYB) based on the MYB domains they contain (Dubos et al., 2010). An R2R3-MYB protein contains two MYB domains and the majority of R2R3-MYB proteins regulate plant-specific functions including immunity against microbial pathogens (Stracke et al., 2001; Dubos et al., 2010). For example, overexpression of the R2R3-MYB gene HbMyb1 from Hevea brasiliensis enhances resistance to B. cinerea in transgenic tobacco (Nicotiana tabacum cultivar Samsun NN; Peng et al., 2011); the wheat R2R3-MYB gene TaPIMP1 regulates plant resistance to the biotrophic bacterial pathogen Ralstonia solanacearum in tobacco (Nicotiana tabacum L.) and to the hemibiotrophic fungal pathogen Bipolaris sorokiniana in wheat (Liu et al., 2011; Zhang et al., 2012); and the R2R3-MYB gene OsJaMyb in rice (O. sativa spp. japonica) is responsive to infection by the blast fungus Magnaporthe oryzae (Lee et al., 2001). Moreover, AtMYB30, the most extensively characterized R2R3-MYB gene in Arabidopsis thaliana, is involved in the regulation of plant immunity to microbial pathogens (Raffaele and Rivas, 2013). Some other R2R3-MYB genes from A. thaliana, such as BOTRYTIS-SUSCEPTIBLE1 BOS1/AtMYB108 (Mengiste et al., 2003), AtMYB72 (Segarra et al., 2009), and AtMYB60 and AtMYB96 (Seo et al., 2009; Seo and Park, 2010), are also associated with the regulation of plant resistance to pathogens.
To unlock the prominent roles played by R2R3-MYB TFs in plant defence against pathogen attack at the molecular level, it is necessary to investigate the details of the interaction between R2R3-MYB TFs and their target genes (Prouse and Campbell, 2013). Investigations have shown that the MYB-core element (C/T)NGTT(G/A) and the AC elements ACC(A/T)A(A/C)(T/C) and ACC(A/T)(A/C/T)(A/C/T) are cis-regulatory elements of R2R3-MYB proteins for transcriptional activation of target genes in yeast and in planta (Romero et al., 1998; Prouse and Campbell, 2012; Kelemen et al., 2015). Grotewold and colleagues first reported the ACC(A/T)ACC(A/C/T) target site of the maize P protein, an R2R3-MYB protein involved in flavonoid biosynthesis (Grotewold et al., 1994). Likewise, the flavonoid biosynthesis-associated proteins AtMYB11, AtMYB12, and AtMYB111 show similar target gene specificity as the maize P protein (Prouse and Campbell, 2012). The Zea mays MYB31 protein has been shown to bind to the sequence ACC(T/A)ACC within promoters of the genes ZmCOMT and ZmF5H (Fornalé et al., 2010). Similarly, pine (Pinus taeda) MYB1 (Patzlaff et al., 2003b ) and MYB4 (Patzlaff et al., 2003a ), eucalyptus (Eucalyptus grandis) MYB2 (Goicoechea et al., 2005), and AtMYB61 (Prouse and Campbell, 2013) also bind to AC elements in promoters of the lignin biosynthetic genes. However, to the best of our knowledge, no one has shown that an MYB protein can bind to a W-box or a Wbl element to regulate plant defence against fungal infection.
The W-box and/or Wbl elements are a major class of cis-acting elements in promoters of many plant genes responsive to pathogen induction (Rushton et al., 1996; Rushton and Somssich, 1998; Rushton et al., 2002; Yamamoto et al., 2004; Gao et al., 2014). The typical nucleotide motif (T/C)TGAC(C/T) of W-box elements is usually bound by the WRKY TFs (Eulgem et al., 2000; Eulgem and Somssich, 2007; Rushton et al., 2010; Chi et al., 2013). For example, rice OsWRKY53 mediates the chitin elicitor-responsiveness by interacting with three tandem W-box elements (Chujo et al., 2009). In our previous study, we identified six Wbl sequences in the chitinase gene BjCHI1 promoter (BjC-P) and designated them as Wbl-1 through Wbl-6 (Wu et al., 2009). Our further study showed that Wbl-4 (GTAGTGACTCAT) in the promoter BjC-P is the core element responsive to fungal infection (Gao et al., 2014). However, the cognate TF interacting with the Wbl-4 element remains unknown. Here, we report the isolation and characterization of an R2R3-MYB TF (BjMYB1) that interacts with the Wbl-4 element to regulate plant defence against the fungus B. cinerea. To our knowledge, this is the first report that an MYB protein can bind to a Wbl element, rather than AC elements, to mediate plant pathogen defence.
Materials and methods
Plant materials and growth conditions
Plants of A. thaliana L. (ecotype Col-0), Nicotiana benthamiana, and B. juncea were grown in a growth chamber at 22°C (light)/19°C (dark) under a 16h light/8h dark cycle. The A. thaliana was used to generate stable transgenic plants, the N. benthamiana for transient expression assays, and the B. juncea for constructing a cDNA library and for endogenous gene expression assays.
Culture of B. cinerea, inoculation, and phenotyping
B. cinerea was cultured on potato agar media plus 1.5% dextrose (potato 200g l−1, glucose 20g l−1, agar 15g l−1, pH 6.0) at 22°C for 10 days. The conidia of well-grown B. cinerea were suspended in sterile distilled water, filtered with two layers of gauze, and diluted to 5×105 cells per millilitre. Four-week-old B. juncea plants and T2 transgenic A. thaliana plants were inoculated with the conidial suspension by spraying. Distilled water was used as a negative control. Disease phenotyping was performed based on B. cinerea biomass quantified by quantitative PCR with B. cinerea-specific internal transcribed spacer (ITS) primers Bc-ITS-F/Bc-ITS-R (Table 1).
Table 1.
The primers used in this study
| Name | Sequence (from 5′ to 3′) | Feature (direction/role) |
|---|---|---|
| Bait1F | TAAAGCTTCTCTGCTAGAGATAGTGTG | Forward, PCR of Bait and Bait-m |
| Bait1R | TAGGATCCGTTTCTCTGAGCTGTATGGTTG | Reverse, PCR of Bait and Bait-m |
| pAbAi-Seq1 | GTTCCTTATATGTAGCTTTCGACAT | Forward, sequencing plasmids of pBait-AbAi and pBait-m-AbAi |
| pAbAi-Seq2 | CATGTTAGGATGGGCAAGGCATTGA | Reverse, sequencing plasmids of pBait-AbAi and pBait-m-AbAi |
| pGADT7-F | TAATACGACTCACTATAGGGC | Forward, sequencing plasmid pGADT7-BjcDNA |
| pGADT7-R | CTGTGCATCGTGCACCATCT | Reverse, sequencing plasmid pGADT7-BjcDNA |
| BjMYB1-F1 | CGGAATTCATGGGAGTGAAAGGCCTCACC | Forward, PCR BjMYB1 for construct plasmid pET28a-BjMYB1 |
| BjMYB1-F2 | CGGGATCCATGGGAGTGAAAGGCCTCACC | Forward, PCR BjMYB1 for construct plasmid |
| pCAMBIA1307-BjMYB1 | ||
| BjMYB1-R | GCGTCGACTTATCCAATGGTACTACTAGG | Reverse, PCR BjMYB1 for construct plasmids |
| pET28a-BjMYB1 and pCAMBIA1307-BjMYB1 | ||
| BjMYB1-F3 | AGCTAGGGAAGAGCTATCAG | Forward, RNA quantification of BjMYB1 |
| BjMYB1-R2 | GAGAGCTTTCAACCGAACAG | Reverse, RNA quantification of BjMYB1 |
| BjCHI1-F1 | GCACCCGATGGAGCAAATACA | Forward, RNA quantification of BjCHI1 |
| BjCHI1-R1 | ATTGGTCCTCGTCCGTAGTAA | Reverse, RNA quantification of BjCHI1 |
| AtACTIN-F | AGTGGTCGTACAACCGGTATTGT | Forward, for internal reference of BjMYB1 in A. thaliana |
| AtACTIN-R | GAGGAAGAGCATTCCCCTCGTA | Reverse, for internal reference of BjMYB1 in A. thaliana |
| BjActin-F | CTTCTTACCGAGGCTCCTCT | Forward, for internal reference of BjMYB1 and BjCHI1 in B. juncea |
| BjActin-R | AAGGATCTTCATGAGGTAATCAGT | Reverse, for internal reference of BjMYB1 and BjCHI1 in B. juncea |
| Bc-ITS-F | TCGAATCTTTGAACGCACATTGCGC | Forward, for B. cinerea biomass quantification |
| Bc-ITS-R | TGGCAGAAGCACACCGAGAACCTG | Reverse, for B. cinerea biomass quantification |
| Bc-actinF | GAGAGCGGTGGTATCCACGTCAC | Forward, for internal reference of Bc-ITS |
| Bc-actinR | CACTTGCGGTGGACAATGGAAGGT | Reverse, for internal reference of Bc-ITS |
Yeast one-hybrid assay to screen TFs binding to the Wbl-4 element
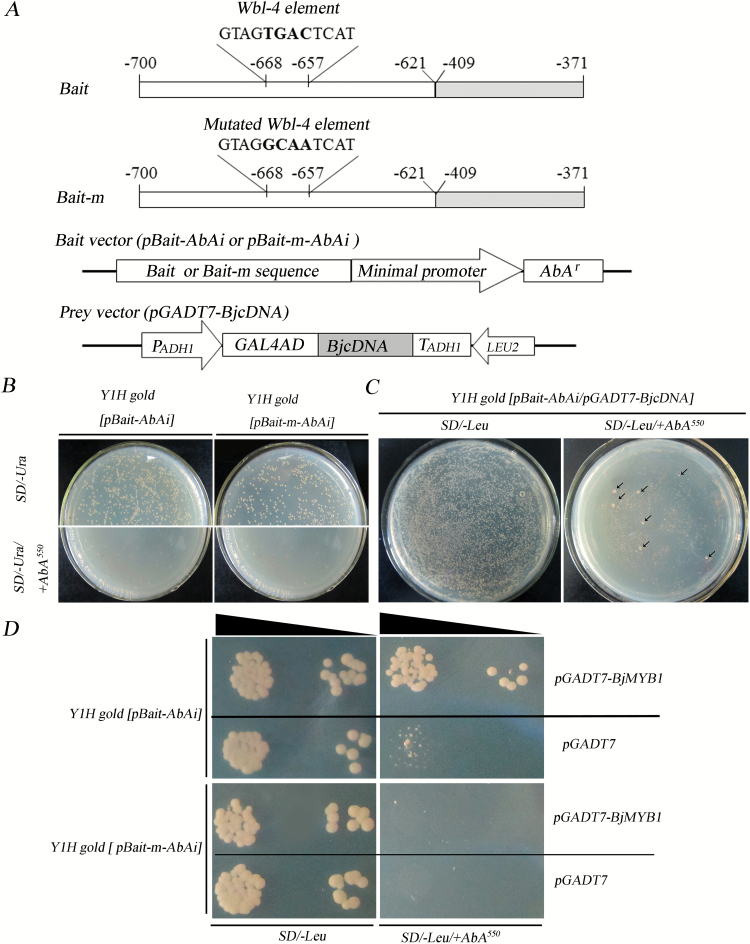
The pathogen-associated molecular patterns of chitin have been intensively studied (Wan et al., 2012). The water-soluble chitin oligomer hexa-N-acetylchitohexaose can be used as a fungal elicitor in research on plant defence against fungi (Raventos et al., 1995; Chen et al., 2010). To avoid the interference of cDNA from B. cinerea in yeast one-hybrid (Y1H) screening, we used this fungal elicitor to treat the B. juncea seedling for cDNA library construction. According to our previous study, spraying of 200 µg ml−1 hexa-N-acetylchitohexaose solution activates expression of the chitinase gene BjCHI1 (Gao et al., 2014). Thus, leaves of B. juncea seedlings were sprayed with 200 µg ml−1 hexa-N-acetylchitohexaose in this study. The sprayed leaves were harvested at 12h, 24h, 48h, and 72h post spraying. Total RNA was extracted from mixture of the harvested leaves for construction of the cDNA library. The B. juncea cDNA was cloned into the Sfi I site of the pGADT7 vector and fused in-frame with GAL4AD (Clontech Laboratories, Inc., a Takara Bio Company), resulting in the cDNA library plasmids, that is, the prey plasmids (Fig. 1A).
Fig. 1.
Yeast one-hybrid screening for factors binding to the Wbl-4 element.
(A) Schematic diagrams of the Bait and Bait-m fragments, and Bait and Prey vectors. The BjC-P promoter fragments -700 to -621 (white box) and -409 to -371 (grey box) were fused as the bait sequence. The numbers above the boxes indicate the nucleotide positions in the BjC-P promoter. The nucleotides in the Wbl-4 element and its mutant are called out wherein the core sequence TGAC and its mutant GCAA are shown in bold. The Bait and Bait-m fragments were, respectively, inserted upstream of the AbA r reporter gene in the pBait-AbAi vector. The cDNA from B. juncea was inserted into the pGADT7 vector and fused in-frame with GAL4AD when preparing the cDNA library. (B) Determination of the minimal inhibitory concentration of AbA by growing the bait-reporter yeast strains on the SD/−Ura media with or without AbA. Images show that 550ng ml−1 (AbA) was the appropriate inhibitory concentration for the reporter strains Y1Hgold [pBait-AbAi] and Y1Hgold [pBait-m-AbAi]. (C) Y1H screening of the B. juncea cDNA library. pGADT7-BjcDNA was transferred into the bait-reporter yeast strain Y1Hgold [pBait-AbAi] and then selected on SD/−Leu agar plates containing 550ng ml−1 AbA (SD/−Leu/+AbA550), using the SD/−Leu agar plate as a control. Arrows indicate the positive clones. (D) BjMYB1 interacts with the Wbl-4 element (Bait), but not the mutated Wbl-4 element (Bait-m). The plasmid pGADT7-BjMYB1 isolated from one of the positive clones in (B) was re-transferred into the bait-reporter yeast strains Y1Hgold [pBait-AbAi] and Y1Hgold [pBait-m-AbAi], respectively, and then selected on SD/−Leu/+AbA550 agar plates. The transformants from the combination Y1Hgold [pBait-AbAi/pGADT7-BjMYB1] could grow healthily on the SD/−Leu/+AbA550 but those from the combination Y1H gold [pBait-m-AbAi/pGADT7-BjMYB1] could not. The empty plasmid pGADT7 and the SD/−Leu agar plate (without AbA) were used as controls.
The BjC-P promoter fragment (-700 to -621) containing the Wbl-4 and the fragment (−409 to −371), coupled for full magnitude of BjC-P fungal induction (Gao et al., 2014), were conjoined as the Bait sequence for Y1H (Fig. 1A). To obtain genuine positive clones that specifically interact with the Wbl-4 element, a mutant Bait (designated Bait-m) was generated as a negative control, by mutating the core nucleotide sequence TGAC in the Wbl-4 element into GCAA (Fig. 1A). The Bait and Bait-m DNA sequences (see Supplementary Table S1 at JXB online) were PCR-amplified from the plasmids P16 and P54 (Gao et al., 2014), respectively, using the primers Bait1F and Bait1R (Table 1). The Bait vectors pBait-AbAi and pBait-m-AbAi were constructed by cloning the Bait or Bait-m sequence into upstream of the AbA r reporter gene in the pAbAi vector (Clontech Laboratories, Inc., a Takara Bio Company; Fig. 1A). The constructed pBait-AbAi and pBait-m-AbAi plasmids were then linearized with BstB I, and homogenously integrated individually into the chromosome of yeast strain Y1Hgold, resulting in the bait-reporter yeast strains Y1Hgold [pBait-AbAi] and Y1Hgold [pBait-m-AbAi] that were later used to screen the B. juncea cDNA library. pBait-AbAi integrations were confirmed by PCR with primers pAbAi-Seq1 and pAbAi-Seq2 (Table 1). Y1H screening experiments were conducted using the Matchmaker Gold Yeast One-hybrid Library Screening System (Clontech Laboratories, Inc., a Takara Bio Company) according to the supplied manual. Y1H positive clones were sequenced with the primers PGADT7-F and PGADT7-R (Table 1).
Bioinformatic analyses of BjMYB1
The software GENSCAN (http://genes.mit.edu/GENSCAN.html, accessed 17 June 2016) was used to predict the open reading frame of BjMYB1 and the deduced amino acid sequence. The NCBI (http://www.ncbi.nlm.nih.gov/, accessed 17 June 2016) protein domain software was used to analyse the conservative structure domain. NCBI BLAST tools (http://www.ncbi.nlm.nih.gov/, accessed 17 June 2016) were adopted to analyse the orthologs of BjMYB1. Clustalx 1.83 and MEGA 6 were used to make the identity comparison and construct the evolutionary tree, respectively.
Subcellular localization of BjMYB1
The coding region of BjMYB1 with the stop codon was inserted into the pCAMBIA1205-YFP vector (Gao et al., 2008) using the BamH I and Sal I restriction sites and fused in-frame with the yellow fluorescent protein (YFP) gene under control of the Cauliflower mosaic virus (CaMV) 35S promoter. The YFP-BjMYB1 fusion protein was transiently expressed in N. benthamiana leaves by agroinfiltration described previously (Gao et al., 2014). About 48h post infiltration, the infiltrated N. benthamiana leaves were stained with DAPI for about 3h in the dark and then the YFP fluorescence was observed under 514nm excitation, using a confocal laser scanning microscope (Zeiss LSM 700, Germany) with a Fluar ×10/0.50 M27 objective lens and a SP640 filter.
Protein expression in Escherichia coli and EMSA
The vector pET-28a (+) [EMD Biosciences (Novagen), USA] harbouring a His tag was used for BjMYB1 expression in Escherichia coli. BjMYB1 was cloned into the pET-28a (+) vector by the EcoR I and Sal I restriction sites. Sequences of primers BjMYB1-F1 and BjMYB1-R used for this cloning were shown in Table 1. The resultant plasmid was transformed into E. coli Rosetta (DE3) for expression of the His-BjMYB1 fusion protein. Overexpression of His-BjMYB1 was induced by 0.6mM isopropyl-D-thiogalactoside when the optical density of the bacteria cell culture at 600nm (OD600) reached 0.8. The cells were grown at 16°C overnight, harvested, and homogenized in a buffer containing 50mM NaH2PO4, 300mM NaCl and 10mM imidazole (pH 8.0). After sonication and centrifugation, the supernatant was applied to Ni2+ affinity resin (Ni-NTA, QIAGEN) as described in the manufacturer’s manual. The purified His-BjMYB1 was used for the EMSA.
The EMSA was performed as described previously (Wang et al., 2015). To identify the binding site of BjMYB1, complementary pairs of non-labelled and 3′-biotin-labelled oligonucleotides of BjC-P fragments containing the Wbl-4 or its mutants were synthesized and annealed to generate the double-stranded DNA fragments. Sequences of the synthesized oligonucleotides of W4 (Wbl-4 element intact), W4-d1 (core sequence TGAC in the Wbl-4 element deleted), W4-d2 (central sequence GTGACT of Wbl-4 changed into the typical W-box element TTGACC), W4-d3 (GTGACT sequence of Wbl-4 changed to the typical AC element sequence ACCTACCA), and PAL2Pro (typical AC element in the promoter of the Phaseolus vulgaris PAL2) are shown in Supplementary Table S1. The binding reactions were performed in a 20 μl reaction mixture containing 1× binding buffer (Pierce, Rockford, IL, USA), 5mM MgCl2, 50ng μl−1 poly (dI-dC), 0.05% NP-40 (v/v), 2.5% glycerol (v/v), 40fmol biotin-labelled DNA, 0 or 4 pmol unlabelled DNA, and 15fmol His-BjMYB1 fusion protein. The binding reactions were kept at room temperature for 30min for competition assays, and for another 30min after biotin-labelled DNA was added. Binding reactions were electrophoresed in 8% native polyacrylamide gel and then electrotransferred onto nylon membrane. The transferred DNA was cross-linked to the membrane at 120 mJ cm−2 for 1min using a CL-1000 Ultraviolet Crosslinker (UVP, LLC, Upland, CA, USA). Immunostaining was performed with the Light ShiftR Chemiluminescent EMSA Kit (Pierce, Rockford, IL, USA) according to the manufacturer’s protocol.
Transient expression assays in tobacco leaves
N. benthamiana plants were grown until the sixth leaf fully expanded. Agrobacterium tumefaciens EHA105 harbouring the construct to be tested was grown on agar-lysogeny broth containing 50 µg ml−1 kanamycin and 30 µg ml−1 rifampicin, then suspended with MMA buffer (10mM MgCl2, 10mM MES pH 5.5, 100 µM acetosyringone) at an OD600 of 0.5, and incubated at 28°C for 3h. The fifth and sixth expanded leaves of N. benthamiana were infiltrated with A. tumefasciens EHA105 containing the construct to be tested at the blade back with a 1ml needless syringe. Infiltrated leaves were harvested for GUS staining, GUS quantitative assays, or confocal imaging of YFP-BjMYB1 at 48h post inoculation. GUS staining and GUS quantitative assays were performed as described previously (Gao et al., 2014).
Overexpression of BjMYB1 in A. thaliana
BjMYB1 cDNA was amplified by RT-PCR, confirmed by sequencing, and then cloned into pCAMBIA1307 at BamH I and Sal I sites under the control of the 35S promoter. Primers BjMYB1-F2 and BjMYB1-R (Table 1) were used for this cloning. The resultant plasmids were introduced into A. thaliana plants through A. tumefasciens EHA105 using the floral dip method (Clough and Bent, 1998). Positive transgenic lines were identified by PCR. Seedlings of three independent T2 transgenic lines growing in Murashige and Skoog medium with 40 µg ml−1 hygromycin were transferred to soil.
Quantitative real-time PCR
Quantitative real-time (qRT-PCR) was performed using SYBR Premix Ex TaqTM II (TAKARA BIO INC, Japan) and a 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with the following conditions: 95°C for 1min, 40 cycles of 95°C for 10s, and 60°C for 34s in 20 μl reaction volumes. A dissociation curve was generated for each reaction to ensure specific amplification. The actin genes of A. thaliana, B. juncea, and B. cinerea were used as their own internal references. The relative expression was quantified using the comparative 2−ΔΔCT method (Livak and Schmittgen, 2001). Primer pairs BjMYB1-F3/BjMYB1-R2, BjCHI1-F1/BjCHI1-R1, and Bc-ITS-F/Bc-ITS-R (Table 1) were used for qRT-PCR analysis of BjMYB1, BjCHI1, and Bc-ITS, respectively. Primer pairs of the corresponding internal reference genes, AtACTIN-F/AtACTIN-R, BjActin-F/BjActin-R, and Bc-actinF/Bc-actinR, are also shown in Table 1.
Results
Molecular cloning of BjMYB1
Our previous work revealed that the Wbl-4 motif (GTAGTGACTCAT) is the core fungus-responsive cis-acting element in BjC-P, the promoter of the unusual chitinase gene BjCHI1 (Gao et al., 2014). To identify TFs that interact with the cis-acting element Wbl-4, we performed Y1H screening of a B. juncea cDNA library. The Bait fragments and Bait and Prey vectors used for the Y1H screening are schematically shown in Fig. 1A.
To perform the Y1H screening, the minimal inhibitory concentration of aureobasidin A (AbA) for the bait-reporter yeast strains Y1Hgold [pBait-AbAi] and Y1Hgold [pBait-m-AbAi] were first determined by growing them on the SD/−Ura/+AbA media, resulting in an appropriate inhibitory concentration of 550ng ml−1 AbA (Fig. 1B). The pGADT7-BjcDNA plasmids (1 µg) from the B. juncea cDNA library were then used to transform the bait-reporter yeast strain Y1Hgold [pBait-AbAi]. Transformants were cultured on SD/−Leu (as a control) and SD/−Leu/+AbA550 (SD/−Leu media containing 550ng ml−1 AbA) agar plates (Fig. 1C). The single-cell clones growing healthy on the SD/−Leu/+AbA550 agar plate were recognized as positive clones. The pGADT7-BjcDNA plasmids in each of the positive clones were isolated and further transformed into the bait-reporter strain Y1Hgold [pBait-m-AbAi], and transformants were screened on the same SD/−Leu/+AbA550 media. Among the pGADT7-BjcDNA plasmids from 28 positive clones, seven could not generate Y1Hgold [pBait-m-AbAi] transformants, suggesting that the inserted BjcDNA in the seven pGADT7-BjcDNA plasmids encode proteins specifically binding to the Wbl-4 element. Sequencing and BLAST analysis revealed that three of the seven pGADT7-BjcDNA plasmids harbour an identical BjcDNA that encodes a putative MYB protein. Because no uniform nomenclature has been proposed for MYB-type genes from B. juncea, we designated the BjcDNA as BjMYB1 (Fig. 1D).
BjMYB1 encodes an R2R3-MYB protein located in the plant cell nucleus
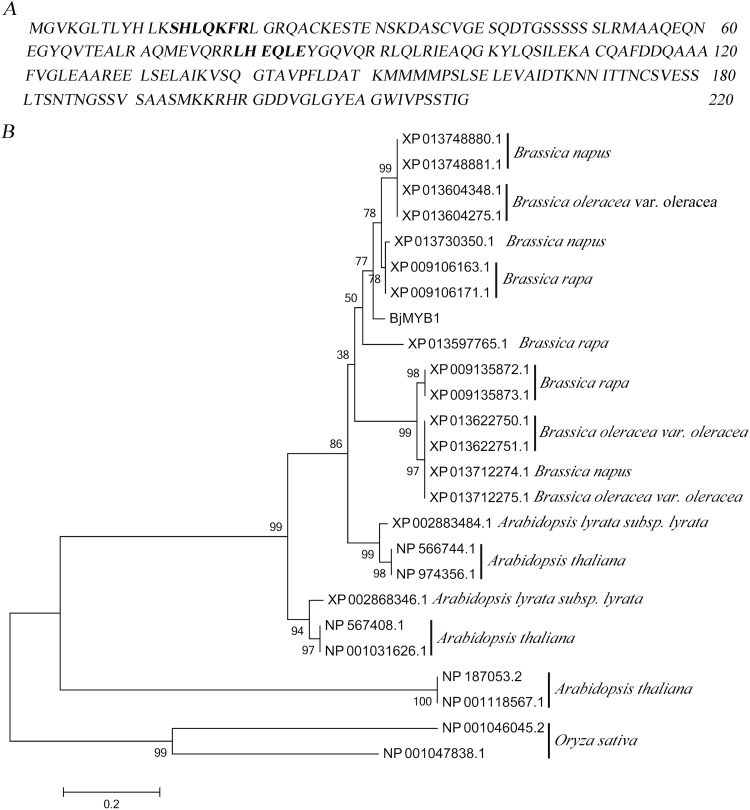
Bioinformatic analysis revealed that the BjMYB1 cDNA isolated by the Y1H screening contains a 663-bp open reading frame that encodes a 220-amino acid protein with two MYB domains (SHLQKFR and LHEQLE, Fig. 2A). We used the BjMYB1 protein as a query sequence to BLAST the NCBI database; the searched orthologs of BjMYB1 were annotated as MYB family members, but none of them has been assigned a function experimentally. The orthologs from species Brassica (with 100% coverage and >80% identity), Arabidopsis (with >80% coverage and >80% identity) and O. sativa (with >80% coverage) were used for multiple sequence alignments (see Supplementary Fig. S1 at JXB online) and maximum-likelihood phylogenetic analysis (Fig. 2B). The phylogenetic analysis allocated BjMYB1 to a distinct subclade, with closest relationship to a cluster containing XP 013730350.1, XP 009106163.1, and XP 009106171.1 from Brassica (Fig. 2B). Three MYB family members (NP 566744.1, NP 974356.1, and XP 002883484.1) from Arabidopsis are relatively close orthologs of BjMYB1. BjMYB1 showed the most distant phylogenetic relationships with orthologs from O. sativa (Fig. 2B and Supplementary Fig. S1).
Fig. 2.
Amino acid sequence of BjMYB1 and phylogenetic analysis. (A) The deduced 220 amino acids and the putative two MYB domains (in bold) of BjMYB1. (B) Phylogenetic analysis of BjMYB1 with the orthologs from Brassica, Arabidopsis, and Oryza. The software Clustalx 1.83 and MEGA 6 were used to make the identity comparison and construct the evolutionary tree, respectively. Node values are percentages of bootstraps generated with 1000 bootstrap replicates. The bar shows an evolutionary distance corresponding to 0.2 amino acid substitutions per site.
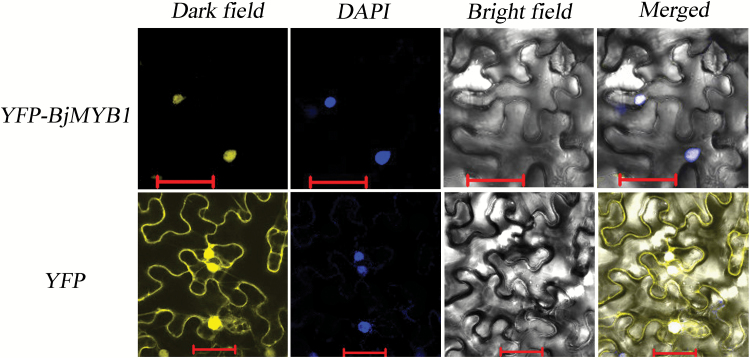
To validate the prediction that BjMYB1 is a TF, we examined its subcellular localization. The chimeric expression vector pCAMBIA1205-YFP-BjMYB1 and the control vector pCAMBIA1205-YFP were constructed and delivered into N. benthamiana leaves via agroinfiltration (Wu et al., 2009). Confocal imaging of the transient expression showed that YFP-BjMYB1 accumulated only in the cell nucleus, whereas YFP alone was present throughout the whole cell as expected, indicating that BjMYB1 is a nucleus-localized protein (Fig. 3), consistent with the fact that TFs typically function in the cell nucleus.
Fig. 3.
BjMYB1 is localized in the nucleus of N. benthamiana cells. Construct pCAMBIA1205-YFP-BjMYB1 was infiltrated into N. benthamiana leaves for transient expression of YFP-BjMYB1. pCAMBIA1205-YFP was infiltrated as a control. Images were taken about 48h after infiltration and the infiltrated tobacco leaves were stained with DAPI before the photo was taken. The experiments were repeated at least three times with similar results. YFP indicates yellow fluorescent protein. Bar = 50 µm.
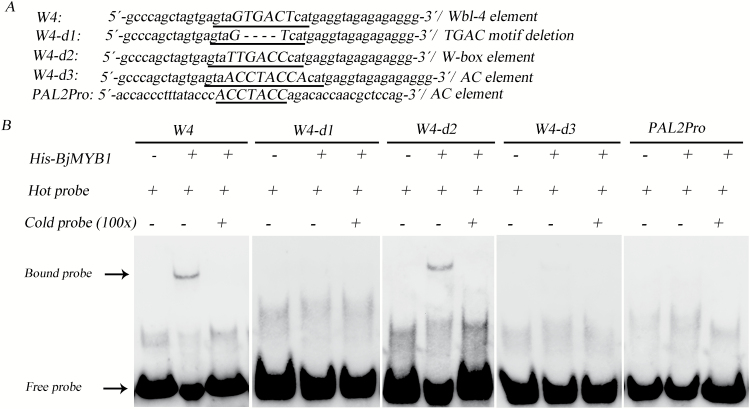
BjMYB1 binds to the Wbl-4 element in vitro
To confirm the binding of BjMYB1 to the Wbl-4 element (GTAGTGACTCAT), a His-tagged BjMYB1 fusion protein was expressed in E. coli Rosetta (DE3) (see Supplementary Fig. S2 at JXB online) and used for an EMSA. An obviously shifted band was observed in the assay with the Wbl-4 element (Fig. 4, W4), indicating the binding between BjMYB1 and the Wbl-4 element. Consistently, when the central sequence GTGACT of Wbl-4 was changed into the typical W-box element TTGACC, an obviously shifted band still appeared (Fig. 4, W4-d2). Conversely, when the core sequence TGAC in the Wbl-4 element was deleted, the shifted band disappeared, indicating that the mutation of the core sequence TGAC abolished the binding between BjMYB1 and the Wbl-4 element (Fig. 4, W4-d1). These results indicate that the isolated BjMYB1 indeed interacts with the Wbl-4 element and that the TGAC core element is essential for binding of BjMYB1 to Wbl-4.
Fig. 4.
BjMYB1 binds to the Wbl-4 and W-box element in vitro. (A) Nucleotide sequences of the probes with central nucleotides in bold and underlined. W4 contains the Wbl-4 element. W4-d1 is a mutant of W4 with the core sequence TGAC in the Wbl-4 element deleted (----). W4-d2 is a mutant of W4 with the GTGACT changed into a typical W-box element motif TTGACC. W4-d3 is another mutant of W4 with the GTGACT changed into the AC element motif ACCTACCA. PAL2Pro is the promoter fragment of the bean PAL2 gene containing the AC element ACCTACC. (B) EMSA for the DNA-binding activity of the His-BjMYB1 fusion protein with W4, W4-d1, W4-d2, W4-d3, and PAL2Pro probes. An equal amount of BjMYB1 protein or hot probe (biotin-labelled) was used in all lanes. There was 100 times the amount of cold probes (without the biotin label) than hot probes for competitive binding. The bound and free hot probes are indicated by arrows on the left.
Because AC elements are the known binding target of some R2R3-MYB proteins (Prouse and Campbell, 2012), we then examined the ability of BjMYB1 to bind to AC elements by changing the GTGACT sequence of Wbl-4 into the typical AC element sequence ACCTACCA (Fig. 4A, W4-d3). EMSA assays showed that BjMYB1 displayed almost no binding affinity to this AC element generated from the Wbl-4 element (Fig. 4B, W4-d3). This observation was further confirmed by EMSA assays with the typical AC element in the promoter of the P. vulgaris PAL2 gene (Patzlaff et al., 2003a ) (Fig. 4, PAL2Pro). PAL2Pro contains the AC element, which is the binding target of the R2R3-MYB protein PtMYB4 (Patzlaff et al., 2003a ). These results clearly showed that the R2R3-MYB protein BjMYB1 could bind to W-box or Wbl elements, but not to the AC elements tested.
BjMYB1 activates promoter BjC-P by binding to the Wbl-4 element in vivo
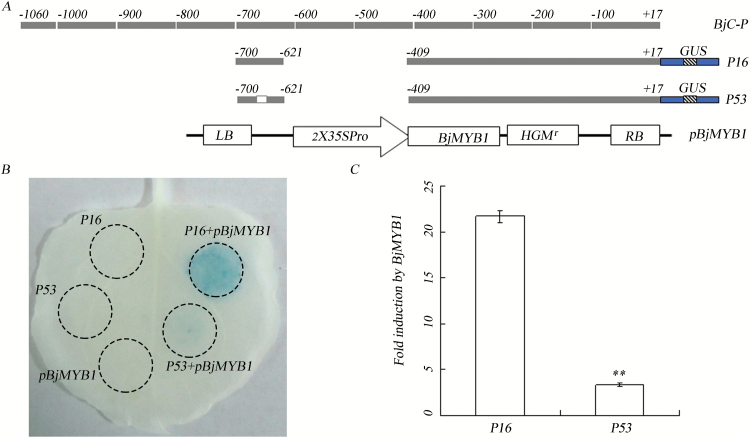
To validate the transcriptional activation of the promoter BjC-P by BjMYB1, we performed transactivation analysis by transient expression in N. benthamiana. Previously, we revealed that the BjC-P deletion derivative P16 containing the Wbl-4 element was sufficient for fungus-response, and this response was abolished by deletion of the core sequence TGAC in the Wbl-4 element as represented by the deletion derivative P53 (Gao et al., 2014). Therefore, we used P16 and its mutant P53 as reporter plasmids (Fig. 5A). To express BjMYB1 in planta, we constructed the plasmid pBjMYB1 by placing the BjMYB1 cDNA downstream of the 35S promoter in the binary vector pCAMBIA1307 (Lin et al., 2014). In the transactivation analysis, N. benthamiana leaves were co-infiltrated with the construct combinations P16/pBjMYB1 and P53/pBjMYB1; the construct P16 or P53 or pBjMYB1 alone were infiltrated as negative controls. In addition, the pBI121-LUCint, a CaMV 35S::LUCint construct, was co-infiltrated in each assay to normalize the GUS activity by its LUC activity (Wu et al., 2009; Gao et al., 2014). GUS staining and GUS quantitative assays of the infiltrated leaves showed that overexpression of BjMYB1 strongly activated the BjC-P promoter derivative P16, but not the mutant P53 (Fig. 5B, C). Given that P53 lacks only the core sequence TGAC in the Wbl-4 element compared with P16, these observations indicate that BjMYB1 could specifically recognize the Wbl-4 element in vivo, consistent with the results of the Y1H and the EMSA assays. Taken together, aforementioned experiments demonstrate that BjMYB1 functions as a transcription activator by interacting with the Wbl-4 element of BjC-P for target gene activation.
Fig. 5.
BjMYB1 activates BjC-P by binding to the Wbl-4 element in vivo. (A) Schematic diagram of BjC-P, GUS-expressing constructs P16, P53, and the BjMYB1-expressing construct pBjMYB1. Grey boxes represent BjC-P and its deletion derivatives. The numbers above the boxes indicate the nucleotide positions relative to the BjCHI1 transcription start site. Blue boxes represent the GUS gene containing an intron indicated by the texture area. The small white box in P53 indicates deletion of the core sequence TGAC in Wbl-4. (B) GUS histochemical staining of a tobacco leave infiltrated or co-infiltrated with the constructs as indicated. The dashed circles indicate the infiltration areas. (C) Quantization of GUS activity of P16 and P53 in transiently transformed tobacco leaves. N. benthamiana leaves were co-infiltrated with P16 or P53 and pBjMYB1 as well as a CaMV 35S::LUCint construct (pBI121-LUCint). Each of the plasmids was adjusted into equal density for the infiltrations. The GUS activity was normalized with the LUC activity. Columns show the ratio of GUS activity induced by pBjMYB1 to that of no induction. Values represent means ± SD from three replicates. The asterisks indicate significant difference (one-tail t-test, compared with control, P < 0.01).
BjMYB1 is involved in positive regulation of plant defence against B. cinerea
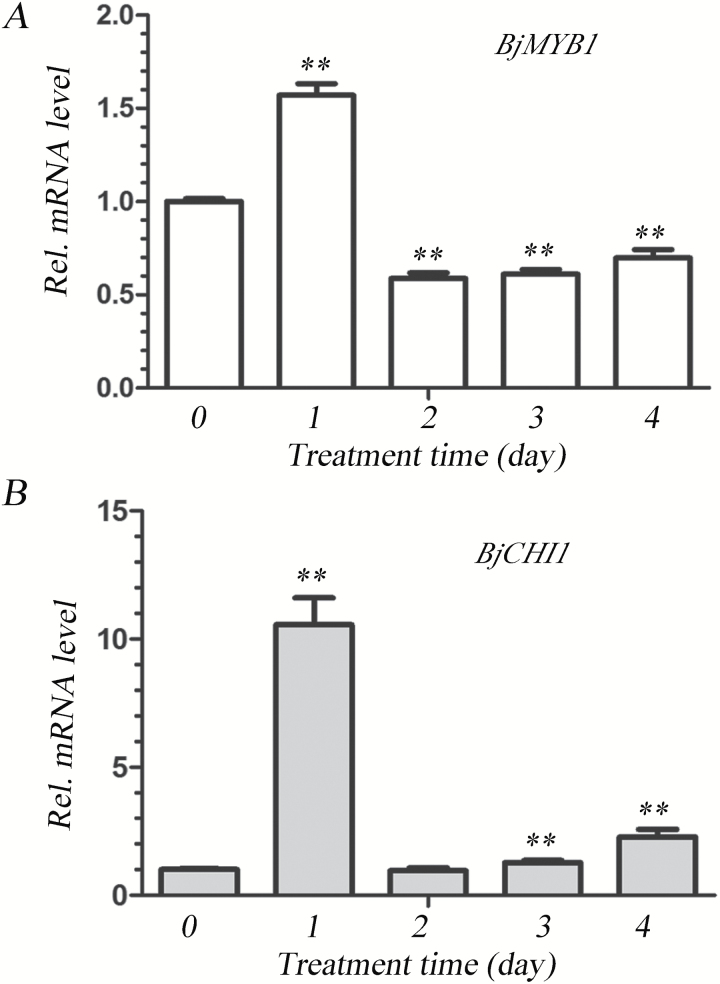
It has been shown that the two-chitin-binding-domains chitinase BjCHI1 has anti-fungal activities against B. cinerea (Guan and Chye, 2008; Guan et al., 2008). To investigate the function of BjMYB1 and its association with BjCHI1 in the regulation of plant defence against B. cinerea, we examined the expression levels of BjMYB1 and BjCHI1 in their native host B. juncea upon infection by B. cinerea. qPCR analysis showed that the transcription of BjMYB1 was strongly induced by B. cinerea, reaching a peak at 1 day post inoculation (Fig. 6A). The expression pattern of BjMYB1 was similar to that of BjCHI1 (Fig. 6B). This result along with the aforementioned in vivo BjC-P promoter activation results suggests that BjMYB1 is potentially involved in host defence against fungal attack by activating the expression of BjCHI1 by binding to the Wbl-4 element in the BjC-P promoter.
Fig. 6.
mRNA expression of BjMYB1 (A) and BjCHI1 (B) in native host plant B. juncea responding to infection by B. cinerea. Leaves of B. juncea seedlings were inoculated with B. cinerea and harvested at 1 d, 2 d, 3 d, and 4 d post inoculation, respectively. Total RNAs were extracted for qPCR analysis. The actin gene of B. juncea was used as an internal control. All real-time results were normalized with respect to the expression level of that at 0 d. The data are shown as mean values ± SD from three replicates. The asterisks indicate significant difference (two-tail t-test, compared with control, P < 0.01).
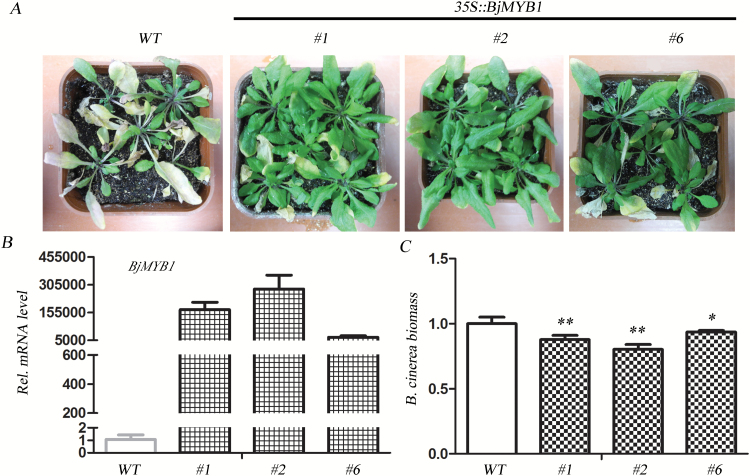
To further evaluate the function of BjMYB1 in plant defence against B. cinerea, we generated stable transgenic A. thaliana plants overexpressing BjMYB1 by Agrobacterium-mediated transformation. Twelve independent transgenic plants were generated and three homozygous T2 lines were used for intensive assays. Seedlings of the transgenic A. thaliana were inoculated with B. cinerea, and the disease was assessed by disease severity and B. cinerea biomass in inoculated leaves (Veronese et al., 2006; Weiberg et al., 2013). As shown in Fig. 7, serious disease symptoms appeared on the leaves of the wild type at 3 weeks post inoculation (wpi) (Fig. 7A). In contrast, the transgenic A. thaliana plants overexpressing BjMYB1 were relatively healthy at 3 wpi, indicating an enhanced resistance to B. cinerea (Fig. 7A). At the mature stage, the leaves of wild type had almost died and the plants produced few pods (see Supplementary Fig. S3 at JXB online). In contrast, the leaves of the BjMYB1-transgenic A. thaliana lines were still green and healthy and the plants produced lots of pods (Supplementary Fig. S3). In concordance with the disease phenotypes, the disease-resistant BjMYB1-expressing transgenic lines showed higher mRNA levels of BjMYB1 (Fig. 7B) and lower B. cinerea biomass compared with the wild type (Fig. 7C). These results demonstrate that BjMYB1 plays a positive role in plant defence against B. cinerea.
Fig. 7.
BjMYB1-overexpressing A. thaliana plants exhibit enhanced disease resistance to B. cinerea. (A) Phenotypes of the wild-type Col-0 (WT) and three BjMYB1-overexpressing A. thaliana lines (#1, #2, and #6) at 3 weeks post inoculation with B. cinerea. (B) qPCR analysis on BjMYB1 expression in transgenic A. thaliana lines inoculated with B. cinerea. The real-time results were normalized with respect to the expression level in wild-type Col-0. (C) The biomass of B. cinerea in wild-type Col-0 and BjMYB1-overexpressing A. thaliana leaves inoculated with B. cinerea was measured by qPCR. The asterisks indicate significant difference (one-tail t-test, compared with WT, **P < 0.01; *P < 0.05).
Discussion
Understanding plant defences at molecular level will help breeders to utilize the resistance mechanisms in crop improvement. Based on our previous findings that the BjCHI1 promoter BjC-P is multi-stress responsive (Wu et al., 2009) and that Wbl-4 is the core element responsive to B. cinerea infection (Gao et al., 2014), we have carried out further analysis on the molecular mechanism of BjC-P-mediated fungal resistance. We have isolated an R2R3-MYB TF, BjMYB1, from B. juncea by Y1H screening and shown that BjMYB1 binds to the Wbl-4 element in vitro, as well as that overexpression of BjMYB1 can activate the BjC-P promoter by acting with the Wbl-4 element in vivo. Deletion of the core sequence TGAC in the Wbl-4 element completely abolished the interaction between BjMYB1 and the Wbl-4 in both yeast and tobacco. In addition, the B. cinerea-induced mRNA expression pattern of BjMYB1 was similar to that of BjCHI1 in the native host plant B. juncea. Overexpression of BjMYB1 in stable, transgenic A. thaliana plants enhanced host plant resistance to B. cinerea. These results suggest that BjMYB1 might be involved in plant defence against fungal infection by interacting with the Wbl-4 element and regulating the expression of BjCHI1 for host plant defence.
Previous investigations have revealed that W-box element (T/C)TGAC(C/T) or Wbl elements (containing a core motif TGAC) are recognized by WRKY transcriptional factors (Eulgem et al., 2000; Eulgem and Somssich, 2007; Rushton et al., 2010; Chi et al., 2013; Lu et al., 2013). In this study, our results showed that the isolated BjMYB1, an R2R3-type MYB transcriptional factor, could bind to the Wbl-4 element. However, most reported DNA targets of plant R2R3-MYB TFs are AC elements (Grotewold et al., 1994; Patzlaff et al., 2003a, b; Goicoechea et al., 2005; Fornalé et al., 2010; Prouse and Campbell, 2012, 2013). To our surprise, when the GTGACT sequence of Wbl-4 was changed into the typical W-box element (TTGACC), BjMYB1 still showed strong binding ability (Fig. 4, W4-d2), but very faint binding affinity to the AC element in W4-d3 (Fig. 4, W4-d3) and no binding affinity to the typical AC element in the PAL2Pro probe (Fig. 4, PAL2Pro). This is consistent with the recent finding that R2R3-MYBs can recognize a variety of DNA motifs and the binding specificities are relevant to their biological roles (Kelemen et al., 2015). Further, Wang and colleagues have demonstrated that the RAV (TaRAV) protein, an AP2/ERF and B3 domain-containing TF, can bind to a W-box motif (CTGACT) in the promoter of TaeIF5A1 (Wang et al., 2012). Based on these emerging factors, it seems conceivable that BjMYB1 binds to the Wbl-4 element to regulate plant defence against fungus.
We also examined the interactions between BjMYB1 and another five Wbl elements in the BjC-P by EMSA assays and found that BjMYB1 also showed faint binding to Wbl-1, Wbl-3, and Wbl-6, but not to Wbl-2 or Wbl-5 (see Supplementary Fig. S4 at JXB online); this variation might be conferred by the delicate differences in the sequences flanking the core motif (TGAC) in the six Wbl elements. Ciolkowski et al. (2008) and Brand et al. (2010) also found that additional nucleotide sequences flanking W-box elements conferred a certain level of specificity of binding. Based on our previous finding that BjC-P is a multi-stress inducible promoter, responsive not only to fungal attack but also to abiotic stresses such as salt, drought, and wounding (Wu et al., 2009), we surmise that BjMYB1 might also be involved in responses to abiotic stresses by interacting with some of the Wbl elements. Furthermore, W-box is involved in the regulation of NaCl-inducible expression of AtWRKY25 and AtWRKY33 (Jiang and Deyholos, 2009) and many Botrytis-induced genes, such as the BOS1 R2R3MYB TF protein in Arabidopsis that is required for biotic and abiotic stress responses (AbuQamar et al., 2006; Mengiste et al., 2003). It is evident that our present data cannot fully explain in vivo functional binding of BjMYB1 to the Wbl elements. Nonetheless, our results provide important information that it is the coupling of the flanking sequence around the W-box core motif (TGAC) that defines a specific functional element.
The ectopic overexpression of BjMYB1 in heterologous A. thaliana strengthened its resistance to B. cinerea. However, it is a pity that our present data cannot fully elucidate the impact of BjMYB1 expression on the native regulatory networks. It is known that plant resistance to B. cinerea is determined by multiple host and environmental factors. In contrast to responses to biotrophic pathogens, which are governed by gene-for-gene interactions, resistance to B. cinerea likely requires contributions from multiple genes for full resistance (Stracke et al., 2001; Windram et al., 2012). Plant resistance to B. cinerea likely involves a complex genetic network which requires plant hormone synthesis and signalling, removal of reactive oxygen species, and biotic and abiotic stress responses (AbuQamar et al., 2006); indeed, Ma and colleagues reported that some genes containing the same motifs could form expression modules in a co-expression network depending on their expression patterns (Ma et al., 2013). For instance, the module I genes of the MYB TF family regulated by the MYB motif CCwACC (with ‘w’ standing for ‘A’ or ‘C’) are involved in the response to the fungal pathogen B. cinerea in a co-expression network (Ma et al., 2013). Thus, whether or not other regulatory factor(s) are coupled with BjMYB1 to co-defend against B. cinerea infection is still unknown. A phylogram analysis showed that A. thaliana harbours BjMYB1 orthologs that would presumably target similar promoters, implying the possibility that BjMYB1 interacts or competes with endogenous MYBs or even WRKYs, resulting in the resistance of the BjMYB1-overexpressing transgenic A. thaliana plants (Fig. 7).
Finally, BjC-P is a multi-stress inducible promoter which is responsive to treatments of jasmonic acid, fungi, drought, and salt (Wu et al., 2009). Detailed characterization of the signalling pathways associated with BjC-P and BjMYB1 might provide new insights into the development of crops with enhanced tolerance to necrotrophic pathogens.
Supplementary data
Supplementary data are available at JXB online.
Table S1. The oligonucleotide sequences used in this study.
Fig. S1. Alignments of amino acid sequences of BjMYB1 and similar MYB-type proteins from Brassica, Arabidopsis, and Oryza.
Fig. S2. Expression of His-BjMYB1 fusion protein in E. coli Rosetta (DE3).
Fig. S3. BjMYB1-overexpressing Arabidopsis plants exhibited enhanced disease resistance to B. cinerea at mature stage.
Fig. S4. EMSA analyses for the interaction between BjMYB1 and five other Wbl elements in BjC-P.
Acknowledgements
We thank Dr Hui Deng (Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences) for providing the B. cinerea strain. We thank Dr Caige Lu (Institute of Plant Protection, Beijing Academy of Agricultural and Forestry Sciences), and Dr Yuhong Yang (Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences) for their help with culturing the B. cinerea. We are grateful to Dr Letian Chen (South China Agricultural University) for help in ordering the fungal elicitor. We are also grateful to Dr Yan Guo (Chinese Agricultural University) for his valuable suggestions for revisions of this manuscript. This work was supported by the National Natural Science Foundation of China (31071484).
Glossary
Abbreviations:
- AbA
aureobasidin A
- B. cinerea
Botrytis cinerea
- B. juncea
Brassica juncea
- EMSA
electrophoretic mobility shift assay
- qRT-PCR
quantitative real-time-PCR
- TF
transcription factor
- Wbl
W-box-like
- Wbl-4
W-box-like-4
- wpi
weeks post inoculation.
References
- AbuQamar S, Chen X, Dhawan R, Bluhm B, Salmeron J, Lam S, Dietrich RA, Mengiste T. 2006. Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to Botrytis infection. The Plant Journal 48, 28–44. [DOI] [PubMed] [Google Scholar]
- Berrocal-Lobo M, Molina A, Solano R. 2002. Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. The Plant Journal 29, 23–32. [DOI] [PubMed] [Google Scholar]
- Brand LH, Fischer NM, Harter K, Kohlbacher O, Wanke D. 2013. Elucidating the evolutionary conserved DNA-binding specificities of WRKY transcription factors by molecular dynamics and in vitro binding assays. Nucleic Acids Research 41, 9764–9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand LH, Kirchler T, Hummel S, Chaban C, Wanke D. 2010. DPI-ELISA: a fast and versatile method to specify the binding of plant transcription factors to DNA in vitro. Plant Methods 6, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen Z. 2002. Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiology 129, 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Hamada S, Fujiwara M, Zhu T, Thao NP, Wong HL, Krishna P, Ueda T, Kaku H, Shibuya N. 2010. The Hop/Sti1-Hsp90 chaperone complex facilitates the maturation and transport of a PAMP receptor in rice innate immunity. Cell Host and Microbe 7, 185–196. [DOI] [PubMed] [Google Scholar]
- Chen W, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T, Mauch F, Luan S, Zou G, Whitham SA. 2002. Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. The Plant Cell 14, 559–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Yang Y, Zhou Y, Zhou J, Fan B, Yu JQ, Chen Z. 2013. Protein–protein interactions in the regulation of WRKY transcription factors. Molecular Plant 6, 287–300. [DOI] [PubMed] [Google Scholar]
- Chujo T, Sugioka N, Masuda Y, Shibuya N, Takemura T, Okada K, Nojiri H, Yamane H. 2009. Promoter analysis of the elicitor-induced WRKY gene OsWRKY53, which is involved in defense responses in rice. Bioscience, Biotechnology, and Biochemistry 73, 1901–1904. [DOI] [PubMed] [Google Scholar]
- Ciolkowski I, Wanke D, Birkenbihl RP, Somssich IE. 2008. Studies on DNA binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Molecular Biology 68, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J. 2012. The top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology 13, 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Chen C, Chen Z. 2003. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Molecular Biology 51, 21–37. [DOI] [PubMed] [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. 2010. MYB transcription factors in Arabidopsis . Trends in Plant Science 15, 573–581. [DOI] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE. 2000. The WRKY superfamily of plant transcription factors. Trends in Plant Science 5, 199–206. [DOI] [PubMed] [Google Scholar]
- Eulgem T, Somssich IE. 2007. Networks of WRKY transcription factors in defense signaling. Current Opinion in Plant Biology 10, 366–371. [DOI] [PubMed] [Google Scholar]
- Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornalé S, Shi X, Chai C, Encina A, Irar S, Capellades M, Fuguet E, Torres JL, Rovira P, Puigdomènech P. 2010. ZmMYB31 directly represses maize lignin genes and redirects the phenylpropanoid metabolic flux. The Plant Journal 64, 633–644. [DOI] [PubMed] [Google Scholar]
- Gao Y, Gong X, Cao W, Zhao J, Fu L, Wang X, Schumaker KS, Guo Y. 2008. SAD2 in Arabidopsis functions in trichome initiation through mediating GL3 function and regulating GL1, TTG1 and GL2 expression. Journal of Integrative Plant Biology 50, 906–917. [DOI] [PubMed] [Google Scholar]
- Gao Y, Zan XL, Wu XF, Yao L, Chen YL, Jia SW, Zhao KJ. 2014. Identification of fungus-responsive cis-acting element in the promoter of Brassica juncea chitinase gene, BjCHI1 . Plant Science 215-216, 190–198. [DOI] [PubMed] [Google Scholar]
- Goicoechea M, Lacombe E, Legay S, Mihaljevic S, Rech P, Jauneau A, Lapierre C, Pollet B, Verhaegen D, Chaubet-Gigot N. 2005. EgMYB2, a new transcriptional activator from Eucalyptus xylem, regulates secondary cell wall formation and lignin biosynthesis. The Plant Journal 43, 553–567. [DOI] [PubMed] [Google Scholar]
- Grotewold E, Drummond BJ, Bowen B, Peterson T. 1994. The myb-homologous P gene controls phlobaphene pigmentation in maize floral organs by directly activating a flavonoid biosynthetic gene subset. Cell 76, 543–553. [DOI] [PubMed] [Google Scholar]
- Guan Y, Chye ML. 2008. A Brassica juncea chitinase with two-chitin binding domains show anti-microbial properties against phytopathogens and Gram-negative bacteria. Plant Signaling and Behavior 3, 1103–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Ramalingam S, Nagegowda D, Taylor PW, Chye ML. 2008. Brassica juncea chitinase BjCHI1inhibits growth of fungal phytopathogens and agglutinates Gram-negative bacteria. Journal of Experimental Botany 59, 3475–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Dong Q, Yu D. 2012. Arabidopsis WRKY46 coordinates with WRKY70 and WRKY53 in basal resistance against pathogen Pseudomonas syringae . Plant Science 185, 288–297. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Deyholos MK. 2009. Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Molecular Biology 69, 91–105. [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. 2006. The plant immune system. Nature 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Katiyar A, Smita S, Lenka SK, Rajwanshi R, Chinnusamy V, Bansal KC. 2012. Genome-wide classification and expression analysis of MYB transcription factor families in rice and Arabidopsis . BMC Genomics 13, 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen Z, Sebastian A, Xu W, et al. 2015. Analysis of the DNA-binding activities of the Arabidopsis R2R3-MYB transcription factor family by one-hybrid experiments in yeast. PLoS One 10, e0141044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z, Vinod K, Zheng Z, Fan B, Chen Z. 2008. Roles of Arabidopsis WRKY3 and WRKY4 transcription factors in plant responses to pathogens. BMC Plant Biology 8, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MW, Qi M, Yang Y. 2001. A novel jasmonic acid-inducible rice myb gene associates with fungal infection and host cell death. Molecular Plant-Microbe Interactions 14, 527–535. [DOI] [PubMed] [Google Scholar]
- Lin H, Du W, Yang Y, Schumaker KS, Guo Y. 2014. A calcium-independent activation of the Arabidopsis SOS2-like protein kinase24 by its interacting SOS3-like calcium binding protein1. Plant Physiology 164, 2197–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhou X, Dong N, Liu X, Zhang H, Zhang Z. 2011. Expression of a wheat MYB gene in transgenic tobacco enhances resistance to Ralstonia solanacearum, and to drought and salt stresses. Functional and Integrative Genomics 11, 431–443. [DOI] [PubMed] [Google Scholar]
- Liu X, Yang L, Zhou X, Zhou M, Lu Y, Ma L, Ma H, Zhang Z. 2013. Transgenic wheat expressing Thinopyrum intermedium MYB transcription factor TiMYB2R-1 shows enhanced resistance to the take-all disease. Journal of Experimental Botany, 64, 2243–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lu X, Jiang W, Zhang L, Zhang F, Zhang F, Shen Q, Wang G, Tang K. 2013. AaERF1 positively regulates the resistance to Botrytis cinerea in Artemisia annua . PloS One 8, e57657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Shah S, Bohnert HJ, Snyder M, Dinesh-Kumar SP. 2013. Incorporating motif analysis into gene co-expression networks reveals novel modular expression pattern and new signaling pathways. PLoS Genetics 9, e1003840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengiste T, Chen X, Salmeron J, Dietrich R. 2003. The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis . The Plant Cell 15, 2551–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzlaff A, McInnis S, Courtenay A, Surman C, Newman LJ, Smith C, Bevan MW, Mansfield S, Whetten RW, Sederoff RR. 2003. a. Characterisation of a pine MYB that regulates lignification. The Plant Journal 36, 743–754. [DOI] [PubMed] [Google Scholar]
- Patzlaff A, Newman LJ, Dubos C, Whetten RW, Smith C, McInnis S, Bevan MW, Sederoff RR, Campbell MM. 2003. b. Characterisation of PtMYB1, an R2R3-MYB from pine xylem. Plant Molecular Biology 53, 597–608. [DOI] [PubMed] [Google Scholar]
- Peng SQ, Wu KX, Huang GX, Chen SC. 2011. HbMyb1, a Myb transcription factor from Hevea brasiliensis, suppresses stress induced cell death in transgenic tobacco. Plant Physiology and Biochemistry 49, 1429–1435. [DOI] [PubMed] [Google Scholar]
- Prouse MB, Campbell MM. 2012. The interaction between MYB proteins and their target DNA binding sites. Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms 1819, 67–77. [DOI] [PubMed] [Google Scholar]
- Prouse MB, Campbell MM. 2013. Interactions between the R2R3-MYB transcription factor, AtMYB61, and target DNA binding sites. PloS One 8, e65132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaele S, Rivas S. 2013. Regulate and be regulated: integration of defense and other signals by the AtMYB30 transcription factor. Frontiers in Plant Science 4, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raventos D, Jensen AB, Rask MB, Casacuberta JM, Mundy J, Segundo B. 1995. A 20bp cis-acting element is both necessary and sufficient to mediate elicitor response of a maize PRms gene. The Plant Journal 7, 147–155. [DOI] [PubMed] [Google Scholar]
- Romero I, Fuertes A, Benito MJ, Malpica JM, Leyva A, Paz-Ares J. 1998. More than 80 R2R3-MYB regulatory genes in the genome of Arabidopsis thaliana . The Plant Journal 14, 273–284. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Reinstädler A, Lipka V, Lippok B, Somssich IE. 2002. Synthetic plant promoters containing defined regulatory elements provide novel insights into pathogen-and wound-induced signaling. The Plant Cell 4, 749–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton PJ, Somssich IE. 1998. Transcriptional control of plant genes responsive to pathogens. Current Opinion in Plant Biology 1, 311–315. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Somssich IE, Ringler P, Shen QJ. 2010. WRKY transcription factors. Trends in Plant Science 15, 247–258. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Torres JT, Parniske M, Wernert P, Hahlbrock K, Somssich I. 1996. Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. The EMBO Journal 15, 5690–5700. [PMC free article] [PubMed] [Google Scholar]
- Segarra G, Van der Ent S, Trillas I, Pieterse C. 2009. MYB72, a node of convergence in induced systemic resistance triggered by a fungal and a bacterial beneficial microbe. Plant Biology 11, 90–96. [DOI] [PubMed] [Google Scholar]
- Seo PJ, Park CM. 2010. MYB96-mediated abscisic acid signals induce pathogen resistance response by promoting salicylic acid biosynthesis in Arabidopsis . New Phytologist 186, 471–483. [DOI] [PubMed] [Google Scholar]
- Seo PJ, Xiang F, Qiao M, Park JY, Lee YN, Kim SG, Lee YH, Park WJ, Park CM. 2009. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis . Plant Physiology 151, 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B. 2001. The R2R3-MYB gene family in Arabidopsis thaliana . Current Opinion in Plant Biology 4, 447–456. [DOI] [PubMed] [Google Scholar]
- Veronese P, Nakagami H, Bluhm B, Abuqamar S, Chen X, Salmeron J, Dietrich RA, Hirt H, Mengiste T. 2006. The membrane-anchored BOTRYTIS-INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. The Plant Cell 18, 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhang X, Fan Y, et al. 2015. XA23 is an executor R protein and confers broad-spectrum disease resistance in rice. Molecular Plant 8, 290–302. [DOI] [PubMed] [Google Scholar]
- Wang L, Xu C, Wang C, Wang Y. 2012. Characterization of a eukaryotic translation initiation factor 5A homolog from Tamarix androssowii involved in plant abiotic stress tolerance. BMC Plant Biology 12, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Tanaka K, Zhang XC, Son GH, Brechenmacher L, Nguyen THN, Stacey G. 2012. LYK4, a lysin motif receptor-like kinase, is important for chitin signaling and plant innate immunity in Arabidopsis . Plant Physiology 160, 396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiberg A, Wang M, Lin FM, Zhao H, Zhang Z, Kaloshian I, Huang HD, Jin H. 2013. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 342, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windram O, Madhou P, McHattie S, Hill C, Hickman R, Cooke E, Jenkins DJ, Penfold CA, Baxter L, Breeze E. 2012. Arabidopsis defense against Botrytis cinerea: chronology and regulation deciphered by high-resolution temporal transcriptomic analysis. The Plant Cell 24, 3530–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XF, Wang CL, Xie EB, Gao Y, Fan YL, Liu PQ, Zhao KJ. 2009. Molecular cloning and characterization of the promoter for the multiple stress-inducible gene BjCHI1 from Brassica juncea . Planta 229, 1231–1242. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Kigawa T, Watanabe S, Inoue M, Yamasaki T, Seki M, Shinozaki K, Yokoyama S. 2012. Structural basis for sequence-specific DNA recognition by an Arabidopsis WRKY transcription factor. Journal of Biological Chemistry 287, 7683–7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Nakano T, Suzuki K, Shinshi H. 2004. Elicitor-induced activation of transcription via W box-related cis-acting elements from a basic chitinase gene by WRKY transcription factors in tobacco. Biochimica et Biophysica Acta (BBA)-Gene Structure and Expression 1679, 279–287. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu X, Wang X, Zhou M, Zhou X, Ye X, Wei X. 2012. An R2R3 MYB transcription factor in wheat, TaPIMP1, mediates host resistance to Bipolaris sorokiniana and drought stresses through regulation of defense-and stress-related genes. New Phytologist 196, 1155–1170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.