ABSTRACT
The pyridine nucleotide nicotinamide adenine dinucleotide phosphate (NADP) is a universal coenzyme in anabolic reactions and also functions in intracellular signaling by serving as a substrate for production of the Ca2+-mobilizing agent nicotinic acid adenine dinucleotide phosphate (NAADP). It has recently been shown that, in mammalian cells, cellular NADP can be released into the extracellular compartment (ECC) upon environmental stresses by active exocytosis or diffusion through transmembrane transporters in living cells or passive leakage across the membrane in dying cells. In the ECC, NADP can either serve as a substrate for production of NAADP or act directly on purinoceptors to activate transmembrane signaling. In the last several years, extracellular NADP has also been suggested to function in plant immune responses. Here, we compared exogenous NADP-induced immune responses against biotrophic and necrotrophic pathogens in the Arabidopsis thaliana ecotype Columbia and found that NADP addition induces salicylic acid-mediated defense signaling but not jasmonic acid/ethylene-mediated defense responses. These results suggest the specificity of exogenous NADP-activated signaling in plants.
KEYWORDS: Arabidopsis, defense response, extracellular NADP, ethylene, jasmonic acid, salicylic acid
We have previously shown in Arabidopsis that exogenous application of NADP (nicotinamide adenine dinucleotide phosphate) induces biosynthesis of the defense signal molecule salicylic acid (SA) and resistance against the hemi-biotrophic bacterial pathogen Pseudomonas syringae pv. maculicola (Psm) ES4326, that cellular NADP is released into the extracellular compartment (ECC) during infection by the avirulent bacterial pathogen P. syringae pv tobacco (Pst) DC3000/avrRpt2, and that the amount of NADP released is sufficient for activating immune responses.1 Importantly, transgenic Arabidopsis plants expressing the human NADP-metabolizing ectoenzyme CD38 depletes extracellular NADP (eNADP) and partially compromises biological induction of the broad-spectrum immune response named systemic acquired resistance (SAR), suggesting that eNADP may play a critical signaling role in activation of SAR.2
Plant pathogens are often divided into biotrophs and necrotrophs according to their lifestyles. Biotrophs obtain their nutrition from living cells, whereas necrotrophs first kill host cells and then feed on the dead tissues. In general, SA signaling is active against biotrophic pathogens, and jasmonic acid (JA)/ethylene (ET) signaling is effective against necrotrophs.3,4 While our previous studies have shown that exogenous application of NADP induces expression of SA-dependent pathogenesis-related (PR) genes and resistance to hemi-biotrophic pathogens,1 it is unclear whether NADP addition induces JA/ET pathway genes and resistance to necrotrophic pathogens. It is also unclear whether JA and/or ET signaling are required for NADP-induced defense gene expression and disease resistance.
To test whether exogenous NADP induces JA/ET pathway genes, we treated Arabidopsis ecotype Columbia (Col-0) leaves by syringe infiltration with 1 mM NADP or 10 mM MgCl2. Twenty-four hours later, the infiltrated leaves were collected and subjected to total RNA extraction. Expression levels of the SA pathway marker genes PR1, PR2, and PR5 and the JA/ET signaling marker genes PLANT DEFENSIN1.2 (PDF1.2), PR4, and BASIC CHITINASE (ChiB) in the samples were examined by real-time quantitative PCR.5-8 Confirming the previous results,1 NADP addition significantly induced the SA pathway marker genes (Fig. 1A). On the other hand, NADP addition did not induce the JA/ET signaling marker genes PDF1.2, PR4, and ChiB (Fig. 1B). A time course experiment confirmed that PDF1.2 was not induced at several times (8, 16, and 24 hr) when the PR1 gene was significanty activated (Fig. 1C). These results suggest that eNADP may not activate JA/ET-mediated defense gene expression.
Figure 1.
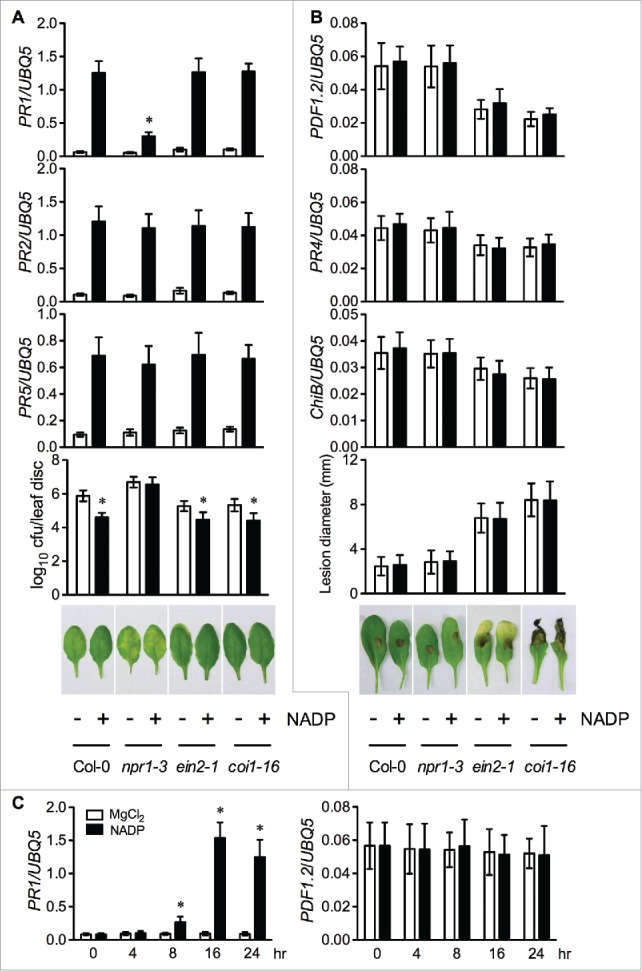
Exogenous NADP-induced immune responses Leaves of 4-week-old soil-grown wild-type Col-0 and the indicated mutant plants were infiltrated with 1 mM NADP or 10 mM MgCl2. Total RNA was extracted from the infiltrated leaf tissues collected at 24 hr (A and B) or at the indicated time points (C and D) and subjected to real-time qPCR analysis. Expression was normalized against constitutively expressed UBQ5. Data represent the mean of 3 independent samples with standard deviation (SD). Psm ES4326 and B. cinerea inoculation was performed 5 hours after NADP treatment following the protocols described previously.1,14 For Psm ES4326, data represent the mean of 8 independent samples with SD, and for B. cinerea, data represent the mean of 24 lesions (one lesion per leaf) with SD. All experiments were repeated at least twice with similar trends. (A) Expression of PR1, PR2, and PR5 and growth of Psm ES4326 in wild-type Col-0, npr1-3, ein2-1, and coi1-16 plants treated with 1 mM NADP or 10 mM MgCl2. The asterisks indicate significant differences between the NADP and the mock treatment (P < 0.05, Student's t test). Photos were taken 3 d post-inoculation. (B) Expression of PDF1.2, PR4, and ChiB and disease severity of B. cinerea on wild-type Col-0, npr1-3, ein2-1, and coi1-16 plants treated with 1 mM NADP or 10 mM MgCl2. Photos were taken 4 d post-inoculation. (C) Expression of PR1 and PDF1.2 in wild-type Col-0 plants at the indicated time points after treatment with 1 mM NADP or 10 mM MgCl2. The asterisks indicate significant differences between the NADP and the mock treatment (P < 0.05, Student's t test).
The JA signaling pathway has 2 major branches. One branch cooperates with ET signaling to synergistically activate defense genes including PDF1.2, PR4, and ChiB, which are associated with resistance to necrotrophic pathogens,9,10 and the other branch regulates the expression of wound-response genes such as VEGATATIVE STORAGE PROTEIN1 (VSP1), VSP2, and JASMONATE RESPONSIVE1 (JR1).11-13 These 2 branches are differentially regulated in response to pathogen infection or wounding. Since cellular NADP is released into the ECC during pathogen infection or upon wounding,1 exogenous NADP might activate the wound response branch of the JA signaling pathway. To test this hypothesis, we examined the expression levels of VSP1, VSP2, and JR1 in the NADP-treated samples and found that these wound-response genes were not up-regulated (Fig. 2). Interestingly, compared with the mock (MgCl2) treatment, NADP addition appeared to suppress the expression of the VSP1 gene at 4 hr (Fig. 2). This observation is consistent with NADP being an elicitor of SA signaling,1 which in turn suppresses JA-mediated wound responses.4 Taken together, these results indicate that exogenous application of NADP does not induce either JA/ET-mediated defense genes or JA-mediated wound-response genes.
Figure 2.
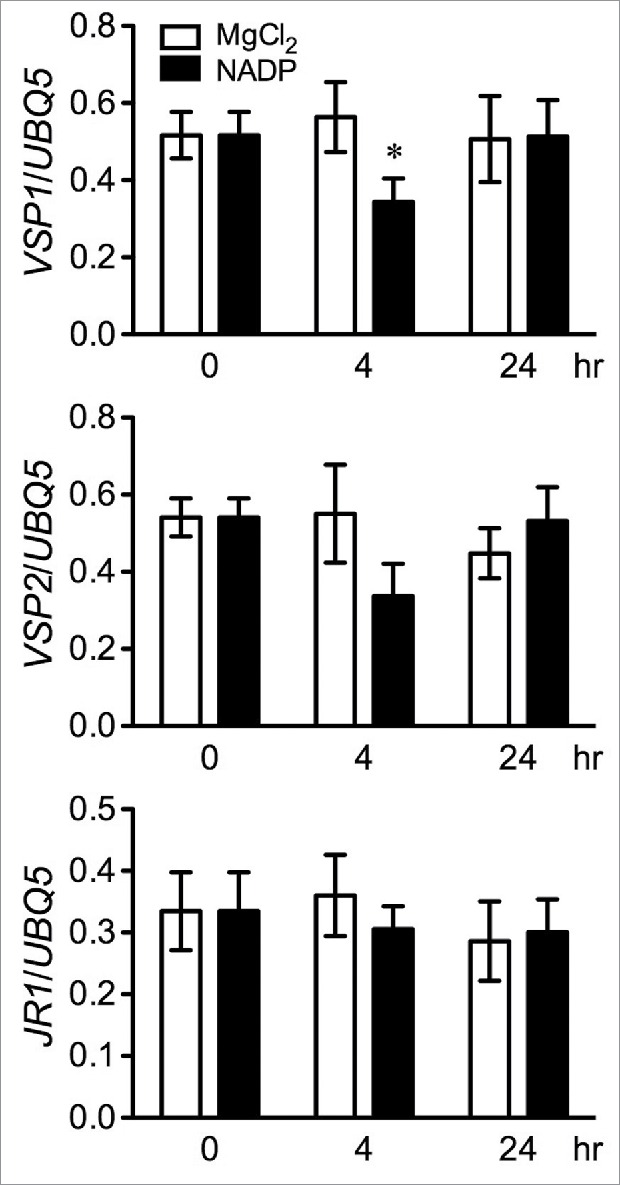
Effect of exogenous NADP on wound-response genes Expression of VSP1, VSP2, and JR1 in wild-type Col-0 plants treated with 1 mM NADP or 10 mM MgCl2. The 0, 4, and 24 hr RNA samples in Fig. 1C were used. Expression was normalized against constitutively expressed UBQ5. Data represent the mean of 3 independent samples with SD. The asterisk indicates a significant difference between the NADP and the mock treatment (P < 0.05, Student's t test). The experiment was repeated twice with simialr trends.
To test whether NADP application induces resistance against necrotrophic pathogens, we treated wild-type Col-0 leaves with 1 mM NADP or 10 mM MgCl2 by syringe infiltration. Five hours later, the MgCl2- or NADP-treated leaves were inoculated with the necrotrophic fungal pathogen Botrytis cinerea. The disease severity of B. cinerea was estimated by measuring lesion sizes 4 d post-inoculation.14 As shown in Fig. 1B, NADP treatment had no effect on the size of the B. cinerea lesions formed on the wild-type Col-0 leaves, indicating that NADP addition did not confer resistance to this necrotrophic fungal pathogen.
To test if disruption of JA and ET signaling affects NADP-induced defense gene expression, the JA signaling mutant coronatine insensitive1-16 (coi1-16) and the ET signaling mutant ethylene insensitive2-1 (ein2-1) were treated together with the wild-type Col-0 by syringe infiltration with 1 mM NADP or 10 mM MgCl2. The SA signaling mutant nonexpressor of PR genes1-3 (npr1-3) was also included as a control. In npr1-3, induction of PR1 was inhibited by ∼76%, while induction of PR2 and PR5 was not significantly affected (Fig. 1A), supporting the previous conclusion that exogenous NADP induces NPR1-dependent and -independent PR gene expression.1 On the other hand, the SA signaling marker genes were similarly induced in coi1-16 and ein2-1 as in the wild-type Col-0 (Fig. 1A). These results indicate that the intactness of the JA/ET signaling pathway is not required for exogenous NADP-induced PR gene expression.
To further test whether disruption of the JA/ET signaling pathway affects NADP-induced disease resistance, we treated wild-type, coi1-16, and ein2-1 leaves with 1 mM NADP or 10 mM MgCl2 by syringe infiltration. The npr1-3 mutant was again included as a control. Five hours later, the MgCl2- or NADP-treated leaves were inoculated with the bacterial pathogen Psm ES4326. The in planta growth of Psm ES4326 in the leaves was determined 2 d post-inoculation. Similar to the previously reported results,1 the growth of Psm ES4326 in the NADP-treated wild-type Col-0 plants was significantly lower than that in the MgCl2-treated wild-type plants, and the growth of Psm ES4326 in the NADP-treated npr1-3 plants was comparable to that in the MgCl2-treated npr1-3 plants (Fig. 1A), confirming that NPR1 is required for NADP-induced resistance to Psm ES4326.1 On the other hand, Psm ES4326 grew to similar levels in the NADP-treated coi1-16, ein2-1, and wild-type Col-0 plants (Fig. 1A), indicating that COI1 and EIN2 are not required for NADP-induced disease resistance.
Exogenous NADP activates the SA pathway marker genes PR1, PR2, and PR5 and resistance to hemi-biotrophic bacterial pathogens (Fig. 1A).1 We show that exogenous NADP does not activate the JA/ET-mediated defense marker genes PDF1.2, PR4, and ChiB (Fig. 1B), the JA-inducible wound-response genes VSP1, VSP2, and JR1 (Fig. 2), and resistance to necrotrophic fungal pathogens (Fig. 1B). In addition, NADP-induced PR gene expression and disease resistance partially or largely depends on SA and NPR1, but not on COI1 and EIN2 (Fig. 1A).1 These results suggest that eNADP may specifically activate the SA signaling pathway. As exogenous added NADP acts in the ECC,1 future studies will focus on how eNADP is perceived on plant cell surface, which then leads to specific activation of the downstream SA pathway.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a grant from National Science Foundation (NSF IOS-0842716) awarded to Z.M.
References
- 1.Zhang X, Mou Z. Extracellular pyridine nucleotides induce PR gene expression and disease resistance in Arabidopsis. Plant J 2009; 57:302-12; PMID:18798871; http://dx.doi.org/ 10.1111/j.1365-313X.2008.03687.x [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Mou Z. Expression of the human NAD(P)-metabolizing ectoenzyme CD38 compromises systemic acquired resistance in Arabidopsis. Mol Plant-Microbe Interact 2012; 25:1209-18; PMID:22670756; http://dx.doi.org/ 10.1094/MPMI-10-11-0278 [DOI] [PubMed] [Google Scholar]
- 3.Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol 2004; 42:185-209; PMID:15283665; http://dx.doi.org/ 10.1146/annurev.phyto.42.040803.140421 [DOI] [PubMed] [Google Scholar]
- 4.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 2005; 43:205-27; PMID:16078883; http://dx.doi.org/ 10.1146/annurev.phyto.43.040204.135923 [DOI] [PubMed] [Google Scholar]
- 5.Uknes S, Mauch-Mani B, Moyer M, Potter S, Williams S, Dincher S, Chandler D, Slusarenko A, Ward E, Ryals J. Acquired resistance in Arabidopsis. Plant Cell 1992; 4:645-56; PMID:1392589; http://dx.doi.org/ 10.1105/tpc.4.6.645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potter S, Uknes S, Lawton K, Winter AM, Chandler D, DiMaio J, Novitzky R, Ward E, Ryals J. Regulation of a hevein-like gene in Arabidopsis. Mol Plant Microbe Interact 1993; 6:680-5; PMID:8118053; http://dx.doi.org/ 10.1094/MPMI-6-680 [DOI] [PubMed] [Google Scholar]
- 7.Chen QG, Bleecker AB. Analysis of ethylene signal-transduction kinetics associated with seedling-growth response and chitinase induction in wild-type and mutant arabidopsis. Plant Physiol 1995; 108:597-607; PMID:7610160; http://dx.doi.org/ 10.1104/pp.108.2.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penninckx IAMA, Thomma BPHJ, Buchala A, Metraux J-P, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 1998; 10:2103-13; PMID:9836748; http://dx.doi.org/ 10.1105/tpc.10.12.2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berrocal-Lobo M, Molina A, Solano R. Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J 2002; 29:23-32; PMID:12060224; http://dx.doi.org/ 10.1046/j.1365-313x.2002.01191.x [DOI] [PubMed] [Google Scholar]
- 10.Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 2003; 15:165-78; PMID:12509529; http://dx.doi.org/ 10.1105/tpc.007468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzo O, Chico JM, Sanchez-Serrano JJ, Solano R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 2004; 16:1938-50; PMID:15208388; http://dx.doi.org/ 10.1105/tpc.022319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazan K, Manners JM. Jasmonate signaling: toward an integrated view. Plant Physiol 2008; 146:1459-68; PMID:18390489; http://dx.doi.org/ 10.1104/pp.107.115717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verhage A, Vlaardingerbroek I, Raaymakers C, Van Dam NM, Dicke M, Van Wees SC, Pieterse CM. Rewiring of the Jasmonate Signaling Pathway in Arabidopsis during Insect Herbivory. Front Plant Sci 2011; 2:47; PMID:22645537; http://dx.doi.org/ 10.3389/fpls.2011.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C, Ding Y, Yao J, Zhang Y, Sun Y, Colee J, Mou Z. Arabidopsis Elongator subunit 2 positively contributes to resistance to the necrotrophic fungal pathogens Botrytis cinerea and Alternaria brassicicola. Plant J 2015; 83:1019-33; PMID:26216741; http://dx.doi.org/ 10.1111/tpj.12946 [DOI] [PubMed] [Google Scholar]


