ABSTRACT
Adventitious rooting is essential for the survival of numerous species from vascular cryptogams to monocots, and is required for successful micropropagation. The tissues involved in AR initiation may differ in planta and in in vitro systems. For example, in Arabidopsis thaliana, ARs originate from the hypocotyl pericycle in planta and the stem endodermis in in vitro cultured thin cell layers. The formation of adventitious roots (ARs) depends on numerous factors, among which the hormones, auxin, in particular. In both primary and lateral roots, growth depends on a functional stem cell niche in the apex, maintained by an active quiescent center (QC), and involving the expression of genes controlled by auxin and cytokinin. This review summarizes current knowledge about auxin and cytokinin control on genes involved in the definition and maintenance of QC, and stem cell niche, in the apex of Arabidopsis ARs in planta and in longitudinal thin cell layers.
KEYWORDS: Adventitious roots, arabidopsis thaliana, auxin, auxin-influx carriers, root apical stem cell niche, scarecrow, short root, trans-zeatin riboside, thin cell layers, YUCCA6
Abbreviations
- AR
adventitious root
- ARP
adventitious root primordium
- ARR1
ARABIDOPSIS RESPONSE REGULATOR1
- AUX1
AUXIN RESISTANT1
- CQ
quiescent center
- IAA
indole acetic acid
- Kin
kinetin
- LR
lateral root
- LAX3
LIKE AUXIN RESISTANT3
- pAGL42
pAGAMOUSLIKE42
- PIN1
PIN-FORMED1
- PLT
PLETHORA
- PR
primary root
- RAM
root apical meristem in the PR
- SCR
SCARECROW
- SHY2
SHORT HYPOCOTYL2
- SHR
SHORT ROOT
- TF
transcription factor
- tTCL
transverse thin cell layer
- lTCL
longitudinal thin cell layer
- WOX5
WUSCHEL-RELATED HOMEOBOX 5
- YUC6
flavin-containing monoxygenase YUCCA6
Introduction
What are the adventitious roots, and what do they do?
Adventitious roots (ARs) are post-embryonic roots formed by the aerial organs, i.e., stem and leaves, and roots in secondary structure.1 ARs are essential for survival in numerous plants, e.g., cereals. In collaboration with lateral roots (LRs), ARs provide anchorage to the substrate, water-use efficiency and extraction of nutrients from the soil, and storage of food reserves. LRs are post-embryonic as the ARs, but, differently from the ARs arise from the primary root (PR) pericycle. Both types of post-embryonic roots are able to form LRs, with this event further contributing to the development of the root system.1 Moreover, the regulation of LR and/or AR organogenesis provides a flexible way for plants to alter form and resource allocation in response to environmental changes or after injury.2 Also the roots formed by in vitro cultured explants are generally adventitious, and their induction is crucial for successful in vitro micropropagation and for breeding programs.3-5
Arabidopsis thaliana, today the main model dicot for molecular plant biology studies, forms one/two ARs in planta by the hypocotyl pericycle cells located at the collet, i.e., the junction region between the hypocotyl and the PR,6 however, mutants overproducing ARs, e.g., superroot2-1, are well known for this species.7 Arabidopsis thaliana forms numerous ARs also in vitro, when cultured under specific conditions, which change with the explant type.8-9 The tissue initiating the ARs also changes in explants coming from the same organ, e.g., entire segments of the inflorescence stem form ARs from the vascular cambium, 9 whereas explants formed by the superficial stem tissues only, i.e. the longitudinal thin cell layers (lTCLs), form ARs from the stem endodermis, as described in a following paragraph.
The stem cells in the primary and lateral root apical meristem: a shared story
The indeterminate growth of the plant is maintained by the activity of stem cells, forming a niche in the shoot and root apical meristems.10 As in animals, the plant stem cells are defined by their ability to both renew themselves and produce daughter cells, fated to develop into the differentiated tissues of the plant primary body.11 Moreover, in comparison with animals, the lack of cell migration in plants allows to easily track the cell lineages coming from a specific initial cell.12 For example, in the root apical meristem (RAM) of the PR, the daughter cells of each cell lineage undergo a definite number of divisions before elongating at a specific distance from the niche, i.e., at the transition zone. The differentiation zone, containing the elongated cells that have acquired their destined cell fates, follows, with its beginning usually marked by the appearance of root hairs.13 Thus, the apical meristems dynamically govern the indeterminate growth, causing at the same time the development of the primary body, by a co-ordinated cell renewal, growth and differentiation, controlled by the stem cell niche.
In Arabidopsis, the RAM in the PR shows a stem niche of initial cells surrounding a small group of rarely dividing cells (usually four cells), termed the quiescent center (QC).14 QC formation occurs in the embryo.15 The QC cells promote the stem cell status in the niche, because they are the source of signals that inhibit differentiation in the contacting initials,14,16 exactly as occurs in nearly every animal stem cell niche system,17 but also behave as stem cells in their own right.18 Numerous experiments have shown that the ectopic specification of QC cells correlates with ectopic respecification of stem cells, sustaining that QC identity may be sufficient to specify stem cell identity in the RAM.19
The stem cell niche definition, and functioning, is repeated in the apical meristem of the LRs, showing that its construction, and activity, is not a prerogative of the RAM of embryonic origin.20 In accordance, in both PR and LRs, the combined activities of phytohormones, transcription factors (TFs), miRNAs, and peptide signaling pathways are involved in the stem cell niche specification and maintenance.16 Among the phytohormones, auxin and cytokinin are the main actors.21-22 Auxin influences the specification of the QC, and the location of the stem cell niche in the RAM of the PR, from embryogenesis onward, and is also necessary for the formation of the entire embryonic root, by causing the expression of numerous TFs necessary for patterned organ specification.23 It has been shown that an antagonistic interaction between auxin and cytokinin is critical for specifying the stem cell niche in the root pole of the embryo.24 In the PR, cytokinin is synthesized in the tip, cap cells in particular, and exported acropetally through the xylem,25 however there is also a basipetal transport, through the phloem, which regulates the polar auxin transport in the RAM.26 Cytokinin transport is essential for maintaining RAM size and for ensuring PR growth, which depends on RAM activity. In fact, cytokinin downregulates the expression of several key regulatory genes in the RAM, e.g., SCARECROW (SCR), AtWUSCHEL RELATED HOMEOBOX5 (WOX5), AUXIN RESISTANT1 (AUX1), and the auxin-efflux carrier PIN-FORMED1 (PIN1),27-28 and causes the transition to elongation/differentiation of the derivative cells. The hormone is perceived by receptors which activate cytokinin response TFs, e.g., ARABIDOPSIS RESPONSE REGULATOR 1 (ARR1), causing the expression of SHORT HYPOCOTYL 2 (SHY2) gene. SHY2, in turn, negatively regulates numerous PIN genes, limiting auxin transport and distribution, and causing cell elongation/differentiation. Conversely, auxin mediates SHY2 degradation, sustaining PIN activities, and cell division in the RAM, and maintains the expression of SCR which represses ARR1 in the QC, sustaining stem cell activity.29-31
Auxin and cytokinin also regulate LR organogenesis positively and negatively, respectively. By the use of a cytokinin-sensitive two-component output sensor reporter and a DR5::GUS auxin-responsive reporter sensor, it has been shown that the two hormones tend to occupy complementary domains and to modulate their activities mutually.32 Auxin promotes the earliest events related to LR organogenesis, including priming in the pericycle cells of the PR,33 and founder cell specification and initiation.34-35 The same hormone is also positively involved in the later phases of primordium formation and emergence.35-36 In contrast, cytokinin contrains both LR initiation and development.32
Critical for the auxin activity is the regulation of its polar transport. The transport of auxin promotes the definition of an auxin maximum centered in the QC and columella cells, with this maximum required for QC functionality, in both PR and LRs.19,35
The auxin efflux carrier PIN1 is involved in generating auxin maximum, and QC positioning, in PR and LRs,37 even if a functional redundancy among various PIN proteins supports the involvement of also other members of the family in the same process.35,37 By regulating PIN1 expression level and the size of the PIN1 expression domain, auxin determines the efficiency of its own transport, and by controlling PIN1 polarity, also the directions of its own streams.38 However, the auxin-efflux carriers alone cannot create the pattern of auxin distribution in the RAM, and AUX1/LAX influx carriers are also required.39 AUX1 and LIKE AUXIN RESISTANT3 (LAX3) of the AUXIN1 (AUX1)-LIKE AUX1 (AUX/LAX) family are auxin influx carriers with specific roles in various plant processes, such as root gravitropism and regulation of vascular patterning, and in Arabidopsis are also necessary for QC organization in the embryonic root pole.40 In the RAM, it has been demonstrated that AUX1 facilitates both the acropetal and the basipetal auxin transport through an activity in different cell types.41 AUX1 and LAX3 are also involved in LR organogenesis, performing distinct functions, i.e., auxin maximum definition and maintenance,42 and LR emergence,36 respectively. Moreover, both PIN1 and LAX3 are auxin inducible.36,43 In addition to transport, a local biosynthesis of auxin is also needed for auxin maximum in the PR and LR tips,44 with YUCCA6 (YUC6), a gene of the tryptophan-dependent indole-3-acetic acid (IAA) biosynthesis, possibly involved.45-46
SCARECROW (SCR) is a TF of the GRAS family controlled by auxin.31 With SHORT ROOT (SHR), another TF of the same family, SCR is involved in the definition of the radial patterning of Arabidopsis PR and LRs. However, both TFs are also implicated in the specification of QC identity and, consequently, in stem cell niche and meristem maintenance of PR and LRs.47-50 SHR activity is necessary for full SCR expression,51 and also controls the expression of the auxin-influx carrier LAX3.52 Two PLETHORA (PLT) genes, i.e., PLT1 and PLT2, encoding AP2 TFs, are expressed in the root pole of the embryo, and in the RAM niche. These genes are also stimulated by auxin, and required for QC and stem cell positioning and maintenance, with an overlap with the expression domains of SHR and SCR.23 Studies on the regeneration of the QC after laser ablation have shown that the re-specification the QC is associated with an auxin-maximum shift, and a re-patterning of the expression of PLTs, SHR and SCR, which in turn regulates the expression and polar localization of PINs, stabilizing the reconstituted auxin distribution in the RAM.53 Interestingly, in addition to directly suppressing ARR1 in the QC, sustaining stem cell activities in the RAM (see above), SCR also controls ARR1 levels at the transition zone via auxin and gibberellin, thus contributing to the definition of the total size of the RAM.31,54 WOX5 is another TF expressed in the QC of the PR, with the function of inhibiting differentiation in the distal initial cells, thus contributing to maintain the stem cell niche and its boundaries.55 Moreover, the expression of WOX5 couples with the QC identity acquisition in the hypophyseal cell in the embryo.15 WOX5 is auxin inducible, acts downstream of auxin distribution,56 and seems to be involved in the maintenance of the auxin maximum at the PR tip.57 Moreover, a relationship between auxin and WOX5 seems to exist also during the specification of the QC in the LRs.58 In fact, it has been demonstrated that PIN1 expression gradually decreases in the outer layers of stage-IV LR primordia, with this event being followed by the beginning of the WOX5 localization in the central cells of the outermost derivatives of the LRP, and the pre-specification of the QC.38,59 WOX5 expression is dependent on SCR,55 and both genes are downregulated by cytokinin in the QC of the RAM.28 WOX5 establishes quiescence by restraining cell division in the QC at the root pole of the embryo and in the RAM. Besides, WOX5 has a role in modulating the expression of tryptophan-dependent auxin biosynthetic genes, thereby contributing to apical auxin production.59-60
In both PR and LRs, the QC identity is also evidenced by the same QC promoter traps, e.g., QC25 and pAGAMOUS LIKE 42 (pAGL42),18-19,61 reinforcing the concept that the same genetic control affects the definition of the QC and stem cell niche in both embryonic-in-origin PR and post-embryonic LRs.
What about the stem cells in the adventitious root apical meristem?
ARs are post-embryonic roots, but, differently from LRs, the establishment of QC and stem cell niche has remained an open question up to recent years. However, as for LRs, auxin is a central player in the control of adventitious rooting.62
In Arabidopsis, as in other plants, mutants affected in both LR and AR formation have been identified, including mutants with regular LR formation, but impaired in AR formation.63-65 Altogether these results have suggested genetic networks in common between the two post-embryonic rooting processes, in particular for stem cell niche and QC definition and maintenance. The following paragraphs summarize recent advances about the establishment and maintenance of a functional QC, and a stem cell niche, and the genetic and hormonal network involved, in the apical meristem of Arabidopsis thaliana ARs, developed in planta and de novo formed in in vitro cultured TCLs.66-67
The adventitious rooting process in planta
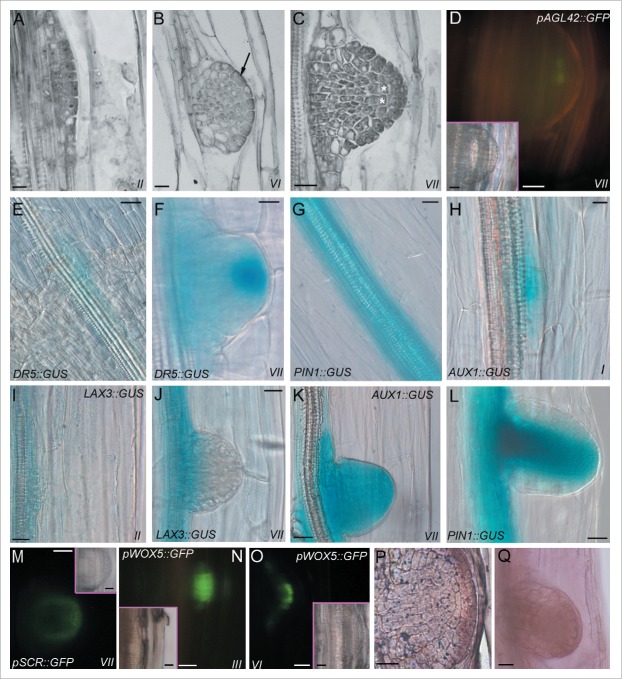
Arabidopsis seedlings generally form one-two ARs at the hypocotyl base.6 These roots are also named anchor roots.50 Even if this AR number is low, it is possible to increase it causing AR formation along the hypocotyl through a seedling growth under specific conditions. These conditions include the sowing of vernalized seeds in Petri dishes containing an agarized medium with MS salts 68 and the application of sucrose (usually 1% w/v). A 6-h exposure to white light is needed to induce germination, and is followed by continuous darkness for two weeks at 22±2°C. The plates must be maintained in a vertical position to cause a constant contact of the hypocotyl with the medium.66,69 Under these conditions, ARs originate from a unique tissue, the pericycle of the hypocotyls.6,66 The development of the ARs repeats the developmental stages of LR formation from the PR pericycle, including the stage at which QC and stem cell niche are defined. The histological stages of AR formation have been indicated by Roman numbers, as in the case of LR formation.20,66 Briefly, the pericycle founder cells divide anticlinally (stage I), and then periclinally (stage II), forming two layers of derivative cells (Fig. 1A). The outer layer divides periclinally before the inner one (stages III and IV, respectively). Anticlinal divisions then occur in the central cells of the de novo formed layers (stage V), and the AR primordium (ARP) acquires a dome shape, specifying the protoderm (stage VI, Fig. 1B). The QC is defined soon after (stage VII, Fig. 1C), and ARP protrusion follows. The identity of the QC cells has been verified by the expression of the same QC promoter traps, e.g., QC25 and pAGL42 (Fig. 1D), previously utilized for identifying the QC in the RAM and LR tip.18-19,61
Figure 1.
Adventitious rooting in the hypocotyl of Arabidopsis seedling, expression of some of the genes involved, and IAA and cytokinin localization. (A) Stage II of the AR process showing two layers of pericycle derivative cells. (B) Stage-VI-ARP with protoderm differentiation (arrow). (C) Definition of the QC (asterisks) at stage VII. (D) Expression of the QC promoter trap pAGL42 (green fluorescence) in the QC at stage VII (pAGL42::GFP line). (E-F) IAA presence (blue color) in the hypocotyl pericycle cells at the transition zone before AR formation (E), and in a stage-VII-ARP (F). The intense staining in the ARP tip in (F) shows the apical auxin maximum (DR5::GUS line). (G) Expression (blue color) of the efflux carrier PIN1 in the hypocotyl vasculature and pericycle (PIN1::GUS line). (H) Expression (blue color) of the influx-carrier AUX1 in the pericycle anticlinal derivatives (stage I, AUX1:: GUS line). (I-J) Expression (blue color) of the influx carrier LAX3 at stage II (I), and at stage VII in the basal part of the ARP only (J) (LAX3:: GUS line). (K-L) Expression pattern (blue color) of AUX1 (K), and of PIN1 (L) in the ARPs from stage VII (K) onwards (L) (AUX1:: GUS and PIN1::GUS line, respectively). (M) SCR expression (green fluorescence) at stage VII in the QC, endodermis/cortical initial cells and their derivatives leading to endodermis formation (pSCR::GFP line). (N-O) WOX5 expression (green fluorescence) at stage III (N), and in the ARP tip, marking the QC and the niche at stage VI (O) (pWOX5::GFP line). (P) Trans-zeatin riboside immunolocalization in an ARP before protrusion. The immunostaining marks the protoderm, in particular. (Q) In situ hybridization showing YUC6 transcription in a not yet protruded ARP. A-C, Histological longitudinal radial sections stained with toluidine blue (A, C, Wassilewskija ecotype, B, Columbia ecotype). Insets in the fluorescence pictures (D, M-O) show corresponding bright-field images. All transgenic lines are in the Columbia background, except for pSCR::GFP, in the Wassilewskija background. 14 days of seedling growth under continuous darkness. Bars = 10 μm (A, H), 20 μm (B-G, I-M, P-Q and insets in D, M, O), 30 μm (N-O and inset in N).
In the PR of Arabidopsis, the accumulation of endogenous indole acetic acid (IAA) is an early event in LR formation. In fact, by the use of the auxin-responsive reporter sensor DR5::GUS, auxin has been detected in the LR founder cells, and in the initial cells anticlinally derived from them.35 This early auxin accumulation also occurs in AR formation. In fact, IAA presence, monitored by the DR5::GUS system, is shown in the hypocotyl pericycle cells before the appearance of AR-forming divisions (Fig. 1E), and is maintained in the founder cells and their anticlinal derivatives. Endogenous auxin continues to be present at further ARP stages, progressively localizing at the ARP tip, where an auxin maximum is defined (stage VII, Fig. 1F), and further maintained.66
An increase of the auxin signal in the pericycle founder cells along the hypocotyl, and an increase in AR production, occur with the addition of natural/synthetic auxins to the medium, i.e., IBA (10 μM) or NAA (2 μM), or by the addition of IBA (10 μM) combined with a cytokinin (0.1 μM Kinetin, Kin). By contrast, auxin signal, and AR formation, highly decrease when the cytokinin is applied alone.66 The intensity of the DR5 signal similarly increases in the formative divisions of LRs after the exogenous application of IAA or NAA.35 The auxin accumulation in the early AR events couples well with the expression pattern of PIN1 and AUX1 auxin carriers. In fact, the efflux carrier PIN1 is initially expressed in the hypocotyl vasculature and in the pericycle AR founder cells (Fig. 1G), and continues to be expressed in first anticlinal derivatives, whereas the expression of the influx-carrier AUX1 begins at stage I (Fig. 1H).66-67 This suggests that PIN1 and AUX1 are coordinated to cause an auxin flux from specific perivascular cells, i.e. those cells located near the protoxylem poles, to the confining pericycle cells, inducing auxin levels sufficient for AR initiation in the latter (Fig. 2A). The expression pattern of the two genes during the following AR-stages sustain their common involvement also in the definition and maintenance of the apical auxin maximum in the ARP (Fig. 2B–C). However, another auxin-influx carrier, LAX3, is active in the AR process. The expression of LAX3 initiates at stage II (Figs. 1I, and 2B), and continues during the following stages. At stage VII, it becomes stronger in the basal part of the ARP, but disappears at the tip (Fig. 1J), where, instead, AUX1 (Fig. 1K), and PIN1 (Fig. 1L) remain expressed. Moreover, LAX3 expression is also present in the hypocotyl peripheral tissues surrounding the protruding ARP (Fig. 2C), exactly as occurs in the PR tissues surrounding the protruding LRP.36,66-67 The treatments with the exogenous auxins increase PIN1 and LAX3 expression, in accordance with their known auxin-inducibility,36,43 whereas the treatment with exogenous cytokinin alone attenuates their expression,66 in accordance with the known cytokinin-induced downregulation of auxin-influx and efflux carriers in the RAM.28
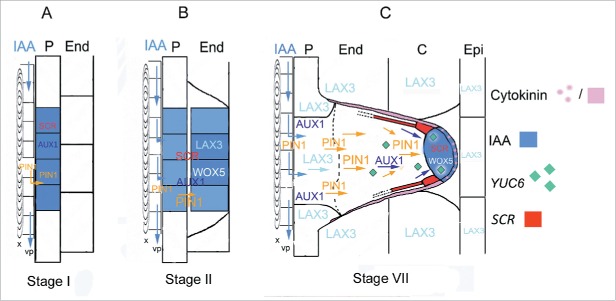
Figure 2.
Model of auxin (IAA) flow and biosynthesis, gene expression and cytokinin (trans-zeatin riboside) localization during AR formation from the hypocotyl of entire seedlings of Arabidopsis thaliana. (A) At stage I auxin is diverted from the basipetal flow along the hypocotyl vascular parenchyma (vp) adjacent to the protoxylem (x) toward the pericycle (P) by the activity of the auxin-efflux-carrier PIN1. The influx of the hormone into the P cells is caused by the activity of the AUX1 auxin-influx-carrier, causing an auxin accumulation (blue color) in the AR-founder cells, their anticlinal division, and SCR expression. (B) At stage II, auxin is maintained in the first-formed inner and outer derivative layers by PIN1 and AUX1 activities, together with the expression of SCR. At this stage, another auxin-influx-carrier, LAX3, and the TF WOX5 are expressed. (C) At stage VII, PIN1 and AUX1 activities in the middle cell files drive the auxin flow toward the ARP tip, whereas cytokinin (pink color) downregulates PIN1 in the protoderm and peripheral ARP layers. Cytokinin also contrasts LAX3, limiting the carrier activity at the ARP base (up to the dotted line). The auxin flow throughout the middle cell files sustains the apical auxin maximum (blue color), limiting WOX5 and SCR expression at the ARP tip, and here establishing the position of the QC in the niche. SCR expression also marks the endodermis/cortical initial cells and the endodermal derivatives (red color). Auxin biosynthesis by YUC6 (green diamonds) contributes to auxin maximum in the tip. LAX3 is also active in the hypocotyl endodermis (End), cortex (C) and epidermis (Epi) around the ARP, possibly for favoring its protrusion. (Modified from Della Rovere et al.66).
In the first anticlinal derivatives of the hypocotyl pericycle founder cells (stage I) SCR signal also appears.67 An early expression of this TF also occurs during LR formation,20 strengthening the similarity in the genetic control of the initiation of the two post-embryonic rooting processes. It is known that SHR activity is necessary for the full expression of SCR in the RAM and in LRs,47-51 and that the AR production is strongly reduced in scr-1 and shr1 mutants.67 Thus, SHR might activate SCR, and the SHR/SCR complex trigger AR formation in the hypocotyl pericycle founder cells (Fig. 2A), as in the PR and LRs (see the previous paragraph). Furthermore, because SCR is induced by auxin,31 and AUX1 is expressed in the AR-initiating cells (Fig. 1H), as SCR, whereas PIN1 also in the confining perivascular cells (Figs. 1G and 2A), it is possible that the priming activity of the SHR/SCR complex in AR formation is affected by AUX1,67 acting downstream to PIN1 (Fig. 2A). At stage VII, SCR is expressed in the ARP tip marking the QC, the endodermis/cortical initial cells and their derivatives leading to endodermis (Fig. 1M), and continues to be expressed in the same cell types, and in the differentiated endodermis, in the mature AR.67 In accordance, the very few macroscopic ARs produced by the scr-1 mutant show no QC definition, altered stem cell niche, and anomalous apical differentiation. The same defects occur in the shr1 mutant.67 The latter mutant is known to be perturbed in auxin abundance, and its incapacity to build up normal roots has been associated with a progressive reduction in the abundance of auxin-efflux carriers, e.g. PIN1.50 Thus, the SHR-SCR activity seems also required for QC specification and maintenance in the AR tip, 67 exactly as in the RAM and LR tip,49-50 but the AR QC also requires AUX1 and PIN1 activities (Fig. 2C).
Another QC-specific gene, WOX5, begins to be expressed at stage II, and reinforces at stage III (Fig. 1N). WOX5 is known to be expressed in the direct precursors of the QC in the early globular embryo,15 but also in the cells founding the LRs.58 In the latter process, it has been suggested that auxin, present in the founder cells, acts very early to specify the QC, through the auxin-inducible WOX5 expression.57 As for SCR, also the expression of WOX5, initially diffuse, becomes progressively restricted toward the ARP tip, marking the QC and the niche at stage VI-VII (Figs. 1O, and 2C). Coupling the expression patterns of WOX5, PIN1 and AUX1, with the localization of the auxin maximum at stage VII, it is possible to hypothesize that an auxin flow, directed toward the ARP tip, causes the restriction of the expression domain of WOX5 up to positioning it in the QC of the ARP. Accordingly, the activity of specific auxin-response factors repress WOX5 transcription, restricting it to the QC in the PR.56 After stage VII, SCR and WOX5 expression are maintained in the QC and niche cells by a persistent auxin maximum requiring the coordinated action of PIN1, AUX1, and LAX3, but also the activity of cytokinin, and the presence of a local auxin biosynthesis.
Trans-zeatin riboside, an important endogenous cytokinin,70 has been immunolocalized in the ARP. It appears at stage IV, but becomes strongly present from stage VII onwards, and mainly in the protoderm (Fig. 1P), where LAX3 and PIN1 are not expressed (Fig. 1J and L).66 In the PR, cytokinin antagonizes auxin by downregulating the PIN proteins (e.g., PIN1) through SHY2 activity.30 Thus, the down-regulation of PIN1 in the protoderm of the ARP (Fig. 1L) could result into a better directioning of auxin to the tip by the PIN1 located along the central longitudinal axis of the ARP, sustaining the auxin maximum at the tip, and QC specification (Fig. 2C). In accordance, cytokinin has been recently demonstrated to control the auxin stream directionality by a differential reduction of PIN1 activity during LR organogenesis.71 On the other hand, cytokinin is capable of promoting auxin biosynthesis in Arabidopsis PR.72 Moreover, the reduction in the cytokinin-stimulated accumulation of auxin in the aux1 mutant sustains that AUX1 is critical to the multifaceted interaction between the two hormones (i.e., redistribution and accumulation).73
To verify whether auxin biosynthesis occurred during AR development, either to compensate the inhibitory action of cytokinin on auxin-carriers or to demonstrate an in loco cytokinin-induced auxin biosynthesis, the transcription of the YUC6 gene, involved in the tryptophan-dependent IAA biosynthesis pathway,74-75 has been investigated.66 YUC6 is detected before QC establishment (stage IV), but its expression increases at stage VII, and in the whole ARP, and is maintained onwards (Fig. 1Q). This result suggests that YUC6 warrants IAA supply for the ARP, contributing to the auxin homeostasis at the tip (Fig. 2C), but also suggests that a prolonged auxin biosynthesis by YUC6 in the apex of the mature AR contributes to the persistence of the auxin maximum at the tip, and QC and stem cell niche maintenance.66
Adventitious rooting from lTCLs cultured in vitro
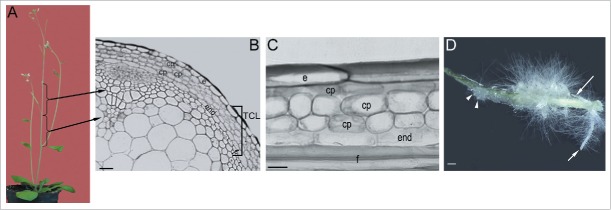
It has been almost 45 years since the technique of in vitro culture of TCLs was first developed to obtain de novo formation of organs, i.e., flowers, vegetative buds, and ARs in numerous plants.76-77 The formation of organs in this system is obtained regulating the auxin/cytokinin ratio, the carbohydrate supply, and the environmental conditions.76 The TCLs are small-sized explants excised from different plant organs, i.e. stem, leaves, cotyledons and hypocotyls and epicotyls, and both transversally (tTCLs) and longitudinally (lTCLs).78 Even being very thin explants, the two TCL types are formed by a different number and types of tissues. Regarding the stem, tTCLs usually consist of epidermis, cortical parenchyma, vascular system including cambium, and pith,79 whereas lTCLs include only the superficial tissues, e.g., the epidermis and the subepidermal chlorenchyma, as the two-layered system of tobacco,80 or all the superficial tissues external to the vascular system, as in Arabidopsis.8,66-67,81 In detail, the lTCLs excised from the inflorescence stem (Fig. 3A) of Arabidopsis consist of epidermis, three layers of cortical parenchyma, a monolayered starch sheath, i.e., stem endodermis,82 and one or two layers of fibers (Fig. 3B–C). The lTCL system has helped to analyze the influence of light regimens, hormones (auxin, cytokinin, jasmonate), and polyamines on the reactivation of cell division and formation of meristemoids and organs, by tissues which are differentiated and unable to express these competences in the context of the entire plant. The latter tissues are also inhibited to express organogenesis in in vitro culture when in the explant are also present meristematic cells with stem activity, as the procambial/cambial cells, because the latter cells are prompt to readily change their activity and fate.3 For example, in Arabidopsis, ARs are formed by the vascular cambium of the petiole in excised leaves 83 and by that of the stem in the inflorescence segments.9 However, in the same plant, also the inflorescence stem explants formed by the tissues external to the vasculature, i.e., the lTCls, produce ARs (Fig. 3D), when cultured with IBA (10 µM) and Kin (0.1 µM) under darkness, with the stem endodermis able to initiate the process in the place of cambium.66 Thus, the stem endodermis re-programmes itself under the exogenous hormonal input, divides periclinally, and organizes meristematic cell clusters (Fig. 4A), further developing into root meristemoids (Fig. 4B). These root meristemoids develop into ARPs (Figs. 3D, and 4C), opening their way through the cortex and epidermis of the lTCL, and then elongating to become mature ARs (Figs. 3D, and 4D). The ARPs and ARs exhibit a QC and a stem cell niche exactly as in planta (Fig. 4E). The QC is defined at a stage equivalent to the stage VII of the development in planta, and shows the expression of the same QC markers, i.e., pAGL42::GFP (Fig. 4F) and QC25::GUS.66 In addition, as for the early phases of AR formation in planta, also in the lTCLs WOX5 and SCR are expressed very early, i.e. in the meristematic cell clusters and the meristemoids (Fig. 4G). Both genes continue to be expressed in protruding and elongating ARs, with the same localization as in the AR apex in planta, i.e., in the QC and niche cells, and, in the case of SCR, also in the root endodermis (Fig. 4H–I). Moreover, the behavior of the lTCLs from scr-1 and shr-1 mutants is of interest. In these mutants the stem endodermis is missing,82 thus their lTCLs lack the tissue responsible for AR initiation. In both mutants, AR response is very low, in accordance with the role of the SHR/SCR complex on AR formation in planta, and the sporadic ARs originate from the innermost cortical layer (Fig. 4J). This means that even if irregular, this cortical tissue is able to re-program itself substituting the endodermis in AR development (Fig. 4K).67
Figure 3.
The lTCL explant system of Arabidopsis and its rhizogenic response. (A) 35-days-old plants from which the inflorescence stem internodes (shown by the parentheses) are excised. (B) Cross section of an internode coming from the inflorescence stem (arrows in A) showing the tissues forming the lTCL explant (parenthesis). (C) Radial longitudinal section of an lTCL at the excision time. (D) Stereomicroscope image of an lTCL with callus and emerged ARPs (arrowheads) and ARs (arrows) at the end (day 22) of culture under continuous darkness on MS'62 medium with 10 μM IBA and 0.1 μM Kin.66 Uniseriate epidermis (e), cortical parenchyma layers (cp), stem endodermis (end), and fibers (f). Bars = 20 μm (C), 30 μm (B), 500 μm (D).
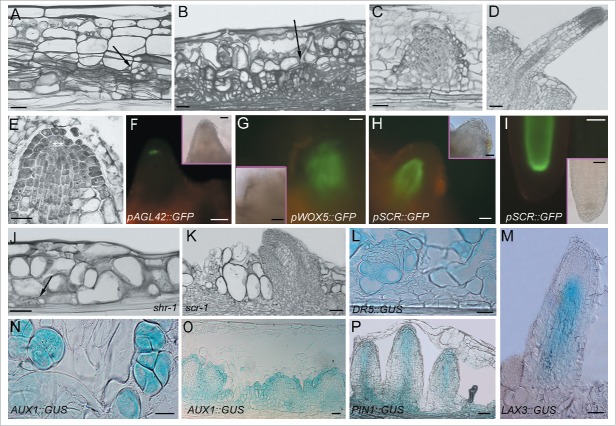
Figure 4.
Adventitious rooting in Arabidopsis lTCLs cultured in the presence of IBA (10 µM) and Kin (0.1 µM), genes involved, and IAA localization and transport. (A-B) Expanded cortical cells and superimposed layers of stem endodermis derivative cells organizing meristematic cell clusters (A, arrow), and meristemoids (B, arrow). (C-D) Not-yet-protruded ARP (C), and elongated AR (D) originated by root meristemoids. (E) QC definition (asterisks) in an ARP. (F) ARP showing the appearance of the expression of the pAGL42 QC-marker (green fluorescence) in the QC cells (pAGL42::GFP line). (G) WOX5 expression (green fluorescence) in a meristemoid (pWOX5::GFP line). (H-I) Protruding ARP (H) and elongated AR (I) showing SCR expression (green fluorescence) in the QC, endodermis/cortical initial cells, their derivatives, and forming endodermis (I) (pSCR::GFP line). (J-K) Initial AR-stages (J) and late stages, i.e., protruding ARPs (K), in lTCLs from the shr-1 (J) and scr-1 (K) mutants. The arrow in (J) shows a meristematic cell cluster originated from the innermost cortical layer which initiates the AR-process in the place of the lacking endodermis. (L) Meristemoid showing IAA presence detected by DR5::GUS expression (DR5::GUS line). (M) AR with LAX3 expression localized in the vasculature and procambium, but excluded by the apex (LAX3:: GUS line). (N-O) AUX1::GUS expression in the meristematic cell clusters (N) and in early-staged ARPs (O) (AUX1::GUS line). (P) Elongated, but not yet protruded, ARPs showing a PIN1 continuous signal from the forming vasculature to the tip (PIN1::GUS line). A-E (Columbia ecotype) and J-K (shr-1 and scr-1) histological longitudinal radial sections stained with toluidine blue. Insets in the fluorescence pictures (F-I) show corresponding bright-field images. All transgenic lines are in the Columbia background, except for pSCR::GFP, in the Wassilewskija background. Wassilewskija is the wild type of homozygous scr-1 and shr-1 null mutants. 22 days of culture under continuous darkness. Bars = 20 μm (E,N), 40 μm (A, H-L and insets in G-I), 60 μm (B-C, F-G, M, O-P and inset in F), 100 μm (D).
By the use of the DR5::GUS lTCLs, it has been demonstrated that IAA accumulates in the stem endodermal cells before the AR-forming divisions, and increases in the meristematic cell clusters/meristemoids (Fig. 4L),66 in concomitance with the appearance of AUX1 expression (Fig. 4N).67 This suggests that AUX1 creates the initial endogenous IAA distribution essential for AR initiation by SHR/SCR complex, as in planta (Fig. 2A). Also LAX3 is active during AR formation in the lTCLs, with a diffuse signal in the meristemoids, and with a signal in the ARPs and ARs localized in the vasculature and procambium up to the elongation zone, but excluded from the apex (Fig. 4M), as in planta. SCR, AUX1 and PIN1 expressions are highly detected in the meristemoids, and, from early-staged ARPs (Fig. 4O) onwards, repeat the expression pattern observed in planta in the same culture condition (Fig. 4P).66-67
Altogether, the activities of the auxin-influx/efflux carriers, and of the TFs (SCR, SHR, WOX5) responsible of the formation of ARs with a functional QC and niche in the apex, are similarly present in planta and in vitro cultured lTCLs, independently of the founding tissue, and with the same requirement of an early auxin accumulation. In the case of the lTCLs, auxin comes from the medium, because endogenous IAA is quite undetectable in these explants at the excision time, as verified in different species, i.e., tobacco 84 and Arabidopsis (Altamura et al., unpublished results). However, as in planta, a local biosynthesis in the AR tips also occurs, and involves YUC6.66
Conclusions and perspectives
In conclusion, Arabidopsis ARs organize a QC and a stem cell niche as the LRs, i.e., the other type of post-embryonic roots. The establishment and maintenance of the QC, and stem cell niche, are independent of the origin of the AR founder cells. A coordinated action of auxin and cytokinin is needed, and involves expression of specific genes, which are the same that control QC and niche definition and functioning in the RAM and LR tip.
The knowledge of the molecular and developmental biology of the adventitious rooting process opens the way to understanding the genetic basis of AR recalcitrance in planta, and in in vitro cultured cuttings, and to optimizing root production in vitro as a tool for a multifaceted biotechnology.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The research was supported by PRIN 2008, Progetto d'Ateneo 2009, Sapienza University, Sapienza Grandi Attrezzature C26G13KJHK/C26G14XYEX and Sapienza Grandi Ricerche Universitarie C26H157ANK (to MMA), and by Progetto d'Ateneo 2014, Sapienza University (prot. C26A14AFZ7) to GF.
References
- 1.Fahn A. Plant Anatomy. 4th ed. Pergamon Press, Oxford, 1990 [Google Scholar]
- 2.Konishi M, Sugiyama M. Genetic analysis of adventitious root formation with a novel series of temperature-sensitive mutants of Arabidopsis thaliana. Development 2003; 130:5637-47; PMID:14522871; http://dx.doi.org/ 10.1242/dev.00794 [DOI] [PubMed] [Google Scholar]
- 3.Altamura MM. Root histogenesis in herbaceous and woody explants cultured in vitro. A critical review. Agronomie 1996; 16:589-602; http://dx.doi.org/ 10.1051/agro:19961001 [DOI] [Google Scholar]
- 4.Altamura MM, Falasca G. Adventitious rooting in model plants and in in vitro systems: an integrated molecular and cytohistological approach In: Adventitious root formation of forest trees and horticultural plants - from genes to applications. Niemi K, Scagel C (Eds.) Research Signpost, Kerala, India: 2009; pp 123-44 [Google Scholar]
- 5.De Klerk GJ, van der Krieken W, de Jong JC. Review the formation of adventitious roots: new concepts, new possibilities. Vitro Cell Dev Biol Plant 1999; 35:189-99; http://dx.doi.org/ 10.1007/s11627-999-0076-z [DOI] [Google Scholar]
- 6.Falasca G, Altamura MM. Histological analysis of adventitious rooting in Arabidopsis thaliana (L.) Heynh seedlings. Plant Biosyst 2003; 137:265-74; http://dx.doi.org/ 10.1080/11263500312331351511 [DOI] [Google Scholar]
- 7.Delarue M, Prinsen E, Onckelen HV, Caboche M, Bellini C. Sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. Plant J 1998; 14:603-11; PMID:9675903; http://dx.doi.org/ 10.1046/j.1365-313X.1998.00163.x [DOI] [PubMed] [Google Scholar]
- 8.Falasca G, Zaghi D, Possenti M, Altamura MM. Adventitious root formation in Arabidopsis thaliana thin cell layers. Plant Cell Rep 2004; 23:17-25; PMID:15118834; http://dx.doi.org/ 10.1007/s00299-004-0801-3 [DOI] [PubMed] [Google Scholar]
- 9.Ludwig-Müller J, Vertocnik A, Town CD. Analysis of indole-3-butyric acid induced adventitious root formation on Arabidopsis stem segments. J Exp Bot 2005; 56:2095-2105; PMID:15955788; http://dx.doi.org/ 10.1093/jxb/eri208 [DOI] [PubMed] [Google Scholar]
- 10.Stahl Y, Rüdiger S. Plant primary meristems: shared functions and regulatory mechanisms. Curr Opin Plant Biol 2010; 13:53-8; PMID:19836993; http://dx.doi.org/ 10.1016/j.pbi.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 11.Sablowski R. Plant stem cell niches: from signalling to execution. Curr Opin Plant Biol 2011; 14:4-9; PMID:20739214; http://dx.doi.org/ 10.1016/j.pbi.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 12.Scheres B. Stem-cell niches: nursery rhymes across kingdoms. Nat Rev Mol Cell Biol 2007; 8:345-54; PMID:17450175; http://dx.doi.org/ 10.1038/nrm2164 [DOI] [PubMed] [Google Scholar]
- 13.Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. Cellular organisation of the Arabidopsis thaliana root. Development 1993; 119:71-84; PMID:8275865 [DOI] [PubMed] [Google Scholar]
- 14.van den Berg C, Willemsen V, Hendriks G, Weisbeek P, Scheres B. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature 1997; 390:287-9; PMID:9384380; http://dx.doi.org/ 10.1038/36856 [DOI] [PubMed] [Google Scholar]
- 15.Haecker A, Groß-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 2004; 131:657-68; PMID:14711878; http://dx.doi.org/ 10.1242/dev.00963 [DOI] [PubMed] [Google Scholar]
- 16.Drisch RC, Stahl Y. Function and regulation of transcription factors involved in root apical meristem and stem cell maintenance. Front Plant Sci 2015; 6:505; PMID:26217359; http://dx.doi.org/ 10.3389/fpls.2015.00505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell 2004; 116:769-78; PMID:15035980; http://dx.doi.org/ 10.1016/S0092-8674(04)00255-7 [DOI] [PubMed] [Google Scholar]
- 18.Nawy T, Lee JY, Colinas J, Wang JY, Thongrod SC, Malamy JE, Birnbaum K, Benfey PN. Transcriptional profile of the Arabidopsis root quiescent center. Plant Cell 2005; 17:1908-25; PMID:15937229; http://dx.doi.org/ 10.1105/tpc.105.031724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, et al.. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 1999; 99:463-72; PMID:10589675; http://dx.doi.org/ 10.1016/S0092-8674(00)81535-4 [DOI] [PubMed] [Google Scholar]
- 20.Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 1997; 124:33-44; PMID:9006065 [DOI] [PubMed] [Google Scholar]
- 21.Slovak R, Ogura T, Satbhai SB, Ristova D, Busch W. Genetic control of root growth: from genes to networks. Ann Bot 2016; 117:9-24; PMID:26558398; http://dx.doi.org/ 10.1093/aob/mcv160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antoniadi I, Plačková L, Simonovik B, Doležal K, Turnbull C, Ljung K, Novák O. Cell-type-specific cytokinin distribution within the Arabidopsis primary root apex. Plant Cell 2015; 27:1955-67; PMID:26152699; http://dx.doi.org/ 10.1105/tpc.15.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 2004; 119:109-20; PMID:15454085; http://dx.doi.org/ 10.1016/j.cell.2004.09.018 [DOI] [PubMed] [Google Scholar]
- 24.Müller B, Sheen J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 2008; 453:1094-7; PMID:18463635; http://dx.doi.org/ 10.1038/nature06943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aloni R, Langhans M, Aloni E, Dreieicher E, Ullrich CI. Root-synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. J Exp Bot 2005; 56:1535-44; PMID:15824073; http://dx.doi.org/ 10.1093/jxb/eri148 [DOI] [PubMed] [Google Scholar]
- 26.Bishopp A, Lehesranta S, Vatén A, Help H, El-Showk S, Scheres B, Helariutta K, Mähönen AP, Sakakibara H, Helariutta Y. Phloem-transported cytokinin regulates polar auxin transport and maintains vascular pattern in the root meristem. Curr Biol 2011; 21:927-32; PMID:21620705; http://dx.doi.org/ 10.1016/j.cub.2011.04.049 [DOI] [PubMed] [Google Scholar]
- 27.Růžička K, Šimášková M, Duclercq J, Petrášek J, Zažímalová E, Simon S, Friml J, Van Montagu MCE, Benková E. Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc Natl Acad Sci USA 2009; 106:4284-9; PMID:19246387; http://dx.doi.org/24120642 10.1073/pnas.0900060106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Swarup R, Bennett M, Schaller GE, Kieber JJ. Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Curr Biol 2013; 23:1979-89; PMID:24120642; http://dx.doi.org/ 10.1016/j.cub.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 29.Dello Ioio R, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S. A genetic framework for the control of cell division and differentiation in the root meristem. Science 2008; 322:1380-4; PMID:19039136; http://dx.doi.org/ 10.1126/science.1164147 [DOI] [PubMed] [Google Scholar]
- 30.Moubayidin L, Di Mambro R, Sabatini S. Cytokinin-auxin crosstalk. Trends Plant Sci 2009; 14:557-62; PMID:19734082; http://dx.doi.org/ 10.1016/j.tplants.2009.06.010 [DOI] [PubMed] [Google Scholar]
- 31.Moubayidin L, Di Mambro R, Sozzani R, Pacifici E, Salvi E, Terpstra I, Bao D, van Dijken A, Dello Ioio R, Perilli S, et al.. Spatial coordination between stem cell activity and cell differentiation in the root meristem. Dev Cell 2013; 26:405-15; PMID:23987513; http://dx.doi.org/ 10.1016/j.devcel.2013.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bielach A, Podlešáková K, Marhavý P, Duclercq J, Cuesta C, Müller B, Grunewald W, Tarkowski P, Benková E. Spatiotemporal regulation of lateral root organogenesis in Arabidopsis by cytokinin. Plant Cell 2012; 24:3967-81; PMID:23054471; http://dx.doi.org/ 10.1105/tpc.112.103044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Smet I, Tetsumura T, De Rybel B, Frei dit Frey N, Laplaze L, Casimiro I, Swarup R, Naudts M, Vanneste S, Audenaert D, et al.. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 2007; 134:681-90; PMID:17215297; http://dx.doi.org/ 10.1242/dev.02753 [DOI] [PubMed] [Google Scholar]
- 34.Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benková E. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci USA 2008; 105:8790-4; PMID:18559858; http://dx.doi.org/ 10.1073/pnas.0712307105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003; 115:591-602; PMID:14651850; http://dx.doi.org/18622388 10.1016/S0092-8674(03)00924-3 [DOI] [PubMed] [Google Scholar]
- 36.Swarup K, Benková E, Swarup R, Casimiro I, Péret B, Yang Y, Parry G, Nielsen E, De Smet I, Vanneste S, et al.. The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol 2008; 10:946-54; PMID:18622388; http://dx.doi.org/ 10.1038/ncb1754 [DOI] [PubMed] [Google Scholar]
- 37.Petrášek J, Friml J. Auxin transport routes in plant development. Development 2009; 136:2675-88; PMID:19633168; http://dx.doi.org/26821586 10.1242/dev.030353 [DOI] [PubMed] [Google Scholar]
- 38.Omelyanchuk NA, Kovrizhnykh VV, Oshchepkova EA, Pasternak T, Palme K, Mironova VV. A detailed expression map of the PIN1 auxin transporter in Arabidopsis thaliana root. BMC Plant Biol 2016; 16(Suppl 1):5; PMID:26821586; http://dx.doi.org/ 10.1186/s12870-015-0685-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Band LR, Wells DM, Fozard JA, Ghetiu T, French AP, Pound MP, Wilson MH, Yu L, Li W, Hijazi HI, et al.. Systems analysis of auxin transport in the Arabidopsis root apex. Plant Cell 2014; 26:862-75; PMID:24632533; http://dx.doi.org/ 10.1105/tpc.113.119495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ugartechea-Chirino Y, Swarup R, Swarup K, Péret B, Whitworth M, Bennett M, Bougourd S. The AUX1 LAX family of auxin influx carriers is required for establishment of embryonic root cell organization in Arabidopsis thaliana. Ann Bot 2010; 105:277-89; PMID:19952011; http://dx.doi.org/ 10.1093/aob/mcp287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Gene Dev 2001; 15:2648-53; PMID:11641271; http://dx.doi.org/ 10.1101/gad.210501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 2002; 14:589-97; PMID:11910006; http://dx.doi.org/ 10.1105/tpc.010354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vieten A, Vanneste S, Wiśniewska J, Benková E, Benjamins R, Beeckman T, Luschnig C, Friml J. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 2005; 132:4521-31; PMID:16192309; http://dx.doi.org/ 10.1242/dev.02027 [DOI] [PubMed] [Google Scholar]
- 44.Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Normanly J, Sandberg G. Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell 2005; 17:1090-104; PMID:15772288; http://dx.doi.org/ 10.1105/tpc.104.029272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JI, Sharkhuu A, Jin JB, Li P, Jeong JC, Baek D, Lee SY, Blakeslee JJ, Murphy AS, Bohnert HJ, et al.. yucca6, a dominant mutation in Arabidopsis, affects auxin accumulation and auxin-related phenotypes. Plant Physiol 2007; 145:722-35; PMID:17885085; http://dx.doi.org/ 10.1104/pp.107.104935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, et al.. The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci USA 2011; 108:18512-7; PMID:22025724; http://dx.doi.org/ 10.1073/pnas.1108434108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn MG, Feldmann KA, Benfey PN. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 1996; 86:423-33; PMID:8756724; http://dx.doi.org/ 10.1016/S0092-8674(00)80115-4 [DOI] [PubMed] [Google Scholar]
- 48.Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser MT, Benfey PN. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 2000; 101:555-67; PMID:10850497; http://dx.doi.org/ 10.1016/S0092-8674(00)80865-X [DOI] [PubMed] [Google Scholar]
- 49.Sabatini S, Heidstra R, Wildwater M, Scheres B. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Gene Dev 2003; 17:354-8; PMID:12569126; http://dx.doi.org/ 10.1101/gad.252503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucas M, Swarup R, Paponov IA, Swarup K, Casimiro I, Lake D, Peret B, Zappala S, Mairhofer S, Whitworth M, et al.. SHORT-ROOT regulates primary, lateral and adventitious root development in Arabidopsis. Plant Physiol 2011; 155:384-98; PMID:21030506; http://dx.doi.org/ 10.1104/pp.110.165126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levesque MP, Vernoux T, Busch W, Cui H, Wang JY, Blilou I, Hassan H, Nakajima K, Matsumoto N, Lohmann JU, et al.. Whole-genome analysis of the SHORT-ROOT developmental pathway in Arabidopsis. PLoS Biol 2006; 4:e143; PMID:16640459; http://dx.doi.org/ 10.1371/journal.pbio.0040143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sozzani R, Cui H, Moreno-Risueno MA, Busch W, Van Norman JM, Vernoux T, Brady SM, Dewitte W, Murray JAH, Benfey PN. Spatiotemporal regulation of cell-cycle genes by SHORT ROOT links patterning and growth. Nature 2010; 466:128-32; PMID:20596025; http://dx.doi.org/ 10.1038/nature09143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu J, Hofhuis H, Heidstra R, Sauer M, Friml J, Scheres B. A molecular framework for plant renegeration. Science 2006; 311:385-8; PMID:16424342; http://dx.doi.org/ 10.1126/science.1121790 [DOI] [PubMed] [Google Scholar]
- 54.Moubayidin L, Salvi E, Giustini L, Terpstra I, Heidstra R, Costantino P, Sabatini S. A SCARECROW-based regulatory circuit controls Arabidopsis thaliana meristem size from the root endodermis. Planta 2016; 243:1159-68; PMID:26848984; http://dx.doi.org/ 10.1007/s00425-016-2471-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 2007; 446:811-4; PMID:17429400; http://dx.doi.org/ 10.1038/nature05703 [DOI] [PubMed] [Google Scholar]
- 56.Ding Z, Friml J. Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc Natl Acad Sci USA 2010; 107:12046-51; PMID:20543136; http://dx.doi.org/ 10.1073/pnas.1000672107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzali S, Novi G, Loreti E, Paolicchi F, Poggi A, Alpi A, Perata P. A turanose-insensitive mutant suggests a role for WOX5 in auxin homeostasis in Arabidopsis thaliana. Plant J 2005; 44:633-45; PMID:16262712; http://dx.doi.org/ 10.1111/j.1365-313X.2005.02555.x [DOI] [PubMed] [Google Scholar]
- 58.Ditengou FA, Teale WD, Kochersperger P, Flittner KA, Kneuper I, van der Graaff E, Nziengui H, Pinosa F, Li X, Nitschke R, et al.. Mechanical induction of lateral root initiation in Arabidopsis thaliana. Proc Natl Acad Sci USA 2008; 105:18818-23; PMID:19033199; http://dx.doi.org/ 10.1073/pnas.0807814105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian H, Wabnik K, Niu T, Li H, Yu Q, Pollmann S, Vanneste S, Govaerts W, Rolčík Geisler M, et al.. WOX5–IAA17 feedback circuit-mediated cellular auxin response is crucial for the patterning of root stem cell niches in Arabidopsis. Mol Plant 2014; 7:277-89; PMID:23939433; http://dx.doi.org/ 10.1093/mp/sst118 [DOI] [PubMed] [Google Scholar]
- 60.Forzani C, Aichinger E, Sornay E, Willemsen V, Laux T, Dewitte W, Murray JAH. WOX5 suppresses CYCLIN D activity to establish quiescence at the center of the root stem cell niche. Curr Biol 2014; 24:1939-44; PMID:25127220; http://dx.doi.org/ 10.1016/j.cub.2014.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Della Rovere F, Airoldi CA, Falasca G, Ghiani A, Fattorini L, Citterio S, Kater M, Altamura MM. The Arabidopsis BET bromodomain factor GTE4 regulates the mitotic cell cycle. Plant Signal Behav 2010; 5:677-80; PMID:20495359; http://dx.doi.org/ 10.4161/psb.5.6.11571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pacurar DI, Perrone I, Bellini C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol Plant 2014; 151:83-96; PMID:24547793; http://dx.doi.org/ 10.1111/ppl.12171 [DOI] [PubMed] [Google Scholar]
- 63.Smith DL, Fedoroff NV. LRP1, a gene expressed in lateral and adventitious root primordia of Arabidopsis. Plant Cell 1995; 7:735-45; PMID:7647564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu H, Wang S, Yu X, Yu J, He X, Zhang S, Shou H, Wu P. ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J 2005; 43:47-56; PMID:15960615; http://dx.doi.org/ 10.1111/j.1365-313X.2005.02434.x [DOI] [PubMed] [Google Scholar]
- 65.Sorin C, Bussell JD, Camus I, Ljung K, Kowalczyk M, Geiss G, McKhann H, Garcion C, Vaucheret H, Sandberg G, et al.. Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell 2005; 17:1343-59; PMID:15829601; http://dx.doi.org/ 10.1105/tpc.105.031625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Della Rovere F, Fattorini L, D'Angeli S, Veloccia A, Falasca G, Altamura MM. Auxin and cytokinin control formation of the quiescent centre in the adventitious root apex of Arabidopsis. Ann Bot 2013; 112:1395-407; PMID:24061489; http://dx.doi.org/ 10.1093/aob/mct215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Della Rovere F, Fattorini L, D'Angeli S, Veloccia A, Del Duca S, Cai G, Falasca G, Altamura MM. Arabidopsis SHR and SCR transcription factors and AUX1 auxin influx carrier control the switch between adventitious rooting and xylogenesis in planta and in in vitro cultured thin cell layers. Ann Bot 2015; 115:617-28; PMID:25617411; http://dx.doi.org/ 10.1093/aob/mcu258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 1962; 15:473-97; http://dx.doi.org/ 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- 69.Takahashi F, Sato-Nara K, Kobayashi K, Suzuki M, Suzuki H. Sugar-induced adventitious roots in Arabidopsis seedlings. J Plant Res 2003; 116:83-91; PMID:12736780; http://dx.doi.org/ 10.1007/s10265-002-0074-2 [DOI] [PubMed] [Google Scholar]
- 70.Kuroha T, Kato H, Asami T, Yoshida S, Kamada H, Satoh S. A trans-zeatin riboside in root xylem sap negatively regulates adventitious root formation on cucumber hypocotyls. J Exp Bot 2002; 53:2193-200; PMID:12379786; http://dx.doi.org/ 10.1093/jxb/erf077 [DOI] [PubMed] [Google Scholar]
- 71.Marhavý P, Duclercq J, Weller B, Feraru E, Bielach A, Offringa R, Friml J, Schwechheimer C, Murphy A, Benková E. Cytokinin controls polarity of PIN1-dependent auxin transport during lateral root organogenesis. Curr Biol 2014; 24:1031-7; PMID:24768050; http://dx.doi.org/ 10.1016/j.cub.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 72.Jones B, Gunnerås SA, Petersson SV, Tarkowski P, Graham N, May S, Dolezal K, Sandberg G, Ljung K. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell 2010; 22:2956-69; PMID:20823193; http://dx.doi.org/ 10.1105/tpc.110.074856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kakani A, Li G, Peng Z. Role of AUX1 in the control of organ identity during in vitro organogenesis and in mediating tissue specific auxin and cytokinin interaction in Arabidopsis. Planta 2009; 229:645-57; PMID:19052775; http://dx.doi.org/ 10.1007/s00425-008-0846-6 [DOI] [PubMed] [Google Scholar]
- 74.Kim JI, Murphy AS, Baek D, Lee SW, Yun D-J, Bressan RA, Narasimhan ML. YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence in Arabidopsis thaliana. J Exp Bot 2011; 62:3981-92; PMID:21511905; http://dx.doi.org/ 10.1093/jxb/err094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao Y. Auxin biosynthesis. Arabidopsis Book 2014; 12:e0173; PMID:24955076; http://dx.doi.org/ 10.1199/tab.0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tran Thanh Van M, Dien NT, Chlyah A. Regulation of organogenesis in small explants of superficial tissue of Nicotiana tabacum L. Planta 1974; 119:149-159; PMID:24442454; http://dx.doi.org/ 10.1007/BF00390888 [DOI] [PubMed] [Google Scholar]
- 77.Teixeira da Silva J, Altamura MM, Dobránszki J. The untapped potential of plant thin cell layers. J Hortic Res 2015; 23:127-31; http://dx.doi.org/ 10.2478/johr-2015-0024 [DOI] [Google Scholar]
- 78.Teixeira da Silva JA, Tran Thanh Van K, Biondi S, Nhut DT, Altamura MM. Thin cell layers: developmental building blocks in ornamental biotechnology. Floriculture Ornamental Biotech 2007; 1:1-13 [Google Scholar]
- 79.Dhir R, Shekhawat GS. In vitro propagation using transverse thin cell layer culture and homogeneity assessment in Ceropegia bulbosa Roxb. J Plant Growth Regul 2014; 33:820-30; http://dx.doi.org/ 10.1007/s00344-014-9432-2 [DOI] [Google Scholar]
- 80.Altamura MM, Torrigiani P, Falasca G, Rossini P, Bagni N. Morpho-functional gradients in superficial and deep tissues along tobacco stem: polyamine levels, biosynthesis and oxidation, and organogenesis in vitro. J Plant Physiol 1993; 142:543-51; http://dx.doi.org/ 10.1016/S0176-1617(11)80396-2 [DOI] [Google Scholar]
- 81.Petroni K, Falasca G, Calvenzani V, Allegra D, Stolfi C, Fabrizi L, Altamura MM, Tonelli C. The AtMYB11 gene from Arabidopsis is expressed in meristematic cells and modulates growth in planta and organogenesis in vitro. J Exp Bot 2008; 59:1201-13; PMID:18359753; http://dx.doi.org/ 10.1093/jxb/ern027 [DOI] [PubMed] [Google Scholar]
- 82.Fukaki H, Wysocka-Diller J, Kato T, Fujisawa H, Benfey PN, Tasaka M. Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J 1998; 14:425-30; PMID:9670559; http://dx.doi.org/ 10.1046/j.1365-313X.1998.00137.x [DOI] [PubMed] [Google Scholar]
- 83.da Rocha Correa L, Troleis J, Mastroberti AA, Mariath JEA, Fett-Neto AG. Distinct modes of adventitious rooting in Arabidopsis thaliana. Plant Biol 2012; 14:100-9; PMID:21974782; http://dx.doi.org/ 10.1007/s00344-014-9432-2 [DOI] [PubMed] [Google Scholar]
- 84.Fattorini L, Falasca G, Kevers C, Mainero Rocca L, Zadra C, Altamura MM. Adventitious rooting is enhanced by methyl jasmonate in tobacco thin cell layers. Planta 2009; 231:155-68; PMID:19885676; http://dx.doi.org/ 10.1007/s00425-009-1035-y [DOI] [PubMed] [Google Scholar]