ABSTRACT
The endophytic fungus Piriformospora indica colonizes Arabidopsis thaliana roots and promotes plant performance, growth and resistance/tolerance against abiotic and biotic stress. Here we demonstrate that the benefits for the plant increase when the two partners are co-cultivated under stress (limited access to nutrient, exposure to heavy metals and salt, light and osmotic stress, pathogen infection). Moreover, physical contact between P. indica and Arabidopsis roots is necessary for optimal growth promotion, and chemical communication cannot replace the physical contact. Lower nutrient availability down-regulates and higher nutrient availability up-regulates the plant defense system including the expression of pathogenesis-related genes in roots. High light, osmotic and salt stresses support the beneficial interaction between the plant and the fungus. P. indica reduces stomata closure and H2O2 production after Alternaria brassicae infection in leaves and suppresses the defense-related accumulation of the phytohormone jasmonic acid. Thus, shifting the growth conditions toward a stress promotes the mutualistic interaction, while optimal supply with nutrients or low stress diminishes the benefits for the plant in the symbiosis.
KEYWORDS: Biophoton, biotic and abiotic stress, defense, light stress, metal resistance, mutualism, osmotic stress, phytohormones, root architecture, reactive oxygen species, salt stress, stomata
Abbreviations
- P
phosphorus
- C
carbon
- N
nitrogen
- Pi
inorganic phosphate
- JA
jasmonic acid
- WT
wild-type
- Nlen
Network length
- SEM
Scanning Electron Microscopy
- ROS
reactive oxygen species
Introduction
Biotic and abiotic stresses cause physiological and hormonal imbalances, nutrition deficiency, ion toxicity, reduction of the defense capacity and thus reduce plant performance and agricultural yields. Stresses can be reduced by different strategies including symbiotic interactions. Besides stress tolerance, a symbiotic interaction can promote the biomass production of the plant1 and ensures a better survival in nature. Fungi and microbes profit from a symbiotic interaction by the photoassimilates from the host.
Arbuscular mycorrhiza (AM) fungi play a key role in ecosystems.2 Inorganic phosphate (Pi) uptake from the soil and carbon transfer from the host to the fungus establish a C-P organic balance which is crucial for symbiotic interactions.2 Pi uptake is energy-consuming for plants and fungi and its uptake by roots results in depletion of the surrounding soil area and consequently requires root growth and development for reaching new areas with enough Pi.3 Plants respond to Pi limitations by increasing the root hair number and length, Pi mining from unsolvable resources by malic and citric acids or acid phosphatases4 and symbiosis with fungi,5 which can increase the solubility of Pi forms.4 In Arabidopsis-Piriformospora indica interaction, the fungus promotes Pi uptake and root development under Pi limitation in wrky6 mutant, which is associated with the stimulation of PHOSPHATE1 expression and ethylene production. Expression profiles from the roots of wrky6 seedlings identified genes involved in hormone metabolism, transport, meristem, cell and plastid proliferation, and growth regulation.6
AM fungal mycelium enhances the hydraulic conductivity in roots. Water deficiency affects growth and plant yield via modification of the plant osmotic potential.7 The plant itself uses anatomical, physiological and cellular mechanisms8 as well as symbiosis9 to reduce the negative effects. Under drought conditions the AM symbiosis increases plant resistance9-11 by increasing nutrient uptake, enlarging the root surface area,10 and adjusting the osmotic potential.7 The fungus preserves the moisture by generating glomalin-soil aggregations,7,11 while the plant responds to the presence of the fungus by altering the gene expression pattern and physiology, e.g. by higher production of antioxidants7 or improvement of water movement into the plant.11 The water potential is much higher in mycorrhizal colonized relative to non-colonized plants.10 P. indica confers drought-stress tolerance to Arabidopsis under in vitro conditions, and this is associated with the priming of the expression of a quite diverse set of stress-related genes in the leaves.12 In Chinese cabbage P. indica retarded the drought-induced decline in the photosynthetic efficiency and the degradation of chlorophylls and thylakoid proteins.13
Reduction of heavy metal uptake in plants via production of metallophytes is another effect of AM fungi interacting with the plant root.14 Different plant species produce metallothionin under heavy metal stress. Hildebrandt et al.14 demonstrated that heavy metal uptake into roots is reduced by the fungus-produced glycoprotein glomalin. Glomalin is highly persistent in soil, affects the soil structure and reduces soil density and indirectly improves plant growth, beside its direct effect on heavy metal tolerance.15 Fungi also precipitate metal ions.16-17
Half of the cultivable lands will become saline in the next 40 years which will result in increasing levels of hyperionic and hyperosmotic stress18 and subsequently in higher oxidative stress.19 Production of antioxidant enzymes helps plants to survive better under salt stress conditions.20 Fungi-mediated antioxidants (ascorbic acid, α-tocopherol, glutathione and carotenoids) cause reduction of hydrogen peroxide and peroxidation of lipids.21 Root dependency of the symbiotic fungi increases under salinity and fungi can alleviate salt stress effects of the plant.22 In salty conditions, plant phosphorus (P) and magnesium (Mg2+) levels are increased in the presence of AM fungi by the accumulation of osmolytes in roots, such as carbohydrates and electrolytes.23 The deficiency of Mg2+ in plants results in reduction of chlorophyll formation and causes adverse effects on photosynthesis.24 The symbiotic plant-fungi interaction also improves NO3−, Ca2+ and K+ uptake under salt stress.25 Gahlot et al.26 obtained 36 salinity-tolerance genes using functional screening, based on random over expression of a P. indica cDNA library in Escherichia coli grown on medium supplemented with 0.6 M NaCl. The expression of these 36 genes was analyzed in P. indica using quantitative RT-PCR and only six genes were up-regulated by salt stress. These six genes are involved in different cellular processes, such as metabolism, energy and biosynthetic processes, DNA repair, regulation of protein turnover, transport and salt stress tolerance.26
The beneficial effect of P. indica association on rice seedlings was analyzed during high salt stress conditions (200 and 300 mM NaCl) by Jogawat et al.27 Salt-stressed seedlings performed much better in the presence of P. indica compared with non-inoculated controls. The photosynthetic pigment content [chlorophyll (Chl) a, Chl b, and carotenoids] was significantly higher in P. indica-inoculated rice seedlings under high salt stress conditions as compared to control seedlings. Proline accumulation was also observed during P. indica colonization, which may help the inoculated plants to become salt tolerant.27
In this study we analyze the P. indica/Arabidopsis thaliana interaction by manipulating stress conditions during their co-cultivation. The beneficial interaction of the root endophyte P. indica with a multitude of horticulturally and agriculturally important plants as well as model plants such as Arabidopsis leads to growth promotion, increased biomass production and enhanced resistance/tolerance against biotic and abiotic stress (cf.28-32). The fungus can be cultivated axenically on synthetic or complex media without a host.29 Once inside the roots, the fungus gets access to photoassimilates and other plant nutrients, which further promotes colonization and proliferation.30 We demonstrate that an increase in various unrelated stresses strengthens the symbiotic interaction of P. indica with Arabidopsis, which results in better performance of the plant.
Results
Nutrient availability in Arabidopsis/P. indica interaction
We first established co-cultivation conditions in which the two symbionts have different access to nutrients from the PNM medium. Arabidopsis seedlings co-cultivated with P. indica in a PNM aquaculture for 30 days accumulate less biomass compared to seedlings grown without the fungus (Fig. 1A, B, C). Growth of the seedlings in the presence of P. indica was also reduced when the two symbionts were co-cultivated on solified PNM medium in Petri dishes. Separation of the biological material from the PNM medium by a nylon membrane restricted the access of the roots and hyphae to the nutrients. Under these conditions, the presence of the fungus is favorable for plant growth which results in a slight, but significant increase in the plant biomass, relative to growth without the fungus. Further restriction of the access to nutrients is achieved by a cellophane membrane which cannot be passed by roots and fungal hyphae. This resulted in an even stronger growth promotion of the plant in the presence of the fungus, relative to the seedlings grown without the fungus (Fig. 1C). This suggests that limited access to the PNM nutrients is crucial for the stimulatory effect of the fungus on plant biomass production.
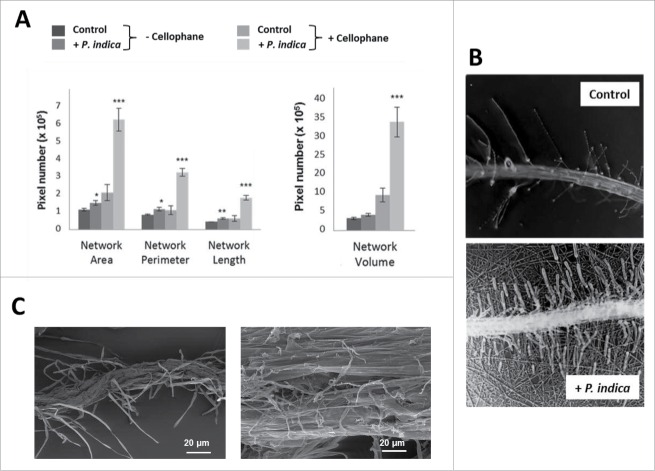
Figure 1.
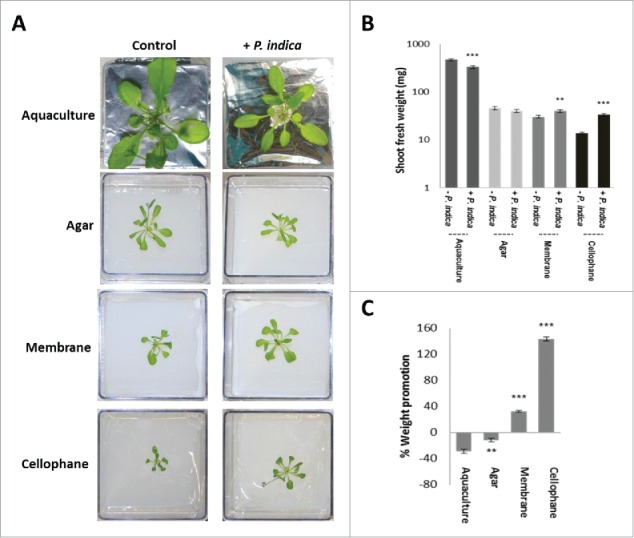
A. thaliana seedlings grown under long day light condition (65 µmol m−2 sec−1) and different access to the nutrients in PNM medium (aquaculture, agar, membrane or cellophane) with or without P. indica. (A) Typical pictures of the seedlings after 30 days of co-cultivation under the four different conditions. (B) Shoot fresh weights of 30 day-old seedlings shown in A. (C) Weight promotion of the shoots by P. indica (in %) relative to the uncolonized control. For statistics, cf. Materials and Methods.
The P. indica-induced changes in plant growth are reflected by the fungus-induced alterations in the root architecture. It is obvious that root growth stimulation by P. indica increases the more the access to the medium is restricted (aquaculture < agar < membrane < cellophane). Using the software program developed by Galkovskyi et al,33 the length (Nlen), perimeter (Perim) and volume of the root system was quantified for the four growth conditions with and without the fungus (Fig. 2A). They confirm an increase in root growth stimulation by P. indica with decrease in accessibility to the nutrients in the medium. The most dramatic increase was observed for roots which were co-cultivated with P. indica on the cellophane membrane (Fig. 2B, C). While barely any lateral roots can be detected in the uncolonised control, lateral root development is strongly promoted by P. indica.
Figure 2.
Effect of P. indica on the root architecture of A. thaliana seedlings grown for 12 days on PNM media under long day light condition (50 ± 15 µmol m−2 sec−1). (A) Root architecture of Arabidopsis seedlings grown on PNM media with and without cellophane. (B) Microscopical view of the roots' network in control and P. indica-treated seedlings (+ P. indica) grown on PNM plates covered with cellophane. Pictures were from the GiaRoot software. (C) SEM images of control and P. indica-colonized Arabidopsis roots after 10 days of colonization. For statistics, cf. Materials and Methods.
Furthermore, physical contact between the two symbionts strongly stimulates P. indica-mediated growth promotion of Arabidopsis seedlings. When the fungus grows under the cellophane membrane (Fig. S1) no physical contact to the host can be established. Under this condition, the growth-promoting effect of the fungus on the seedlings was more than 10-times lower compared to co-cultivation conditions on the surface of the cellophane membrane (data not shown). We conclude that a physical contact cannot be replaced by chemical communication between the two symbionts. In addition, restriction in nutrient access strengthens the physical contact between the two symbionts: Scanning Electron Microscopy (SEM) (Fig. 2C) shows the mycelial network when the two symbionts are in physical contact. The fungal mycelium grows on the root surface, covers the surrounding area of the roots and penetrates into the root epidermal cells. The root-associated mycelial network extends to distantly located cellophane areas. The capillary activity from the root cells is supported by this fungal network, which allows a more efficient nutrient and water uptake from the accessible medium, compared to roots growing without the fungus.
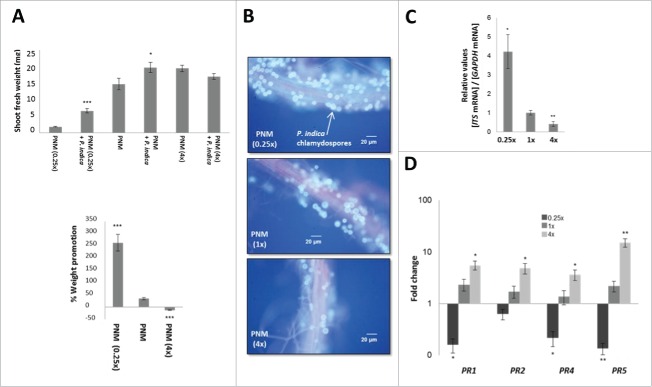
To further support the concept that limitations in nutrient availability promotes plant growth by P. indica, Arabidopsis seedlings with and without the fungus were grown on different PNM concentrations (0.25x, 1x, 4x). The strongest growth-promoting effect of the fungus was observed on 0.25x PNM medium and the smallest effect on 4x PNM medium (Fig. 3A). Microscopic analyses and determination of the ratio of fungal ITS mRNA/plant GAPDH mRNA34 demonstrate that root colonization is higher on low PNM medium and reduced of high PNM medium (Fig. 3B, C). This suggests that root colonization increases with decrease in nutrient availability.
Figure 3.
Effect of P. indica on A. thaliana shoot growth (A), root colonization (B and C), and expression of defense genes (D) in different PNM concentration [7 days co-cultivation under long day light (50 ± 15 µmol m−2 sec−1)]. (A) Shoot fresh weight (up) and promotion in % relative to the uncolonized control (down). (B) Fluorescent microscopy of Arabidopsis roots stained with trypan blue and fuchsin acid. (C) P. indica ITS mRNA level (relative to plant GAPDH mRNA level) from colonized roots grown on different PNM concentrations. (D) Fold changes of PR genes (+ P. indica / - P. indica) in Arabidopsis shoots grown on different PNM concentrations. For statistics, cf. Materials and Methods.
Lower nutrient availability resulted also in the down-regulation and higher nutrient availability in the up-regulation of the pathogenesis-related genes PR1, PR2, PR4 and PR5 in shoots indicating a general repression of the plant defense system (Fig. 3D). This might be caused by less resource availability for the plant defense machinery under nutrient-limiting growth conditions, or higher root colonization results in a more efficient downregulation of the plant defense machinery.
In conclusion, lower nutrient availability promotes the mutualistic interaction. The role of P. indica in promoting the plant´s excess to the nutrients is manifested by higher root colonization and root and plant growth stimulation. On the other hand, the plant´s investment in defense is reduced under nutrient-limiting conditions.
Heavy metal and osmotic stress in Arabidopsis/P. indica interaction
To further support the idea that stress conditions promote the P. indica/Arabidopsis interaction, we exposed colonized and control seedlings to various heavy metal and osmotic stresses.
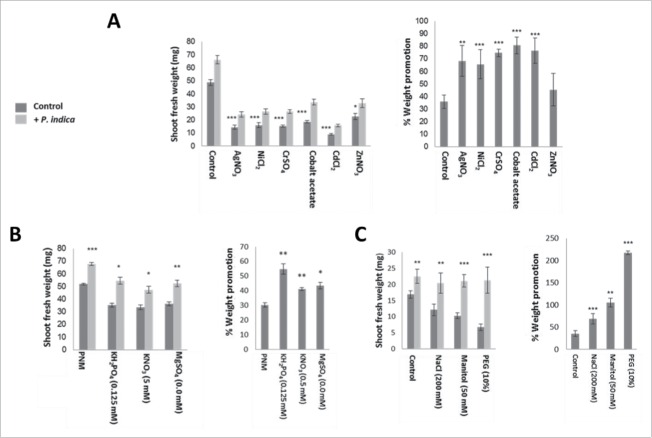
As expected, exposure of colonized and uncolonized Arabidopsis seedlings to heavy metal stress severely reduced their shoot fresh weights. However, when the % increase in shoot weight of colonized versus uncolonized seedlings is considered, the colonized seedlings perform better than the uncolonized controls (Fig. 4A). It appears that the benefits for the plants induced by the fungus increase with increasing toxicity of the metal ions (Zn < Ag = Ni < Cr = Co = Cd).
Figure 4.
Effect of heavy metals and salts on A. thaliana growth in the presence/absence of P. indica under long day light (50 ± 15 µmol m−2 sec−1). (A) Shoot fresh weights (left) and % weight promotion by P. indica (relative to the uncolonized control) (right) after 10 days of co-cultivation or mock-treatment. 1 mM of different metal salts was used. (B) Shoot fresh weights (left) and % weight promotion by P. indica (right) of seedlings grown for 10 days with/without P. indica on different salt concentrations. (C) Shoot fresh weights (left) and % weight promotion (right) of 10-day old seedlings co-cultivated with/without P. indica under different osmotic and salt conditions. For statistics, cf. Materials and Methods.
Similar effects were observed for seedlings exposed to osmotic stress, i.e. PNM medium plus 200 mM NaCl or 50 mM mannitol or 10% PEG. The strongest effect was observed for PEG-exposed seedlings (Fig. 4C).
These examples demonstrate that the seedlings profit more from the symbiosis when they are exposed to a heavy metal or osmotic stress.
Role of Pi, NO3− and SO42− in the P. indica/Arabidopsis interaction
Pi availability is a classical model to study the role of beneficial fungi in helping the plant to obtain better access to this growth-limiting nutrient. Several studies have demonstrated that Pi limitation restricts Arabidopsis growth and promotes the Arabidopsis/P. indica interaction,35 Bakshi and Oelmüller, unpublished results). Under our growth conditions, the shoot fresh weight of P. indica-colonized seedlings grown on full PNM medium on the nylon membrane is increased by 31 ± 3% compared to the uncolonized control (Fig. 4B). If the KH2PO4 concentration in the PNM medium is reduced 10-fold, the growth-stimulating effect increased to 58 ± 4% (Fig. 4B). Similar results were also obtained for other ions: 10-fold reduction of the KNO3 concentration leads to an increase in the fresh weight from 31 ± 3% to 46 ± 2%, and of the MgSO4 concentration from 31 ± 3% to 54 ± 5% (Fig. 4B). These examples support the concept that limitations in Pi and other essential ions promote the benefits for the plant in the interaction.
Light intensity in Arabidopsis/P. indica interaction
While minerals are taken up from the soil, light stress acts directly on leaves. Since plant performance is strongly affected by light quality and intensity, we tested whether root colonization by P. indica has an effect on light stress to the leaves.
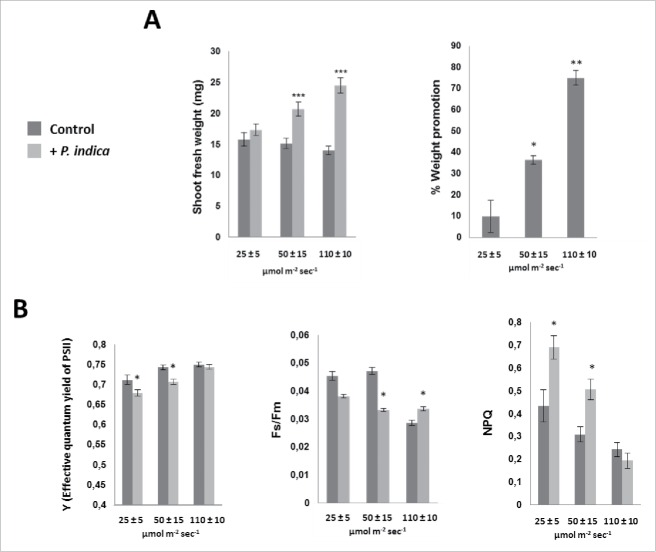
P. indica-colonized and control plants were grown under 25, 50 and 110 µmol m−2 sec−1 continuous illumination. Fig. 5A demonstrates that the increase in the light intensity results in reduced shoot biomass of uncolonized seedlings, while the opposite is observed for colonized seedlings. This demonstrates that continuous illumination with higher light intensities is stressful for the seedlings, and that root colonization counteracts this stress by root-to-shoot signaling.
Figure 5.
Effect of different light intensities on A. thaliana growth in the presence/absence of P. indica. (A) Shoot fresh weights (left) and % weight promotion by P. indica (right) under continuous light and different light intensities (25–110 µmol m−2 sec−1). Seedlings were grown for 10 days with/without P. indica. (B) Fluorescence parameters [Y (effective quantum yield of photosystem II), Fs/Fm and NPQ] in Arabidopsis shoots after 10 days co-cultivation with P. indica under continuous light (25 ± 5, 50 ± 15 and 110 ± 10 µmol m−2 sec−1). All results are based on 3 independent experiments with 10 individual seedlings. Bars represent SE. Asterisks indicate significant differences (relative to its own control) as determined by t-test (* P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001).
To gain more inside into these processes, we measured fluorescence parameters for the efficiency of the photosynthetic electron flow under the different conditions. The photosynthetic yield represented by the Fs/Fm parameters in low light (25 and 50 µmol m−2 sec−1) is higher in uncolonized than in colonized plants. The opposite effect was observed for high light-grown plants (110 µmol m−2 sec−1). Consistent with these observations, the NPQ values representing energy loss by heat dissipation gave the opposite results (Fig. 5B). The data demonstrate that the efficiency of the photosynthetic electron flow decrease gradually with increasing light intensity in uncolonized plants, and that fungal signals from the roots counteract the efficiency loss (Fig. 5B).
Response to pathogen infection in Arabidopsis/P. indica interaction
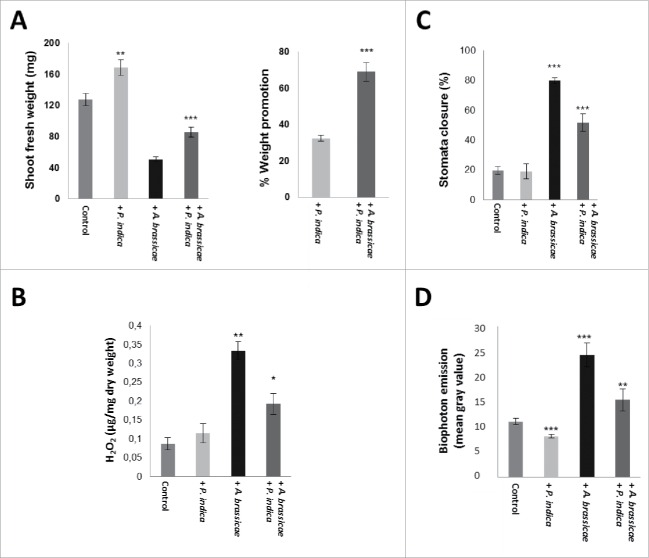
Finally, we exposed Arabidopsis leaves to dosis of the pathogenic fungus A. brassicae (Fig. 6) and tested whether this has an effect on P. indica-induced growth promotion. Fig. 6A demonstrates that growth promotion by the beneficial fungus was stronger in pathogen-exposed seedlings when compared to seedlings which were not treated with A. brassicae. Infection of the leaves with A. brassicae induced H2O2 production, and seedlings which were co-cultivated with P. indica produced significantly less H2O2 (Fig. 6B). Similarly, A. brassicae infection to the leaves resulted in stomata closure and this effect was also reduced in P. indica-exposed seedlings (Fig. 6C). The latter two observations suggest an efficient root-to-shoot communication.
Figure 6.
(A) Shoot fresh weights (left) and % weight promotion by P. indica (right) in A. thaliana leaves of seedling with/without P. indica and 10 days after leaf infection with A. brassicae. The seedlings were grown under long day light condition (50 ± 15 µmol m−2 sec−1). (B) H2O2 levels in leaves of Arabidopsis seedlings after infection with A. brassicae in the presence/absence of P. indica after 10 days. (C) Stomata closure after Alternaria leaf infection of colonized and uncolonized seedlings after 10 days. (D) Relative gray values of the images (biophoton records) of Alternaria-infected Arabidopsis leaves treated with/without P. indica. For statistics, cf. Materials and Methods.
Biophotons are ultra-weak emission of photons which mainly result from the generation of reactive oxygen species (ROS) and metabolic activities as by-products of cellular respiration.36 Biophoton measurements have been used for demonstration of stress conditions in various organisms.36 Biophoton emission after A. brassicae infection was lower in P. indica-colonized seedlings relative to the seedlings not exposed to P. indica. This confirms that P. indica reduces the stress induced by A. brassicae infection (Fig. 6D).
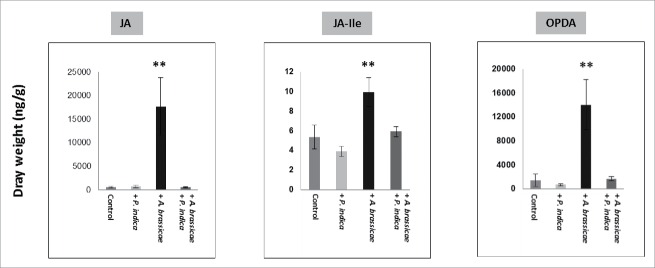
The phytohormones jasmonic acid (JA), JA-isoleucine, and oxophytodienoic acid are crucial for the activation of defense responses. Fig. 7 demonstrates that JA, JA-isoleucine, and oxophytodienoic acid accumulate in the shoots of Arabidopsis seedlings after infection of the leaves with the pathogenic fungus A. brassicae. Pre-treatment of the seedlings with P. indica reduced significantly hormone accumulation. We conclude that P. indica represses Alternaria-induced phytohormone accumulation in Arabidopsis shoots.
Figure 7.
JA, JA-Ile and oxophytodienoic acid (OPDA) levels in the shoots with/without P. indica and 10 days after leaf infection with A. brassicae. The seedlings were grown under long day light condition (50 ± 15 µmol m−2 sec−1). For statistics, cf. Materials and Methods.
Taken together, increased abiotic and biotic stress results in the promotion of plant biomass and performance by P. indica.
Discussion
We demonstrate that an increase in quite different stresses promotes the symbiotic interaction between P. indica and Arabidopsis roots, which ultimately results in better plant performance. Our experimental setup included abiotic and biotic stress experiments, both to roots and shoots, in which the two symbionts had access to different nutrient concentrations, were exposed to heavy metals with different toxicities, to different osmotic and light stress conditions and a pathogen. We measured different well characterized responses of the plants to these stresses which describe the performance of the plant in the presence and absence of root colonization by P. indica. We optimized the conditions such that the highest applied stress reduced plant performance but did not cause irreversible damage. All experiments demonstrate that the fungal effect on plant growth and performance increases with an increase in the applied stress. These experiments also highlight the importance of the experimental setup for studying plant/microbe interaction. For instance, co-cultivation of the two symbionts on PNM medium does not result in growth promotion, unless access to nutrients in the PNM medium is restricted by a nylon or cellophane membrane.
Limited access to or availability of nutrients strengthens the mutualistic interaction which also results in the stimulation of root growth and severe alterations in the root architecture. Similar results for the P. indica/Arabidopsis interaction were obtained when specific ion (Pi, NO3−, SO42−) concentrations were reduced in the PNM medium. Likewise, AM symbiosis is stimulated by P limitation.37 A number of studies have demonstrated that mycorrhizal fungi and plant growth promoting rhizobacteria improve crop productivity under stressful environments.38 In AM symbiosis, a more intense interaction of the two partners under nutrient-limiting conditions has been intensively studied for Pi, NO3− and SO42−. Smith and Smith39 proposed that the level of colonization is crucial for a beneficial interaction in AM symbiosis, and in Pi− and NO3− rich soil the level is lower in AM plants. This results in growth depression because the beneficial traits in the symbiosis decrease. The higher fungal RNA/plant RNA ratio indicates that the roots are more dependent on the fungus under low PNM medium. The degree of root colonization is crucial for mutualistic interactions,40 and might be under fungal41 and/or plant control.42 Down-regulation of PR gene expression in low PNM medium provides optimal condition for colonization by P. indica. Conversely, in higher nutrient concentration, the plant does not need the fungus for nutrient acquisition and root colonization is reduced. Smith and Smith39 showed that Pi and NO3− rich soil reduces AM root colonization. Up-regulation of the PR genes and induction of the plant defense system under these conditions shows that the plant produces anti-fungal compounds to restrict or even prevent root colonization. Thus, the level of stress is defining the level of biotic interaction between the 2 symbionts.
Under nutrient-limiting conditions, the fungus stimulates root growth more than under conditions with sufficient nutrient supply, as shown by the Nlen and the root surface volume values (Fig. 2A).
We also demonstrate that heavy metal tolerance of Arabidopsis seedlings is stimulated by P. indica, and the protective role of the fungus increases with increasing toxicity of the heavy metal ion. Similarly, plants in mycorrhizal associations are less sensitive to heavy metal stress than non-mycorrhizal plants,43 and cadmium-induced changes in mycorrhizal roots were absent or smaller than those in non-mycorrhizal roots.44 Several mechanisms can account for these observations. The heavy metals could accumulate primarily in the fungal hyphae and thus to a lesser extent in the roots. Alternatively, metal chelators or organic acids could be secreted by fungi or mycorrhizal roots.4,45 The antioxidant activity becomes activated after exposure to heavy metal stress and participates in protecting the roots against this stress.46 Detoxification of heavy metals by fungi occurs also through the generation of nanoparticles, extracellular particles, which scavenge the metal ions and reduces their concentrations prior to the up-take of the roots.16 Considering that these detoxification mechanisms do not distinguish between different heavy metal ions, it is reasonable to assume that detoxification of highly toxic ions has a stronger effect for the plant than detoxification of less toxic heavy metals. Taken together, better plant growth and performance include also P. indica-mediated detoxification or sedimentation of toxic metal cations outside or inside of plant and fungal cells.
Similar results were obtained when Arabidopsis seedlings were exposed to osmotic stress. The growth-promoting effect of P. indica increased with increasing osmotic stress. Also Augé et al.47 found that the efficiency of mycorrhiza on plant perfomance depends on the stress severity.
Finally, we applied two types of stress to the leaves and checked how they are affected by signals from colonized roots: light and infection with Alternaria spores. With increase in light intensity, colonized plants perform better compared to those grown under low light conditions. Under high light conditions, the ROS level in the leaves increase, and ROS is also produced after Alternaria infection. The ROS levels in the leaves are reduced in P. indica-colonized plants, e.g. by the stimulation of the antioxidant capacity in the plant.31,48
Furthermore, the root-associated fungus promotes the performance of the aerial part by signals from the roots. We introduced the new and highly sensitive biophoton technology, which detects ultra-weak emission of photons mainly from the generation of ROS and metabolic activities as by-products of cellular respiration.36 This technology is appropriate to quantitatively describe stress and thus plant fitness in plants.
Infection of Arabidopsis leaves with A. brassicae spores results in JA, JA-isoleucine, and oxophytodienoic acid accumulation in shoots in response to infection. The pathogen-induced phytohormone accumulation in leaves is suppressed by the beneficial fungus P. indica when roots are pre-treated with this fungus. A comparable systemic effect was also shown for Arabidopsis leaves infected with Verticillium dahliae.49
Collectively, these results demonstrate that increase in stress within a reasonable and non-toxic scale stimulates the symbiotic interaction between P. indica and Arabidopsis which in turn results in better performance of the plant.
Materials and methods
Co-cultivation of A. thaliana with P. indica in different conditions, data analyses
A modified Kaefer medium50 was used for the propagation of P. indica on a Petri dish. After growing for 14 days at room temperature, a P. indica plaque with 5 mm diameter (control: plaque without hyphae) was placed on plant nutrient media (PNM) with a nylon membrane (pore size 70 μm) on the top. The fungal culture was kept at the same condition for 1 week until the P. indica mycelium covered the entire membrane surface.32 For co-cultivation, 12 days-old seedlings of A. thaliana were grown on MS media as described in Johnson et al.32 Four Arabidopsis seedlings were transferred to the plates colonized by P. indica as well as to control plates and grown for 10 days in long day condition [50 (± 15) μmol m−2 sec−1 light from the top]. Different concentrations of PNM (0.25x, 1x and 4x) were used to study the nutrition effect on the P. indica/Arabidopsis interaction. To investigate the effect of N, P and S, the respective salts in the PNM medium were reduced to the final concentrations of 0.5 mM, 0.25 mM and 0.125 mM for KNO3, MgSO4 and KH2PO4, respectively. Continuous light of 10 ± 5, 50 ± 15 and 110 ± 10 μmol m−2 sec−1 was given from the top to analyze the effect of the light intensity on the symbiotic interaction. Biotic stress was investigated with the pathogenic fungus Alternaria brassicae. After infection of Arabidopsis seedlings co-cultivated with or without P. indica for 7 days, the leaves were infected with a spore solution. A. brassicae was cultured on PDA media for two weeks at room condition. The spores were collected with a sterile scalpel and three times washed with distilled sterile water. Spore concentration was adjusted to the 1×106 spore/ml and 2 µl of this suspension was used for leaf infection. As control, sterile water was used instead of the A. brassicae inoculum. In all experiments fresh weight of the seedlings was used as a system of reference according to Sherameti et al.44 The pH of PNM media was adjusted to 5.6 and all experiments were performed at 23°C. Except for the fluorescence and biophoton measurements, all results are based on 3 independent experiments with 10 replicate (10 individual seedlings each). Error bars represent standard error (SE). Asterisks indicate significant differences (relative to its own control) as determined by t-test (* P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001).
Nutrition availability study
Sterilized Arabidopsis seeds were placed on PNM plates with different nutrition concentrations, and inoculated with 2 µl of the P. indica spore solution (1×106 spore/ml). Distilled water was used as control and the seedlings were kept for 2 days at 4°C before transfer to the respective light conditions for four weeks under long day condition (18 h light and 6 h dark) and a light intensity of 50 ± 15 μmol m−2 sec−1, applied from the top. Different access to nutrient supply was achieved by growing the symbionts under four different conditions: an aquaculture system,17 growth of the symbionts on solid PNM media, on solid PNM media covered with a nylon membrane (70 µm pore size), or on solid PNM media covered with a cellophane membrane (<200 nm pore size which does not allow the penetration of roots and mycelia).
Co-cultivation of the symbionts under aquaculture condition was performed in Magenta boxes with 300 ml sterile PNM liquid media. The boxes were covered by aluminum foil and a microtube from which the tip was removed, filled with solid PNM media placed in a hole in the middle of the aluminum foil as described in Vahabi et al.17 For solid PNM media, 300 ml PNM media containing 1% agar in a Magenta box was used. A 70 µm pore-size nylon membrane was placed on top of 300 ml PNM medium solidified with agar. For the co-cultivation conditions with cellophane, the nylon membrane was replaced by a cellophane membrane.
Root architecture
Root architecture studies were performed by co-cultivation of Arabidopsis seedlings with P. indica in vertical square plates. Twelve days-old seedlings grown on MS media were transferred to the PNM media prepared with gelrite and kept vertically for 10 days under long day conditions (50 ± 15 μmol m−2 sec−1, light from the top). P. indica inoculation was done at the same time32 by placing 2 agar plaques of 14 day-old fungus in KM media to the center of the square plates, in 5 cm distance to each other and 2.5 cm distance from the edge of plates.
To study how the root hair architecture of the seedlings is affected by P. indica, Arabidopsis seeds were placed on the surface of PNM media covered by a cellophane membrane together with P. indica spores. After 4°C for 48 h, they were kept under long day condition for three weeks. 2 µl of a P. indica spore solution (as described above) was used for inoculation of the seeds on the surface of the cellophane membrane. Control seeds were treated with sterile water instead of the P. indica inoculum. The GiA Roots software was used to analyze the differences in the root architecture between control and P. indica-treated plants according to Galkovskyi et al.33
For more detailed analyses of the root architecture, the Network area parameter (the number of root or root hair pixels in the image), the Network length parameter (Nlen, the total number of pixels in the network skeleton), the Perimeter parameter (Perim, the total number of root or root hair pixels connected to a background pixel), the Network volume (the sum of the root or root hair volume in pixel as approximated by a tubular shape whose radius was estimated from the image) were calculated as described by Galkovskyi et al.33
To check physical contact of the roots with P. indica, the 2 symbionts (Arabidopsis seedlings and 2 µl of a P. indica spore suspension) were co-cultivated on a cellophane membrane as described above and compared with conditions in which 2 µl of the spore suspension of P. indica was applied under the cellophane membrane. Microscopic and PCR analyses confirmed that the 2 symbionts did not have physical contact when they are growing on opposite sides of the cellophane membrane. The seedlings were harvested after 3 weeks of co-cultivation, and the fresh weights of seedlings grown on the membrane alone and with P. indica on the same or opposite sides of the membrane were compared.
Light stress, fluorescence parameters
Co-cultivation of the 2 symbionts was performed as described above on a nylon membrane on PNM medium, except that the plates were exposed to continuous illuminations with different light intensities (25 ± 5 µmol m−2 sec−1, 50 ± 15 µmol m−2 sec−1 and 110 ± 10 µmol m−2 sec−1) for 10 days. The fluorescence parameters were measured with a FluorCam 700MF instrument and analysed with the Flucam 5.0 software. The data are averages for 30 seedlings and 3 independent biological experiments.
Heavy metal experiments
Arabidopsis seedlings were co-cultivated with P. indica (or mock-treated) for 7 days on PNM medium supplemented with NiCl2, CrSO4, CdCl2, Co-acetate, ZnNO3 or AgNO3 (concentrations are given in the Result section) and compared with the PNM control.
Osmotic and salt stress
Twelve day-old seedling grown on MS media were transferred to the PNM plates containing either NaCl (200 mM), PEG (10%) or mannitol (50 mM). These concentrations correspond to osmotic potential of −0.78, −1.24 and −1.69 MPa, respectively76 The seedlings were inoculated with a P. indica plaque (control: KM medium without the fungus) for one week before the fresh weights of the seedlings were determined.
Biophoton measurement
Biophoton investigation of Arabidopsis leaves was performed with a Zeiss LSM 710 microscope in darkness with the ZEN-software and without laser excitation of the samples. Detached leaves on the microscope glass slides in 100 µl glycerol (50%) matrix were analyzed using a 10x magnification objective (EC Plan-Neofluar 10x/0.30 M27) and an image scanning area of 512 × 512 pixel. The image acquisition setup was selected for three different dyes via a smart setup button, and the biofluorescence of the leaves was measured from 382 to 596 nm. To minimize the crosstalk among each fluorophore, the “Best signal mode” sequential scanning was chosen. The emission detection range of each emission filter had been modified around the maximum of the filters to a 20 nm spectral range. Due to the weak autofluorescence of the leaves, the pinhole of each channel was adjusted to the maximum value, to collect more light emission of the leaves by the detector. The voltage on the photomultiplier tube which detects the emitted light from the sample was set to 800 to increase the sensitivity to obtain optimal intensity and background signals. Grays scale was used for image processing of the 512 × 512 pixel area by the ImageJ 1.48 v software and the mean gray value was used for comparison of relative amounts of biophotons recorded on the images. Background noises induced by high-energy particles were recorded by imaging of biophoton blank samples and their mean gray values were subtracted from all sample measurements. The results show an average of images recorded from 30 leaves of 3 independent biological experiments.
RNA analysis
RNA was isolated from shoots by adding 1 ml Trizol to 100 mg tissue powdered in liquid nitrogen and shacking for 10 min in a microtube. After the addition of 200 µl chloroform, the tubes were shaken for 10 more min and centrifuged for 30 min at 10.000 rpm at 4°C. The supernatant was transferred to a new microtube and mixed with half volume of ice-cold isopropanol and extraction buffer (0.8 M Na-citrate and 1.2 M NaCl). After 10 min incubation at room temperature, followed by centrifugation for 30 min at 11.000 rpm at 4°C, the pellet was washed with 1 ml 70% ethanol, dried and dissolved in 50 µl distilled H2O. Oligo dT primers were used for reverse transcription of 1 µg of total RNA with the Qiagen kit (Omniscript, Qiagen, Hilden, Germany). RT-PCR was conducted with the primer pairs given in S1 Table. Arabidopsis GAPDH and P. indica ITS were used as housekeeping genes for the two organisms.
The “Bio-Rad CFX connect real-time system” and “Bio-Rad CFX manager version 3.1” (Bio-Rad, Munich, Germany) were used for preforming real-time quantitative RT-PCR with Taq DNA polymerase and Eva green (Bio-Rad) in a final volume of 20 µl for the amplification of the PCR products. The reactions were performed with 95°C 2 min, 39× (95°C 30 s, 60°C 40 s, 72°C 45 s), 72°C 8 min followed by a melting curve program (55–95°C in increasing steps of 0.5°C). Annealing temperature was adjusted according to the primers. Three amplification repetitions for at least three independently isolated RNAs were used for all biological repetitions. Bio-Rad CFX Manager software was used for fold induction value calculations.
H2O2 measurements and ROS staining
Arabidopsis seedlings co-cultivated with P. indica for 15 days were infected with A. brassicae spores. After 10 days, they were stained with 3,3′-diaminobenzidine (DAB) as described by Daudi et al.51 H2O2 level in stained leaves was quantified according to Vahabi et al.52 As a result of staining a brown precipitate upon oxidation was formed, which is insoluble in aqueous and organic solvents. For the detection/quantification of H2O2 inside the plant material, 100 mg of stained tissue was washed with acetone three times, ground to a fine powder and - after drying - dissolved in 1 ml DMSO at 90°C for 1 h. The supernatant was separated from the precipitate by centrifugation at 10,000 rpm for 5 min and further used for spectrophotometric measurements at 270 nm (Perkin Elmer, Lambda 12) as described by Greenfield et al.53 The poly-DAB concentration of the plant tissue was correlated to the H2O2 concentration using a standard curve which was generated by the application of four different concentrations of H2O2 (0.1, 1, 10, 100 µg).
Microscopy of roots and stomata
Fluorescent staining of root samples was performed with fuchsin acid and trypan blue and the material was analyzed with a Zeiss fluorescent microscope Oxiovert.16
Stomata staining was done according to Vahabi et al.54 The data are averages of 20 leaves from 10 seedlings with 3 independent biological repetitions.
Scanning Electron Microscopy analysis
For SEM analysis, samples were fixed overnight with 2.5% glutaraldehyde in sodium cacodylate buffer (0.1 M, pH 7.0). They were dehydrated with ethanol in serially increased concentration, followed by critical point drying in a Leica EM CPD300 Automated Critical Point Dryer (Leica, Wetzlar, Germany). The samples were coated with carbon (20 nm) in a BAL-TEC SCD005 Sputter Coater (BAL-TEC, Liechtenstein) and analyzed at different magnifications in a LEO 1530 Gemini field emission scanning electron microscope (Zeiss, Oberkochen, Germany) at 12 kV acceleration voltage and a working distance of 6 mm using an inlense secondary electron detector and a scintillation type backscatter electron detector (Centaurus detector, K.E. Developments, Cambridge, UK).
Plant hormone measurements
Ten day after inoculation of control and P. indica treated seedling with A. brasicae, the shoots were used for quantification of JA, JA-isoleucine and oxophytodienoic acid. The phytohormone levels are expressed relative to the dry weight according to Sun et al.49
Supplementary Material
Disclosure of potential closure of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Sarah Mußbach and Claudia Röppischer for their excellent technical assistance, and Susanne Linde and Frank Steiniger (Electron Microscopy Center, University of Jena) for their help with the SEM studies.
Author contributions
KV, SKD and RO designed all the experiments. KV did most of the experiments. SKD contributed to the light microscopy. SM and PH contributed to the biophoton measurements. MW did the electron microscopy. MR did the phytohormone analysis. DF contributed on light stress experiments. SKD and IS contributed to the discussion. KV and RO wrote the article. RO supervised the research. All authors read and approved the final manuscript.
Funding
Research was supported by a grant from the German Research Foundation (CRC 1127).
References
- 1.Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 2013; 32(2):429-48 pii:S0734-9750(13)00222-X; PMID:24380797; http://dx.doi.org/ 10.1016/j.biotechadv.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 2.Smith SE, Smith FA. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosytems scales. Ann Rev Plant Biol 2011; 62:227-50; PMID: 21391813; http://dx.doi.org/10819202 10.1146/annurev-arplant-042110-103846 [DOI] [PubMed] [Google Scholar]
- 3.Tinker PBH, Nye PH. Solute movement in the rhizosphere. Oxford, UK: Oxford Univ Press; 2010; p. 464 [Google Scholar]
- 4.Khan AG, Kuek C, Chaudhry TM, Khoo C, Hayes WJ. Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 2000; 41:197-207; PMID:10819202; http://dx.doi.org/ 10.1016/S0045-6535(99)00412-9 [DOI] [PubMed] [Google Scholar]
- 5.Lambers H, Raven JA, Shaver G, Smith SE. Plant nutrient acquisiton strategies change with soil age. Trends Ecol Evol 2008; 23:95-103; PMID:18191280; http://dx.doi.org/ 10.1016/j.tree.2007.10.008 [DOI] [PubMed] [Google Scholar]
- 6.Bakshi M, Vahabi K, Bhattacharya S, Sherameti I, Varma A, Wun Yeh KW, Baldwin IT, Johri AT, Oelmüller R. WRKY6 restricts Piriformospora indica-stimulated and phosphate-induced root development in Arabidopsis. BMC Plant Biol 2015; 15(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz-Lozano JM. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 2003; 13:309-17; PMID:12690537; http://dx.doi.org/ 10.1007/s00572-003-0237-6 [DOI] [PubMed] [Google Scholar]
- 8.Bray EA. Plant responses to water deficit. Trends Plant Sci 1997; 2:48-54; http://dx.doi.org/ 10.1016/S1360-1385(97)82562-9 [DOI] [Google Scholar]
- 9.Sylvia DM, Hammond LC, Bennet JM, Hass JH, Linda SB. Field response of maize to a VAM fungus and water management. Agronomy J 1993; 85:93-198; http://dx.doi.org/ 10.2134/agronj1993.00021962008500020006x [DOI] [Google Scholar]
- 10.Subramanian K, Santhanakrishnan P, Balasubramanian P. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci Hort 2006; 107:245-53; http://dx.doi.org/ 10.1016/j.scienta.2005.07.006 [DOI] [Google Scholar]
- 11.Augé RM. Water relations, drought and vesicular arbuscular mycorrhizal symbiosis. Mycorrhiza 2001; 11:3-42; http://dx.doi.org/ 10.1007/s005720100097 [DOI] [Google Scholar]
- 12.Sherameti I, Tripathi S, Varma A, Oelmüller R. The root-colonizing endophyte Pirifomospora indica confers drought tolerance in Arabidopsis by stimulating the expression of drought stress-related genes in leaves. Mol Plant-Microbe Interact. 2008; 21(6):799-807.; PMID:18624643; http://dx.doi.org/ 10.1094/MPMI-21-6-0799 [DOI] [PubMed] [Google Scholar]
- 13.Sun C, Johnson JM, Cai D, Sherameti I, Oelmüller R, Lou B. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought‐related genes and the plastid‐localized CAS protein. J Plant Physiol 2010; 167:1009‐17; PMID:20471134; http://dx.doi.org/17078985 10.1016/j.jplph.2010.02.013 [DOI] [PubMed] [Google Scholar]
- 14.Hildebrandt U, Regvar M, Bothe H. Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 2007; 68:139-46; PMID:17078985; http://dx.doi.org/ 10.1016/j.phytochem.2006.09.023 [DOI] [PubMed] [Google Scholar]
- 15.Rillig MC, Wright SF, Nichols KA, Schmidt WF, Torn MS. Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant Soil 2001; 233:67-177; http://dx.doi.org/ 10.1023/A:1010364221169 [DOI] [Google Scholar]
- 16.Vahabi K, Johnson JM, Drzewiecki C, Oelmüller R. Fungal staining tools to study the interaction between the beneficial endophyte Piriformospora indica with Arabidopsis thaliana roots. Endocyt Cell Res 2011; 21:77-88 [Google Scholar]
- 17.Vahabi K, Meichsner D, Oelmüller R. Interaction of Arabidopsis and Piriformospora indica in a hydroponic system. Endocyt Cell Res 2014; 5:53-55 [Google Scholar]
- 18.Porcel R, Aroca R, Ruiz-Lozano JM. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron Sustain Dev 2012; 32:181-200; PMID:22553287; http://dx.doi.org/20870416 10.1007/s13593-011-0029-x [DOI] [Google Scholar]
- 19.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 2010; 48:909-30; PMID:20870416; http://dx.doi.org/ 10.1016/j.plaphy.2010.08.016 [DOI] [PubMed] [Google Scholar]
- 20.Sekmen AH, Turkan I, Takio S. Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt tolerant Plantago maritima and salt-sensitive Plantago media. Physiol Plant 2007; 131:399-411; PMID:18251879; http://dx.doi.org/ 10.1111/j.1399-3054.2007.00970.x [DOI] [PubMed] [Google Scholar]
- 21.Evelin H, Kapoor R. Arbuscular mycorrhizal symbiosis modulates antioxidant response in salt-stressed Trigonella foenum-graecum plants. Mycorrhiza 2013; 24(3):197-208; PMID: 24113907; http://dx.doi.org/25982746 10.1007/s00572-013-0529-4 [DOI] [PubMed] [Google Scholar]
- 22.Tian CY, Feng G, Li XL, Zhang FS. Different effects of arbuscular mycorrhizal fungal isolates from saline or non-saline soil on salinity tolerance of plants. Appl Soil Ecol 2004; 26:143-8; http://dx.doi.org/ 10.1016/j.apsoil.2003.10.010 [DOI] [Google Scholar]
- 23.Daei G, Ardekani M, Rejali F, Teimuri S, Miransari M. Alleviation of salinity stress on wheat yield, yield components, and nutrient uptake using arbuscular mycorrhizal fungi under field conditions. J Plant Physiol 2009; 166:217-25; PMID: 19100656; http://dx.doi.org/25982746 10.1016/j.jplph.2008.09.013 [DOI] [PubMed] [Google Scholar]
- 24.Giri B, Kapoor R, Mukerji KG. Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass, and mineral nutrition of Acacia auriculiformis. Biol Fertil Soils 2003; 38:170-5; http://dx.doi.org/ 10.1007/s00374-003-0636-z [DOI] [Google Scholar]
- 25.Vicente-Sánchez J, Nicolás E, Pedrero F, Alarcón JJ, Maestre-Valero JF; Fernández F. Arbuscular mycorrhizal symbiosis alleviates detrimental effects of saline reclaimed water in lettuce plants. Mycorrhiza 2014; 24(5):339-48; http://dx.doi.org/ 10.1007/s00572-013-0542-7 [DOI] [PubMed] [Google Scholar]
- 26.Gahlot S, Joshi A, Singh P, Tuteja R, Dua M, Jogawat A, Kumar M, Raj S, Dayaman V, Johri AK, et al.. Isolation of genes conferring salt tolerance from Piriformospora indica by random overexpression in Escherichia coli. World J Microbiol Biotechnol 2015; 31(8):1195-209; PMID:25982746; http://dx.doi.org/ 10.1007/s11274-015-1867-5 [DOI] [PubMed] [Google Scholar]
- 27.Jogawat A, Saha S, Bakshi M, Dayaman V, Kumar M, Dua M, Varma A, Oelmüller R, Tuteja N, Johri AK. Piriformospora indica rescues growth diminution of rice seedlings during high salt stress. Plant Signal Behav 2013; e26891; PMID:24494239; http://dx.doi.org/ 10.4161/psb.26891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pham GH, Kumari R, Singh An, Sachdev M, Prasad R, Kaldorf M, Buscot F, Oelmüller R, et al.. Axenic cultures of Piriformospora indica in: Varma A, Abbott L, Werner D, Hampp R eds. Plant Surface Microbiology, Springer-Verlag, Germany: 2004; 593-616 [Google Scholar]
- 29.Peškan‐Berghöfer T, Shahollari B, Giong PH, Hehl S, Markert C, Blanke V, Kost G, Varma AK, Oelmüller R. Association of Piriformospora indica with Arabidopsis thaliana roots represents a novel system to study beneficial plant‐microbe interactions and involves early plant protein modifications in the endoplasmatic reticulum and at the plasma membrane. Physiol Plant 2004; 122:465‐77; http://dx.doi.org/ 10.1111/j.1399-3054.2004.00424.x [DOI] [Google Scholar]
- 30.Sherameti I, Shahollari B, Venus Y, Altschmied L, Varma A, Oelmüller R. The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch degrading enzyme glucan‐water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. J Biol Chem 2005; 280:26241‐7; PMID:15710607; http://dx.doi.org/22834569 10.1074/jbc.M500447200 [DOI] [PubMed] [Google Scholar]
- 31.Vadassery J, Tripathi S, Prasad R, Varma A, Oelmüller R. Monodehydroascorbate reductase 2 and dehydroascorbate reductase 5 are crucial for a mutualistic interaction between Piriformospora indica and Arabidopsis. J Plant Physiol 2009; 166:1263‐74; PMID:19386380; http://dx.doi.org/22834569 10.1016/j.jplph.2008.12.016 [DOI] [PubMed] [Google Scholar]
- 32.Johnson JM, Sherameti I, Ludwig A, Nongbri PL, Sun C, Lou B, Varma A, Oelmüller R. Protocols for Arabidopsis thaliana and Piriformospora indica co-cultivation - A model system to study plant beneficial traits. Endocyt Cell Res 2011; 21:101-13 [Google Scholar]
- 33.Galkovskyi T, Mileyko Y, Bucksch A, Moore B, Symonova O, Price CA, Topp CN, Iyer-Pascuzzi AS, Zurek PR, Fang S, et al.. GiA Roots: software for the high-throughput analysis of plant root system architecture. BMC Plant Biol 2012; 12:116; PMID:22834569; http://dx.doi.org/ 10.1186/1471-2229-12-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobs S, Zechmann B, Molitor A, Trujillo M, Petutschnig E, Lipka V, Kogel KH, Schäfer P. Broad-spectrum suppression of innate immunity is required for colonization of Arabidopsis roots by the fungus Piriformospora indica. Plant Physiol 2011; 156(2):726-40; PMID:21474434; http://dx.doi.org/ 10.1104/pp.111.176446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vadassery J, Ritter C, Venus Y, Camehl I, Varma A, Shahollari B, Novak O, Strnad M, Ludwig-Müller J, Oelmüller R. The role of auxins and cytokinins in the mutualistic interaction between Arabidopsis and Piriformospora indica. Mol Plant-Microbe Interact 2008; 21:1371-83; PMID:18785832; http://dx.doi.org/ 10.1094/MPMI-21-10-1371 [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi M. Highly sensitive imaging for ultra-weak photon emission from living organisms. J Photochem Photobiol 2014; 139:34-8; PMID:24360927; http://dx.doi.org/24380797 10.1016/j.jphotobiol.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 37.Bonneau L, Huguet S, Wipf D, Pauly N, Truong HN. Combined phosphate and nitrogen limitation generates a nutrient stress transcriptome favorable for arbuscular mycorrhizal symbiosis in Medicago truncatula. New Phytol 2013; 1991:188-202; PMID:23506613; http://dx.doi.org/24380797 10.1111/nph.12234 [DOI] [PubMed] [Google Scholar]
- 38.Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 2014; 32(2):429-48; PMID:24380797; http://dx.doi.org/ 10.1016/j.biotechadv.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 39.Smith SE, Smith FA. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 2012; 104:1-13; PMID:21933929; http://dx.doi.org/ 10.3852/11-229 [DOI] [PubMed] [Google Scholar]
- 40.Harrison MJ. Cellular programs for arbuscular mycorrhizal symbiosis. Curr Opin Plant Biol 2012; 15:691-8; PMID:23036821; http://dx.doi.org/ 10.1016/j.pbi.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 41.Campos-Soriano L, Segundo BS. New insights into the signaling pathways controlling defense gene expression in rice roots during the arbuscular mycorrhizal symbiosis. Plant Signal Behav 2011; 6:553-7; PMID:21422823; http://dx.doi.org/ 10.4161/psb.6.4.14914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nehls U, Göhringer F, Wittulsky S, Dietz S. Fungal carbohydrate support in the ectomycorrhizal symbiosis: a review. Plant Biol (Stuttg) 2010; 12:292-301; PMID:20398236; http://dx.doi.org/ 10.1111/j.1438-8677.2009.00312.x [DOI] [PubMed] [Google Scholar]
- 43.Schützendübel A, Polle A. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 2002; 53(372):1351-65; PMID:11997381; http://dx.doi.org/ 10.1093/jexbot/53.372.1351 [DOI] [PubMed] [Google Scholar]
- 44.Repetto O, Bestel-Corre G, Dumas-Gaudot E, Berta G, Gianinazzi-Pearson V, Gianinazzi S. Targeted proteomics to identify cadmium-induced protein modifications in Glomus mosseae-inoculated pea roots. New Phytol 2003; 157(3):555-67; http://dx.doi.org/ 10.1046/j.1469-8137.2003.00682.x [DOI] [PubMed] [Google Scholar]
- 45.Ahonen-Jonnarth U, Van Hees P, Lundström US, Finlay RD. Organic acids produced by mycorrhizal Pinus sylvestris exposed to elevated aluminium and heavy metal concentrations. New Phytol 2000; 146:557-67; http://dx.doi.org/ 10.1046/j.1469-8137.2000.00653.x [DOI] [Google Scholar]
- 46.Abdel Latef AA. Influence of arbuscular mycorrhizal fungi and copper on growth, accumulation of osmolyte, mineral nutrition and antioxidant enzyme activity of pepper (Capsicum annuum L.). Mycorrhiza 2011; 21:495-503; PMID:21221660; http://dx.doi.org/ 10.1007/s00572-010-0360-0 [DOI] [PubMed] [Google Scholar]
- 47.Augé RM, Toler HD, Saxton AM. Arbuscular mycorrhizal symbiosis and osmotic adjustment in response to NaCl stress: a meta-analysis. Front Plant Sci 2014; 5:562; PMID: 25368626; http://dx.doi.org/18681935 10.3389/fpls.2014.00562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baltruschat H, Fodor J, Harrach BD, Niemczyk E, Barna B, Gullner G, Janeczko A, Kogel KH, Schäfer P, Schwarczinger I, et al.. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol 2008; 180:501-10; PMID:18681935; http://dx.doi.org/ 10.1111/j.1469-8137.2008.02583.x [DOI] [PubMed] [Google Scholar]
- 49.Sun C, Shao Y, Vahabi K, Lu J, Bhattacharya S, Dong S, Yeh KW, Sherameti I, Lou B, Baldwin IT, et al.. The beneficial fungus Piriformospora indica protects Arabidopsis from Verticillium dahliae infection by downregulation plant defense responses. BMC Plant Biol 2014; 14:268; PMID:25297988; http://dx.doi.org/ 10.1186/s12870-014-0268-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill TW, Kaefer E. Improved protocols for Aspergillus medium: trace elements and minimum media salt stock solutions. Fungal Genet New 2001; 48:20-1 [Google Scholar]
- 51.Daudi A, Cheng Z, O'Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 2012; 24:275-87; PMID:22247251; http://dx.doi.org/ 10.1105/tpc.111.093039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vahabi K, Sherameti I, Bakshi M, Mrozinska A, Ludwig A, Reichelt M, Oelmüller R. The interaction of Arabidopsis with Piriformospora indica shifts from initial transient stress induced by fungus-released chemical mediators to a mutualistic interaction after physical contact of the two symbionts. BMC Plant Biol 2015; 15:58; PMID:25849363; http://dx.doi.org/ 10.1186/s12870-015-0419-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenfield L, Starkenburg S, Shallice M, Nyhus J, Leong L.. Stable compositions comprising chromogenic compounds and methods of use. Patent, United States 2010
- 54.Vahabi K, Sun C, Govindaswamy J, Falkenberg D, Venus T, Oelmüller R. Stomata staining in Arabidopsis. Endocyt Cell Res 2015; 26:21-4 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.