ABSTRACT
In the past, a conventional wisdom has been that abscisic acid (ABA) is a xylem-transported hormone that is synthesized in the roots, while acting in the shoot to close stomata in response to a decrease in plant water status. Now, however, evidence from two studies, which we have conducted independently, challenges this root-sourced ABA paradigm. We show that foliage-derived ABA has a major influence over root development and that leaves are the predominant location for ABA biosynthesis during drought stress.
KEYWORDS: Abscisic acid (ABA), root growth, shoot to root signaling, stomata, water stress
Stomatal closure by abscisic acid (ABA) is essential for seed plant survival during water stress and thus an understanding of where plants synthesize this hormone is of paramount importance. Arguably one of the most influential studies investigating this question is that of Zhang et al.1 The central conclusion drawn from this study was that roots are the primary source of ABA (Fig. 1). This model for ABA biosynthesis has permeated the literature, forming the basis for some of the most widely-cited leaf gas exchange models2, and leading to the popular practice of measuring xylem sap ABA level in studies of drought responses.3 However, doubts surrounding the generality of root-sourced ABA were raised by grafting studies with ABA biosynthetic mutants4,5 as well as evidence for significant ABA biosynthesis in leaves.6,7 This led our two groups to undertake investigations into the conventional wisdom.8,9 Our results contrast strongly with the established dogma and together suggest a new paradigm for the ABA biosynthesis and translocation in water-stressed plants (Fig. 1). Using a combination of experimental approaches, including foliar application of labeled ABA, reciprocal grafting between ABA biosynthetic mutant and wild-type plants, as well as stem girdling to inhibit basipetal phloem transport, we found that functional foliage-derived ABA is readily transported to the roots where it is critical for not only maintaining normal root ABA levels but also for determining root architecture and growth.8,9
Figure 1.
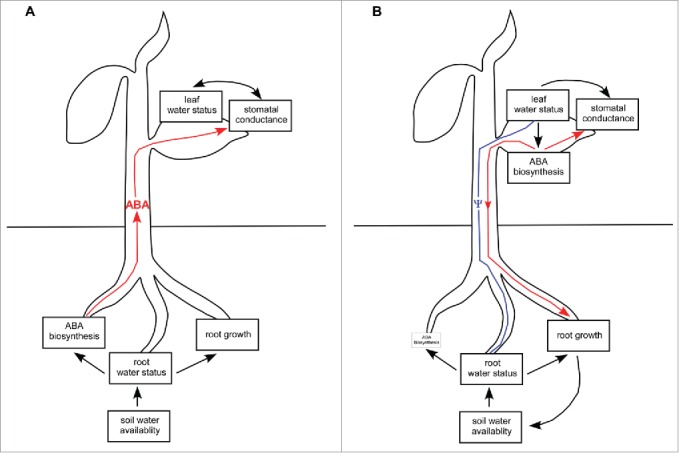
Diagrammatic representations of (A) the classical 'root-sourced' model for ABA biosynthesis and transport (red) in a plant experiencing soil water limitation (adapted from Davies and Zhang12) and (B) a schematic synthesis of the findings from our two recent studies.8,9 We propose a new, ‘leaf-sourced’ model whereby soil water limitation reduces root water status and thus plant water potential (Ψ, blue). A decline in water potential in the roots provides an instantaneous signal through the internal water column in the xylem to the leaves, directly influencing leaf water status. A decline in leaf water status triggers foliar ABA biosynthesis which in turn closes stomata.6 Foliage-derived ABA is basipetally transported from the leaves to the roots where it promotes root growth under both well-watered and water-stressed conditions. The promotion of root growth by foliage-derived ABA feeds back on the soil water available to the plant. Root ABA biosynthesis is minimal, scarcely influencing shoot physiology.
Through reciprocal grafting of ABA biosynthetic mutant and wild-type plants we found that foliar ABA strongly promotes root growth while inhibiting the development of lateral roots, with the overall effect of increasing root biomass investment.8 This finding is in agreement with earlier studies showing drought-induced root growth through enhanced ABA levels.10 Foliar ABA levels fluctuate dynamically in response to changes in both atmospheric and soil water status,11 providing a signal that could link root growth with foliar water stress through the phloem transport of ABA. Thus water limitation sensed in the leaves triggers foliar synthesis of ABA which is transported in the phloem to the roots, promoting root growth to enhance soil water uptake. Furthermore, by observing girdled plants that were exposed to cycles of drought and recovery we found that root ABA accumulation was dependent on a supply of foliage-derived ABA.9 Contrary to the ‘root-sourced’ model, droughted plants with roots that are unable to synthesize ABA, either because of an exhaustion of carotenoid precursor reserves or because of a biochemcial inability to synthesize ABA, were found to have normal increases in foliar ABA level and displayed normal stomatal responses to drought.9
In conclusion, the results of our two studies provide a new model for ABA synthesis and transport in the plant body. This new ‘leaf-sourced model’ views foliage-dervied ABA as predominant, determining not only the stomatal response to drought but also root growth and biomass allocation. ABA biosynthesis in roots occurs to a limited extent only.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Zhang J, Schurr U, Davies WJ.. Control of stomatal behaviour by abscisic acid which apparently originates in the roots. J Exp Bot 1987; 38:1174-81; http://dx.doi.org/ 10.1093/jxb/38.7.1174 [DOI] [Google Scholar]
- 2.Tardieu F, Davies WJ. Integration of hydraulic and chemical signalling in the control of stomatal conductance and water status of droughted plants. Plant Cell Environ 1993; 16:341-9; http://dx.doi.org/ 10.1111/j.1365-3040.1993.tb00880.x [DOI] [Google Scholar]
- 3.Zhang J, Davies WJ.. Changes in the concentration of ABA in xylem sap as a function of changing soil water status can account for changes in leaf conductance and growth. Plant Cell Environ 1990; 13:277-85; http://dx.doi.org/ 10.1111/j.1365-3040.1990.tb01312.x [DOI] [Google Scholar]
- 4.Holbrook NM, Shashidhar VR, James RA, Munns R.. Stomatal control in tomato with ABA-deficient roots: response of grafted plants to soil drying. J Exp Bot 2002; 53:1503-14; PMID:12021298; http://dx.doi.org/ 10.1093/jexbot/53.373.1503 [DOI] [PubMed] [Google Scholar]
- 5.Christmann A, Weiler EW, Steudle E, Grill E.. A hydraulic signal in root-to-shoot signalling of water shortage. Plant J 2007; 52:167-74; PMID:17711416; http://dx.doi.org/ 10.1111/j.1365-313X.2007.03234.x [DOI] [PubMed] [Google Scholar]
- 6.McAdam SAM, Sussmilch FC, Brodribb TJ.. Stomatal responses to vapour pressure deficit are regulated by high speed gene expression in angiosperms. Plant Cell Environ 2016; 39:485-91; PMID:26353082; http://dx.doi.org/ 10.1111/pce.12633 [DOI] [PubMed] [Google Scholar]
- 7.Kuromori T, Sugimoto E, Shinozaki K.. Intertissue signal transfer of abscisic acid from vascular cells to guard cells. Plant Physiol 2014; 164:1587-92; PMID:24521878; http://dx.doi.org/ 10.1104/pp.114.235556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAdam SAM, Brodribb TJ, Ross JJ.. Shoot-derived abscisic acid promotes root growth. Plant Cell Environ 2016; 39:652-9; PMID:26514625; http://dx.doi.org/ 10.1111/pce.12669 [DOI] [PubMed] [Google Scholar]
- 9.Manzi M, Lado J, Rodrigo MJ, Zacarías L, Arbona V, Gómez-Cadenas A. Root ABA accumulation in long-term water-stressed plants is sustained by hormone transport from aerial organs. Plant Cell Physiol 2015; 56:2457-66; PMID:26542111; http://dx.doi.org/ 10.1093/pcp/pcv161 [DOI] [PubMed] [Google Scholar]
- 10.Sharp RE, LeNoble ME.. ABA, ethylene and the control of shoot and root growth under water stress. J Exp Bot 2002; 53:33-7; PMID:11741038; http://dx.doi.org/ 10.1093/jexbot/53.366.33 [DOI] [PubMed] [Google Scholar]
- 11.McAdam SAM, Brodribb TJ.. The evolution of mechanisms driving the stomatal response to vapour pressure deficit. Plant Physiol; 2015. 167:833-43; PMID:25637454; http://dx.doi.org/ 10.1104/pp.114.252940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies WJ, Zhang J.. Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 1991; 42:55-76http://dx.doi.org/ 10.1146/annurev.pp.42.060191.000415 [DOI] [Google Scholar]


