ABSTRACT
Hydrogen sulphide (H2S) has traditionally been considered as a phytotoxin, having deleterious effects on the plant growth and survival. Recently, it was recongnized as a potential signaling molecule involving in physiological regulation similar to nitric oxide (NO) and carbon monoxide (CO) in plants. In a recent study, we mainly focused on the signaling function of H2S in improving adaptation of Zea mays seedlings to iron deficiency. We reported that H2S was closely related to iron uptake, transport, and accumulation, and consequently increased chlorophyll biosynthesis, chloroplast development, and photosynthesis in Z. mays seedlings. Here, we provide more commentary on the signaling roles of H2S in coping with Fe deficiency in plants through increasing sulfur containing metabolites and regulating the expression level of iron homeostasis and sulfur metabolism-related genes in maize seedlings.
KEYWORDS: Gene expression, hydrogen sulphide, iron deficiency, iron homeostasis, sulfur metabolism, Zea mays
Iron (Fe) is an essential microelement for plants and all other living organisms, which is a component of a number of proteins and enzymes with functions in key metabolic process and Fe.1 Despite being the fourth most abundant element in the earth's crust, Fe deficiency is one of the major limiting factors for crop production in calcareous soils all over the world.2 Higher plants have two strategies for the uptake of Fe(III) from the rhizosphere. Strategy I plant species respond to lack of Fe by three steps including acidification of rhizosphere by an H+-ATPase, reduction of Fe (III) to Fe (II) by ferric-chelate reductase and uptake of Fe(II) by iron transporters in the roots.3-5 In contrast, in Strategy II plants, iron acquisition includes biosynthesis of phytosiderophores (mugineic acids, MAs) inside the roots; secretion of phytosiderophores to the rhizosphere; solubilization of insoluble iron in soils by chelation of phytosiderophores; and uptake of the ferric-phytosiderophore complex by the roots.6,7 However, strategies I and II are not sufficient to support the iron requirement for plant development when iron availability is under a threshold level, thus stress symptoms become evident in iron-deficient plants.
In the last few years, there has been a renewed interest in the effect of hydrogen sulphide (H2S) on plant physiology.8 Literatures published from the last 30 y showed that this gas can affect the growth of plants, but more recent works suggested H2S can act as a signaling molecule similar to nitric oxide (NO) and carbon monoxide (CO) in plants at low concentrations by participating in various biological process.9,10 For instance, previous studies showed that H2S promoted seed germination, alleviated oxidative damage, inhibited boron toxicity, salt toxicity, and aluminum toxicity and so on in plants.11-13
H2S is endogenously generated during the metabolism of L-cysteine by the catalysis of cystathionine β-synthase and cystathionine γ-lyase in plants.14 Besides, H2S is thought to be released from cysteine via a reversible O-acetyl-L-serine(thiol)lyase (OAS-TL) reaction in plants.15 Moreover, the uptake of H2S is largely dependent on its rate of metabolism into cysteine by OAS-TL and subsequent assimilation into other organic sulfur compounds.16 Therefore, H2S as an important compound involved in plant sulfur metabolism. It is noteworthy that S supply could help plants cope with the Fe shortage.17-20 For instance, Astolfi et al.,18 reported that barley exhibited a positive correlation between the S nutritional status and its capability of coping with Fe deficiency emerged. Moreover, one of the responses to Fe deficiency in strategy II plant is the extrusion of phytosiderophores in the root rhizosphere in order to chelate and solubilize Fe3+.18,19 Phytosiderophores are derived from nicotianamine that is synthesized from three molcules of S-adenosyl-methionine, thus representing another possible junction between Fe and S metabolism. Under S deficiency condition the release of phytosiderophores was reduced.19,21 However, it is not clear whether H2S as sulfur compound or signaling molecule play a key role in response to Fe deficiency in plants?
In our recent published study, we presented compelling data that revealed a novel effect of H2S on iron nutrition.22 In our experiment, the S content by exogenously applied H2S was much lower than that of nutrition solution itself which was only 1%. However, a profound change in chlorophyll content, iron uptake, and iron homeostasis-related gene expression including S-adenosyl homocysteine nucleosidase (ZmMTN), nicotianamine synthase (ZmNAS1 and ZmNAS3), deoxymugineic acid synthase 1 (ZmDMAS1), transporter of MAs (ZmTOM2 and ZmTOM3), iron-regulated transporter (ZmIRT), iron binding protein (ZmIBP), ferric-chelate reductase (ZmFRO1), and yellow stripe 1 (ZmYS1) happened in iron-deficiency Z. mays seedlings when treated by exogenous H2S (Fig. 1). Therefore, we concluded that H2S as a signaling molecule played a vital role in improving adaptation of maize seedlings to iron deficiency rather than sulfur nutrition.
Figure 1.
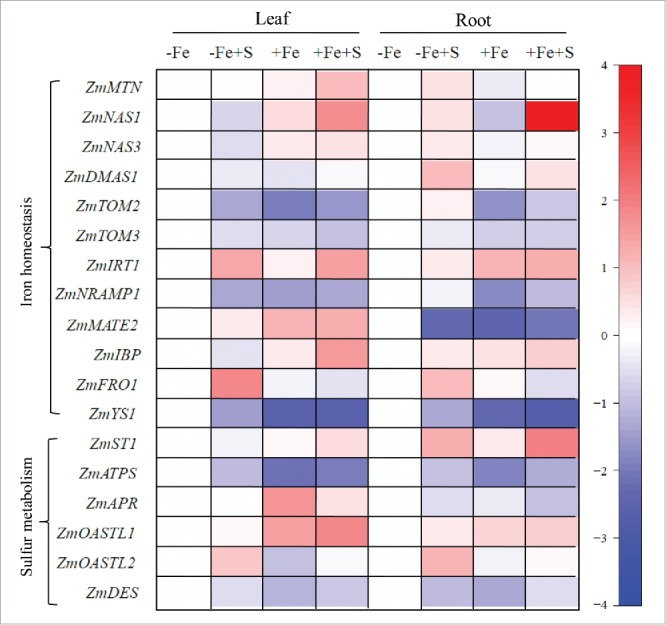
Heat map of the transcripts of iron homeostasis-related genes and sulfur metabolism-related genes of maize seedling leaves. Maize seedlings were pre-treated with 100 µM NaHS for 8 d and then grown in a nutrient solution containing 1 µM Fe(III)-EDTA or 50 µM Fe(III)-EDTA for 12 d. Red color represents higher relative expression and blue color represents lower relative expression when compared with the control samples (−Fe). Scale is the log2 of the mean concentration values after normalization (n = 4).
The supply of H2S would directly feed into cysteine and glutathione biosynthesis. Many studies have reported that H2S exposure generally results in an increased content of water-soluble non-protein thiol compounds including GSH and cysteine in shoot, particularly, in some species an increase of sulfate content in shoot has been observed.16,23–25 In our study, a high accumulation of endogenous H2S in maize seedling leaves and roots caused by exogenously applied NaHS was observed under –Fe (0.1 μM FeIII-EDTA) or +Fe (50 μM FeIII-EDTA) conditions. Meanwhile, NaHS treatment caused GSH and NPTs increase in roots and leaves under –Fe or +Fe conditions. Besides, H2S also could regulate sulfur metabolism-related genes expression including sulfate transporter (ZmST1), sulfate reduction-related genes (ZmATPS and ZmAPR), O-acetyl-L-serine(thiol)lyase (ZmOASTL1 and ZmOASTL2), and cysteine desulfhydrase (ZmDES) (Fig. 1). These results indicated exogenously applied NaHS was not only directly feed into cysteine and glutathione biosynthesis by regulating sulfur metabolism-related enzymes activities and genes expression, but also increased the content of endogenous H2S in plants.22
Therefore, our results suggested that H2S as a signaling molecule could cope with iron deficiency through increasing sulfur containing metabolites including GSH and NPTs and regulating the expression level of iron homeostasis and sulfur metabolism-related genes in maize seedlings. The detailed signaling pathway of H2S-regulated iron assimilation need to further study.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was financially supported by the Natural Science Foundation of China (NSFC No 31501822, 31570586, 31300505, 31260057), the Postdoctoral Science Foundation of China (2015M580876), the foundation of doctoral Fig. 1. Heat map of the transcripts of iron homeostasis-related genes and sulfur metabolism-related genes of maize seedling leaves. Maize seedlings were pre-treated with 100 mM NaHS for 8 d and then grown in a nutrient solution containing 1 mM Fe(III)-EDTA or 50 mM Fe(III)-EDTA for 12 d. Red color represents higher relative expression and blue color represents lower relative expression when compared with the control samples (iFe). Scale is the log2 of the mean concentration values after normalization (n D 4). 2 J. CHEN ET AL. scientific research in Northwest A&F University (Z109021409), and West Light PhD Project Foundation of the Chinese Academy of Sciences, Chinese Universities Scientific Fund (K318021510).
References
- 1.Pavlovic J, Samardzic J, Maksimović V, Timotijevic G, Stevic N, Laursen KH, Hansen TH, Husted S, Schjoerring JK, Liang Y, et al.. Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytol 2013; 198:1096-107; PMID:23496257; http://dx.doi.org/ 10.1111/nph.12213 [DOI] [PubMed] [Google Scholar]
- 2.Ramirez L, Simontacchi M, Murgia I, Zabaleta E, Lamattina L. Nitric oxide, nitrosyl iron complexes, ferritin and frataxin: A well equipped team to preserve plant iron homeostasis. Plant Sci 2011; 181:582-92; PMID:21893255; http://dx.doi.org/ 10.1016/j.plantsci.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 3.Curie C, Briat JF. Iron transport and signaling in plants. Annu Rev Plant Biol 2003; 54:183-206; PMID:14509968; http://dx.doi.org/ 10.1146/annurev.arplant.54.031902.135018 [DOI] [PubMed] [Google Scholar]
- 4.Walker EL, Connolly EL. Time to pump iron: iron-deficiency-signaling mechanisms of higher plants. Curr Opin Plant Biol 2008; 11:530-5; PMID:18722804; http://dx.doi.org/ 10.1016/j.pbi.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 5.Morrissey J, Guerinot ML. Iron uptake and transport in plants: the food, the bad, and the Ionome. Chem Rev 2009; 109:4553-67; PMID:19754138; http://dx.doi.org/ 10.1021/cr900112r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma JF. Plant root responses to three abundant soil minerals: silicon, aluminum and iron. Crit Rev Plant Sci 2005; 24:267-81; http://dx.doi.org/ 10.1080/07352680500196017 [DOI] [Google Scholar]
- 7.Ueno D, Yamaji N, Ma JF. Further characterization of ferric-phytosiderophore transporters ZmYS1 and HvYS1 in maize and barley. J Exp Bot 2009; 60:3513-20; PMID:19549626; http://dx.doi.org/ 10.1093/jxb/erp191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lisjak M, Teklic T, Wilson ID, Whiteman M, Hancock JT. Hydrogen sulfide: environmental factor or signalling molecule? Plan Cell Environ 2013; 36:1607-16; http://dx.doi.org/ 10.1111/pce.12073 [DOI] [PubMed] [Google Scholar]
- 9.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, et al.. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine r-lyase. Science 2008; 322:587-90; PMID:18948540; http://dx.doi.org/ 10.1126/science.1162667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter?. FASEB J 2002; 16:1792-8; PMID:12409322; http://dx.doi.org/ 10.1096/fj.02-0211hyp [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Hu LY, Hu KD, He YD, Wang SH, Luo J-P. Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J Integr Plant Biol 2008; 50:1518-29; PMID:19093970; http://dx.doi.org/ 10.1111/j.1744-7909.2008.00769.x [DOI] [PubMed] [Google Scholar]
- 12.Wang BL, Shi L, Li YX, Zhang WH. Boron toxicity is alleviated by hydrogen sulfide in cucumber (Cucumis sativus L.) seedlings. Planta 2010; 231:1301-9; PMID:20224946; http://dx.doi.org/ 10.1007/s00425-010-1134-9 [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Wang WH, Wu FH, He EM, Liu X, Shangguan ZP, Zheng HL. Hydrogen sulfide enhances salt tolerance through nitric oxide-mediated maintenance of ion homeostasis in barley seedling roots. Sci Rep 2015; 5:12516; PMID:26213372; http://dx.doi.org/ 10.1038/srep12516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: A review. Free Radical Biol Med 2009; 47:1346-53; PMID:19770036; http://dx.doi.org/25416292 10.1016/j.freeradbiomed.2009.09.018 [DOI] [PubMed] [Google Scholar]
- 15.Birke H, De Kok LJ, Wirtz M, Hell R. The role of compartment-specific cysteine synthesis for sulfur homeostasis during H2S exposure in Arabidopsis. Plant Cell Physiol 2015; 56:358-67; PMID:25416292; http://dx.doi.org/ 10.1093/pcp/pcu166 [DOI] [PubMed] [Google Scholar]
- 16.Durenkamp M, De Kok LJ, Kopriva S. Adenosine 5'-phosphosulphate reductase is regulated differently in Allium cepa L. and Brassica oleracea L. upon exposure to H2S. J Exp Bot 2007; 58:1571-9; PMID:17332418; http://dx.doi.org/ 10.1093/jxb/erm031 [DOI] [PubMed] [Google Scholar]
- 17.Astolfi S, Zuchi S, Cesco S, Varanini Z, Pinton R. Influence of iron nutrition on sulphur uptake and metabolism in maize (Zea mays L.) roots. Soil Sci Plant Nutr 2004; 50:1079-83; http://dx.doi.org/ 10.1080/00380768.2004.10408577 [DOI] [Google Scholar]
- 18.Astolfi S, Zuchi S, Hubberten H-M, Pinton R, Hoefgen R. Supply of sulphur to S-deficient young barley seedlings restores their capability to cope with iron shortage. J Exp Bot 2010; 61:799-806; PMID:20018904; http://dx.doi.org/ 10.1093/jxb/erp346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astolfi S, Zuchi S, Neumann G, Cesco S, di Toppi LS, Pinton R. Response of barley plants to Fe deficiency and Cd contamination as affected by S starvation. J Exp Bot 2012; 63:1241-50; PMID:22090437; http://dx.doi.org/ 10.1093/jxb/err344 [DOI] [PubMed] [Google Scholar]
- 20.Zuchi S, Cesco S, Astolfi S. High S supply improves Fe accumulation in durum wheat plants grown under Fe limitation. Environ Exper Bot 2012; 77:25-32; http://dx.doi.org/ 10.1016/j.envexpbot.2011.11.001 [DOI] [Google Scholar]
- 21.Astolfi S, Zuchi S, Cesco S, Sanità di Toppi L, Pirazzi D, Badiani M, et al.. Iron deficiency induces sulfate uptake and modulates redistribution of reduced sulfur pool in barley plants. Funct Plant Biol 2006; 33:1055-61; http://dx.doi.org/ 10.1071/FP06179 [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Wu FH, Shang YT, Wang WH, Hu WJ, Simon M, Liu X, Shangguan ZP, Zheng HL. Hydrogen sulphide improves adaptation of Zea mays seedlings to iron deficiency. J Exp Bot 2015; 66(21):6605-22; PMID:26208645; http://dx.doi.org/ 10.1093/jxb/erv368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forieri I, Wirtz M, Hell R. Toward new perspectives on the interaction of iron and sulfur metabolism in plants. Front Plant Sci 2013; 4:357; PMID:24106494; http://dx.doi.org/ 10.3389/fpls.2013.00357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riemenschneider A, Nikiforova V, Hoefgen R, De Kok LJ, Papenbrock J. Impact of elevated H2S on metabolite levels, activity of enzymes and expression of genes involved in cysteine metabolism. Plant Physiol Biochem 2005; 43:473-83; PMID:15914014; http://dx.doi.org/ 10.1016/j.plaphy.2005.04.001 [DOI] [PubMed] [Google Scholar]
- 25.Bloem E, Riemenschneider A, Volker J, Papenbrock J, Schmidt A, Salac I, Haneklaus S, Schnug E. Sulphur supply and infection with Pyrenopeziza brassicae influence L-cysteine desulphydrase activity in Brassica napus L. J Exp Bot 2004; 55:2305-12; PMID:15310816; http://dx.doi.org/ 10.1093/jxb/erh236 [DOI] [PubMed] [Google Scholar]


