ABSTRACT
Mitochondria are double-membrane organelles that move around and change their shapes dynamically. In plants, the dynamics of the outer membrane is not well understood. We recently demonstrated that mitochondria had tubular protrusions of the outer membrane with little or no matrix, called MOPs (mitochondrial outer-membrane protrusions; MOPs). Here we show that a MOP can form a bridge between two mitochondria in Arabidopsis thaliana. The bridge does not appear to involve the inner membranes. Live imaging revealed stretching of the MOP bridge, demonstrating the flexibility of the outer membrane. Mitochondria frequently undergo fission and fusion. These observations raise the possibility that MOPs bridges have a role in these processes.
KEYWORDS: Arabidopsis, ELM1, live-imaging, mitochondria, MOPs, outer membrane
In eukaryotic cells, mitochondria are essential organelles that have roles in producing energy, controlling diverse metabolic pathways and regulating cell death.1,2 Mitochondria are also dynamic organelles that move around, change their shape and frequently undergo fusion and fission.3,4 In particular, plant mitochondria are more spherical than animal mitochondria and their morphology depends on the developmental stage and growth conditions.5-8 In previous works, plant mitochondrial morphology and dynamics were usually studied by staining the matrix with a fluorescent dye or by visualizing fluorescently-labeled proteins in the matrix.5,6,8 As a result, the behavior of the mitochondrial outer membrane has received little attention. As the outermost layer of mitochondria, the mitochondrial outer membrane mediates the import of proteins and lipids, the passage of small molecules and conduction of signaling events between the mitochondrion and the cytosol.9,10 Therefore, the dynamics of the outer membrane also needs to be carefully observed.
We recently reported that Arabidopsis cells have mitochondrial outer membrane-derived structures, some of which protrude from the main body of mitochondria (mitochondrial outer-membrane protrusions; MOPs), while others form vesicle-like structures.11 The morphology and the dynamics of MOPs are similar to those of the outer membrane protrusions of mammalian mitochondria,11,12 and the vesicle-like structures are similar to some mammalian MDVs (mitochondrial-derived vesicles).13,14 Both plant MDVs and MOPs did not contain a matrix marker.11 In addition, the numbers of plant MDVs and MOPs increased in senescent leaves and after dark treatment.11 Plant mitochondria were previously shown to have tubular protrusions of the matrix, called matrixules.15-17 However, it was not known whether the outer membrane just surrounded the matrixule.15,16 Recent studies have demonstrated that outer membrane proteins localize not only to the surface of the matrixule,11,17 but also to the more extended protrusion (MOP) from the tip of the matrixule,11 which suggests that the outer membrane changes their shape flexibly. In this addendum, we show another type of MOP structure that forms a bridge between two mitochondria.
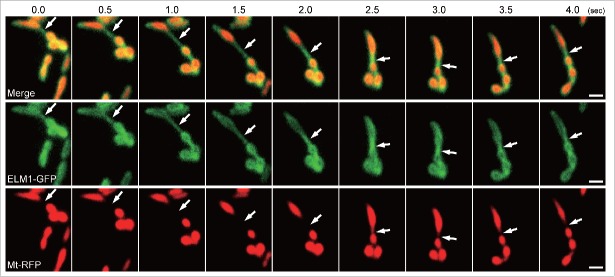
To determine the dynamics of MOPs, we observed the outer membrane and the matrix simultaneously in epidermal cells of Arabidopsis cotyledons. The outer membrane was labeled with GFP fused with ELM1 under the control of its own promoter (ELM1-GFP) and the matrix was labeled with RFP fused to the mitochondrial matrix localization signal of the Arabidopsis mitochondrial F1-ATPase delta-prime subunit (Mt-RFP).11,18 In a time series (Fig. 1), one of the MOP-like protrusions formed a bridge (arrows) between two mitochondria. This is also shown in Supplemental Movie 1. These mitochondria move around and change their morphology, but the bridge stretched and remained for at least a few seconds. In the bridging region, Mt-RFP was below the limit of detection by fluorescence, indicating that the region was composed of outer membrane and had little or no matrix. These results suggest that the mitochondrial outer membrane sometimes forms a bridge to another mitochondrion but that the inner membranes do not connect. Because plant mitochondria undergo fusion and fission frequently,19 the MOP bridge can be regarded as an intermediate stage of mitochondrial fusion, which is just before the latter inner membrane fusion, or as another intermediate stage of mitochondrial fission just after the inner membrane fission. Although the outer membrane is usually thought to just surround the matrix,9 the present results show that it has other potential roles. Further studies are needed to better elucidate the dynamics of mitochondrial outer surface and the mechanism of the generation of MOPs.
Figure 1.
Time-course observation of the bridging of two mitochondria by a mitochondrial outer-membrane protrusion (MOP). These are representative images from a movie (Supplemental Movie 1) that was recorded at 10 frames per second. The movie was obtained by VIAFM (Variable incidence angle fluorescent microscopy20) using leaf epidermal cells from 10-day-old transgenic Arabidopsis plants expressing ELM1-GFP and Mt-RFP. The arrows indicate a MOP bridge, and the bridge was stretched for a few seconds. The time stamp is in half seconds. Bars = 1 µm.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Yun J, Finkel T. Mitohormesis. Cell Metab 2014; 19:757-66; PMID:24561260; http://dx.doi.org/ 10.1016/j.cmet.2014.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasahara A, Scorrano L. Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol 2014; 24:761-70; PMID:25189346; http://dx.doi.org/ 10.1016/j.tcb.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 3.Bleazard W, McCaffery JM, King EJ, Bale S, Mozdy A, Tieu Q, Nunnari J, Shaw JM. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat Cell Biol 1999; 1:298-304; PMID:10559943; http://dx.doi.org/ 10.1038/13014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sesaki H, Jensen RE. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J Cell Biol 1999; 147:699-706; PMID:10562274; http://dx.doi.org/ 10.1083/jcb.147.4.699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logan DC, Leaver CJ. Mitochondria-targeted GFP highlights the heterogeneity of mitochondrial shape, size and movement within living plant cells. J Exp Bot 2000; 51:865-71; PMID:10948212; http://dx.doi.org/ 10.1093/jexbot/51.346.865 [DOI] [PubMed] [Google Scholar]
- 6.Van Gestel K, Köhler RH, Verbelen JP. Plant mitochondria move on F-actin, but their positioning in the cortical cytoplasm depends on both F-actin and microtubules. J Exp Bot 2002; 53:659-67; PMID:11886885; http://dx.doi.org/ 10.1093/jexbot/53.369.659 [DOI] [PubMed] [Google Scholar]
- 7.Seguí-Simarro JM, Coronado MJ, Staehelin LA. The mitochondrial cycle of Arabidopsis shoot apical meristem and leaf primordium meristematic cells is defined by a perinuclear tentaculate/cage-like mitochondrion. Plant Physiol 2008; 148:1380-93; PMID:18799659; http://dx.doi.org/23291162 10.1104/pp.108.126953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruberti C, Barizza E, Bodner M, La Rocca N, De Michele R, Carimi F, Lo Schiavo F, Zottini M. Mitochondria change dynamics and morphology during grapevine leaf senescence. PLoS One 2014; 9:1-12; PMID:25009991; http://dx.doi.org/23291162 10.1371/journal.pone.0102012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan O, van der Merwe MJ, Daley DO, Whelan J. The outer mitochondrial membrane in higher plants. Trends Plant Sci 2013; 18:207-17; PMID:23291162; http://dx.doi.org/ 10.1016/j.tplants.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 10.Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, DeBono A, Durrett TP, et al.. Acyl-lipid metabolism. Arab B 2013; 11:e0161; PMID:22303259; http://dx.doi.org/26752045 10.1199/tab.0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita A, Fujimoto M, Katayama K, Yamaoka S, Tsutsumi N, Arimura S. Formation of mitochondrial outer membrane derived protrusions and vesicles in Arabidopsis thaliana. PLoS One 2016; 11:e0146717; PMID:26752045; http://dx.doi.org/ 10.1371/journal.pone.0146717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi S, Okada Y. Ultrafast superresolution fluorescence imaging with spinning disk confocal microscope optics. Mol. Biol. Cell 2015; 26:1743-51; PMID:25717185; http://dx.doi.org/18207745 10.1091/mbc.E14-08-1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, Andrade-Navarro MA, McBride HM. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr Biol 2008; 18:102-8; PMID:18207745; http://dx.doi.org/ 10.1016/j.cub.2007.12.038 [DOI] [PubMed] [Google Scholar]
- 14.Sugiura A, McLelland GL, Fon EA, McBride HM. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J 2014; 33:2142-56; PMID:25107473; http://dx.doi.org/ 10.15252/embj.201488104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan DC, Scott I, Tobin AK. ADL2a, like ADL2b, is involved in the control of higher plant mitochondrial morphology. J Exp Bot 2004; 55:783-5; PMID:14754924; http://dx.doi.org/ 10.1093/jxb/erh073 [DOI] [PubMed] [Google Scholar]
- 16.Mathur J, Mammone A, Barton KA. Organelle extensions in plant cells. J Integr Plant Biol 2012; 54:851-67; PMID:23046073; http://dx.doi.org/ 10.1111/j.1744-7909.2012.01175.x [DOI] [PubMed] [Google Scholar]
- 17.Ruberti C, Costa A, Pedrazzini E, Lo Schiavo F, Zottini M. FISSION1A, an Arabidopsis tail-anchored protein, is localized to three subcellular compartments. Mol Plant 2014; 7:1393-6; PMID:24658461; http://dx.doi.org/ 10.1093/mp/ssu027 [DOI] [PubMed] [Google Scholar]
- 18.Arimura S, Fujimoto M, Doniwa Y, Kadoya N, Nakazono M, Sakamoto W, Tsutsumi N. Arabidopsis elongated mitochondria1 is required for localization of dynamin-related protein3A to mitochondrial fission sites. Plant Cell 2008; 20:1555-66; PMID:18559960; http://dx.doi.org/ 10.1105/tpc.108.058578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arimura S, Yamamoto J, Aida GP, Nakazono M, Tsutsumi N. Frequent fusion and fission of plant mitochondria with unequal nucleoid distribution. Proc Natl Acad Sci USA 2004; 101:7805-8; PMID:15136720; http://dx.doi.org/ 10.1073/pnas.0401077101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujimoto M, Arimura S, Ueda T, Takanashi H, Hayashi Y, Nakano A, Tsutsumi N. Arabidopsis dynamin-related proteins DRP2B and DRP1A participate together in clathrin-coated vesicle formation during endocytosis. Proc Natl Acad Sci USA 2010; 107:6094-9; PMID:2023146; http://dx.doi.org/ 10.1073/pnas.09135621075 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.