Abstract
Background
Is the timing of dosing for amlodipine and atorvastatin important with regard to therapeutic efficacy? To answer this question, we designed an outpatient, practice-based, case-control study lasting 8 weeks.
Material/Methods
Two hundred patients were divided into 2 groups: in Group I, patients were provided with a single pill containing amlodipine/atorvastatin (5/20 mg) to be taken each night at 10 pm, and in Group II, patients were taking amlodipine (5 mg) and atorvastatin (20 mg) each morning at 7 am.
Results
Our results indicated no obvious difference in blood pressure control between the 2 groups. Taking amlodipine at night not only lowered blood pressure, but it also provided better control during the peak blood pressure in the morning. Hypercholesterolemia control in the 2 groups was also not significantly different, taking atorvastatin in the morning was as effective as dosing at night in patients with hypercholesterolemia. While the carotid IMT, hs-CRP, and LVMI were significantly lower after treatment, no differences were found between the 2 groups. Although no obvious difference was found in adverse drug reactions between the 2 groups, compliance was much better in the single-pill group than in patients taking the 2 medications separately.
Conclusions
In conclusion, single-pill amlodipine-atorvastatin taken at night can lower blood pressure and reduce the morning peak blood pressure levels the next day. Additionally, this dosing method could improve patient adherence to the therapy.
MeSH Keywords: Amlodipine, Hypercholesterolemia, Hypertension
Background
According to the data from the 2002 National Hypertension Survey of China [1], the prevalence rate of hypertension in Chinese adults over 18 years of age is 18.8%, and estimates are that there are more than 200 million hypertensive people in China. This means that 1 in 10 adults have hypertension, and that approximately 1/5 of the total global population is hypertensive. However, the awareness, treatment, and control of hypertension in developing countries are still quite poor when compared with those of developed countries, especially in rural or remote areas. The death rate related to stroke in rural areas exceeds that of stroke deaths in cities. At present, approximately 130 million hypertensive people in China do not know they have hypertension, and there are also close to 3 million patients diagnosed with hypertension who are not receiving any treatment. Of the patients receiving antihypertensive therapy, 75% have not reached target blood pressure levels. Prevention and treatment of hypertension in China is still an arduous task.
At the same time, with the development of the social economy, the improvements in standard of living and changes in lifestyle have led to the gradual increase of the blood lipid levels of Chinese people and the prevalence of dyslipidemia. Elevated total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) are independent risk factors for coronary heart disease and ischemic stroke. Many studies have confirmed that patients with hypertension and primary hyperlipidemia are at high risk for experiencing cardiovascular events [2]. Therefore, these patients need to simultaneously control both hyperlipidemia and hypertension.
A common question with regard to treatment with medication is, “when is the best time to take the medication?” Blood pressure tends to be highest between 8:00 and 11:00 in the morning or 3:00 to 5:00 in the afternoon and may be too low at night. The onset of the effect of medication commonly occurs 30 min after taking it, and its efficacy peaks 2 to 3 h thereafter. If patients with hypertension often forget to take their medicine in the daytime and instead take their medication at night, the result may be low blood pressure. For the majority of patients, taking antihypertensive medications at 7:00 in the morning or at 2:00 in the afternoon is the most appropriate. Statins work via the inhibition of cholesterol synthesis by hydroxyl-methylglutaric-acyl coenzyme A (HMG-CoA) reductase, which mainly exists in the liver, with its typical circadian rhythm creating the highest levels at night and lower levels during the day. The best time for taking statins, therefore, may be at night. Statins also have demonstrated a capability to reduce the rate of cardiovascular events [3–6]. Data from the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) support the view that statins protect hypertensive patients from end-organ damage, not only through cholesterol reduction, but also through other pathways [7].
Given this background, there arose another question regarding the use of the fixed-dose combination of amlodipine and atorvastatin. The best time to take amlodipine is 7:00 am or 2:00 pm, while the best time for dosing atorvastatin may be at night. Therefore, what is the best time of day for taking the single-pill combination of amlodipine and atorvastatin? There are 3 questions that need to be answered: Is taking amlodipine at night safe?; Are the antihypertensive effects the same between day and night?; and Does taking atorvastatin in the daytime lead to better lipid-lowering effects?
Material and Methods
Ethics statement
The study was conducted with prior institutional ethics approval under the requirements of the Chinese Prevention of Cruelty to Human Subjects and the Code of Practice for the Care and Use of Human Subjects for Scientific Purposes. All human subjects of this study were inspected by members of the Human Subject Ethics Committee of West China Medical Centre (HSPC20120407-17468) and in compliance with the Helsinki Declaration.
Patients
Patients who were seen in the outpatient department of the West China Hospital from May 2012 to September 2014 and who were at least 18 years of age were eligible if they had the primary diagnoses of hypertension and hypercholesterolemia (TC >5.70 mmol/L). The diagnosis of hypertension was made following ambulatory blood pressure monitoring (ABPM) [8], with the following levels considered to be hypertensive: average blood pressure >130/80 mmHg, daytime blood pressure >135/85 mmHg or nocturnal blood pressure >125/75 mmHg. To ensure the safety of the single-pill therapy, the daytime blood pressure was required to be lower than 160/100 mmHg. Patients were excluded if they were previously treated with any lipid-lowering therapy or if they had a diagnosis of secondary hypertension, any cerebrovascular disease within the past 6 months, an acute myocardial infarction, congestive heart failure, congenital or rheumatic heart disease, autoimmune disease, severely abnormal liver or kidney values, severe trauma, infection, major surgery, or secondary hyperlipidemia caused by drug therapy. Before entering the study, all patients signed informed consent forms approved by the institutional review boards. A total of 200 patients were enrolled in the study.
Study design and conduct
The clinical trial was an outpatient, practice-based, case-control study lasting 8 weeks. To evaluate the efficacy and safety of single-pill combination of amlodipine and atorvastatin, 200 patients were divided into 2 groups. In Group I, patients were provided with single-pill amlodipine/atorvastatin (5/20 mg, Hisun-Pfizer Pharmaceutical, LTD, Tai Zhou, China, which is also the only dose could be used in China) at 10 pm every night. In Group II, patients were instructed to take the amlodipine tablet (5 mg, Pfizer Chinese Pharmaceutical, LTD., Beijing, China) every morning at 7 am. At the same time, atorvastatin (20 mg, Pfizer Chinese Pharmaceutical, LTD., Beijing, China) was also been given. The patients were allowed to take the necessary drugs for cardiovascular indications other than hypertension. All patients from Group I and II then returned for the final visit after 8 weeks.
Study procedures
At first visit, the average, daytime, nocturnal, and morning blood pressures were measured and recorded by ABPM. Other variables collected at this visit included age, sex, smoking status, serum lipid levels, presence of diabetes mellitus, and coronary heart disease (CHD). Other laboratory examination indexes, including high-sensitivity C-reaction protein (hs-CRP), left ventricular mass index (LVMI), carotid intima-media thickness (IMT), and concurrent medications, were also recorded. At the second visit, BP measurements by ABPM, serum lipid levels, hs-CRP, LVMI, and IMT were repeated. Other variables collected at this visit included the occurrence of adverse drug reactions (ADRs) and the number of pills missing from their prescription bottle (see flow program below in Figure 1).
Figure 1.
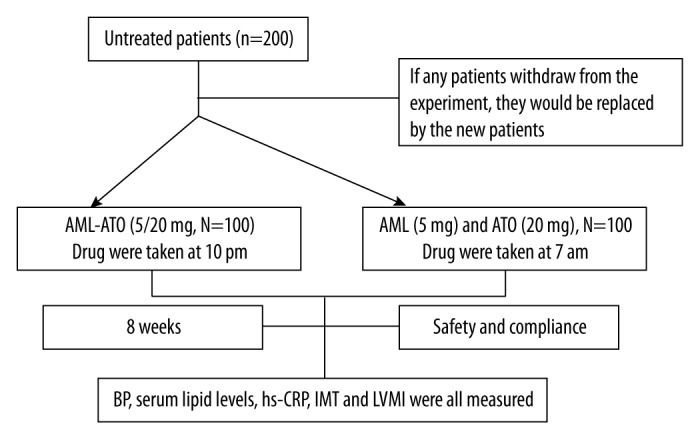
Flow diagram of this research.
AML – Amlodipine; ATO – Atorvastatin.
Measurement of laboratory examination index before and after the experiment
Serum lipid levels were measured by fasting blood samples, which were collected in the early morning. Total cholesterol (TC) and triglycerides (TG), as well as low- and high-density lipoprotein cholesterol (LDL-C and HDL-C) were all included and analyzed by routine enzymatic assays. hs-CRP was also determined by the same blood samples with double-antibody avidin-biotin complex-ELISA (ABC ELISA) method.
Carotid IMT was detected by the same highly qualified doctor in our hospital. For bilateral carotid artery detection, we used the IE 33 type ultrasonic diagnosis instrument, produced by PHILIPS Company, with probe frequency 7. 0 MHz. We put the probe at the distal carotid artery, 1–1.5 cm below the fork level, avoiding the plaques in accordance with the synchronization ECG in end-diastolic, measured 5 times on every side, and the average index was recorded as the measured value of carotid IMT.
LVMI was inspected by using the HP 5500 color Doppler ultrasonic diagnostic instrument, with probe frequency 3.5 MHz. We measured left ventricular end-diastolic ventricular septal thickness (IVST), left ventricular posterior wall thickness (PWT), left ventricular end-diastolic diameter (LVIDd), and left ventricular mass (LVM), according to the formula of Devereux: the LVM (g) = 1.04 × [(IVST + LVIDd + PWT)3 − (LVIDd)3] − 13.6. LVMI = LVM/body surface area (S), S=0.0061×Height (cm) + 0.0128 × Weight (kg) − 0.1529.
Data analyses
All statistical tests were two-tailed, and all analyses were considered statistically significant if p<0.05. Baseline demographic and clinical characteristics, as well as efficacy variables, were compared between Group I and Group II using the paired t test.
Results
Baseline demographics of patients
The baseline demographic characteristics for the 200 patients included in this analysis are shown in Table 1. In Group I, 52% of the patients were male and 48% were female. The average age was 53.1 years, and the overall average, daytime, nocturnal, and morning blood pressures were 143.5/86.2 mmHg, 155.2/96.8 mmHg, 130.6/75.3 mm Hg, and 157.3/98.6 mmHg, respectively. The mean baseline serum lipid levels were: TG (2.02 mmol/L), TC (5.99 mmol/L), LDL-C (3.96 mmol/L), and HDL-C (1.19 mmol/L). The frequency of the other risk factors included smoking (40%), diabetes mellitus (42%), and CHD (37%). In Group II, 51% of the patients were male and 49% were female. The average age of the patients was 52.7 years, and the average overall, daytime, nocturnal, and morning blood pressures were 145.7/85.1 mmHg, 156.5/94.7 mmHg, 133.0/76.2 mmHg, and 158.0/97.9 mmHg, respectively. The mean baseline serum lipid levels were: TG (1.98 mmol/L), TC (6.01 mmol/L), LDL-C (4.11 mmol/L), and HDL-C (1.22 mmol/L). The frequency of the other risk factors included smoking (38%), diabetes mellitus (48%), and CHD (32%).
Table 1.
Baseline demographics of patients.
| Group I | Group II | p-value | |||
|---|---|---|---|---|---|
| Characteristics | Sex (number) | Male (52) Female (48) |
Male (51) Female (49) |
||
| Age, years, mean ±SD | 53.1±9.7 | 52.7±10.6 | p=0.486 | ||
| Blood pressure | Average, mmHg (mean ±SD) | SBP | 143.5±11.8 | 145.7±10.9 | p=0.541 |
| DBP | 86.2±7.2 | 85.1±8.6 | p=0.379 | ||
| Daytime (mmHg) | SBP | 155.2±10.7 | 156.5±11.8 | p=0.492 | |
| DBP | 96.8±8.1 | 94.7±7.7 | p=0.358 | ||
| Nocturnal (mmHg) | SBP | 130.6±12.4 | 133.0±11.7 | p=0.621 | |
| DBP | 75.3± 6.8 | 76.2±7.1 | p=0.577 | ||
| Morning (mmHg) | SBP | 157.3±11.6 | 158.0±10.4 | p=0.396 | |
| DBP | 98.6±7.3 | 97.9±7.5 | p=0.439 | ||
| Serum lipid levels | TC (mmol/L) | 2.02±1.34 | 1.98±1.72 | p=0.386 | |
| TG (mmol/L) | 5.99±1.62 | 6.01±1.36 | p=0.692 | ||
| LDL-C (mmol/L) | 3.96±1.45 | 4.11±1.54 | p=0.414 | ||
| HDL-C (mmol/L) | 1.19±0.49 | 1.22±0.55 | p=0.583 | ||
| Other risk factors | Smokers, number | 40 | 38 | ||
| Diabetes mellitus, number | 42 | 48 | |||
| CHD, number | 37 | 32 |
Changes in blood pressure in Group I and Group II
In Group I, the overall average, daytime, nocturnal, and morning peak blood pressures from baseline to week 8 declined from 143.5/86.2 mmHg, 155.2/96.8 mmHg, 130.6/75.3 mmHg, and 157.3/98.6 mmHg to 129.4/75.9 mmHg, 139.7/85.4 mmHg, 117.9/66.2 mmHg, and 139.5/85.2 mmHg, respectively. In Group II, the overall average, daytime, nocturnal, and morning peak blood pressures from baseline to week 8 declined from 145.7/85.1 mmHg, 156.5/94.7 mmHg, 133.0/76.2 mmHg, and 158.0/97.9 mmHg to 131.2/74.4 mmHg, 138.9/82.1 mmHg, 121.6/68.4 mmHg, and 149.9/92.7 mmHg, respectively. The BP reduction from baseline at week 8 was statistically significant in both groups (p<0.001), but there was no statistically significant change in the D-value except for in the morning peak group. In Group I, the morning peak BP reduction in SBP and DBP at week 8 was markedly less than in Group II (see Table 2, Figure 2).
Table 2.
Comparison of blood pressure before and after treatment in both groups.
| Baseline | Change to 8 weeks | D-value | P-value | |||
|---|---|---|---|---|---|---|
| Group I | Average (mmHg) | SBP | 143.5±11.8 | 129.4±12.1 | 14.1±4.6 | 0.0006 |
| DBP | 86.2±7.2 | 75.9±7.7 | 10.3±3.3 | 0.0010 | ||
| Daytime (mmHg) | SBP | 155.2±10.7 | 139.7±12.3 | 15.5±5.1 | 0.0018 | |
| DBP | 96.8±8.1 | 85.4±8.8 | 11.4±2.7 | 0.0005 | ||
| Nocturnal (mmHg) | SBP | 130.6±12.4 | 117.9±11.6 | 12.7±4.4 | 0.0016 | |
| DBP | 75.3±6.8 | 66.2±7.0 | 9.1±2.9 | 0.0004 | ||
| Morning (mmHg) | SBP | 157.3±11.6 | 139.5±12.1 | 17.8±4.1* | 0.0003 | |
| DBP | 98.6±7.3 | 85.2±6.7 | 13.4±3.6# | 0.0006 | ||
| Group II | Average (mmHg) | SBP | 145.7±10.9 | 131.2±11.5 | 14.5±4.7 | 0.0025 |
| DBP | 85.1±8.6 | 74.4±8.8 | 10.7±3.1 | 0.0004 | ||
| Daytime (mmHg) | SBP | 156.5±11.8 | 138.9±10.8 | 17.6±4.2 | 0.0015 | |
| DBP | 94.7±7.7 | 82.1±8.3 | 12.6±3.0 | 0.0008 | ||
| Nocturnal (mmHg) | SBP | 133.0±11.7 | 121.6±10.2 | 11.4±4.6 | 0.0002 | |
| DBP | 76.2±7.1 | 68.4±6.9 | 8.8±2.7 | 0.0008 | ||
| Morning(mmHg) | SBP | 158.0±13.4 | 149.9±11.8 | 9.1±2.3 | 0.0170 | |
| DBP | 97.9±7.5 | 92.7±6.6 | 5.2±1.9 | 0.0104 |
Compared with Group II, P-value=0.0003;
Compared with Group II, P-value=0.0001.
Figure 2.
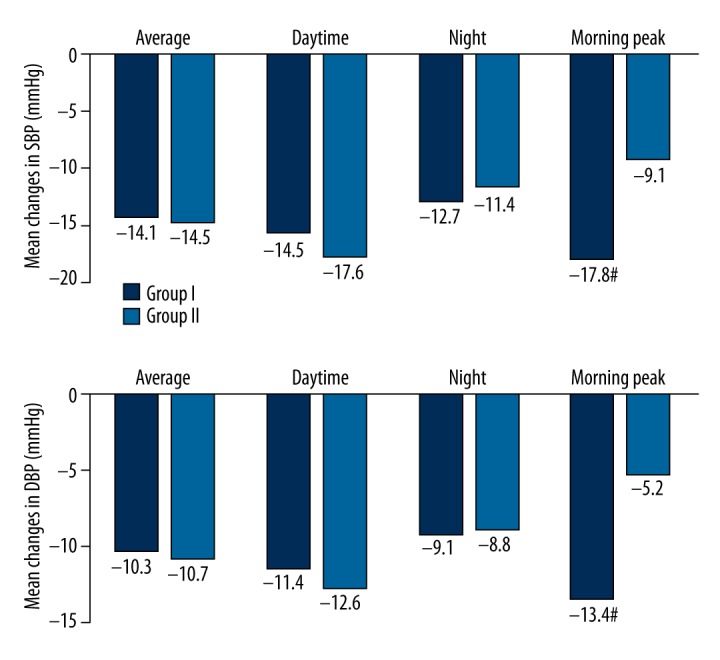
Comparison of blood pressure before and after treatment in both groups. There was no statistically significant change in D-value except in the morning peak group. In Group I, the morning peak BP reduction in SBP and DBP at week 8 was markedly less than in Group II (P<0.05).
Changes in serum lipid levels, hs-CRP, LVMI, and carotid-IMT
Serum concentrations of TC, TG, and LDL-C were significantly lower after 8-week treatment (P<0.01), but HDL-C was increased (P<0.05) in both groups. However, there was no significant difference in D-value (P>0.05) after 8-week treatment compared with index before (see Table 3, Figure 3). Also, there were no significant differences between before and after 8-week treatment in either group with regard to the hs-CRP, LVMI, and carotid -IMT (P>0.05) (see Table 4, Figure 4).
Table 3.
Comparison of serum lipid levels before and after treatment in both groups.
| Baseline | Change to 8 weeks | D-value | P-value | ||
|---|---|---|---|---|---|
| Group I | TC (mmol/L) | 5.99±1.62 | 4.23±1.18 | 1.76±0.95 | 0.0003 |
| TG (mmol/L) | 2.02±1.34 | 1.32±0.61 | 0.70±0.82 | 0.0006 | |
| LDL-C (mmol/L) | 3.96±1.45 | 2.72±1.77 | 1.24±0.69 | 0.0004 | |
| HDL-C (mmol/L) | 1.19±0.49 | 1.54±0.78 | 0.35±0.47 | 0.0017 | |
| Group II | TC (mmol/L) | 6.01±1.26 | 4.13±1.33 | 1.88±1.12 | 0.0003 |
| TG (mmol/L) | 1.98±1.72 | 1.36±1.21 | 0.62±0.99 | 0.0007 | |
| LDL-C (mmol/L) | 4.11±1.54 | 2.95±1.05 | 1.16±0.76 | 0.0004 | |
| HDL-C (mmol/L) | 1.22±0.55 | 1.52±0.78 | 0.30±0.84 | 0.0018 |
Figure 3.
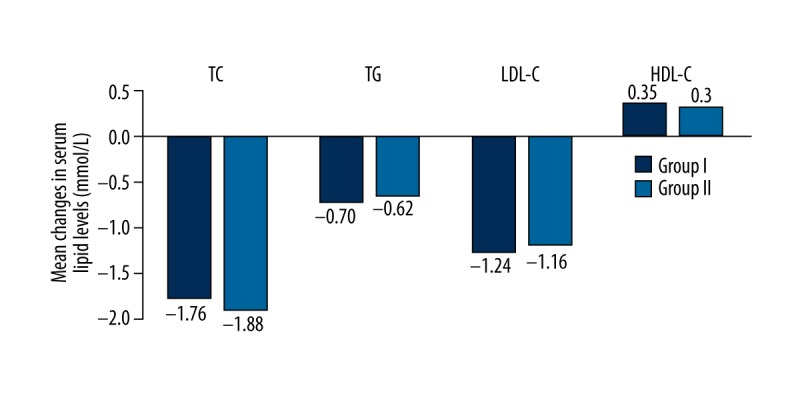
Comparison of serum lipid levels before and after treatment in both groups. There were no significant differences in the D-values of TC, TG, and LDL-C or HDL-C before and after 8-week treatment (P>0.05).
Table 4.
Comparison of serum hs-CRP, LVMI, and carotid IMT before and after treatment.
| Baseline | Change to 8 weeks | D-value | P-value | ||
|---|---|---|---|---|---|
| Group I | hs-CRP (mg/L) | 4.15±1.08 | 3.22±1.46 | 0.93±0.66 | 0.0005 |
| IMT (mm) | 1.07±0.39 | 0.82±0.25 | 0.25±0.14 | 0.0009 | |
| LVMI (g/m2) | 124.83±36.77 | 111.46±37.08 | 13.37±9.85 | 0.0061 | |
| Group II | hs-CRP (mg/L) | 3.99±1.77 | 3.04±1.38 | 0.95±0.72 | 0.0005 |
| IMT(mm) | 1.11±0.21 | 0.88±0.68 | 0.23±0.11 | 0.0011 | |
| LVMI (g/m2) | 121.65±35.19 | 108.07±36.92 | 13.58±8.71 | 0.0059 |
Figure 4.
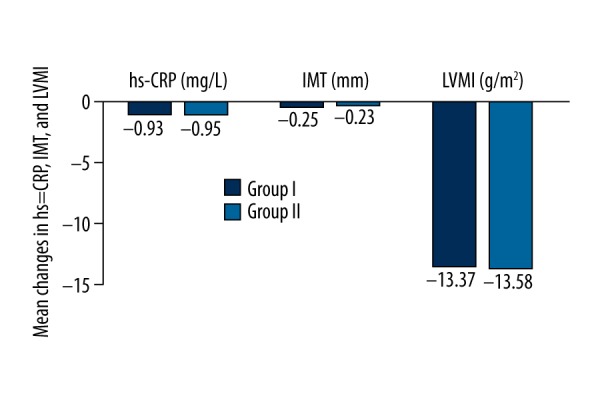
Comparison of hs-CRP, LVMI, and carotid -IMT before and after treatment in both groups. There were no significant differences in the D-values of hs-CRP, LVMI, and IMT before and after 8-week treatment (P>0.05).
Safety and compliance
The overall percent of patients with adverse events was 10% (20/200). Ankle edema, the most frequent adverse event, was experienced by 7% (7/100) in Group I and 8% (8/100) in Group II. The incidence of headache was 1% (1/100) in Group I and 2% (2/100) in Group II. Facial flushing (1% in Group II) and palpitations (1% in Group I) had the lowest incidences. No serious adverse drug reactions were found in our research. The number of pills missing was much higher in Group II (Once: 29; Twice: 16; 3 times: 10; More than 3 times: 7) than in Group I (Once: 7; Twice: 5; 3 times: 2; More than 3 times: 1).
Discussion
To address whether the timing of dosing for amlodipine and atorvastatin is important with regard to therapeutic efficacy, our results showed no obvious differences between the different dosing times for amlodipine-atorvastatin in blood pressure control between the 2 groups. Taking amlodipine at night not only lowered blood pressure, but it also provided better control during the peak blood pressures in the morning. Hypercholesterolemia control in the 2 groups was also not significantly different; taking atorvastatin in the morning was equally as effective as taking it at night in patients with hypercholesterolemia. Although no obvious difference was found in adverse drug reactions between the 2 groups, compliance was much better in the single-pill group than in those patients taking the 2 drugs separately.
Hypertension is one of the most important causes of and risk factors for a variety of cardiovascular and cerebrovascular diseases, and also affects important organs such as the structure and function of the heart, brain, and kidneys, eventually leading to organ failure. In recent years, studies have determined that there is a close relationship between endothelial dysfunction and hypertension. Hypertension is regarded as one of the initiating factors of endothelial injury [9,10]. Impaired endothelial function could promote the occurrence and development of atherosclerosis. Carotid IMT could be used to evaluate early atherosclerosis. A prospective follow-up study has confirmed that carotid IMT can strongly and independently forecast heart or cerebrovascular disease, with the increase of carotid IMT corresponding to an increase in the incidence of myocardial infarction and stroke [11]. Leiboritz [12] has confirmed that atorvastatin can improve the small artery elasticity of patients with hyperlipidemia and reduce the diastolic and systolic blood pressures. The PREVENT study observed that the carotid IMT in the amlodipine group was reduced by 0.013 mm on average, while it increased by an average of 0.033 mm in the placebo group; this difference was statistically significant [11]. Amlodipine is a long-acting calcium antagonist with powerful, stable, and long-lasting effects on hypertension; it promotes arterial endothelial function, may effectively improve arterial elasticity, and reduces carotid IMT [13,14], thus inhibiting the progression of atherosclerosis. The results of our study corroborated these findings. Compared with before treatment, carotid IMT was significantly lower after treatment, but no difference was found between the 2 groups.
Hypertension and its related complications are often attended by vascular epithelial damnification and other pathological changes. The effect of inflammation may be related to its pathological role. As one of the important inflammatory markers, serum hs-CRP is considered to participate in the process of left ventricular hypertrophy and hypertension [15. During the treatment of patients with hypertension, we need not only effectively control blood pressure, but also must monitor and improve endothelial inflammation and left ventricular remodeling. The effect of the oxidative stress induced by endothelial dysfunction can destroy the function of nitric oxide. Many studies have proven that combination therapy can effectively stimulate the endothelial cells to release nitric oxide and maintain normal vascular function. Statins can reduce the hardening of the arteries, improve arterial compliance, and reverse the remodeling of the aorta, which could reverse the left ventricular hypertrophy caused by changes in arterial structure and function [16–18]. In addition, atorvastatin can reduce the levels of serum CRP and other inflammatory markers [19], reduce inflammation, and improve endothelial function [20]. Atorvastatin can inhibit hypertrophy and cell proliferation by reducing angiotensin I [21,22] and improve left ventricular hypertrophy. Our experiment achieved similar results: compared to before treatment, the hs-CRP and LVMI were significantly lower after treatment, but no significant difference was found between the 2 groups.
There is another problem which deserves attention: The blood pressure was decreased significantly but not normalized in some patients by using amlodipine 5 mg per day, which indicated that the daily dose of 5 mg of amlodipine was probably too low for these patients. For solving this problem, we designed and conducted Part II of this experiment, in which all patients in Part I were divided into 4 groups and different doses of SPC were been given to these patients. This clinic research may provide answers allowing us to know how to correct and reasonably use the SPC of amlodipine-atorvastatin.
Conclusions
Taking the single-pill amlodipine-atorvastatin combination at night lowered blood pressure and reduced the morning peak blood pressure levels on the second day. This dosing strategy may also improve patient adherence to therapy. In combination with amlodipine, atorvastatin reduced systolic blood pressure, inhibited cardiac hypertrophy in left ventricle and inflammation, then the whole progress of left ventricular remodeling during hypertension could be affected. All suggests that this combination could be a better choice for hypertensive patients with primary hypercholesterolemia.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
Source of support: This study was funded by the National Natural Science Foundation of China (No. 81200153), the Science Foundation of Health Department, Sichuan Province (No. 20120213), the Science Foundation of Science and Technology Department, Sichuan Province (No. 2015SZ0180), and the Science Foundation of Science and Technology Department, Sichuan Province (No. 2014JY0204)
References
- 1.Liu LS Writing Group of Chinese Guidelines for the Management of H. [2010 Chinese guidelines for the management of hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39:579–615. [in Chinese] [PubMed] [Google Scholar]
- 2.Glorioso N, Troffa C, Filigheddu F, et al. Effect of the HMG-CoA reductase inhibitors on blood pressure in patients with essential hypertension and primary hypercholesterolemia. Hypertension. 1999;34:1281–86. doi: 10.1161/01.hyp.34.6.1281. [DOI] [PubMed] [Google Scholar]
- 3.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 4.Byington RP, Davis BR, Plehn JF, et al. Reduction of stroke events with pravastatin: the Prospective Pravastatin Pooling (PPP) Project. Circulation. 2001;103:387–92. doi: 10.1161/01.cir.103.3.387. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GG, Olsson AG, Ezekowitz MD, et al. Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering Study I. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: The MIRACL study: A randomized controlled trial. JAMA. 2001;285:1711–18. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 6.Yu JM, Kong QY, Schoenhagen P, et al. The prognostic value of long-term visit-to-visit blood pressure variability on stroke in real-world practice: A dynamic cohort study in a large representative sample of Chinese hypertensive population. Int J Cardiol. 2014;177:995–1000. doi: 10.1016/j.ijcard.2014.09.149. [DOI] [PubMed] [Google Scholar]
- 7.Sever PS, Dahlof B, Poulter NR, et al. ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): A multicentre randomised controlled trial. Lancet. 2003;361:1149–58. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 8.Mancia G, Laurent S, Agabiti-Rosei E, et al. European Society of Hypertension. Reappraisal of European guidelines on hypertension management: A European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–58. doi: 10.1097/HJH.0b013e328333146d. [DOI] [PubMed] [Google Scholar]
- 9.Spencer CG, Martin SC, Felmeden DC, et al. Relationship of homocysteine to markers of platelet and endothelial activation in “high risk” hypertensives: A substudy of the Anglo-Scandinavian Cardiac Outcomes Trial. Int J Cardiol. 2004;94:293–300. doi: 10.1016/j.ijcard.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Hu CS, Han YL, Ge JB, et al. A novel management program for hypertension. Cardiovasc Diagn Ther. 2015;5:316–22. doi: 10.3978/j.issn.2223-3652.2015.05.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitt B, Byington RP, Furberg CD, et al. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. PREVENT Investigators. Circulation. 2000;102:1503–10. doi: 10.1161/01.cir.102.13.1503. [DOI] [PubMed] [Google Scholar]
- 12.Leibovitz E, Hazanov N, Zimlichman R, et al. Treatment with atorvastatin improves small artery compliance in patients with severe hypercholesterolemia. Am J Hypertens. 2001;14:1096–98. doi: 10.1016/s0895-7061(01)02210-5. [DOI] [PubMed] [Google Scholar]
- 13.Messerli FH, Bakris GL, Ferrera D, et al. Efficacy and safety of coadministered amlodipine and atorvastatin in patients with hypertension and dyslipidemia: Results of the AVALON trial. J Clin Hypertens. 2006;8:571–81. doi: 10.1111/j.1524-6175.2006.05636.x. quiz 582–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda H, Minamikawa J, Nakamura Y, et al. Comparison of effects of amlodipine and angiotensin receptor blockers on the intima-media thickness of carotid arterial wall (AAA study: amlodipine vs. ARB in atherosclerosis study) Diabetes Res Clin Pract. 2009;83:50–53. doi: 10.1016/j.diabres.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Liao JK. Endothelium and acute coronary syndromes. Clin Chem. 1998;44:1799–808. [PubMed] [Google Scholar]
- 16.Matsuo T, Iwade K, Hirata N, et al. Improvement of arterial stiffness by the antioxidant and anti-inflammatory effects of short-term statin therapy in patients with hypercholesterolemia. Heart Vessels. 2005;20:8–12. doi: 10.1007/s00380-004-0793-5. [DOI] [PubMed] [Google Scholar]
- 17.Leibovitz E, Beniashvili M, Zimlichman R, et al. Treatment with amlodipine and atorvastatin have additive effect in improvement of arterial compliance in hypertensive hyperlipidemic patients. Am J Hypertens. 2003;16:715–18. doi: 10.1016/s0895-7061(03)00949-x. [DOI] [PubMed] [Google Scholar]
- 18.Ge CJ, Hu SJ, Wu YS, Chen NY. Effects of atorvastatin on vascular remodeling in spontaneously hypertensive rats. J Zhejiang Univ Sci. 2003;4:612–15. doi: 10.1631/jzus.2003.0612. [DOI] [PubMed] [Google Scholar]
- 19.Fogari R, Preti P, Zoppi A, et al. Effects of amlodipine-atorvastatin combination on inflammation markers and insulin sensitivity in normocholesterolemic obese hypertensive patients. Eur J Clin Pharmacol. 2006;62:817–22. doi: 10.1007/s00228-006-0176-1. [DOI] [PubMed] [Google Scholar]
- 20.Sugiyama M, Ohashi M, Takase H, et al. Effects of atorvastatin on inflammation and oxidative stress. Heart Vessels. 2005;20:133–36. doi: 10.1007/s00380-005-0833-9. [DOI] [PubMed] [Google Scholar]
- 21.Wassmann S, Laufs U, Baumer AT, et al. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–57. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- 22.Nickenig G, Baumer AT, Temur Y, et al. Statin-sensitive dysregulated AT1 receptor function and density in hypercholesterolemic men. Circulation. 1999;100:2131–34. doi: 10.1161/01.cir.100.21.2131. [DOI] [PubMed] [Google Scholar]


