Abstract
Background
The prognostic role of serum liver fibrosis markers in cirrhotic patients remains unclear. We performed a prospective observational study to evaluate the effect of amino-terminal pro-peptide of type III pro-collagen (PIIINP), collagen IV (CIV), laminin (LN), and hyaluronic acid (HA) on the prognosis of liver cirrhosis.
Material/Methods
All patients who were diagnosed with liver cirrhosis and admitted to our department were prospectively enrolled. PIIINP, CIV, LN, and HA levels were tested.
Results
Overall, 108 cirrhotic patients were included. Correlation analysis demonstrated that CIV (coefficient r: 0.658, p<0.001; coefficient r: 0.368, p<0.001), LN (coefficient r: 0.450, p<0.001; coefficient r: 0.343, p<0.001), and HA (coefficient r: 0.325, p=0.001; coefficient r: 0.282, p=0.004) levels, but not PIIINP level (coefficient r: 0.081, p=0.414; coefficient r: 0.090, p=0.363), significantly correlated with Child-Pugh and MELD scores. Logistic regression analysis demonstrated that HA (odds ratio=1.00003, 95% confidence interval [CI]=1.000004–1.000056, p=0.022) was significantly associated with the 6-month mortality. Receiver operating characteristics analysis demonstrated that the area under the curve (AUC) of HA for predicting the 6-month mortality was 0.612 (95%CI=0.508–0.709, p=0.1531).
Conclusions
CIV, LN, and HA levels were significantly associated with the severity of liver dysfunction, but might be inappropriate for the prognostic assessment of liver cirrhosis.
MeSH Keywords: Fibrosis, Liver Cirrhosis, Survival
Background
Amino-terminal pro-peptide of type III pro-collagen (PIIINP), collagen IV (CIV), laminin (LN), and hyaluronic acid (HA) are 4 major serum markers for the non-invasive assessment of liver fibrosis [1–4]. Numerous studies have confirmed their diagnostic performance. Some examples have been shown as follows. In 1996, Murawaki et al. demonstrated a close relationship of elevated HA with the severity of liver fibrosis in patients with chronic viral liver diseases [5]. In 2000, Plevris et al. found that HA alone could reliably identify the presence of liver cirrhosis in patients with chronic liver diseases of mixed etiologies [6]. In 2000, the consensus interferon study group also found that HA alone may be effective in non-invasively assessing the degree of liver fibrosis and cirrhosis in patients with chronic hepatitis C virus infection [7]. In 2001, Murawaki et al. suggested the usefulness of PIIINP and HA for staging liver fibrosis in chronic hepatitis C [8]. In 2002, Xie et al. confirmed the relationship of HA and CIV with the degree of hepatic fibrosis in patients with chronic viral hepatitis [9]. In 2004, Patel et al. found that HA in combination with tissue inhibitor of matrix metalloproteinase 1 and alpha 2-macroglobulin can reliably differentiate between moderate/severe and no/mild fibrosis in patients with chronic hepatitis C infection [10]. In 2004, the European Liver Fibrosis Group reported that HA and PIIINP in combination with age and tissue inhibitor of matrix metalloproteinase 1 can accurately identify the absence of liver fibrosis [11]. In 2005, Cale et al. reported that HA in combination with platelet count, prothrombin index, aspartate aminotransferase, alpha 2-macroglobulin, urea, and age can predict the presence of clinically significant fibrosis in patients with viral hepatitis; HA in combination with prothrombin index, alpha 2-macroglobulin, and age could predict the presence of clinically significant fibrosis in patients with alcoholic liver diseases; HA in combination with gamma-glutamyltransferase, bilirubin, platelet count, and apolipoprotein A1 could predict the area of fibrosis in patients with viral hepatitis; and HA in combination with prothrombin index, alpha 2-macroglobulin, and platelets could predict the area of fibrosis in patients with alcoholic liver diseases [12]. In 2011, Seven et al. confirmed the correlation of PIIINP, CIV, LN, and HA with advanced fibrosis in patients with chronic hepatitis B and D [13]. In 2015, El-Mezayen et al. found that CIV and LN in combination with aspartate aminotransferase-to-platelet ratio index and albumin can be used to identify a very low risk of significant fibrosis in patients with chronic hepatitis C virus infection [14]. Theoretically, the grade of liver fibrosis is positively associated with the severity of liver dysfunction, thereby influencing the survival conditions. However, it remains unclear whether serum liver fibrosis markers can predict the prognosis of patients with liver cirrhosis. We conducted the present prospective observational study to explore this issue.
Material and Methods
This was a prospective observational study, which was registered with clinicaltrials.gov (NCT02335073). The study was conceived by 2 researchers (XSQ and XZG). The study protocol was written by XSQ, discussed with our study group, and approved by the Medical Ethics Committee of our hospital. The approval number was k(2014)28. The written informed consent was signed by every participant before liver fibrosis tests were performed. Inclusion criteria were: 1) patients were admitted to our department; 2) patients were diagnosed with liver cirrhosis; and 3) patients signed the informed consent and agreed to testing of serum liver fibrosis markers (PIIINP, CIV, LN, and HA). Major exclusion criteria were: 1) non-cirrhotic patients; 2) malignancy; and 3) repeated admission.
The participants were prospectively enrolled by our study group (ZMC n=1, YH n=17, XYL n=1, HL n=2, XL n=22, LNR n=14, DW n=6, HZW n=2, JLW n=2, CYW n=10, YLZ n=3, XZ n=3, YGZ n=18, and JJZ n=7). Three researchers (YP, HD, and FFH) recorded the regular clinical and laboratory data of participants from the electronic medical charts of our hospital in the printed case report forms. The primary data at admissions were: age, sex, hepatic encephalopathy, ascites, hydrothorax on chest X ray or CT scans, etiology of liver cirrhosis, red blood cell (RBC), hemoglobin (Hb), white blood cell (WBC), platelets count (PLT), total bilirubin (TBIL), albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamine transferase (GGT), blood urea nitrogen (BUN), creatinine (Cr), prothrombin time (PT), international normalized ratio (INR), potassium (K), and sodium (Na). Child-Pugh and MELD scores were calculated [15,16]. Survival condition at the 6th month was obtained by collecting the re-admission and outpatient information in the electronic medical charts and telephone follow-up. Three researchers (XYW, FFH, and XSQ) were responsible for the telephone follow-up. The last telephone follow-up date was April 1, 2016.
As previously mentioned [17,18], a 3-ml fasting venous blood sample was obtained from every participant and then centrifuged. Two laboratory researchers (JC and CLX) tested the PIIINP, CIV, LN, and HA levels by using the chemiluminescent immunoassay in the LUmo Microplate Luminometer equipment at the lab of our department. The diagnostic kits for the PIIINP, CIV, LN, and HA were provided by the Autobio Diagnostics Co., Ltd. (Zhengzhou, Henan Province, China). According to the directions of diagnostic kits, the reference values were defined by the samples from 546 healthy volunteers. The reference values were: PIIINP <15 ng/mL, CIV <95 ng/mL, LN <130 ng/mL, and HA <120 ng/mL.
Statistical analyses were performed by using the SPSS and MedCalc software. Categorical data are expressed as frequencies. Continuous data are expressed as means with standard deviations and medians with ranges. Spearman non-parametric tests were employed to test the correlation of PIIINP, CIV, LN, and HA levels with clinical and laboratory data. Logistic regression analysis was used to test the statistically significant prognostic factors. Odds ratio (OR) with 95% confidence interval (CI) was calculated. Receiver operating characteristics (ROC) analysis was used to test the prognostic accuracy. The AUC was calculated and compared by the De Long test. Two-tailed p<0.05 was considered statistically significant.
Results
Patients characteristics
Between January and June 2015, 108 cirrhotic patients were included in this prospective observational study. Patient characteristics are shown in Table 1. A majority of included patients had hepatitis B virus infection and alcohol abuse as the major etiology of liver cirrhosis. Child-Pugh score was calculated for 104 of them. Mean Child-Pugh score was 7.5±1.9. Eighty-four percent of patients had Child-Pugh classes A and B. MELD score could be calculated in 104 of them. Mean MELD score was 7.1±5.6.
Table 1.
Patient characteristics.
| Variables | No. Pts available | Mean or frequency | Std. deviation | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Age (years) | 108 | 59.030 | 11.498 | 59.065 | 26.74 | 83.16 |
| Sex (Male/Female) – n. | 108 | 67/41 | ||||
| Hepatic encephalopathy – n. | 108 | 8 | ||||
| Ascites – n. | 108 | 63 | ||||
| Hydrothorax on chest X ray or CT scans – n. | 88 | 8 | ||||
| Etiology of liver cirrhosis – n. | 108 | |||||
| Hepatitis B virus alone | 22 | |||||
| Hepatitis C virus alone | 7 | |||||
| Alcohol alone | 27 | |||||
| Drug alone | 4 | |||||
| Hepatitis B virus+Alcohol | 8 | |||||
| Autoimmune | 5 | |||||
| Cholestatic | 2 | |||||
| Hepatitis B virus+Fatty Liver | 1 | |||||
| Alcohol+Budd-Chiari Syndrome | 1 | |||||
| Unknown | 31 | |||||
| Red blood cell (1012/L) | 108 | 3.239 | 0.856 | 3.225 | 1.38 | 5.36 |
| Hemoglobin (g/L) | 108 | 94.148 | 29.253 | 93 | 33 | 153 |
| White blood cell (109/L) | 108 | 4.257 | 2.315 | 4.1 | 0.9 | 15.7 |
| Platelet (109/L) | 108 | 96.185 | 62.805 | 78.5 | 22 | 316 |
| Total bilirubin (umol/L) | 108 | 33.418 | 36.361 | 22.65 | 5.2 | 234.8 |
| Albumin (g/L) | 107 | 31.665 | 6.276 | 31.5 | 16.8 | 46 |
| Alanine aminotransferase (U/L) | 108 | 35.306 | 33.980 | 25 | 5 | 249 |
| Aspartate aminotransferase (U/L) | 108 | 48.046 | 37.442 | 36 | 10 | 227 |
| Alkaline phosphatase (U/L) | 108 | 123.741 | 81.544 | 102.5 | 24 | 543 |
| Gamma-glutamyl transpeptidase (U/L) | 108 | 93.046 | 156.625 | 50.5 | 9 | 1377 |
| Blood urea nitrogen (mmol/L) | 106 | 6.676 | 3.645 | 5.62 | 1.47 | 20.46 |
| Creatinine (umol/L) | 106 | 86.243 | 133.729 | 62.45 | 34.5 | 1092 |
| Potassium (mmol/L) | 108 | 3.849 | 0.584 | 3.81 | 2.53 | 6.13 |
| Sodium (mmol/L) | 108 | 138.232 | 4.112 | 138.9 | 115.7 | 144.4 |
| Prothrombin time (seconds) | 105 | 14.663 | 3.648 | 14 | 10.4 | 38.8 |
| International normalized ratio | 105 | 1.262 | 0.313 | 1.2 | 0.9 | 3.37 |
| Amino-terminal pro-peptide of type III pro-collagen (ng/mL) | 108 | 31.373 | 37.246 | 13.14 | 2.18 | 192.35 |
| IV-collagen (ng/mL) | 108 | 225.882 | 333.062 | 149.69 | 28.79 | 2990.01 |
| Laminin (ng/mL) | 108 | 182.016 | 429.861 | 92.045 | 16.09 | 4184.99 |
| Hyaluronic acid (ng/mL) | 108 | 5275.794 | 21185.907 | 636.885 | 66.69 | 145053.94 |
| Child-Pugh score | 104 | 7.538 | 1.920 | 7 | 5 | 12 |
| Child-Pugh class – n. | 104 | |||||
| A | 33 | |||||
| B | 55 | |||||
| C | 16 | |||||
| Model for end stage liver diseases (MELD) score | 104 | 7.106 | 5.588 | 6.672 | −3.16 | 31.4 |
Correlation of serum liver fibrosis markers with clinical and laboratory data
PIIINP level. No variables significantly correlated with PIIINP level (Table 2).
Table 2.
Correlation of PIIINP with clinical and laboratory data by Spearman non-parametric tests.
| Variables | No. Pts available | Correlation coefficient | Sig. (2-tailed) |
|---|---|---|---|
| Age | 108 | 0.028 | 0.771 |
| Sex | 108 | −0.047 | 0.628 |
| Hepatic encephalopathy | 108 | 0.079 | 0.414 |
| Ascites | 108 | 0.046 | 0.636 |
| Hydrothorax on chest X ray or CT scans | 88 | 0.114 | 0.292 |
| Red blood cell | 108 | 0.014 | 0.890 |
| Hemoglobin | 108 | 0.055 | 0.574 |
| White blood cell | 108 | 0.133 | 0.170 |
| Platelet | 108 | −0.075 | 0.443 |
| Total bilirubin | 108 | 0.074 | 0.448 |
| Albumin | 107 | −0.122 | 0.212 |
| Alanine aminotransferase | 108 | −0.019 | 0.843 |
| Aspartate aminotransferase | 108 | −0.016 | 0.871 |
| Alkaline phosphatase | 108 | −0.113 | 0.246 |
| Gamma-glutamyl transpeptidase | 108 | 0.006 | 0.955 |
| Blood urea nitrogen | 106 | −0.018 | 0.858 |
| Creatinine | 106 | 0.038 | 0.700 |
| Potassium | 108 | −0.047 | 0.629 |
| Sodium | 108 | −0.058 | 0.554 |
| Prothrombin time | 105 | 0.019 | 0.844 |
| International normalized ratio | 105 | 0.065 | 0.512 |
| Child-Pugh score | 104 | 0.081 | 0.414 |
| Model for end stage liver diseases (MELD) score | 104 | 0.090 | 0.363 |
CIV level. Ascites, RBC, WBC, TBIL, ALB, ALT, AST, GGT, Na, PT, INR, Child-Pugh score, and MELD score significantly correlated with CIV level (Table 3).
Table 3.
Correlation of CIV with clinical and laboratory data by Spearman non-parametric tests.
| Variables | No. Pts available | Correlation coefficient | Sig. (2-tailed) |
|---|---|---|---|
| Age | 108 | 0.063 | 0.519 |
| Sex | 108 | −0.126 | 0.192 |
| Hepatic encephalopathy | 108 | 0.027 | 0.78 |
| Ascites | 108 | 0.438 | <0.001 |
| Hydrothorax on chest X ray or CT scans | 88 | 0.204 | 0.057 |
| Red blood cell | 108 | −0.225 | 0.019 |
| Hemoglobin | 108 | −0.073 | 0.453 |
| White blood cell | 108 | 0.225 | 0.019 |
| Platelet | 108 | −0.131 | 0.176 |
| Total bilirubin | 108 | 0.434 | <0.001 |
| Albumin | 107 | −0.567 | <0.001 |
| Alanine aminotransferase | 108 | 0.235 | 0.014 |
| Aspartate aminotransferase | 108 | 0.321 | 0.001 |
| Alkaline phosphatase | 108 | 0.174 | 0.072 |
| Gamma-glutamyl transpeptidase | 108 | 0.289 | 0.002 |
| Blood urea nitrogen | 106 | −0.074 | 0.453 |
| Creatinine | 106 | 0.026 | 0.793 |
| Potassium | 108 | −0.212 | 0.028 |
| Sodium | 108 | −0.339 | <0.001 |
| Prothrombin time | 105 | 0.356 | <0.001 |
| International normalized ratio | 105 | 0.37 | <0.001 |
| Child-Pugh score | 104 | 0.658 | <0.001 |
| Model for end stage liver diseases (MELD) score | 104 | 0.368 | <0.001 |
LN level. Ascites, WBC, TBIL, ALB, ALT, AST, ALP, GGT, Na, INR, Child-Pugh score, and MELD score significantly correlated with LN level (Table 4).
Table 4.
Correlation of LN with clinical and laboratory data by Spearman non-parametric tests.
| Variables | No. Pts available | Correlation coefficient | Sig. (2-tailed) |
|---|---|---|---|
| Age | 108 | 0.059 | 0.544 |
| Sex | 108 | 0.022 | 0.821 |
| Hepatic encephalopathy | 108 | 0.100 | 0.301 |
| Ascites | 108 | 0.296 | 0.002 |
| Hydrothorax on chest X ray or CT scans | 88 | 0.178 | 0.097 |
| Red blood cell | 108 | 0.050 | 0.609 |
| Hemoglobin | 108 | 0.173 | 0.073 |
| White blood cell | 108 | 0.268 | 0.005 |
| Platelet | 108 | −0.015 | 0.881 |
| Total bilirubin | 108 | 0.461 | <0.001 |
| Albumin | 107 | −0.324 | 0.001 |
| Alanine aminotransferase | 108 | 0.298 | 0.002 |
| Aspartate aminotransferase | 108 | 0.421 | <0.001 |
| Alkaline phosphatase | 108 | 0.232 | 0.016 |
| Gamma-glutamyl transpeptidase | 108 | 0.254 | 0.008 |
| Blood urea nitrogen | 106 | −0.179 | 0.066 |
| Creatinine | 106 | 0.060 | 0.543 |
| Potassium | 108 | −0.158 | 0.102 |
| Sodium | 108 | −0.238 | 0.013 |
| Prothrombin time | 105 | 0.153 | 0.120 |
| International normalized ratio | 105 | 0.199 | 0.041 |
| Child-Pugh score | 104 | 0.450 | <0.001 |
| Model for end stage liver diseases (MELD) score | 104 | 0.343 | <0.001 |
HA level. Ascites, hydrothorax, RBC, PLT, TBIL, ALB, PT, INR, Child-Pugh score, and MELD score significantly correlated with HA level (Table 5).
Table 5.
Correlation of HA with clinical and laboratory data by Spearman non-parametric tests.
| Variables | No. Pts available | Correlation coefficient | Sig. (2-tailed) |
|---|---|---|---|
| Age | 108 | 0.062 | 0.525 |
| Sex | 108 | −0.038 | 0.694 |
| Hepatic encephalopathy | 108 | 0.164 | 0.089 |
| Ascites | 108 | 0.300 | 0.002 |
| Hydrothorax on chest X ray or CT scans | 88 | 0.274 | 0.010 |
| Red blood cell | 108 | −0.258 | 0.007 |
| Hemoglobin | 108 | −0.104 | 0.286 |
| White blood cell | 108 | 0.000 | 1.000 |
| Platelet | 108 | −0.268 | 0.005 |
| Total bilirubin | 108 | 0.240 | 0.012 |
| Albumin | 107 | −0.248 | 0.010 |
| Alanine aminotransferase | 108 | 0.046 | 0.636 |
| Aspartate aminotransferase | 108 | 0.041 | 0.671 |
| Alkaline phosphatase | 108 | −0.060 | 0.536 |
| Gamma-glutamyl transpeptidase | 108 | 0.075 | 0.442 |
| Blood urea nitrogen | 106 | 0.004 | 0.965 |
| Creatinine | 106 | 0.144 | 0.141 |
| Potassium | 108 | −0.180 | 0.062 |
| Sodium | 108 | −0.022 | 0.824 |
| Prothrombin time | 105 | 0.239 | 0.014 |
| International normalized ratio | 105 | 0.229 | 0.019 |
| Child-Pugh score | 104 | 0.325 | 0.001 |
| Model for end stage liver diseases (MELD) score | 104 | 0.282 | 0.004 |
Prognostic factors
The 6-month survival data was available in 97 patients. The 6-month mortality was 14.4% (14/97). The logistic regression univariate analysis of factors associated with the 6-month mortality included the absence of ascites (OR=0.184, 95%CI=0.038–0.899, p=0.037) and increased RBC (OR=0.355, 95%CI=0.159–0.795, p= 0.012), TBIL (OR=1.023, 95%CI=1.008–1.039, p=0.003), HA (OR=1.00003, 95%CI=1.000004–1.000056, p=0.022), Child-Pugh score (OR=1.561, 95%CI=1.113–2.152, p=0.007), and MELD score (OR=1.29, 95%CI=1.122–1.483, p<0.001) (Table 6).
Table 6.
Logistics regression analysis of factors associated with 6-month death.
| Variables | No. Pts available | 6-month death vs. alive | P value | OR | 95%CI |
|---|---|---|---|---|---|
| Age | 97 | 14 vs. 83 | 0.237 | 1.031 | 0.980–1.085 |
| Sex (Male vs. Female) | 97 (57 vs. 40) | 14 vs. 83 | 0.894 | 0.925 | 0.294–2.908 |
| Hepatic encephalopathy (No vs. Yes) | 97 (91 vs. 6) | 14 vs. 83 | 0.999 | 2.937 | NA |
| Ascites (No vs. Yes) | 97 (39 vs. 58) | 14 vs. 83 | 0.037 | 0.184 | 0.038–0.899 |
| Hydrothorax on chest X ray or CT scans (No vs. Yes) | 79 (72 vs. 7) | 11 vs. 68 | 0.258 | 0.357 | 0.060–2.123 |
| Red blood cell | 97 | 14 vs. 83 | 0.012 | 0.355 | 0.159–0.795 |
| Hemoglobin | 97 | 14 vs. 83 | 0.062 | 0.980 | 0.959–1.001 |
| White blood cell | 97 | 14 vs. 83 | 0.99 | 0.998 | 0.775–1.285 |
| Platelet | 97 | 14 vs. 83 | 0.93 | 1.000 | 0.992–1.009 |
| Total bilirubin | 97 | 14 vs. 83 | 0.003 | 1.023 | 1.008–1.039 |
| Albumin | 96 | 14 vs. 82 | 0.08 | 0.916 | 0.831–1.011 |
| Alanine aminotransferase | 97 | 14 vs. 83 | 0.57 | 0.993 | 0.971–1.016 |
| Aspartate aminotransferase | 97 | 14 vs. 83 | 0.996 | 1.000 | 0.985–1.015 |
| Alkaline phosphatase | 97 | 14 vs. 83 | 0.488 | 1.002 | 0.996–1.008 |
| Gamma-glutamyl transpeptidase | 97 | 14 vs. 83 | 0.424 | 0.997 | 0.989–1.005 |
| Blood urea nitrogen | 95 | 14 vs. 81 | 0.102 | 1.121 | 0.978–1.286 |
| Creatinine | 93 | 13 vs. 80 | 0.111 | 1.002 | 0.999–1.006 |
| Potassium | 97 | 14 vs. 83 | 0.075 | 0.338 | 0.103–1.114 |
| Sodium | 97 | 14 vs. 83 | 0.069 | 0.896 | 0.795–1.009 |
| Prothrombin time | 94 | 14 vs. 80 | 0.161 | 1.093 | 0.965–1.237 |
| International normalized ratio | 94 | 14 vs. 80 | 0.128 | 3.084 | 0.723–13.155 |
| Amino-terminal pro-peptide of type III pro-collagen | 97 | 14 vs. 83 | 0.265 | 1.007 | 0.995–1.020 |
| IV-collagen | 97 | 14 vs. 83 | 0.235 | 1.001 | 0.999–1.004 |
| Laminin | 97 | 14 vs. 83 | 0.899 | 1.000 | 0.999–1.001 |
| Hyaluronic acid | 97 | 14 vs. 83 | 0.022 | 1.00003 | 1.000004–1.000056 |
| Child-Pugh score | 93 | 14 vs. 79 | 0.007 | 1.561 | 1.113–2.152 |
| Model for end stage liver diseases (MELD) score | 93 | 14 vs. 79 | <0.001 | 1.290 | 1.122–1.483 |
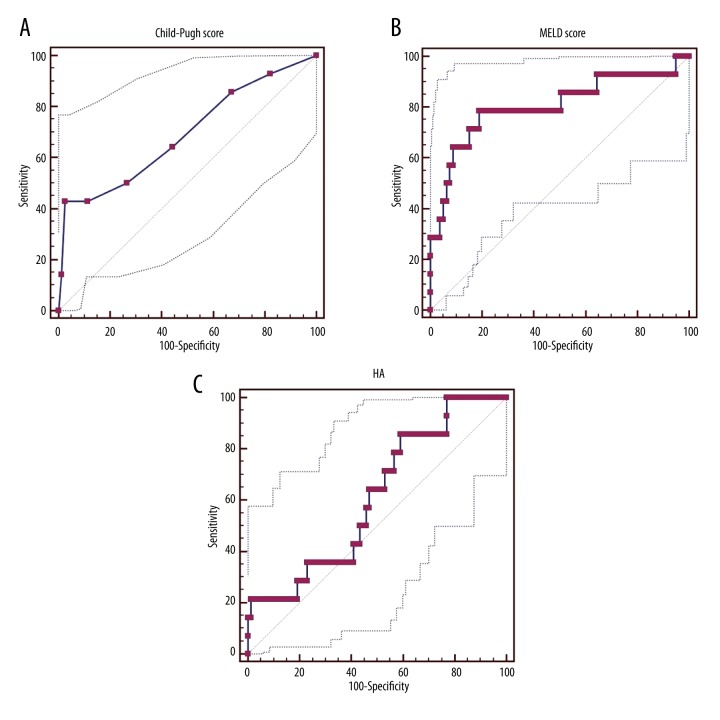
ROC analysis
The AUC of Child-Pugh score, MELD score, and HA level for predicting the 6-month mortality was 0.692 (95%CI=0.587–0.783, p=0.0276), 0.803 (95%CI=0.707–0.878, p=0.0002), and 0.612 (95%CI=0.508–0.709, p=0.1531), respectively (Figure 1). There was a statistically significant difference between Child-Pugh score and HA level (p=0.0124), but there was not a statistically significant difference between Child-Pugh score and HA level (p=0.3421).
Figure 1.
ROC analysis of Child-Pugh score (A), MELD score (B), and HA level (C) for predicting the 6-month mortality rate of cirrhotic patients.
Discussion
The present study had 2 primary objectives. The first objective was to validate our previous retrospective observational study regarding the correlation of the 4 serum liver fibrosis markers with the severity of liver dysfunction 17. The major similarity and discrepancy are summarized as follows. First, our previous study demonstrated that CIV (coefficient r: 0.2361, p=0.0006), LN (coefficient r: 0.2445, p=0.0004), and HA (coefficient r: 0.1612, p=0.0203) levels significantly correlated with Child-Pugh score, but not PIIINP level (coefficient r: 0.02665, p=0.7031). Similarly, the present prospective observational study confirmed that CIV (coefficient r: 0.658, p<0.001), LN (coefficient r: 0.450, p<0.001), and HA (coefficient r: 0.325, p=0.001) levels significantly correlated with Child-Pugh score, but not PIIINP level (coefficient r: 0.081, p=0.414). Second, our previous study also demonstrated that CIV (coefficient r: 0.1795, p=0.0108) and LN (coefficient r: 0.2588, p=0.0002) levels significantly correlated with MELD score, but not PIIINP (coefficient r: 0.04573, p=0.5191) or HA (coefficient r: 0.07926, p=0.2633) level. In contrast, the present study showed that CIV (coefficient r: 0.368, p<0.001), LN (coefficient r: 0.343, p<0.001), and HA (coefficient r: 0.282, p=0.004) levels significantly correlated with MELD score, but not PIIINP (coefficient r: 0.090, p=0.363) level. The possible causes for such a discrepancy could be: 1) the patient characteristics were different between the 2 studies; 2) Pearson chi-square test was used in the previous study, but Spearman non-parametric test was used in the present study; and 3) the correlation of HA level with MELD score might be unstable.
The second objective was to explore the effect of the 4 serum liver fibrosis markers on the survival of liver cirrhosis patients. Logistic regression analysis showed that only HA level, but not PIIINP, CIV, or LN level, was significantly associated with the 6-month mortality in cirrhotic patients. However, this association was very weak. When we used the ROC analysis to evaluate the effect of HA level for the predicting the 6-month mortality, the significance disappeared. Indeed, the prognostic value of HA level may be inferior to those of the traditional prognostic models (i.e., Child-Pugh and MELD scores). Therefore, we do not recommend the prognostic values of the 4 serum liver fibrosis markers in liver cirrhosis.
Several limitations of the present study should be mentioned. First, the in-hospital mortality was very low (0.9%, 1/108), and the logistic regression analysis of factors associated with the in-hospital mortality was not performed. Second, the information on 6-month mortality was missing in 11 patients (10.1%, 11/108). Third, long-term follow-up was lacking. Fourth, the causes of admission were heterogeneous.
Conclusions
CIV, LN, and HA levels significantly correlated with the severity of liver dysfunction, but they could not predict the 6-month mortality rate of cirrhotic patients. Therefore, the current evidence does not recommend the prognostic value of serum liver fibrosis markers in liver cirrhosis patients.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Papastergiou V, Tsochatzis E, Burroughs AK. Non-invasive assessment of liver fibrosis. Ann Gastroenterol. 2012;25(3):218–31. [PMC free article] [PubMed] [Google Scholar]
- 2.Jarcuska P, Janicko M, Veseliny E, Skladany L. Circulating markers of liver fibrosis progression. Clin Chim Acta. 2010;411(15–16):1009–17. doi: 10.1016/j.cca.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Oh S, Afdhal NH. Hepatic fibrosis: Are any of the serum markers useful? Curr Gastroenterol Rep. 2001;3(1):12–18. doi: 10.1007/s11894-001-0035-2. [DOI] [PubMed] [Google Scholar]
- 4.Lichtinghagen R, Bahr MJ. Noninvasive diagnosis of fibrosis in chronic liver disease. Expert Rev Mol Diagn. 2004;4(5):715–26. doi: 10.1586/14737159.4.5.715. [DOI] [PubMed] [Google Scholar]
- 5.Murawaki Y, Ikuta Y, Koda M, et al. Clinical significance of serum hyaluronan in patients with chronic viral liver disease. J Gastroenterol Hepatol. 1996;11(5):459–65. doi: 10.1111/j.1440-1746.1996.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 6.Plevris JN, Haydon GH, Simpson KJ, et al. Serum hyaluronan – a non-invasive test for diagnosing liver cirrhosis. Eur J Gastroenterol Hepatol. 2000;12(10):1121–27. doi: 10.1097/00042737-200012100-00009. [DOI] [PubMed] [Google Scholar]
- 7.McHutchison JG, Blatt LM, de Medina M, et al. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J Gastroenterol Hepatol. 2000;15(8):945–51. doi: 10.1046/j.1440-1746.2000.02233.x. [DOI] [PubMed] [Google Scholar]
- 8.Murawaki Y, Ikuta Y, Okamoto K, et al. Diagnostic value of serum markers of connective tissue turnover for predicting histological staging and grading in patients with chronic hepatitis C. J Gastroenterol. 2001;36(6):399–406. doi: 10.1007/s005350170084. [DOI] [PubMed] [Google Scholar]
- 9.Xie SB, Yao JL, Zheng SS, et al. The levels of serum fibrosis marks and morphometric quantitative measurement of hepatic fibrosis. Hepatobiliary Pancreat Dis Int. 2002;1(2):202–6. [PubMed] [Google Scholar]
- 10.Patel K, Gordon SC, Jacobson I, et al. Evaluation of a panel of non-invasive serum markers to differentiate mild from moderate-to-advanced liver fibrosis in chronic hepatitis C patients. J Hepatol. 2004;41(6):935–42. doi: 10.1016/j.jhep.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg WM, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: A cohort study. Gastroenterology. 2004;127(6):1704–13. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 12.Cales P, Oberti F, Michalak S, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42(6):1373–81. doi: 10.1002/hep.20935. [DOI] [PubMed] [Google Scholar]
- 13.Seven G, Karatayli SC, Kose SK, et al. Serum connective tissue markers as predictors of advanced fibrosis in patients with chronic hepatitis B and D. Turk J Gastroenterol. 2011;22(3):305–14. doi: 10.4318/tjg.2011.0217. [DOI] [PubMed] [Google Scholar]
- 14.El-Mezayen HA, Habib S, Marzok HF, Saad MH. Diagnostic performance of collagen IV and laminin for the prediction of fibrosis and cirrhosis in chronic hepatitis C patients: A multicenter study. Eur J Gastroenterol Hepatol. 2015;27(4):378–85. doi: 10.1097/MEG.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 15.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–49. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 16.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45(3):797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 17.Zhu C, Qi X, Li H, et al. Correlation of serum liver fibrosis markers with severity of liver dysfunction in liver cirrhosis: A retrospective cross-sectional study. Int J Clin Exp Med. 2015;8(4):5989–98. [PMC free article] [PubMed] [Google Scholar]
- 18.Qi X, Li H, Chen J, et al. Serum liver fibrosis markers for predicting the presence of gastroesophageal varices in liver cirrhosis: A retrospective cross-sectional study. Gastroenterol Res Pract. 2015;2015:274534. doi: 10.1155/2015/274534. [DOI] [PMC free article] [PubMed] [Google Scholar]