Abstract
Wine has been used since the dawn of human civilization. Despite many health benefits, there is still a lot of discussion about the real properties of its components and its actions on cells and molecular interactions. A large part of these issues permeate the fine line between the amount of alcohol that causes problems to organic systems and the amount that could be beneficial for the health. However, even after the process of fermentation, wine conserves different organic compounds from grapes, such as polysaccharides, acids, and phenolic compounds, such as flavonoids and nonflavonoids. These substances have known anti-inflammatory and antioxidant capacities, and are considered as regulatory agents in cardiometabolic process. In this study, the main chemical components present in the wine, its interaction with molecules and biological mechanisms, and their interference with intra- and extracellular signaling are reviewed. Finally, the properties of wine that may benefit cardiovascular system are also revised.
Keywords: wine, ethanol, flavonoids, cardiovascular system
Introduction
Wine is a traditional alcoholic beverage of high commercial importance, obtained by fermentation of grape must. By this definition, the quality of wine is related to the composition and variety of grape.1 Moreover, wines can be distinguished by the geographic location of vineyards, variations in the same vineyard, different viticultural practices, and winemaking and aging techniques.2
Wine is a complex mixture of several hundred compounds, many of them found at very low concentrations; however, they play an important role in its evolution and quality.3 In general, the average concentrations of the major components of wine are water, 86%; ethanol, 12%; glycerol and polysaccharides or other trace elements, 1%; different types of acids, 0.5%; and volatile compounds, 0.5%.4
Wine may be classified as red, white, and rosé wines based on sweetness, alcohol content, carbon dioxide content, color, grape variety, fermentation, and maturation process or geographic origin.5 While red wines are obtained by the alcoholic fermentation of musts in the presence of the solid parts of the berry (skins and seeds), white wines are exclusively produced by the fermentation of grape juice.6
Red wine is known to contain 10-fold more phenolic compounds than white wine, resulting from the fermentation of grape juice with skins, grape pieces, and seeds (Table 1).1 Although the antioxidant property of red wines is correlated with their phenol content, no single compound sufficiently defines the total antioxidant capacity, because of the potential synergistic antioxidant effect of other compounds.7
Table 1.
Content of majority phenolic compounds of red and white wines, expressed in milligrams of gallic acid equivalent (mg/GAE/L).78,79
| PHENOLIC COMPOUNDS | RED WINE (mg/GAE/L) | WHITE WINE (mg/GAE/L) |
|---|---|---|
| Catequin | 191 | 35 |
| Epigallocatechin | 82 | 21 |
| Gallic acid | 95 | 7 |
| Cyanidin-3-glucoside | 3 | 0 |
| Malvidin-3-glucoside | 24 | 1 |
| Rutine | 9 | 0 |
| Quercetin | 8 | 0 |
| Myricetin | 9 | 0 |
| Caffeic acid | 7.1 | 2.8 |
| Resveratrol | 1.5 | 0 |
| Total content of phenolics | 2567 | 239 |
Abbreviation: GAE, gallic acid equivalent.
Studies have shown the effect of alcohol and wine consumption on the improvement of cardiometabolic risk factors (blood pressure, serum glucose, low-density lipoprotein [LDL] and high-density lipoprotein [HDL] levels, inflammation, and endothelial function).8–10 Hyperglycemia and hypertension may contribute to the development of endothelial dysfunction, and high serum levels of LDL oxidized by reactive oxygen species (ROS) play the main role in the initiation and progression of atherosclerosis. On the other hand, HDL exerts a protective effect in coronary heart disease by suppressing endothelial damage, LDL oxidation, vascular-LDL accumulation, inflammation, and thrombosis.11 Although international guidelines suggest a light-to-moderate alcoholic beverage consumption (15–30 g/day of ethanol, about 130–250 mL of wine/day)12–14 for cardiovascular risk reduction, it is known that high alcohol intake (>31 g/day) may have negative effects on the cardiovascular system, including an increase in blood pressure, activation of the sympathetic system,15–17 and an increase in the incidence of atrial fibrillation, cardiomyopathy, and hemorrhagic stroke.18 Thus, the relationship between alcohol consumption and the risk for many cardiovascular conditions is characterized by a U- or J-shaped curve.
The aim of this mini review is to highlight the main cardioprotective molecules present in red wine and how these compounds interact with cellular systems, particularly those involving antioxidation and anti-inflammatory activities. Furthermore, we discuss how these molecules can benefit human health by pointing out results from clinical studies.
Chemical Properties of Phenolic Compounds in Red Wine
The pulp, skin, seeds, and stems of grapes of the Vitis genus are relatively rich in nonflavonoid compounds.19 Polyphenols are the main phenolic compounds extracted from grapes during the winemaking process, initially obtained by the crushing of the fruit, and intensified by the maceration and pumping-over processes during fermentation.
The total amount of polyphenols in red wines has been estimated to range from 2000 to 6000 mg/L,20 as shown in Table 1. The main bioactive polyphenols in red wines are notably flavanols, flavonols, anthocyanins, and resveratrol.21 Flavonoids, which account for over 85% of the phenolic components in red wine, include different molecular families such as flavonols [eg, monomeric (catechin, epicatechin), oligomeric, and polymeric compounds (proanthocyanidins, also called condensed tannins)], flavones, anthocyanins,1 flavan-3-ols, catechins, and epicatechins.22,23
Catechin and epicatechin are usually the most important flavanols in both grape skins and seeds and can represent up to 60% of total phenolic compounds present in the seed.24 Both are responsible for the astringency, bitterness, and structure of wines.25 Catechin and epicatechin can be extracted from grape pomace by using aqueous solutions, reaching similar level of extraction than using ethanol/water as extraction solvent.22 Red wines from Cabernet Sauvignon and Refosco grapes showed the highest polyphenol and catechin contents.26
Flavonols comprise compounds such as myricetin, quercetin, kaempferol, and rutin. Quercetin is very common in different grapes; for example, it is the most abundant flavonol found in Sangiovese grapes.27 Flavonols and their glycosides are important components in wine because of their impact on color, taste, and health properties.28
Anthocyanins are responsible for the red color of wines and are extracted from grape skins during the winemaking process. The anthocyanins most commonly found in wines are delphinidin-3-glucoside, cyanidin-3-glucoside, and malvidin-3-glucoside,29 with recognized antioxidant capacity.19–21
Resveratrol is a phenolic compound of the stilbene family present in grape skin and seeds, and hence, constituent of grape juice and wines.30 Although resveratrol has been considered as the major functional compound in red wine, its concentration is lower than other polyphenols.7
Tannins, another subgroup of phenols found in the skins and seeds of grapes, can be classified as monomeric, oligomeric, and polymeric flavan-3-ols (condensed tannins).31 Tannins play an important role in the quality of wine, since they contribute to sensory aspects such as color, bitterness, and astringency and structure of the wine.
The composition of wine mainly depends on grape variety, followed by the winemaking techniques. The sugar, acid, tannin, anthocyanin, phenolic, and aromatic compound contents of the grapes and their interactions play key roles in the composition of wines.32 Enological practices in winemaking can affect wine production, composition, and quality.
In summary, wine characteristics are mainly determined by the combination and interaction of phenolic compounds of grapes and its changes during the winemaking process. The main constituents of red wine, with important effects on pathophysiological mechanisms, are reported below.
Effect of Wine Constituents on Biological Functions
Wine has a varying concentration of water, alcohol, and phenolic compounds, of which tannins, resveratrol, and quercetin have been the most studied. These polyphenols have positive effects on cardiac function and prevention of cardiovascular diseases,33 by modulating cellular and molecular mechanisms that lead to anti-inflammatory, antioxidant, and hypotensive responses.34 Some of these mechanisms have been well described and explored in therapeutic and preventive approaches for cardiovascular diseases.
The effects of alcohol
High consumption of alcohol may lead to lipid peroxidation, in which ROS cause damage to the cell membranes, sometimes irreversible to the cell.35 Alcohol stimulates the activity of the enzyme cytochrome P450 and alters the levels of some metals in the body, contributing to ROS production.36 In tissues, exacerbated ROS generation triggers a cascading inflammatory response, which affects homeostasis and culminates in tissue injury and establishment of a disease. In this context, the negative effect of alcohol has been well described, particularly on the liver, causing severe alcohol-related liver diseases.37
On the other hand, light-to-moderate consumption of alcohol can bring benefits to health. Chronic intake of light-to-moderate doses of alcohol may increase HDL levels and decrease LDL oxidation.38 In addition, prior ethanol administration (ethanol preconditioning) induces a mild oxidative stress that has a protective effect against ischemia/reperfusion-induced brain damage.39 In fact, the level of alcohol intake is closely related to ROS production and their deleterious effects—a low concentration is essential for the physiological degradation of polyunsaturated fatty acids, whereas high concentrations of ROS cause potential damages to cellular components, giving rise to endothelial dysfunction and other conditions.40 Also, Agarwal pointed out the influence of moderate consumption of alcohol on preventing blood coagulation and reducing platelet aggregation.38,41
Moderate alcohol consumption is also related to decreased insulin resistance in skeletal muscle, and such insulin-sensitizing activity may be related to improved production of AMP-activated protein kinase, generated by the metabolism of acetate in peripheral tissues, and involved in glucose uptake (among other functions).42 Finally, moderate alcohol intake also raises the paraoxonase 1 (PON1) levels,43 an enzyme that, among other functions, prevents the oxidation of LDL and increases levels of homocysteine.38 These beneficial effects of alcohol have been mostly associated with the phenolic compounds present in red wine.
The role of polyphenols
In vitro studies and preclinical models have demonstrated the association of wine polyphenols with activation of antioxidant and anti-inflammatory mechanisms. Flavonoids, particularly quercetins, catechins, tannins, and resveratrol,44 also act against free radicals, allergies, inflammation, ulcers, viruses, tumors, and hepatotoxins, inhibit platelet aggregation, reduce heart disease and stroke risk, and is involved in the synthesis of estrogen. Additionally, these molecules, present in almost all varieties of red wine, have their action in cells and tissues adjacent to blood vessels, mainly in the endothelium. In addition to the already mentioned functions, they have a direct role in the reduction of cell proliferation,45 which can be exploited for cancer therapy.
Anthoxanthins, flavans, and anthocyanidins
The main antioxidant mechanism of catechins, a flavan-3-ol quite abundant in grape and red wine, is related to the inhibition of nuclear factor kappa-B (NF-κB), a transcription factor that activates inflammatory cytokines in tissue injury or ischemia.46 These cytokines are released in tissue oxidative damage affecting the liver, heart, lungs, kidney, and vascular endothelium, related to chronic diseases or aging.
Epigallocatechin has been shown to mitigate the proliferation of vascular smooth muscle cells, induced by interleukin-1-beta (IL-1β, a potent proinflammatory cytokine), and that contributes to atherosclerosis. Besides, this polyphenolic catechin also reduces the release of ROS and activates the synthesis of antioxidant enzymes.47 In vitro experiments also showed the potential role of epigallocatechin in preventing skin aging, as it protects against oxidative stress-induced apoptosis in fibroblasts by inhibiting phosphorylation of p38 and c-Jun N-terminal kinases.48
The hydroxylation of the catechin monomer results in proanthocyanidin polymers (the so-called condensed tannins). Proanthocyanidins have beneficial effects on human health due to antioxidant, antimicrobial, and antiallergic properties.49 In addition, these molecules inhibit the angiotensin-converting enzyme, preventing the formation of angiotensin II, a potent vasoconstrictor.50 Interestingly, a preclinical study carried out on dyslipidemic obese rats showed that proanthocyanidins, in association with docosahexaenoic acid, were able to modulate the expression of microRNAs, such as miR-33a and miR-122, which are the major regulators of lipid metabolism in the liver.51
Quercetin strongly induces the activity of antioxidant enzymes such as heme oxygenase, glutathione S-transferase, and thioredoxin reductase.52 Besides, this flavonol is able to upregulate nitric oxide synthase (NOS) expression and decrease oxidative stress. Quercetin was also reported as an anti-inflammatory compound, due to its role in mediating the reduction of the expression of Toll-like receptors (TLR2 and TLR4) by inhibiting NF-κB translocation to the nucleus.53 In addition, the reduction of excessive production of nitric oxide by phenolic compounds has also been analyzed and evidenced in aorta of rats subjected to a diet with alcohol-free red wine,54 suggesting that both quercetin and catechin not only activate antioxidant mechanisms but are also capable to modulate them. Moreover, quercetin also seems to be associated with the inhibition of cell proliferation, attenuating the progression of some cancers,55 and with reduction of blood pressure56 and obesity.57 Shimizu et al57 showed that quercetin reduces the gene expression of apolipoproteins, including apolipoprotein B (apoB), in human enterocytes.
Stilbenoids
In terms of health effects of wine constituents, resveratrol has been the most studied element, in both animal models and clinical trials. First, resveratrol is a key regulator of homeostasis, acting on gene regulation (chromatin remodeling), protein synthesis, posttranslation modifications, enzymatic function, apoptosis, signal transduction (kinase activation/inhibition), and modulation of intracellular calcium concentration.44,58 Considering its mechanism of action, resveratrol is able to modulate the inflammatory response in a balanced way: inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), IL-1β, and interleukin-6 (IL-6), have already been demonstrated to be either induced or repressed by resveratrol.59 In this scenario, resveratrol is also able to inhibit inflammatory enzymes, such as the inducible isoforms of NOS (iNOS) and cyclooxygenase-1 (COX-1), adhesion molecules, and the NF-κB. Olas and Wachowicz showed that resveratrol is capable of inhibiting the synthesis of thromboxane and reducing platelet aggregation.60 Further, in addition to its influence on cell signaling, inflammatory, and antioxidant profile,61 resveratrol can suppress acute and chronic pain by inhibiting the mammalian target of rapamycin (mTOR) and the extracellular signal-regulated kinase signaling in neuronal cells.62 Finally, Peltz et al demonstrated distinct and dynamic actions of resveratrol on human mesenchymal stem cells,63 highlighting its role in tissue repair, which is very attractive to regenerative medicine.64 Furthermore, resveratrol activates sirtuins, a class of protein deacetylases that regulate metabolism, stress responses, and aging processes.65 In this way, resveratrol, in a dosage-dependent manner, regulates the expression of genes associated with cell cycle, cell senescence, and longevity, implicated on both cell self-renewal and differentiation capacity of mesenchymal stem cells. Together, these data corroborate the potential use of red wine as a functional food, supported by its anti-inflammatory and antioxidant functions, and its contribution to tissue repair processes.
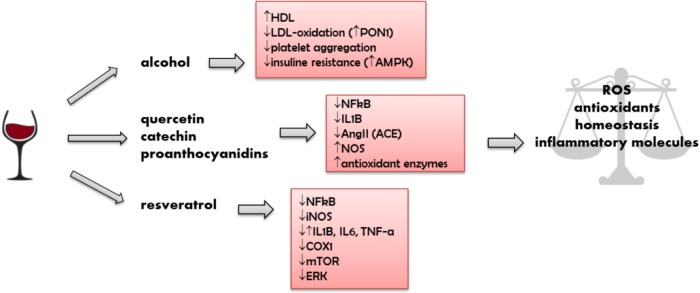
The elucidation of cellular and molecular mechanisms modulated by phenolic constituents present in red wine contributes to the understanding of the potential beneficial effects of these compounds on the prevention and treatment of several chronic diseases such as cardiovascular and inflammatory diseases and cancer (Fig. 1).33,44,66 These effects become even more pronounced when a light-to-moderate wine consumption is associated with a healthy lifestyle and habits, such as the adoption of the Mediterranean diet and physical activity.67,68
Figure 1.
Red wine constituents and their action on biological mechanisms. Phenolic compounds and alcohol obtained from light-to-moderate consumption of wine may balance organic functions related to homeostasis, inflammation, and oxidation, leading to benefits that may assist in the recovery of cardiovascular pathologies and other chronic diseases.
Studies in Humans Regarding Cardiometabolic Factors and Wine
Several clinical studies have been made in both healthy volunteers and individuals with chronic diseases (dyslipidemia, hypertension, type 2 diabetes mellitus [T2DM], metabolic syndrome [MS], and coronary heart disease), regarding the effects of wine consumption on metabolic, inflammatory, and cardiovascular parameters. However, it is noteworthy that these effects are dependent on the bioavailability of the phenolic compounds, which may be affected by many factors, such as environmental, food processing (thermal treatments, cooking techniques, storage), and dietary factors (presence of positive or negative effectors of absorption, such as meals rich in fats and fibers), interactions with other compounds (polyphenols with similar mechanism of absorption), chemical structure of polyphenols and their concentrations in food, and host-related factors (intestinal factors such as enzyme activity, transit time, and microbiota; age; gender; presence of diseases; and genetic condition).69 Some clinical trials that evaluated the beneficial effects of wine consumption (for a minimum of 15 days) on cardiometabolic factors in nonhealthy subjects are described below.
In individuals with dyslipidemia, a trend toward significance for decreased LDL/HDL ratio levels (P = 0.05) was detected after red wine consumption for 30 days,70 and in hypercholesterolemic postmenopausal women, chronic consumption of red wine significantly reduced the LDL levels by 8% and increased the HDL levels by 17%.71 In patients with well-controlled T2DM, the consumption of 150 mL/day of red wine at dinner for two years significantly increased HDL and apolipoprotein A1 levels, and decreased the total cholesterol/HDL ratio.72 Apolipoprotein A1 and A2 and HDL levels increased in men at high cardiovascular risk who consumed 30 g alcohol/day of red wine for four weeks.73
As previously mentioned, white wine is composed of a minor amount of phenolic compounds when compared to red wine, but its effects on metabolic parameters regarding lipidic, glycidic, and inflammatory profile in nonhealthy individuals have also been evaluated. Eighteen patients with MS consumed white wine for four weeks, and no changes were detected regarding total cholesterol, LDL, triglyceride, and fasting plasma glucose levels; however, homeostasis model assessment of insulin release decreased significantly (P = 0.002).74 The impact of white wine in combination with extra-virgin olive oil on inflammatory profile was evaluated in patients with chronic kidney disease KDOQI stages III–IV. Subjects were allocated to two weeks of treatment with extra-virgin olive oil alone or white wine (4 mL/kg body weight, 0.48 g/kg of alcohol 12%, corresponding to 2–3 glasses/daily) plus extra-virgin olive oil. Plasma C-reactive protein (CRP) and IL-6 levels decreased after wine plus olive oil consumption, but no difference was detected after the treatment with olive oil alone.75
Ventricular dyssynchrony and inflammatory markers were evaluated in 115 individuals with T2DM who had sustained a first nonfatal myocardial infarction and were randomized to receive red wine (during a meal) or not (control group). After one year of intervention, compared to the treatment group, all inflammatory markers (CRP, TNF-α, IL-6, IL-18, and nitrotyrosine) were increased, and echo-cardiographic parameters indicated ventricular dyssynchrony in the control group.76 In another study that evaluated metabolic, autonomic, hemodynamic, and endothelial responses in subjects with hypercholesterolemia or arterial hypertension, 250 mL/day of red wine for 15 days decreased blood pressure levels and vascular resistance, enhanced muscle sympathetic fibular nerve activity in hypertensive and hypercholesterolemic individuals, and restored brachial artery flow-mediated dilation in hypercholesterolemic patients.77
In this review, we described that alcohol and specific phenolic compounds may have different effects on different metabolic factors. Although the beneficial effects of these compounds on cardiometabolic traits have been indicated by several studies, the results of clinical studies should be interpreted with caution. Limitations of many of these studies include small sample size, short-term evaluation of wine consumption (making the extrapolation of the results to longer periods of wine consumption difficult), and lack of measurements of phenolic compounds in plasma, urine, or even in the wines used as intervention. Besides, several issues in these studies deserve careful consideration, including the heterogeneity and genetic variability of the populations, the use of medications and their interactions with phenolic compounds, the different amounts of wine used as intervention, the lack of data regarding other dietary sources of polyphenols consumed by the subjects, and different methods used to evaluate the same outcome. Thus, further randomized, clinical trials evaluating the effects of long-term consumption of red wine are necessary, taking into account the safe limits of alcohol intake for each group. Additionally, although much has been known about the properties of wine, how different compounds of different grape varieties might help in therapeutic approaches need to be explored.
Conclusion
Studies conducted in humans have evidenced that phenolic compounds, as well as ethanol present in red wine, can have beneficial effects on health, due to its anti-inflammatory and antioxidant properties and their role in tissue repair processes. These processes are modulated due to antioxidant and anti-inflammatory capabilities of the components of the wine. Such mechanisms help the organic systems in bringing assistance to cellular and tissue functions. However, despite the protective effects of these phenolic constituents, the amount of wine consumed deserves attention, since a chronic excessive intake may lead to an exacerbated response, oxidative stress, endothelial dysfunction, and cardiovascular disease.
Footnotes
ACADEMIC EDITOR: Joseph Zhou, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,139 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Contributed to the writing of the manuscript, made critical revisions, and approved the final version: MMM, JG, AO, JO, and AM.
REFERENCES
- 1.Artero A, Artero A, Tarín JJ, et al. The impact of moderate wine consumption on health. Maturitas. 2015;80(1):3–13. doi: 10.1016/j.maturitas.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Dennis EG, Keyzers RA, Kalua CM, Maffei SM, Nicholson EL, Boss PK. Grape contribution to wine aroma: production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must. J Agric Food Chem. 2012;60(10):2638–2646. doi: 10.1021/jf2042517. [DOI] [PubMed] [Google Scholar]
- 3.García-Guzmán JJ, Hernández-Artiga MP, Palacios-Ponce de León L, Bellido-Milla D. Selective methods for polyphenols and sulphur dioxide determination in wines. Food Chem. 2015;182:47–54. doi: 10.1016/j.foodchem.2015.02.101. [DOI] [PubMed] [Google Scholar]
- 4.Sumby KM, Grbin PR, Jiranek V. Microbial modulation of aromatic esters in wine: current knowledge and future prospects. Food Chem. 2010;121:1–16. [Google Scholar]
- 5.Jackson RS. Wine Science: Principles, Practice, Perception. 2nd ed. Cambridge: Academic Press; 2000. p. 645. [Google Scholar]
- 6.Ribéreau-Gayon P, Dubourdieu D, Donèche B, Lonvaud A. Handbook of Enology: The Microbiology of Wine and Vinifications. 2nd ed. Vol. 1. Chichester: John Wiley & Sons; 2006. p. 497. [Google Scholar]
- 7.Xiang L, Xiao L, Wang Y, Li H, Huang Z, He X. Health benefits of wine: don’t expect resveratrol too much. Food Chem. 2014;156:258–263. doi: 10.1016/j.foodchem.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Opie LH, Lecour S. The red wine hypothesis: from concepts to protective signalling molecules. Eur Heart J. 2007;28:1683–1693. doi: 10.1093/eurheartj/ehm149. [DOI] [PubMed] [Google Scholar]
- 9.Di Castelnuovo A, Costanzo S, di Giuseppe R, de Gaetano G, Iacoviello L. Alcohol consumption and cardiovascular risk: mechanisms of action and epidemiologic perspectives. Future Cardiol. 2009;5(5):467–477. doi: 10.2217/fca.09.36. [DOI] [PubMed] [Google Scholar]
- 10.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badimon L, Vilahur G. LDL-cholesterol versus HDL-cholesterol in the atherosclerotic plaque: inflammatory resolution versus thrombotic chaos. Ann N Y Acad Sci. 2012;1254:18–32. doi: 10.1111/j.1749-6632.2012.06480.x. [DOI] [PubMed] [Google Scholar]
- 12.Piepoli MF, Hoes AW, Agewall S, et al. European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 20162016 doi: 10.1093/eurheartj/ehw106. pii: ehw106. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Sociedade Brasileira de Cardiologia, Sociedade Brasileira de Hipertensão, Sociedade Brasileira de Nefrologia VI Brazilian guidelines on hypertension. Arq Bras Cardiol. 2010;95(1 Sul):1–51. [PubMed] [Google Scholar]
- 15.Mori TA, Burke V, Beilin LJ, Puddey IB. Randomized controlled intervention of the effects of alcohol on blood pressure in premenopausal women. Hypertension. 2015;66(3):517–523. doi: 10.1161/HYPERTENSIONAHA.115.05773. [DOI] [PubMed] [Google Scholar]
- 16.McFadden CB, Brensinger CM, Berlin JA, Townsend RR. Systematic review of the effect of daily alcohol intake on blood pressure. Am J Hypertens. 2005;18:276–286. doi: 10.1016/j.amjhyper.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Grassi GM, Somers VK, Renk WS, Abboud FM, Mark AL. Effects of alcohol intake on blood pressure and sympathetic nerve activity in normotensive humans: a preliminary report. J Hypertens Suppl. 1989;7(6):S20–S21. doi: 10.1097/00004872-198900076-00007. [DOI] [PubMed] [Google Scholar]
- 18.Mukamal K. Alcohol intake and noncoronary cardiovascular diseases. Ann Epidemiol. 2007;17(5 suppl):S8–S12. doi: 10.1016/j.annepidem.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulero J, Martínez G, Oliva J, et al. Phenolic compounds and antioxidant activity of red wine made from grapes treated with different fungicides. Food Chem. 2015;180:25–31. doi: 10.1016/j.foodchem.2015.01.141. [DOI] [PubMed] [Google Scholar]
- 20.Quideau S, Deffieux D, Douat-Casassus C, Pouysegu L. Plant polyphenols: Chemical properties, biological activities and synthesis. Angewandte Chemie (International ed. in English) 2011;50:586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 21.Vallverdú-Queralt A, Boix N, Piqué E, et al. Identification of phenolic compounds in red wine extract samples and zebrafish embryos by HPLC-ESI-LTQ-Orbitrap-MS. Food Chem. 2015;181:146–151. doi: 10.1016/j.foodchem.2015.02.098. [DOI] [PubMed] [Google Scholar]
- 22.López-Miranda S, Serrano-Martínez A, Hernández-Sánchez P, et al. Use of cyclodextrins to recover catechin and epicatechin from red grape pomace. Food Chem. 2016;203:379–385. doi: 10.1016/j.foodchem.2016.02.100. [DOI] [PubMed] [Google Scholar]
- 23.Pascual-Teresa S, Moreno DA, García-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci. 2010;11:1679–1703. doi: 10.3390/ijms11041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chedea VS, Braicu C, Socaciu C. Antioxidant/prooxidant activity of a polyphenolic grape seed extract. Food Chem. 2010;121:132–139. [Google Scholar]
- 25.Dias FS, Lovillo MP, Barroso CG, David JM. Optimization and validation of a method for the direct determination of catechin and epicatechin in red wines by HPLC/fluorescence. Microchem J. 2010;96:17–20. [Google Scholar]
- 26.Garaguso I, Nardini M. Polyphenols content, phenolics profile and antioxidant activity of organic red wines produced without sulfur dioxide/sulfites addition in comparison to conventional red wines. Food Chem. 2015;179:336–342. doi: 10.1016/j.foodchem.2015.01.144. [DOI] [PubMed] [Google Scholar]
- 27.Romboli Y, Mangani S, Buscioni G, Granchi L, Vincenzini M. Effect of Saccharomyces cerevisiae and Candida zemplinina on quercetin, vitisin A and hydroxytyrosol contents in Sangiovese wines. World J Microbiol Biotechnol. 2015;31(7):1137–1145. doi: 10.1007/s11274-015-1863-9. [DOI] [PubMed] [Google Scholar]
- 28.Jeffery DW, Parker M, Smith PA. Flavonol composition of Australian red and white wines determined by high-performance liquid chromatography. Aust J Grape Wine Res. 2008;14:153–161. [Google Scholar]
- 29.Ribéreau-Gayon P, Bertrand A. Simultaneous determination of organic acids, polyalcohols and sugars in wine. Applications. C R Acad Sci Hebd Seances Acad Sci D. 1971;273(19):1761–1762. [PubMed] [Google Scholar]
- 30.Fernández-Mar MI, Mateos R, García-Parrilla MC, et al. Bioactive compounds in wine: resveratrol, hydroxytyrosol and melatonin: a review. Food Chem. 2012;130:797–813. [Google Scholar]
- 31.Rinaldi A, Blaiotta G, Aponte M, Moio L. Effect of yeast strain and some nutritional factors on tannin composition and potential astringency of model wines. Food Microbiol. 2016;53(pt B):128–134. doi: 10.1016/j.fm.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Wang R, Sun Q, Chang Q. Soil types effect on grape and wine composition in Helan Mountain area of Ningxia. PLoS One. 2015;10(2):e0116690. doi: 10.1371/journal.pone.0116690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das S, Santani DD, Dhalla NS. Experimental evidence for the cardioprotective effects of red wine. Exp Clin Cardiol. 2007;12(1):5–10. [PMC free article] [PubMed] [Google Scholar]
- 34.Arranz S, Chiva-Blanch G, Valderas-Martínez P, Medina-Remón A, Lamuela-Raventós RM, Estruch R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients. 2012;4(7):759–781. doi: 10.3390/nu4070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meagher EA, Barry OP, Burke A, et al. Alcohol-induced generation of lipid peroxidation products in humans. J Clin Invest. 1999;104(6):805–813. doi: 10.1172/JCI5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27(4):277–284. [PMC free article] [PubMed] [Google Scholar]
- 37.Shield KD, Parry C, Rehm J. Chronic diseases and conditions related to alcohol use. Alcohol Res. 2013;35(2):155–173. [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal DP. Cardioprotective effects of light-moderate consumption of alcohol: a review of putative mechanisms. Alcohol Alcohol. 2002;37(5):409–415. doi: 10.1093/alcalc/37.5.409. [DOI] [PubMed] [Google Scholar]
- 39.Wang Q, Sun AY, Simonyi A, et al. Ethanol preconditioning protects against ischemia/reperfusion-induced brain damage: role of NADPH oxidase-derived ROS. Free Radic Biol Med. 2007;43(7):1048–1060. doi: 10.1016/j.freeradbiomed.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem J. 2012;442(3):453–464. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruf JC. Wine and polyphenols related to platelet aggregation and atherothrombosis. Drugs Exp Clin Res. 1999;25(2–3):125–131. [PubMed] [Google Scholar]
- 42.McCarty MF. Does regular ethanol consumption promote insulin sensitivity and leanness by stimulating AMP-activated protein kinase? Med Hypotheses. 2001;57(3):405–407. doi: 10.1054/mehy.2001.1404. [DOI] [PubMed] [Google Scholar]
- 43.Leckey LC, Garige M, Varatharajalu R, et al. Quercetin and ethanol attenuate the progression of atherosclerotic plaques with concomitant up regulation of paraoxonase1 (PON1) gene expression and PON1 activity in LDLR-/- mice. Alcohol Clin Exp Res. 2010;34(9):1535–1542. doi: 10.1111/j.1530-0277.2010.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khurana S, Venkataraman K, Hollingsworth A, Piche M, Tai TC. Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients. 2013;5(10):3779–3827. doi: 10.3390/nu5103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia E-Q, Deng G-F, Guo Y-J, Li HB. Biological activities of polyphenols from grapes. Int J Mol Sci. 2010;11(2):622–646. doi: 10.3390/ijms11020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bharrhan S, Koul A, Chopra K, Rishi P. Catechin suppresses an array of signalling molecules and modulates alcohol-induced endotoxin mediated liver injury in a rat model. PLoS One. 2011;6(6):e20635. doi: 10.1371/journal.pone.0020635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu PL, Liu JT, Kuo HF, Chong IW, Hsieh CC. Epigallocatechin gallate attenuates proliferation and oxidative stress in human vascular smooth muscle cells induced by interleukin-1β via heme oxygenase-1. Mediators Inflamm. 2014;2014:523684. doi: 10.1155/2014/523684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanigawa T, Kanazawa S, Ichibori R, et al. (+)-Catechin protects dermal fibroblasts against oxidative stress-induced apoptosis. BMC Complement Altern Med. 2014;14:133. doi: 10.1186/1472-6882-14-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aron PM, Kennedy JA. Flavan-3-ols: nature, occurrence and biological activity. Mol Nutr Food Res. 2008;52(1):79–104. doi: 10.1002/mnfr.200700137. [DOI] [PubMed] [Google Scholar]
- 50.Ottaviani JI, Actis-Goretta L, Villordo JJ, Fraga CG. Procyanidin structure defines the extent and specificity of angiotensin I converting enzyme inhibition. Biochimie. 2006;88(3–4):359–365. doi: 10.1016/j.biochi.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Baselga-Escudero L, Arola-Arnal A, Pascual-Serrano A, et al. Chronic administration of proanthocyanidins or docosahexaenoic acid reversess the increase of miR-33a and miR-122 in dyslipidemic obese rats. PLoS One. 2013;8(7):e69817. doi: 10.1371/journal.pone.0069817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angeloni C, Leoncini E, Malaguti M, Angelini S, Hrelia P, Hrelia S. Role of quercetin in modulating rat cardiomyocyte gene expression profile. Am J Physiol Heart Circ Physiol. 2008;294(3):H1233–H1243. doi: 10.1152/ajpheart.01091.2007. [DOI] [PubMed] [Google Scholar]
- 53.Chirumbolo S. Role of quercetin in vascular physiology. Can J Physiol Pharmacol. 2012;90(12):1652–1657. doi: 10.1139/y2012-137. [DOI] [PubMed] [Google Scholar]
- 54.Benito S, Lopez D, Sáiz MP, et al. A flavonoid-rich diet increases nitric oxide production in rat aorta. Br J Pharmacol. 2002;135(4):910–916. doi: 10.1038/sj.bjp.0704534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Velázquez KT, Enos RT, Narsale AA, et al. Quercetin supplementation attenuates the progression of cancer cachexia in ApcMin/+ mice. J Nutr. 2014;144(6):868–875. doi: 10.3945/jn.113.188367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yi L, Zongyuan Y, Cheng G, Lingyun Z, GuiLian Y, Wei G. Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer-binding protein homologous protein (CHOP)-death receptor 5 pathway. Cancer Sci. 2014;105(5):520–527. doi: 10.1111/cas.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimizu M, Li J, Inoue J, Sato R. Quercetin represses apolipoprotein B expression by inhibiting the transcriptional activity of C/EBPβ. PLoS One. 2015;10(4):e0121784. doi: 10.1371/journal.pone.0121784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCalley AE, Kaja S, Payne AJ, Koulen P. Resveratrol and calcium signaling: molecular mechanisms and clinical relevance. Molecules. 2014;19(6):7327–7340. doi: 10.3390/molecules19067327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vang O, Ahmad N, Baile CA, et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One. 2011;6(6):e19881. doi: 10.1371/journal.pone.0019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olas B, Wachowicz B. Resveratrol, a phenolic antioxidant with effects on blood platelet functions. Platelets. 2005;16(5):251–260. doi: 10.1080/09537100400020591. [DOI] [PubMed] [Google Scholar]
- 61.Kovacic P, Somanathan R. Multifaceted approach to resveratrol bioactivity: focus on antioxidant action, cell signaling and safety. Oxid Med Cell Longev. 2010;3(2):86–100. doi: 10.4161/oxim.3.2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tillu DV, Melemedjian OK, Asiedu MN, et al. Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision-induced acute and chronic pain. Mol Pain. 2012;8:5. doi: 10.1186/1744-8069-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peltz L, Gomez J, Marquez M, et al. Resveratrol exerts dosage and duration dependent effect on human mesenchymal stem cell development. PLoS One. 2012;7(5):e37162. doi: 10.1371/journal.pone.0037162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaun MI, Eibel B, Kristocheck M, et al. Cell therapy in ischemic heart disease: interventions that modulate cardiac regeneration. Stem Cells Int. 2016;2016:2171035. doi: 10.1155/2016/2171035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gertz M, Nguyen GTT, Fischer F, et al. A molecular mechanism for firect sirtuin activation by resveratrol. PLoS One. 2012;7(11):e49761. doi: 10.1371/journal.pone.0049761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tomé-Carneiro J, Larrosa M, González-Sarrías A, Tomás-Barberán FA, García-Conesa MT, Espín JC. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr Pharm Des. 2013;19(34):6064–6093. doi: 10.2174/13816128113199990407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tresserra-Rimbau A, Medina-Remón A, Lamuela-Raventós RM, et al. Moderate red wine consumption is associated with a lower prevalence of the metabolic syndrome in the PREDIMED population. Br J Nutr. 2015;113(suppl 2):S121–S130. doi: 10.1017/S0007114514003262. [DOI] [PubMed] [Google Scholar]
- 68.Soares Filho PR, Castro I, Stahlschmidt A. Effect of red wine associated with physical exercise in the cardiovascular system of spontaneously hipertensive rats. Arq Bras Cardiol. 2011;96(4):277–283. doi: 10.1590/s0066-782x2011005000020. [DOI] [PubMed] [Google Scholar]
- 69.D’Archivio M, Filesi C, Varì R, Scazzocchio B, Masella R. Bioavailability of the polyphenols: status and controversies. Int J Mol Sci. 2010;11(4):1321–1342. doi: 10.3390/ijms11041321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Apostolidou C, Adamopoulos K, Lymperaki E, et al. Cardiovascular risk and benefits from antioxidant dietary intervention with red wine in asymptomatic hypercholesterolemics. Clin Nutr ESPEN. 2015;10(6):e224–e233. doi: 10.1016/j.clnesp.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 71.Naissides M, Mamo JC, James AP, Pal S. The effect of chronic consumption of red wine on cardiovascular disease risk factors in postmenopausal women. Atherosclerosis. 2006;185(2):438–445. doi: 10.1016/j.atherosclerosis.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 72.Gepner Y, Golan R, Harman-Boehm I, et al. Effects of initiating moderate alcohol intake on cardiometabolic risk in adults with type 2 diabetes: a 2-year randomized, controlled trial. Ann Intern Med. 2015;163(8):569–579. doi: 10.7326/M14-1650. [DOI] [PubMed] [Google Scholar]
- 73.Chiva-Blanch G, Urpi-Sarda M, Ros E, et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: a randomized clinical trial. Clin Nutr. 2013;32(2):200–206. doi: 10.1016/j.clnu.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 74.Abel T, Blázovics A, Wimmer A, et al. Effect of “Pintes” white wine on metabolic parameters in patients with metabolic syndrome. Orv Hetil. 2012;153(22):861–865. doi: 10.1556/OH.2012.29389. [DOI] [PubMed] [Google Scholar]
- 75.Migliori M, Panichi V, de la Torre R, et al. Anti-inflammatory effect of white wine in CKD patients and healthy volunteers. Blood Purif. 2015;39(1–3):218–223. doi: 10.1159/000371570. [DOI] [PubMed] [Google Scholar]
- 76.Marfella R, Cacciapuoti F, Siniscalchi M, et al. Effect of moderate red wine intake on cardiac prognosis after recent acute myocardial infarction of subjects with type 2 diabetes mellitus. Diabet Med. 2006;23(9):974–981. doi: 10.1111/j.1464-5491.2006.01886.x. [DOI] [PubMed] [Google Scholar]
- 77.Andrade ACM, Cesena FHY, Consolim-Colombo FM, et al. Short-term red wine consumption promotes differential effects on plasma levels of high-density lipoprotein cholesterol, sympathetic activity, and endothelial function in hypercholesterolemic, hypertensive, and healthy subjects. Clinics. 2009;64(5):435–442. doi: 10.1590/S1807-59322009000500011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lingua MS, Fabani MP, Wunderlin DA, Baroni MV. From grape to wine: changes in phenolic composition and its influence on antioxidant activity. Food Chem. 2016;208:228–238. doi: 10.1016/j.foodchem.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 79.Frankel EN, Waterhouse AL, Teissedre PL. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. J Agric Food Chem. 1995;43:890–894. [Google Scholar]