ABSTRACT
AtNHX5 and AtNHX6, endosomal Na+,K+/H+ antiporters in Arabidopsis, are localized in the Golgi, trans-Golgi network, and prevacuolear compartment. It becomes evident that AtNHX5 and AtNHX6 play an important role in protein transport toward the vacuole. Studies have shown that AtNHX5 and AtNHX6 regulate the transport of seed storage proteins as well as the biogenesis of the protein storage vacuoles. Three distinct mechanisms have been revealed for the roles of AtNHX5 and AtNHX6 in protein transport. AtNHX5 and AtNHX6 control: (i) the binding of VSR to its cargoes; (ii) the recycling of VSRs; and (iii) subcellular localization of the SNARE complex. Moreover, it has been found that the endosomal pH homeostasis maintained by AtNHX5 and AtNHX6 is critical for the transport of seed storage proteins. Taken together, AtNHX5 and AtNHX6 regulate the trafficking of seed storage proteins into the vacuole; the H+ leak pathway conducted by AtNHX5 and AtNHX6 is critical for protein transport.
KEYWORDS: Arabidopsis, AtNHX5 and AtNHX6, endosomal Na+, H+ leak pathway, K+/H+ antiporters, pH homeostasis, protein transport
AtNHX5 and AtNHX6 are endosomal NHXs in Arabidopsis.1-3 They are localized in the Golgi, trans-Golgi network (TGN), and prevacuolear compartment (PVC).4-6 Studies have shown that AtNHX5 and AtNHX6 facilitate ion and pH homeostasis, and are crucial for growth and development.4-6 Accumulating evidence indicates that AtNHX5 and AtNHX6 play an important role in protein transport.5,7-9
AtNHX5 and AtNHX6 have diversified roles in protein transport
Reguera et al. examined the role of AtNHX5 and AtNHX6 in protein transport.5 They found that nhx5 nhx6 produced large, heavy seeds with a dark coat. Additionally, protein storage vacuoles (PSVs) were smaller but their number was increased in nhx5 nhx6. The precursors of seed storage proteins, 2S albumin and 12S globulin, were missorted to the apoplast in nhx5 nhx6. The interaction was reduced between VSR2;1 and its cargoes in nhx5 nhx6. Luminal pH was reduced in the VSR compartments, TGN and PVCs in nhx5 nhx6.5 These results indicate that AtNHX5 and AtNHX6 may regulate protein transport by controlling the binding of VSR to its cargoes. These results also suggest that endosomal pH homeostasis is central to the trafficking of seed storage proteins.5
Ashnest et al. confirmed the notion that AtNHX5 and AtNHX6 regulate the transport and processing of seed storage proteins as well as the biogenesis of the PSVs.7 In addition, they found that nhx5 nhx6 accumulated in the seeds a high level of the 52 kDa precursor of βVPE, a vacuolar processing enzyme. The catalytic activity of βVPE was reduced in nhx5 nhx6. Moreover, they found that AtNHX6 interacted via its C-terminal with SNX1, a component of the Retromer. Retromer is the cellular sorting machinery that recycles VSRs back to the TGN from the PVC.7 Therefore, these findings suggest that AtNHX5 and AtNHX6 regulate protein transport by controlling the recycling of VSRs.7
A recent study by Wu et al. examined the role of AtNHX5 and AtNHX6 in regulating a SNARE complex and its function in protein trafficking.8 This SNARE complex, which is composed of VAMP727, SYP22, VTI11 and SYP51, is important for protein transport and PSV biogenesis in Arabidopsis.10 The SNARE complex directs membrane fusion between the PVC and vacuole, and thus it facilitates protein transport to the vacuole. Wu et al. found that the nhx5 nhx6 syp22 triple mutant was defective in seedling growth and seed development.8 The triple mutant had short siliques, reduced seed sets and larger seeds. They further found that the triple mutant accumulated the precursors of seed storage proteins and had numerous smaller PSVs. In addition, they showed that a large amount of SYP22 and VAMP727 was trapped in the Golgi and TGN in nhx5 nhx6, indicating repression of the localization of SYP22 and VAMP727 in the PVC. However, AtNHX5 and AtNHX6 do not interact physically with the SNARE complex. They showed that 3 conserved acidic residues, D164, E188, and D193 in AtNHX5 and D165, E189, and D194 in AtNHX6, were necessary for the transport of the storage proteins, indicating the importance of exchange activity in protein transport.6,8,9 These results suggest that AtNHX5 and AtNHX6 may regulate protein transport by controlling subcellular localization of the SNARE complex.8
The studies mentioned above by 3 groups demonstrate that AtNHX5 and AtNHX6 regulate protein transport. However, these studies revealed 3 distinct mechanisms underlying AtNHX5 and AtNHX6's action (Fig. 1): (i) controlling the binding of VSR to its cargoes;5 (ii) controlling the recycling of VSRs;7 and (iii) controlling subcellular localization of the SNARE complex.8
Figure 1.
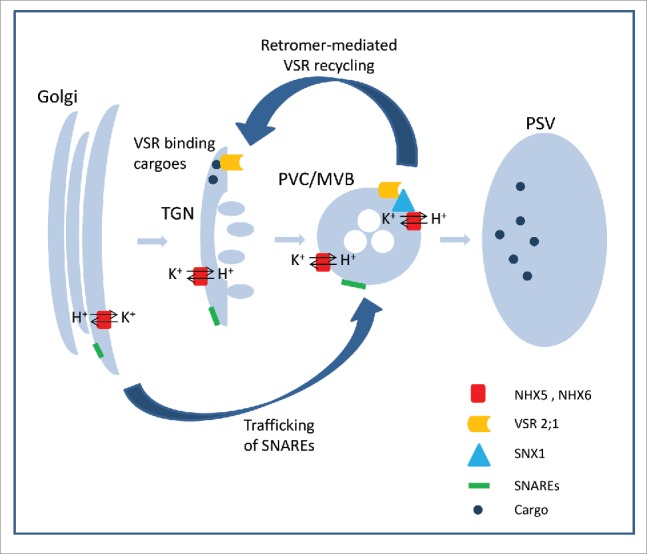
A schematic model for the roles of AtNHX5 and AtNHX6 in protein transport into the vacuole. Three distinct mechanisms have been revealed. AtNHX5 and AtNHX6 control: (i) the binding of VSR to its cargoes; (ii) the recycling of VSRs; and (iii) subcellular localization of the SNARE complex. TGN, trans-Golgi network; PVC, prevacuolear compartment; MVB, multivesicular body; PSV, protein storage vacuole; VSR, vacuolar sorting receptor.
Why do AtNHX5 and AtNHX6 have so diversified roles in protein transport? In plants, seed storage proteins are transported into the PSVs to convert to mature forms after they are synthesized as precursors in the endoplasmic reticulum (ER).11-13 Proteins are transported into the vacuole via a vesicle-mediated trafficking pathway, including the ER, Golgi, TGN, and MVB/PVC.14 Therefore, the Golgi, TGN and MVB/PVC are sorting stations in protein transport pathway.15,16 Since AtNHX5 and AtNHX6 are localized to the Golgi, TGN and PVC,4-6 where they overlap with the protein transport pathway, they thus may regulate the protein transport activities carried out in these organelles, including the function of the SNARE complex and the receptor protein VSR.
H+ leak is vital for protein transport
Cellular or organelle pH is vital for protein transport in both the secretory and endocytic pathways.17-20 In the exocytic or endocytic pathways, the organelles become more acidic along the process of maturation.17,18,21 In plants, the acidic pH of the organelles is maintained by the proton pumps V-ATPases and pyrophosphatase.20,21 Studies have shown that AtNHX5 and AtNHX6 may act as a H+-leak pathway to counter the luminal acidification.21 Reguera et al. found that the endosomal pH homeostasis maintained by AtNHX5 and AtNHX6 is critical for the transport of seed storage proteins.5 Consistently, Wu et al. found that the exchange activity of AtNHX5 and AtNHX6 was crucial for protein transport, indicating the importance of pH homeostasis regulated by AtNHX5 and AtNHX6 in protein transport.8 Therefore, these results demonstrate that H+ leak pathway plays an important role in protein transport in plants.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Basic Research Program of China (973 project, 2013CB429904 to QSQ), the National Natural Science Foundation of China (NSFC) (31571464, 31371438, 31070222 to QSQ), the Research Fund for the Doctoral Program of Higher Education of China (RFDP) (20130211110001 to QSQ).
References
- 1.Pardo JM, Cubero B, Leidi EO, Quintero FJ. Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot 2006; 57:1181-99; PMID:16513813; http://dx.doi.org/ 10.1093/jxb/erj114 [DOI] [PubMed] [Google Scholar]
- 2.Bassil E, Coku A, Blumwald E. Cellular ion homeostasis: emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J Exp Bot 2012; 63:5727-40; PMID:22991159; http://dx.doi.org/ 10.1093/jxb/ers250 [DOI] [PubMed] [Google Scholar]
- 3.Chanroj S, Wang G, Venema K, Zhang MW, Delwiche CF, Sze H. Conserved and diversified gene families of monovalent cation/H+ antiporters from algae to flowering plants. Front Plant Sci 2012; 3:25; PMID:22639643; http://dx.doi.org/ 10.3389/fpls.2012.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassil E, Ohto MA, Esumi T, Tajima H, Zhu Z, Cagnac O, Belmonte M, Peleg Z, Yamaguchi T, Blumwald E. The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. The Plant cell 2011; 23:224-39; PMID:21278129; http://dx.doi.org/ 10.1105/tpc.110.079426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reguera M, Bassil E, Tajima H, Wimmer M, Chanoca A, Otegui MS, Paris N, Blumwald E. pH regulation by NHX-type antiporters is required for receptor-mediated protein trafficking to the vacuole in Arabidopsis. Plant Cell 2015; 27:1200-17; PMID:25829439; http://dx.doi.org/ 10.1105/tpc.114.135699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Wu X, Liu Y, Qiu QS. AtNHX5 and AtNHX6 control cellular K+ and pH Homeostasis in Arabidopsis: three conserved acidic residues are essential for K+ transport. PLoS One 2015; 10:e0144716; PMID:26650539; http://dx.doi.org/26416852 10.1371/journal.pone.0144716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashnest JR, Huynh DL, Dragwidge JM, Ford BA, Gendall AR. Arabidopsis intracellular NHX-type sodium-proton antiporters are required for seed storage protein processing. Plant Cell Physiol 2015; 56:2220-33; PMID:26416852; http://dx.doi.org/ 10.1093/pcp/pcv138 [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Ebine K, Ueda T, Qiu QS. AtNHX5 and AtNHX6 are required for the subcellular localization of the SNARE complex that mediates the trafficking of seed storage proteins in Arabidopsis. PLoS One 2016; 11:e0151658; PMID:26986836; http://dx.doi.org/18984676 10.1371/journal.pone.0151658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu QS. Plant endosomal NHX antiporters: activity and function. Plant Signal Behav 2016; 18:0; PMID:26890367; http;//dx.doi.org/18984676 10.1080/15592324.2016.1147643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebine K, Okatani Y, Uemura T, Goh T, Shoda K, Niihama M, Morita MT, Spitzer C, Otegui MS, Nakano A, et al.. A SNARE complex unique to seed plants is required for protein storage vacuole biogenesis and seed development of Arabidopsis thaliana. Plant Cell 2016;11:e1147643; PMID:18984676; http://dx.doi.org/ 10.1105/tpc.107.057711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara-Hishimura I, Takeuchi Y, Inoue K, Nishimura M. Vesicle transport and processing of the precursor to 2S albumin in pumpkin. Plant J 1993; 4:793-800; PMID:8275099; http://dx.doi.org/ 10.1046/j.1365-313X.1993.04050793.x [DOI] [PubMed] [Google Scholar]
- 12.Shimada T, Fuji K, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I. Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc Natl Acad Sci USA 2003; 100:16095-100; PMID:14657332; http://dx.doi.org/17293568 10.1073/pnas.2530568100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuji K, Shimada T, Takahashi H, Tamura K, Koumoto Y, Utsumi S, Nishizawa K, Maruyama N, Hara-Nishimura I. Arabidopsis vacuolar sorting mutants (green fluorescent seed) can be identified efficiently by secretion of vacuole-targeted green fluorescent protein in their seeds. Plant Cell 2007; 19:597-609; PMID:17293568; http://dx.doi.org/ 10.1105/tpc.106.045997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassham DC, Raikhel NV. Unique features of the plant vacuolar sorting machinery. Curr Opin Cell Biol 2000; 12:491-5; PMID:10873819; http://dx.doi.org/ 10.1016/S0955-0674(00)00121-6 [DOI] [PubMed] [Google Scholar]
- 15.Otegui MS, Spitzer C. Endosomal functions in plants. Traffic 2008; 9:1589-98; PMID:18627577; http://dx.doi.org/ 10.1111/j.1600-0854.2008.00787.x [DOI] [PubMed] [Google Scholar]
- 16.Richter S, Voss U, Jurgens G. Post-Golgi traffic in plants. Traffic 2009; 10:819-28; PMID:19416470; http://dx.doi.org/ 10.1111/j.1600-0854.2009.00916.x [DOI] [PubMed] [Google Scholar]
- 17.Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol 2008; 20:415-26; PMID:18511251; http://dx.doi.org/ 10.1016/j.ceb.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 2010; 11:50-61; PMID:19997129; http://dx.doi.org/ 10.1038/nrm2820 [DOI] [PubMed] [Google Scholar]
- 19.Shen J, Zeng Y, Zhuang X, Sun L, Yao X, Pimpl P, Jiang L. Organelle pH in the Arabidopsis endomembrane system. Mol Plant 2013; 6:1419-37; PMID:23702593; http://dx.doi.org/ 10.1093/mp/sst079 [DOI] [PubMed] [Google Scholar]
- 20.Schumacher K. pH in the plant endomembrane system-an import and export business. Curr Opin Plant Biol 2014; 22:71-6; PMID:25282587; http://dx.doi.org/ 10.1016/j.pbi.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 21.Martiniere A, Bassil E, Jublanc E, Alcon C, Reguera M, Sentenac H, Blumwald E, Paris N. In vivo intracellular pH measurements in tobacco and Arabidopsis reveal an unexpected pH gradient in the endomembrane system. Plant Cell 2013; 25:4028-43; PMID:24104564; http://dx.doi.org/ 10.1105/tpc.113.116897 [DOI] [PMC free article] [PubMed] [Google Scholar]


