ABSTRACT
GTPase activity accelerating proteins (GAPs) are key regulators of the G-protein signaling cycle. By facilitating effective hydrolysis of the GTP bound on Gα proteins, GAPs control the timing and amplitude of the signaling cycle and ascertain the availability of the inactive heterotrimer for the next round of activation. Until very recently, the studies of GAPs in plants were focused exclusively on the regulator of G-protein signaling (RGS) protein. We now show that phospholipase Dα1 (PLDα1) is also a bona fide GAP in plants and together with the RGS protein controls the level of active Gα protein.
KEYWORDS: GAP activity, heterotrimeric G-proteins, PLDα1, phospholipases, RGS proteins
Heterotrimeric G-protein signaling mechanisms: The role of GAPs in modulating G-protein cycle
Signaling pathways mediated by heterotrimeric G-proteins are prevalent in all eukaryotes.1-3 The core of the heterotrimeric protein consists of a complex made of one Gα, one Gβ and one Gγ protein. The Gα protein acts as a bimodal molecular switch controlling the ‘on’ or ‘off’ stages of signaling (Fig. 1). When Gα is GDP-bound, it exists as a trimeric complex with the Gβγ proteins and represents the inactive or ‘off’ state of the signaling complex. Conversely, when GDP on Gα is replaced with GTP, it results in the dissociation of heterotrimer to monomeric Gα and dimeric Gβγ. Both these entities can interact with the downstream effectors to transduce the signal.4,5 The Gα protein possesses an inherent GTPase activity, which causes hydrolysis of bound GTP and regeneration of GDP-bound Gα, leading to its association with the Gβγ dimer and reassembly of the inactive trimeric complex.4,5 Because it is a cyclic process, the precise regulation of both the rate of activation and deactivation is required for proper signaling.6 The activation of G-protein cycle i.e. the exchange of GTP for GDP on Gα is usually facilitated by the guanine nucleotide exchange activity (GEF) of G-protein coupled receptors (GPCRs).7 The deactivation of G-protein cycle is achieved via the inherent GTPase activity of Gα (Fig. 1). However, the GTP hydrolysis rates are relatively slower than the GTP/GDP exchange rates; therefore continued signaling is achieved via assistance with proteins that have GTPase activity accelerating properties.6,8 In mammalian systems, diverse proteins containing a conserved RGS domain or specific phospholipase Cβ isoforms bind and stabilize catalytically active form of Gα and accelerate its GTPase activity, facilitating efficient deactivation.6,8
Figure 1.
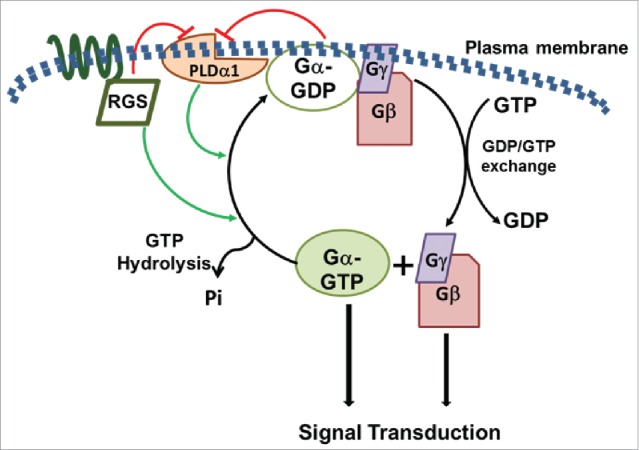
Model showing heterotrimeric G-protein signaling mechanism and its proposed regulatory modes. Green and red arrows represent activation and inhibition of the specific activities, respectively. A complex regulatory loop is formed between the Gα, RGS and PLDα1 proteins that control the level of active Gα and duration of the G-protein cycle. Additional proteins such as extra-large Gα proteins, additional phospholipases or other regulatory proteins may also be involved which are not shown in this model.
Unique aspects of the plant G-protein signaling
Extensive biochemical and structure-function characterization of the Arabidopsis Gα protein, GPA1, has suggested that it may undergo spontaneous GDP/GTP exchange without the activity of a GPCR,9 which seems to be lacking in plants. Additionally, GPA1 is a very slow GTPase, at least an order of magnitude slower than the slowest mammalian Gα10. A fast (or spontaneous) acquisition of GTP combined with its very slow hydrolysis implies that GPA1 stays in its GTP-bound, active form, unless the hydrolysis is enabled by the GAP activity of another protein. This unusual kinetics imply that GAPs are the most critical regulatory proteins of the plant G-protein cycle.
Until very recently, the RGS domain containing 7-transmembrane proteins, typified by AtRGS1, were thought to be the only GAPs in plants.11,12 Therefore, it was surprising that many plant species, especially those of the monocot lineage, do not have an obvious RGS homolog in their genomes.1 The commonly studied monocots such as rice, maize, sorghum and Brachypodium possess the complete repertoire of G-proteins, but no RGS protein. This apparent discrepancy can be explained in 2 possible ways; one the G-protein cycle in plants with no RGS is regulated differently from the ones that have RGS protein i.e., the Gα proteins from plants without RGS proteins exhibit significantly faster inherent rates of GTP hydrolysis. However, biochemical analysis of the rice Gα protein shows that it exhibits GTP-binding and GTPase activities similar to those of Arabidopsis GPA1.13 Another, more logical possibility is that RGS homologs are not the exclusive GAPs for plant Gα and there are additional proteins, found in all plants, which can act as GAPs. Our recent work and some of the work previously published by the Wang group establishes PLDα1 as one such GAP in plants.14,15 We have recently shown, based on genetic and biochemical data that both RGS1 and PLDα1 act as efficient GAPs for Arabidopsis GPA1.14 While the idea of phospholipases acting as GAPs is well-established in mammalian systems, where PLCβ isoforms have been shown to act both as GAPs and as effectors of Gα proteins,6 the lack of classical PLCβ homologs in plants16 has precluded such a possibility till now. Our results imply that this role is likely fulfilled by the PLD family, which is more elaborate in plants.16
We show that the G-protein complex and their regulators form multiprotein complexes and affect each other's activities depending on specific signaling or developmental pathways.14 For example, both RGS1 and PLDα1 accelerate the GTPase activity of GPA1, RGS1 also inhibits the activity of PLDα1, and GPA1 affects the activity of PLDα1.14 The net outcome of such complex interactions are exquisitely controlled levels and duration of active Gα, which result in specificity of response regulation (Fig. 1). It is clear from our data that distinct regulatory modes are operative when comparing phenotypes such as leaf shape, hypocotyl length and ABA sensitivity, to primary root length, lateral root density, stomatal development and silique shapes.14
Significance of PLDs as GAPs in plants
The establishment of PLDα1 as a GAP, an RGS target, and an effector of G-protein cycle is significant at many levels. First, it offers some insight into how the G-protein cycle might be regulated in plants that do not possess an obvious RGS homolog, but do require accelerated GAP activity for efficient deactivation of the cycle. All plants have an elaborate network of phospholipases which may suffice for regulating the G-protein deactivation.16 Second, the extant multiplicity of phospholipases in plants e.g., 12 PLDs, 9 PLCs, 6 non-specific PLCs, 12 PLA1, 4 PLA2 and 10 pPLAs in Arabidopsis; may significantly expand the diversity of G-protein regulatory mechanisms. A detailed biochemical analysis of the role of other PLDs (and addition phospholipases) will give insight in to whether other proteins can also function as GAPs and/or are regulated by RGS protein.17,18 If this is the case, it would predict that the multiplicity of response regulation occurs is not only at the level of various effectors but also at the level of regulatory proteins. Finally, the involvement of PLDα1 (and potentially of other phospholipases) as a regulator of G-protein cycle also suggests a link between the lipid-based signaling and G-protein signaling. Phospholipases are primarily the lipid hydrolyzing enzymes and their activity may have an impact on membrane permeability or dynamics, thereby affecting the G-proteins' localization or complex formation. In addition, the secondary messengers generated by the phospholipases, especially phosphatidic acid (PA), diacyl glycerol (DAG) and inositol triphosphate (IP3) are known to have multifaceted effects on diverse growth and development pathways, some of which are already linked to G-proteins.16,18-25 Detailed genetic and biochemical characterization of phospholipases will validate their roles as effectors and regulators in the context of plant G-protein signaling.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Research in the Pandey lab is supported by NIFA/AFRI (2015-67013-22964) and NSF (MCB-1157944) grants to SP.
References
- 1.Urano D, Jones AM. Heterotrimeric G protein-coupled signaling in plants. Annu Rev Plant Biol 2014; 65:365-84; PMID:24313842; http://dx.doi.org/ 10.1146/annurev-arplant-050213-040133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev 2005; 85:1159-204; PMID:16183910; http://dx.doi.org/ 10.1152/physrev.00003.2005 [DOI] [PubMed] [Google Scholar]
- 3.Hackenberg D, Pandey S. Heterotrimeric G proteins in green algae: an early innovation in the evolution of the plant lineage. Plant Signal Behav 2014; 9:e28457; PMID:25764428; http://dx.doi.org/ 10.4161/psb.28457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Offermanns S. G-proteins as transducers in transmembrane signalling. Prog Biophys Mol Biol 2003; 83:101-30; PMID:12865075; http://dx.doi.org/ 10.1016/S0079-6107(03)00052-X [DOI] [PubMed] [Google Scholar]
- 5.Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Insights into G protein structure, function, and regulation. Endocr Rev 2003; 24:765-81; PMID:14671004; http://dx.doi.org/ 10.1210/er.2000-0026 [DOI] [PubMed] [Google Scholar]
- 6.Ross EM. Coordinating speed and amplitude in G-protein signaling. Curr Biol 2008; 18:R777-R83; PMID:18786383; http://dx.doi.org/ 10.1016/j.cub.2008.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol 2008; 9:60-71; PMID:18043707; http://dx.doi.org/ 10.1038/nrm2299 [DOI] [PubMed] [Google Scholar]
- 8.Siderovski DP, Willard FS. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci 2005; 1:51-66; PMID:15951850; http://dx.doi.org/ 10.7150/ijbs.1.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones JC, Duffy JW, Machius M, Temple BR, Dohlman HG, Jones AM. The crystal structure of a self-activating G protein alpha subunit reveals its distinct mechanism of signal initiation. Sci Signal 2011; 4:ra8; PMID:21304159; http://dx.doi.org/ 10.1126/scisignal.2001446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston CA, Taylor JP, Gao Y, Kimple AJ, Grigston JC, Chen JG, Siderovski DP, Jones AM, Willard FS. GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling. Proc Natl Acad Sci U S A 2007; 104:17317-22; PMID:17951432; http://dx.doi.org/ 10.1073/pnas.0704751104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy Choudhury S, Westfall CS, Laborde JP, Bisht NC, Jez JM, Pandey S. Two chimeric regulators of G-protein signaling (RGS) proteins differentially modulate soybean heterotrimeric G-protein cycle. J Biol Chem 2012; 287:17870-81; PMID:22474294; http://dx.doi.org/ 10.1074/jbc.M112.353219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen JG, Willard FS, Huang J, Liang J, Chasse SA, Jones AM, Siderovski DP. A seven-transmembrane RGS protein that modulates plant cell proliferation. Science 2003; 301:1728-31; PMID:14500984; http://dx.doi.org/ 10.1126/science.1087790 [DOI] [PubMed] [Google Scholar]
- 13.Urano D, Dong T, Bennetzen JL, Jones AM. Adaptive evolution of signaling partners. Mol Biol Evol 2015; 32:998-1007; PMID:25568345; http://dx.doi.org/ 10.1093/molbev/msu404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy Choudhury S, Pandey S. The role of PLDalpha1 in providing specificity to signal-response coupling by heterotrimeric G-protein components in Arabidopsis. Plant J 2016; 86:50-61; PMID:26935351; http://dx.doi.org/ 10.1111/tpj.13151 [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Wang X. Arabidopsis phospholipase Dalpha1 interacts with the heterotrimeric G-protein alpha-subunit through a motif analogous to the DRY motif in G-protein-coupled receptors. J Biol Chem 2004; 279:1794-800; PMID:14594812; http://dx.doi.org/ 10.1074/jbc.M309529200 [DOI] [PubMed] [Google Scholar]
- 16.Wang G, Ryu S, Wang X. Plant phospholipases: an overview. Methods Mol Biol 2012; 861:123-37; PMID:22426716; http://dx.doi.org/ 10.1007/978-1-61779-600-5_8 [DOI] [PubMed] [Google Scholar]
- 17.Zhao J, Wang X. Biochemical analysis of the interaction between phospholipase Dalpha1 and GTP-binding protein alpha-subunit from Arabidopsis thaliana. Methods Mol Biol 2013; 1043:21-35; PMID:23913032; http://dx.doi.org/ 10.1007/978-1-62703-532-3_3 [DOI] [PubMed] [Google Scholar]
- 18.Peters C, Li M, Narasimhan R, Roth M, Welti R, Wang X. Nonspecific phospholipase C NPC4 promotes responses to abscisic acid and tolerance to hyperosmotic stress in Arabidopsis. Plant Cell 2010; 22:2642-59; PMID:20699393; http://dx.doi.org/ 10.1105/tpc.109.071720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M, Wei F, Tawfall A, Tang M, Saettele A, Wang X. Overexpression of patatin-related phospholipase AIIIdelta altered plant growth and increased seed oil content in camelina. Plant Biotechnol J 2015; 13:766-78; PMID:25557877; http://dx.doi.org/ 10.1111/pbi.12304 [DOI] [PubMed] [Google Scholar]
- 20.Lu S, Bahn SC, Qu G, Qin H, Hong Y, Xu Q, Zhou Y, Hong Y, Wang X. Increased expression of phospholipase Da1 in guard cells decreases water loss with improved seed production under drought in Brassica napus. Plant Biotechnol J 2013; 11:380-9; PMID:23279050; http://dx.doi.org/ 10.1111/pbi.12028 [DOI] [PubMed] [Google Scholar]
- 21.Guo L, Wang X. Crosstalk between Phospholipase D and Sphingosine Kinase in Plant Stress Signaling. Front Plant Sci 2012; 3:51; PMID:22639650; http://dx.doi.org/ 10.3389/fpls.2012.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Zhu H, Zhang Q, Li M, Yan M, Wang R, Wang L, Welti R, Zhang W, Wang X. Phospholipase dalpha1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 2009; 21:2357-77; PMID:19690149; http://dx.doi.org/ 10.1105/tpc.108.062992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M, Hong Y, Wang X. Phospholipase D- and phosphatidic acid-mediated signaling in plants. Biochim Biophys Acta 2009; 1791:927-35; PMID:19289179; http://dx.doi.org/ 10.1016/j.bbalip.2009.02.017 [DOI] [PubMed] [Google Scholar]
- 24.Peleg-Grossman S, Volpin H, Levine A. Root hair curling and Rhizobium infection in Medicago truncatula are mediated by phosphatidylinositide-regulated endocytosis and reactive oxygen species. J Exp Bot 2007; 58:1637-49; PMID:17420174; http://dx.doi.org/ 10.1093/jxb/erm013 [DOI] [PubMed] [Google Scholar]
- 25.Mane SP, Vasquez-Robinet C, Sioson AA, Heath LS, Grene R. Early PLDalpha-mediated events in response to progressive drought stress in Arabidopsis: a transcriptome analysis. J Exp Bot 2007; 58:241-52; PMID:17261695; http://dx.doi.org/ 10.1093/jxb/erl262 [DOI] [PubMed] [Google Scholar]


