ABSTRACT
The Bcl-2–associated athanogene (BAG) family is an evolutionarily conserved, multifunctional group of cytoprotective co-chaperones. Using structural bioinformatic approaches we identified 7 homologs of the Arabidopsis BAG family. Evaluating knockouts in Arabidopsis of individual BAG family members, we noted that Arabidopsis BAG6 (AtBAG6) knockout lines exhibited a pronounced enhancement of susceptibility to the necrotrophic fungal pathogen Botrytis cinerea. Moreover, we identified a single predicted caspase-1 site that was cleaved by an aspartyl protease (AtAPCB1). Finally, we showed AtBAG6 forms a complex with AtAPCB1 via coupling to a C2 GRAM domain protein (AtBAGP1). This complex and its activation is necessary for triggering pathogen mediated autophagic cell death and host resistance.
KEYWORDS: Autophagy, aspartyl protease, AtBAG6, C2-GRAM domain protein, fungal resistance, programmed cell death
In response to stress, multicellular organisms have developed various strategies for cytoprotection. Among those, altruistic cellular suicide cell or programmed cell death (PCD) involves the orchestration of cellular demise that under non-stressed conditions is a beneficial cell death to the organism, maintaining cell homeostasis for growth and development. While purported to be a conserved regulatory circuit, core regulators or functional equivalents of apoptotic-like PCD (e.g. Bcl-2 family and caspases), while well characterized in mammalian cells, have in general, not been identified in plants; at least at the primary sequence level. We reasoned that if some degree of conservation occurs between plant and animal PCD, it might be evident at the structural level, independent of sequence. Based on structural homology and computational searches, we uncovered the Bcl-2 athanogene (BAG) family in Arabidopsis, a family of co-chaperone regulators distinguished by a common conserved region known as the BAG domain which can mediate direct interaction with the ATPase domain of heat-shock protein 70 (Hsp70)/heat-shock cognate 70 (Hsc70).1
Although the mammalian BAGs localize either to the nucleus or cytoplasm, the 7 Arabidopsis BAGs are individually differentially localized to the nucleus, mitochondria, cytoplasm, endoplasmic reticulum (ER), and vacuole.2,3 Several plant BAGs were evaluated and shown to be cytoprotective in response to biotic and abiotic stresses.1-3 In particular, AtBAG6 knockout lines and no others, exhibited an enhanced susceptibility phenotype when challenged by the necrotrophic fungus Botrytis cinerea, suggesting AtBAG6 may impact basal resistance.1 We recently uncovered the role by which AtBAG6 effects plant immunity.4 We found that AtBAG6 is processed at a single caspase 1-like cleavage site through association with protein partners that include a C2-GRAM protein (AtBAGP1), and an aspartyl protease (AtAPCB1). Both AtBAGP1 and AtAPCB1 are required for AtBAG6 cleavage and the cleavage is required for subsequent host resistance. Knock-out of AtBAGP1 or AtAPCB1 resulted in the blocking of AtBAG6 processing coincident with loss of resistance. Expressing cleavage site-mutated AtBAG6 led to inhibited autophagy and unimpeded fungal growth.
To determine the subcellular location of AtBAG6, YFP-tagged AtBAG6 was co-transformed with different RFP-tagged organelle markers into Arabidopsis protoplasts. Our results indicated YFP-AtBAG6 particularly merged with the vacuole signal (Vac-RFP) (Fig. 1). The result suggests the plant vacuole is a relevant location for AtBAG6 and accords with the occurrence of autophagy.
Figure 1.
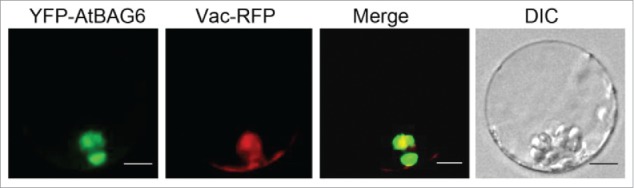
AtBAG6 localizes to the plant vacuole. Arabidopsis protoplasts were co-transfected with the fluorescent constructs YFP-AtBAG6 (yellow) and Vac-RFP (red). Fluorescence was visualized using an Olympus IX81 inverted fluorescence confocal microscope All images were collected using an Olympus DP controller and processed using Olympus FLUOVIEW software. Scale bar = 5 μm.
Autophagy is an evolutionary conserved catabolic process originally noted during yeast starvation, and is known to trigger pathways that non-selectively degrade cytosolic molecules and maintain homeostasis when resources are limiting. Autophagy is characterized by double membrane-bound vesicles or autophagosomes that sequester cytoplasmic components and damaged organelles which are then delivered to plant vacuoles for degradation and recycling.
Numerous studies have demonstrated important roles for autophagy in the regulation of nutrient starvation, cell differentiation and aging as well as abiotic as well as biotic stresses.5-9 Like a double edged sword, autophagy has been shown to activate pro-death or pro-survival pathways, leading to much discussion as to whether autophagy is pro-life, pro-death, or both. Consistent with our observations, autophagy correlated with basal resistance to necrotrophs.10-12 In contrast to biotrophs, necrotrophic pathogens kill host cells relying on a range of virulence factors, including toxins, reactive oxygen species (ROS) and hydrolytic enzymes for nutrient acquisition.11-14 Dead tissue via autophagy could be construed to promote and enhance invasion and colonization by necrotrophic pathogens. However, Arabidopsis plants defective in autophagy genes (atg) were more susceptible to B. cinerea and Alternaria brassicicola, suggesting autophagy promotes host resistance (pro-survival) to necrotrophic pathogens.11,12 In accordance, atbag6 mutants have a similar phenotype to those described in other autophagy defective systems.4,11,12 We investigated whether a functional autophagy system was present in atbag6 mutants. Importantly, atbag6 mutants retained functional autophagic responses when treated with chemical autophagy inducers (Trehalose, Tunicamycin)15 in an atbag6 backgound; thus atbag6 was dispensable for general autophagy and the observed phenotype is a direct result of B. cinerea challenge. The chemical inducers restored the ability of the plant to respond successfully to fungal attack.4 These results indicate AtBAG6 is not directly required for the process of autophagy. Thus we consider this to be a pathogen-induced autophagy (PIA) and AtBAG6 is likely a link between pathogen perception and the execution of PCD resulting in restricted fungal growth and resistance.4 As a result of such specificity, we suggest the PIA may be involved with selective autophagy rather than a non-specific bulk degradation of cell components. For example, starvation-induced autophagy is a non-selective process that degrades undefined cellular materials providing the cell building blocks. Conversely, selective autophagy removes specific cytoplasmic constituents, including damaged organelles, protein aggregates and bacteria. Selectivity is regulated via cargo receptors and ATG8, a ubiquitin like protein involved in autophagosome formation.16 While well studied in mammals, the relatively large family of ATG8 proteins8,17 may represent an evolutionary adaptation by plants to promote flexibility in selective autophagy for a range of stress stimuli. We thus suggest that ATG8 harbors more complex and/or additional specialized functions specifically associated with plant selective-autophagy. This may be needed due to the sessile nature of plants and unpredictable environmental conditions.18 If and how this complex selectively captures appropriate cargo material and the identity of cargo materials that in this case triggers autophagy induced resistance is a topic of keen interest.
To date, bona fide strictly defined caspase genes have not been identified in plants. However, caspase-like protease activities are widely detected in plants.19-22.The aspartyl protease AtAPCB1 cleaves the single caspase 1-like cleavage site in AtBAG6.4 Caspase-1 enzymes are inflammatory; thus it is unclear how the aspartyl proteases are regulated in this system. In addition, aspartyl proteases are associated with plant development23 and systemic acquired resistance.24 AtAPCB1 appears to be AtBAG6 specific as 2 unrelated Arabidopsis aspartyl proteases were unable to complement the atacpb1 mutant line with respect to AtBAG6 cleavage. The C2-GRAM proteins are only found in plants and are typically endomembrane localized; thus far their functions are largely unknown. The C2 domain is a Ca2+-dependent membrane targeting module found in many cellular proteins involved in signal transduction or membrane trafficking25 and is coupled to enzymatic domains.26 AtBAG6 is a calmodulin (CaM)-binding protein (CaMBP) which is selectively induced by Ca2+ but not other divalent cations and modulated by CaM through transducing Ca2+ signals.27 It is reasonable to hypothesize that the C2-GRAM protein AtBAGP1, recruits AtBAG6 as a consequence of Ca2+-regulation, and couples the enzymatic domain of AtAPCB1 via the C2 domain to degrade the substrate AtBAG6 under specific stimulation (Fig. 2).
Figure 2.
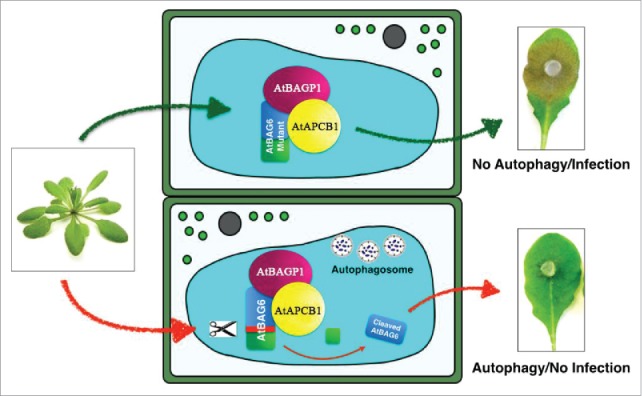
Autophagy induced by processed AtBAG6 is required for plant defense. In response to the fungal phyopathogen B. cinerea, a complex (AtBAG6-AtBAGP1-AtAPCB1) is formed, and processing of AtBAG6 occurs. Autophagy is then triggered fungal growth is restricted and plant resistance occurs. If cleavage is blocked, autophagy is suppressed and PCD runaway cell death occurs.
In summary, Arabidopsis AtBAG6 is a cytoprotective co-chaperone that we suggest is involved with selective autophagy. As a result of fungal interaction with the necrotrophic pathogen B. cinerea, a complex is formed, and processing of AtBAG6 occurs. The processed AtBAG6 subsequently triggers autophagy, resulting in restricted fungal growth and resistance. If this process is blocked, autophagy is suppressed and PCD runaway cell death occurs (Fig. 2).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Doukhanina E. V, Chen S, van der Zalm E, Godzik A, Reed J, Dickman M. B. Identification and functional characterization of the BAG protein family in Arabidopsis thaliana. J Biol Chem 2006; 281:18793-801; PMID:16636050; http://dx.doi.org/ 10.1074/jbc.M511794200 [DOI] [PubMed] [Google Scholar]
- 2.Kabbage M, Dickman M. B. The BAG proteins: a ubiquitous family of chaperone regulators. Cell Mol Life Sci 2008; 65:1390-402; PMID:18264803; http://dx.doi.org/ 10.1007/s00018-008-7535-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams B, Kabbage M, Britt R, Dickman M. B. AtBAG7, an Arabidopsis Bcl-2-associated athanogene, resides in the endoplasmic reticulum and is involved in the unfolded protein response. Proc Natl Acad Sci USA 2010; 107:6088-93; PMID:20231441; http://dx.doi.org/ 10.1073/pnas.0912670107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y. R, Kabbage M, Liu W. D, Dickman M. B. Aspartyl protease-mediated cleavage of BAG6 is necessary for autophagy and fungal resistance in plants. Plant Cell 2016; 28:233-47; PMID:26739014; http://dx.doi.org/ 10.1105/tpc.15.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Schiff M, Czymmek K, Talloczy Z, Levine B, Dinesh-Kumar S. P. Autophagy regulates programmed cell death during the plant innate immune response. Cell 2005; 121:567-77; PMID:15907470; http://dx.doi.org/ 10.1016/j.cell.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 6.Bassham D. C. Plant autophagy-more than a starvation response. Curr Opin Plant Biol 2007; 10:587-93; PMID:17702643; http://dx.doi.org/ 10.1016/j.pbi.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 7.Hofius D, Schultz-Larsen T, Joensen J, Tsitsigiannis D. I, Petersen N. H, Mattsson O, Jørgensen L. B, Jones J. D, Mundy J, Petersen M. Autophagic components contribute to hypersensitive cell death in Arabidopsis. Cell 2009; 137:773-83; PMID:19450522; http://dx.doi.org/ 10.1016/j.cell.2009.02.036 [DOI] [PubMed] [Google Scholar]
- 8.Liu Y. M, Bassham D. C. Autophagy: pathways for self-eating in plant cells. Annu Rev Plant Biol 2012; 63:215-37; PMID:22242963; http://dx.doi.org/ 10.1146/annurev-arplant-042811-105441 [DOI] [PubMed] [Google Scholar]
- 9.Bassham D. C, Crespo J. L. Autophagy in plants and algae. Front Plant Sci 2014; 5:679; PMID:25520731; http://dx.doi.org/ 10.3389/fpls.2014.00679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabbage M, Williams B, Dickman M. B. Cell Death Control: The interplay of apoptosis and autophagy in the pathogenicity of Sclerotinia sclerotiorum. Plos Pathog 2013; 9:e1003287; PMID:23592997; http://dx.doi.org/ 10.1371/journal.ppat.1003287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai Z, Wang F, Zheng Z, Fan B, Chen Z. A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J 2011; 66:953-68; PMID:21395886; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04553.x [DOI] [PubMed] [Google Scholar]
- 12.Lenz H. D, Haller E, Melzer E, Kober K, Wurster K, Stahl M, Bassham D. C, Vierstra R. D, Parker J. E, Bautor J, et al.. Autophagy differentially controls plant basal immunity to biotrophic and necrotrophic pathogens. Plant J 2011; 66:818-30; PMID:21332848; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04546.x [DOI] [PubMed] [Google Scholar]
- 13.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 2005; 43:205-27; PMID:16078883; http://dx.doi.org/ 10.1146/annurev.phyto.43.040204.135923 [DOI] [PubMed] [Google Scholar]
- 14.van Kan JAL. Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci 2006; 11:247-53; PMID:16616579; http://dx.doi.org/ 10.1016/j.tplants.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 15.Williams B, Njaci I, Moghaddam L, Long H, Dickman M. B, Zhang X. R, Mundree S. Trehalose accumulation triggers autophagy during plant desiccation. Plos Genet 2015; 11:e1005705; PMID:26633550; http://dx.doi.org/ 10.1371/journal.pgen.1005705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 2011; 7:279-96; PMID:21189453; http://dx.doi.org/ 10.4161/auto.7.3.14487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizushima N, Yoshimori T, Ohsumi Y. The role of atg proteins in autophagosome formation. Annu Rev Cell Dev Bi 2011; 27:107-32; PMID:21801009; http://dx.doi.org/ 10.1146/annurev-cellbio-092910-154005 [DOI] [PubMed] [Google Scholar]
- 18.Michaeli S, Galili G. Degradation of organelles or specific organelle components via selective autophagy in plant cells. Int J Mol Sci 2014; 15:7624-38; PMID:24802874; http://dx.doi.org/ 10.3390/ijms15057624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon M, Belenghi B, Delledonne M, Menachem E, Levine A. The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 1999; 11:431-43; PMID:10072402; http://dx.doi.org/ 10.1105/tpc.11.3.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woltering E. J, van der Bent A, Hoeberichts F. A. Do plant caspases exist? Plant Physiol 2002; 130:1764-9; PMID:12481059; http://dx.doi.org/ 10.1104/pp.006338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chichkova N. V, Shaw J, Galiullina R. A, Drury G. E, Tuzhikov A. I, Kim S. H, Kalkum M, Hong T. B, Gorshkova E. N, Torrance L, et al.. Phytaspase, a relocalisable cell death promoting plant protease with caspase specificity. EMBO J 2010; 29:1149-61; PMID:20111004; http://dx.doi.org/ 10.1038/emboj.2010.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanmartin M, Jaroszewski L, Raikhel N. V, Rojo E. Caspases. Regulating death since the origin of life. Plant Physiol 2005; 137:841-7; PMID:15761210; http://dx.doi.org/ 10.1104/pp.104.058552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niu N. N, Liang W. Q, Yang X. J, Jin W. L, Wilson Z. A, Hu J. P, Zhang D. EAT1 promotes tapetal cell death by regulating aspartic proteases during male reproductive development in rice. Nat Commun 2013; 4:1445; PMID:23385589; http://dx.doi.org/ 10.1038/ncomms2396 [DOI] [PubMed] [Google Scholar]
- 24.Breitenbach H. H, Wenig M, Wittek F, Jorda L, Maldonado-Alconada A. M, Sarioglu H, Colby T, Knappe C, Bichlmeier M, Pabst E, et al.. Contrasting Roles of the Apoplastic Aspartyl Protease APOPLASTIC, ENHANCED DISEASE SUSCEPTIBILITY1-DEPENDENT1 and LEGUME LECTIN-LIKE PROTEIN1 in Arabidopsis systemic acquired resistance. Plant Physiol 2014; 165:791-809; PMID:24755512; http://dx.doi.org/ 10.1104/pp.114.239665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponting C. P, Parker P. J. Extending the C2 domain family: C2s in PKCs delta, epsilon, eta, theta, phospholipases, GAPs, and perforin. Protein Sci 1996; 5:162-6; PMID:8771209; http://dx.doi.org/ 10.1002/pro.5560050120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odriozola L, Singh G, Hoang T, Chan A. M. Regulation of PTEN activity by its carboxyl-terminal autoinhibitory domain. J Biol Chem 2007; 282:23306-15; PMID:17565999; http://dx.doi.org/ 10.1074/jbc.M611240200 [DOI] [PubMed] [Google Scholar]
- 27.Kang C. H, Jung W. Y, Kang Y. H, Kim J. Y, Kim D. G, Jeong J. C, Baek D. W, Jin J. B, Lee J. Y, Kim M. O, et al.. AtBAG6, a novel calmodulin-binding protein, induces programmed cell death in yeast and plants. Cell Death Diff 2006; 13:84-95; PMID:16003391; http://dx.doi.org/ 10.1038/sj.cdd.4401712 [DOI] [PubMed] [Google Scholar]


