ABSTRACT
Red/Far Red (R/FR) sensing positively influences the arbuscular mycorrhizal (AM) symbiosis of both legume and nonlegume plants through jasmonic acid (JA) and strigolactone signaling. We previously reported that root exudates obtained from high R/FR-grown plants contained more strigolactone than low R/FR-grown plants. To determine whether JA and JA derivatives were secreted from roots, we investigated the expression levels of JA-responsive genes in L. japonicus Miyakojima MG20 plants treated with root exudates prepared from either high or low R/FR light-treated plants. The root exudates from high R/FR light-treated plants were found to enhance the expression levels of JA-responsive genes significantly. Moreover, exogenous JA increased AM fungal hyphal elongation as did the root exudates derived from high R/FR-grown L. japonicus plants. We conclude that increased JA accumulation and secretion into root exudates from high R/FR light-grown plants is the best explanation for increased colonization and enhanced mycorrhization under these conditions.
KEYWORDS: Arbuscular mycorrhizal fungi, jasmonic acid, root exudate
Arbuscular Mycorrhizal (AM) fungi establish a phosphate-acquiring symbiosis with the majority of higher plants, and thus this symbiosis represents the most widespread association between plant roots and fungi in natural and agricultural ecosystems. AM fungi populate root cortical cells and obtain carbon provided by the plant while transferring mineral nutrients from the soil to the roots. AM fungi persist in soil as spores, and upon germinating, the hyphal germ tube grows through the soil in search of a host plant root. Once the AM fungal hyphae contact the root epidermal cells, they enter the apoplast of the host plant root. These intraradical hyphae extend into the intercellular spaces of root cortical cells adjacent to the stele, and the symbiotic structures, arbuscules, are formed by hyphae being enveloped by the host membrane of the cortical cells. Extraradical hyphae remain outside the root to explore the soil environment to capture and transfer nutrients. The complex network of AM fungal hyphae, both extra- and intraradical, facilitates the uptake of water and phosphate from soil, in return for carbon from the host plant.1-3
It is well known that plants monitor both light quality and quantity for survival. Plants have photoreceptors that sense the presence of their neighbors by monitoring the R/FR ratio. A low R/FR ratio indicates the presence of neighbors that may compete for photosynthetically active radiation (PAR). Low R/FR perception initiates the shade avoidance syndrome (SAS), whereby crowding causes plants to grow taller or bend toward the light to avoid shade.4-7 These low R/FR light conditions, however, are suboptimal for establishing both root nodule and AM symbioses. For the root-nodule symbiosis, we previously showed that JA-responsive gene expression was decreased in low R/FR light-treated L. japonicus plants and was correlated with reduced jasmonoyl-isoleucine (JA-Ile) content in phyB mutants.8 We determined in AM-inoculated Solanum lycopersicum that the expression of SlAOS (allene oxide synthase) and SlLOX (lipoxygenase), which are involved in JA biosynthesis, increased in high R/FR light-grown plant roots compared to low R/FR light-grown plant roots.9 An additional finding was that the root exudates derived from high R/FR light-treated plants contained more strigolactone, an AM-fungal hyphal branching inducer, than did the low R/FR light-grown plants.9
Because previous studies reported that wheat seedling root exudates contain JA,10 we predicted that root exudates from L. japonicus also contain JA. Moreover, because JA affects AM fungal colonization,14 we further predicted that light would influence the amount of JA secreted into the rhizosphere. Earlier, we tried to measure the amounts of JA and JA derivatives directly from root exudates prepared from either high or low R/FR light-treated uninoculated plants.9 However, even though exudates from 50 plants were analyzed by LC/MS, we could not definitely find any JA-related compounds, most likely due to their very low concentrations and the presence of impurities. Therefore, to evaluate JA and JA derivative content, we took an indirect approach and studied the expression levels of JA-responsive genes in WT MG20 plants treated with root exudates prepared from either high or low R/FR light-treated plants. First, L. japonicus Miyakojima MG20 plants were cultivated in 1/2 strength Hoagland's liquid medium for 3 weeks under low or high R/FR light regimes. The liquid media from the different treatments were concentrated 50-fold and filtrated through a 0.45 μm nylon filter membrane, after which the filtrate was used as a root exudate preparation. When root exudates from high R/FR and low R/FR light-grown plants were added to WT MG20 roots, the expression levels of LjJAR1 and LjPDF1.2, 2 well known JA-responsive genes, were significantly enhanced above the levels compared to plants treated with the low R/FR light-grown exudates (Fig. 1A, B).8 The LjPDF1.2 gene encodes a plant defensin that is implicated in JA-dependent defense responses.11 These data strongly suggest that JA levels are elevated in response to root exudates of high R/FR light-grown plants, and not in response to exudates from low R/FR light-grown plants.
Figure 1.
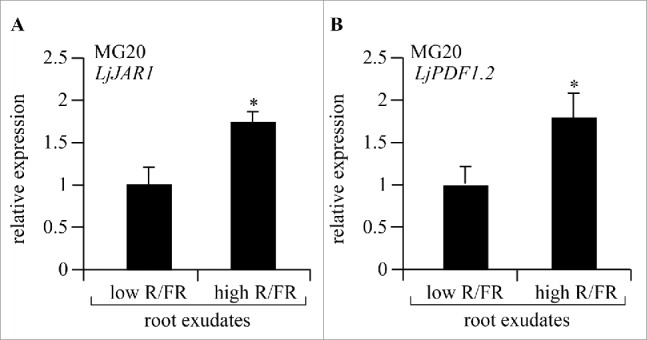
Expression of L. japonicus JA-responsive genes after treatment with root exudates. Relative expression of (A) LjJAR1 (n = 9) and (B) LjPDF1.2 (n = 9) 48 hours after treatment with root exudates derived from either high or low R/FR light-grown plants. Transcript amounts were normalized against ATP synthase transcripts. The mean value of expression in plants grown with root exudates prepared from low R/FR light-treated plants was set as one. The data represent averages ± SE of 3 independent experiments. Error bars represent SE and a statistically significant difference is indicated by asterisks (p < 0.05 by Student's t-test).
Next, we examined the effect of low and high R/FR light-grown plant-derived exudates on hyphal elongation to test whether the exudates might have an effect on AM fungi (Rhizophagus irregularis, Premier Tech Biotechnologies) in the rhizosphere. As shown in Fig. 2, no significant difference in hyphal length was observed between mock- and low R/FR-grown plant-derived root exudates. However, the root exudates prepared from the high R/FR light-grown plants significantly increased hyphal length compared to root exudates derived from low R/FR light-grown plants, strongly suggesting that high R/FR light-grown plant root exudates contain more compound(s) that stimulate(s) hyphal elongation.
Figure 2.
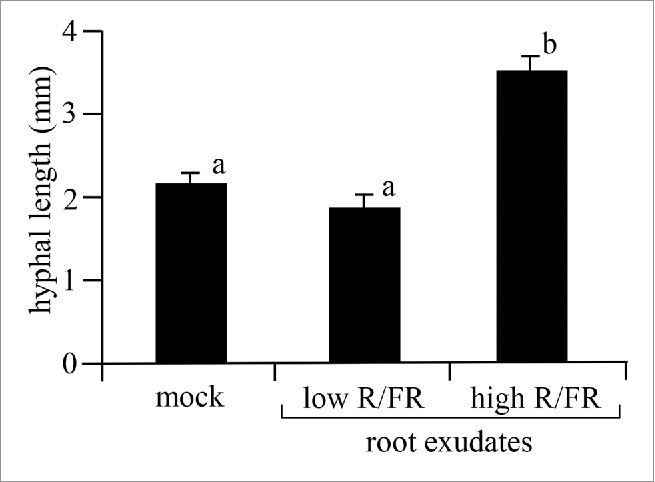
Effect of root exudates derived from plants grown in low and high R/FR light on hyphal elongation. The increase in hyphal length was measured 2 weeks after treatment on M medium containing root exudate from either low (n = 50) or high R/FR (n = 50) light-grown plants. The mock (n = 50) treatment consists of 50-fold concentrated 1/2 strength Hoagland's liquid medium. The data represent averages ± SE of independent experiments. Error bars represent SE. Means denoted by the same letter do not differ significantly at P < 0.01 (Tukey multiple comparison test).
We also studied the direct effect of JA on AM-fungal hyphal elongation under aseptic conditions, i.e. on M medium.12 As shown in Fig. 3, hyphal length significantly increased when 0.5 μM JA was added and at all the tested incubation times. Taken together, the combination of results strongly supports the possibility that JA in root exudates derived from high R/FR light-grown plants induced hyphal elongataion. In our previous study,9 we showed that root exudates obtained from high R/FR-grown plants contained more strigolactone than exudates from low R/FR-grown plants. However, crude root exudates contain many compounds including amino acids, organic acids, sugars, phenolics, and other secondary metabolites.13 Thus, we cannot dismiss the possibility that compound(s) other than or in combination with JA stimulate hyphal elongation. This hypothesis needs further testing.
Figure 3.
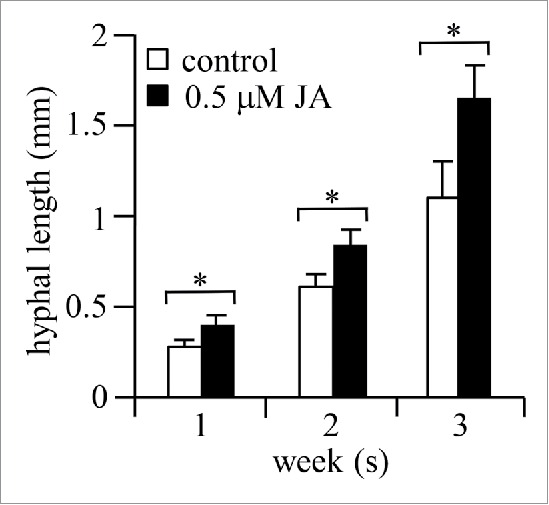
Effect of 0.5 μM JA on hyphal elongation. Extent of hyphal elongation (mm) observed in the dark 1, 2 and 3 weeks after plating spores on M medium containing 0.5 μM JA (n = 118). The data represent averages ± SE of independent experiments. Error bars represent SE and a statistically significant difference is indicated by asterisks (p < 0.05 by Student's t-test).
Based on the data, we conclude that JA accumulates in high R/FR light-grown plants and is most likely secreted from roots into the rhizosphere at higher levels compared to plants growing under low R/FR light conditions. These root exudates promote hyphal elongation of AM fungi in the rhizosphere. Under low R/FR light conditions, the levels of accumulated and secreted JA are reduced compared to those found in high R/FR light-grown plants. As a result, AM-colonization is reduced because exudates from low R/FR light-grown plants do not promote fungal hyphal growth. Previous studies support this conclusion. JA-defective mutants exhibited reduced mycorrhizal colonization14 and furthermore, suppression of JA biosynthetic gene expression in Medicago truncatula roots resulted in reduced JA levels, which negatively affected both mycorrhizal colonization and arbuscule formation.15 JA also functions as a positive regulator of hyphal elongation (Fig. 3). Therefore, a reduction in JA synthesis and secretion by low R/FR light-grown plants is the most parsimonious explanation for the poor colonization by fungal hyphae and the resulting reduction in mycorrhization.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
L. japonicus Miyakojima MG20 seeds were provided by the National BioResource Project of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Funding
This work was supported by a Grant-in-Aid for Challenging Exploratory Research from the Japan Society for the Promotion of Science (grant no. 24658020 to A.S.).
References
- 1.Harrison MJ. Signaling in the arbuscular mycorrhizal symbiosis. Annu Rev Microbiol 2005; 59:19-42; PMID:16153162; http://dx.doi.org/ 10.1146/annurev.micro.58.030603.123749 [DOI] [PubMed] [Google Scholar]
- 2.Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005; 435:824-7; PMID:15944706; http://dx.doi.org/ 10.1038/nature03608 [DOI] [PubMed] [Google Scholar]
- 3.Bolan NS. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 1991; 134:189-207; http://dx.doi.org/ 10.1007/BF00012037 [DOI] [Google Scholar]
- 4.Flanklin KA, Quail PH. Phytochrome functions in Arabidopsis development. J Exp Bot 2010; 61:11-24; PMID:19815685; http://dx.doi.org/ 10.1093/jxb/erp304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith H, Whitelam GC. The shade avoidance syndrome: Multiple responses mediated by multiple phytochromes. Plant Cell Environ 1997; 20:840-4; PMID:17238911; http://dx.doi.org/ 10.1046/j.1365-3040.1997.d01-104.x17238911 [DOI] [Google Scholar]
- 6.Neff M, M Fankhauser C, Chory J. Light: An indicator of time and place. Genes Dev 2000; 14:257-71; PMID:10673498; http://dx.doi.org/ 10.1101/gad.14.3.257 [DOI] [PubMed] [Google Scholar]
- 7.Flanklin KA. Shade avoidance. New Phytol 2008; 179:930-44; PMID:18537892; http://dx.doi.org/ 10.1111/j.1469-8137.2008.02507.x [DOI] [PubMed] [Google Scholar]
- 8.Suzuki A, Suriyagoda L, Shigeyama T, Tominaga A, Sasaki M, Hiratsuka Y, Yoshinaga A, Arima S, Agarie S, Sakai T, et al.. Lotus japonicus nodulation is photomorphogenetically controlled by sensing the red/far red (R/FR) ratio through jasmonic acid (JA) signaling. Proc Natl Acad Sci USA 2011; 108:16837-42; PMID:21930895; http://dx.doi.org/ 10.1073/pnas.1105892108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagata M, Yamamoto N, Shigeyama T, Terasawa Y, Anai T, Sakai T, Inada S, Arima S, Hashiguchi M, Akashi R, et al.. Red/far red light controls arbuscular mycorrhizal colonization via jasmonic acid and strigolactone signaling. Plant Cell Physiol 2015; 56:2100-9; PMID:26412782; http://dx.doi.org/ 10.1093/pcp/pcv135 [DOI] [PubMed] [Google Scholar]
- 10.Dathe W, Parry AD, Heald JK, Scott IM, Miersch O, et al.. Jasmonic acid and abscisic acid in shoots, coleoptiles, and roots of wheat seedlings. J Plant Growth Regul 1994; 13:59-62; http://dx.doi.org/ 10.1007/BF00210947 [DOI] [Google Scholar]
- 11.Brown RL, Kazan K, McGrath KC, Maclean DJ, Manners JM. A role for the GCC-box in jasmonate-mediated activation of the PDF1.2 gene of Arabidopsis. Plant Physiol 2003; 132:1020-32; PMID:12805630; http://dx.doi.org/ 10.1104/pp.102.017814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becard G, Fortin JA. Early events of vesicular-arbuscular mycorrhizal formation on Ri T-DNA transformed roots. New Phytol 1988; 108:211-8; http://dx.doi.org/ 10.1111/j.1469-8137.1988.tb03698.x [DOI] [PubMed] [Google Scholar]
- 13.Narula N, Kothe E, Behl RK. Role of root exudates in plant-microbe interactions. J Appl Bot Food Qual 2009; 82:122-30 [Google Scholar]
- 14.Tejeda-Sartorius M, Martínez de la Vega O, Délano-Frier JP. Jasmonic acid influences mycorrhizal colonization in tomato plants by modifying the expression of genes involved in carbohydrate partitioning. Physiol Plant 2008; 133:339-53; PMID:18331402; http://dx.doi.org/ 10.1111/j.1399-3054.2008.01081.x [DOI] [PubMed] [Google Scholar]
- 15.Isayenkov S, Mrosk C, Stenzel I, Strack S, Hause B. Suppression of allene oxide cyclase in hairy roots of Medicago truncatula reduces jasmonate levels and the degree of mycorrhization with Glomus intraradices. Plant Physiol 2005; 1329:1401-10; PMID:16244141; http://dx.doi.org/ 10.1104/pp.105.069054 [DOI] [PMC free article] [PubMed] [Google Scholar]


