Abstract
Background
Vasa (a DEAD-box helicase, also known as Ddx4) is an ATP-dependent RNA helicase highly conserved among all animals. Research on the presence and function of DDX4 in female mammals is limited. To gain greater insight into its distribution and role in female mice, we detected the expression of DDX4 protein in the ovaries and analyzed its expression pattern.
Material/Methods
MVH was detected in the cytoplasm of oocytes in all non-apoptotic follicles.
Results
In the present study, we found that higher expression levels of ~55–60 kDa MVH isoform in the ovaries were followed by the accumulations of preovulatory follicles.
Conclusions
Higher levels of MVH protein in the ovaries might prepare oocytes for the competence to resume meiosis.
MeSH Keywords: Mice, Inbred C57BL; Ovarian Follicle; RecQ Helicases
Background
Vasa (also known as Ddx4) is an ATP-dependent RNA helicase, which is a fundamental member of the DEAD-box protein family [1]. Vasa was first identified in Drosophila and was soon shown to be critical for the process of primordial germ cell specification and embryonic posterior patterning [2,3]. Its expression was subsequently characterized in a wide range of species and revealed to be highly conserved among various organisms [4]. Because of its definite function in germ lineage development in several insects and vertebrates [5], Vasa has long and widely been considered as a reliable germline marker. While at the same time, data also demonstrated its expression in multipotent somatic cell types and its potential function in regeneration activities beyond germline development [6]. Planarian worms are a valuable model for the study of somatic stem cell biology because of the existence of a population of proliferative somatic cells called neoblasts. Vasa mRNA is localized to the chromatoid bodies in neoblasts [7] and RNA interference leads to defects in cell differentiation [8]. In the polychaete annelid, Vasa is expressed in the highly proliferative stem cells – progenitor mesodermal posterior growth zone cells [9].
Researches on the expression patterns and underlying mechanisms of Vasa mostly focused on non-mammals, and in mammals it mostly focused on males. Although mouse Vasa homologue (MVH) mutant female mice are totally fertile [10], few studies have examined how MVH protein behaves in female mice, and the very few studies were involved in the very controversial hypothesis of postnatal oogenesis in female mammals. A study in 2005 detected a series of germline markers including MVH in the bone marrow of C57BL/6 female mice and presumed that the bone marrow might be a potential source of postnatal oogenesis [11]. The hypothesis of postnatal oogenesis in mammals is so contentious that all related research focuses on whether it exists or not. No one has reexamined the presence of Vasa in the somatic cells (the bone marrow cells), nor explored its potential functions.
Vasa has long and widely been considered to be specifically expressed in germ cell lineage, so we are concerned with the presence and role of MVH in somatic cells – the bone marrow cells of C57BL/6 female mice. In our study, we confirmed the presence of MVH in bone marrow. Because Vasa is a widely used germ line marker, its role in reproduction would be our first thought. However, MVH mutant female mice showed no difference in reproduction vs. wild types. Therefore, we aimed to find out whether MVH in bone marrow acted in germ line context based on the investigation of its expressional behavior. We analyzed its expression patterns in bone marrow of C57BL/6 female mice in different stages of estrous cycle, different ages, and different number of days after cyclophosphamide treatment. The ovarian expression patterns were set as comparisons.
Vasa is a germline marker. However, emerging data show its potential functions in somatic multipotent cell types. We would like to draw a relatively detailed picture of MVH in bone marrow of C57BL/6 female mice, and thus to provide more evidence for further research about the role of Vasa in somatic cells.
Material and Methods
Sources of material
All experimental procedures were performed in accordance with the Institutional Animal Care and Use Committee at Tongji Medical Collage, Huazhong University of Science and Technology ([2015] IACUC Number: 431). Female C57Bl/6 mice aged 3, 12, and 48 weeks were obtained from Hubei Research Center of Laboratory Animals. Vaginal smears of 12-week-old and 48-week-old mice were checked daily to confirm the stage of the estrous cycle.
Western blot analysis
Total proteins were extracted from homogenized ovaries using RIPA lysis buffer (Guge Biotechnology Co. Ltd., Wuhan, China), containing 1/50 protease inhibitor cocktail. Protein concentration was determined by BCA protein Assay Kit (Guge Biotechnology Co. Ltd., Wuhan, China). Equal amounts of proteins were separated by 10% SDS-PAGE gel electrophoresis after boiling for 5–10 min and then transferred onto polyvinylidene fluoride membranes. The membranes were blocked with 5% skimmed milk powder in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 h at RT, followed by an overnight incubation at 4°C with the primary antibody (Anti-MVH, rabbit, 1:1000, Cell Signaling Technology, #8761; Anti-β-actin, rabbit, 1:1000, Guge Biotechnology Co. Ltd., Wuhan, China) diluted in 5% skimmed milk powder in TBST. After washing 3 times in TBST for 10 min, the membranes were incubated with horseradish peroxidase-linked anti-rabbit secondary antibodies (1:3000, Guge Biotechnology Co. Ltd., Wuhan, China) for 30 min at RT. Membranes were washed 3 times in TBST for 5 min and detected by enhanced chemoluminescence (ECL) (Guge Biotechnology Co. Ltd., Wuhan, China). Images were analyzed by AlphaEaseFC software version 4.0.
Ovary preparation and follicular morphology
Ovaries collected for follicular morphology analysis were fixed in 4% paraformaldehyde for 12–24 h at 4°C and then embedded in paraffin wax for sectioning. Three-micrometer step sections were mounted at 60-μm intervals onto slides to avoid double counting of follicles, after which slides were stained with hematoxylin and eosin using standard methods for follicular histology. Only follicles in which the nucleus of the oocyte was identified were scored. A primordial follicle was counted when the oocyte was encapsulated by a single layer of flattened pregranulosa cells. A primary follicle was defined as an oocyte encapsulated by a single layer of cubic granulosa cells. A secondary follicle consisted of 2 or more layers of cubic granulosa cells, but no antrum. An antral follicle was defined when the oocyte was surrounded by more than 2 layers of cubic granulosa cells with an antrum forming.
Immunofluorescence
Tissue sections were dewaxed and rehydrated with a graded series of alcohols using standard methods. Before staining, sections were immersed in ethylene diamine tetraacetic acid buffer (PH8.0, Guge Biotechnology Co. Ltd., Wuhan, China) in a microwave oven to accomplish antigen retrieval (medium heat for 8 min and 7 min with an interval of 8 min) and then allowed to cool. After washing 3 times in PBS for 5 min, tissue sections were blocked in 3% bovine serum albumin (BSA) in PBS for 30 min at RT, followed by an overnight incubation at 4°C with a primary antibody (Anti-MVH, rabbit, 1:50, Cell Signaling Technology, #8761). Negative control samples were treated with solutions without the primary antibody. After 3 washes, sections were subsequently treated with a secondary antibody (anti-rabbit, 1:400, Guge Biotechnology Co. Ltd., Wuhan, China) for 50 min at RT. Cell nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI) for 10 min. Sections were washed thereafter, air dried, mounted with antifade mounting medium (Guge Biotechnology Co. Ltd., Wuhan, China), and covered with cover slips. Images were obtained using a fluorescence microscope (NIKON ECLIPSE TI-SR).
Statistical analysis
Data are presented as mean values ± SEM of at least 4 independent experiments. One-way ANOVA with post hoc tests was used for multiple comparisons. SPSS software version 17.0 was used for statistical analysis. The statistical significance was considered as P<0.05.
Results
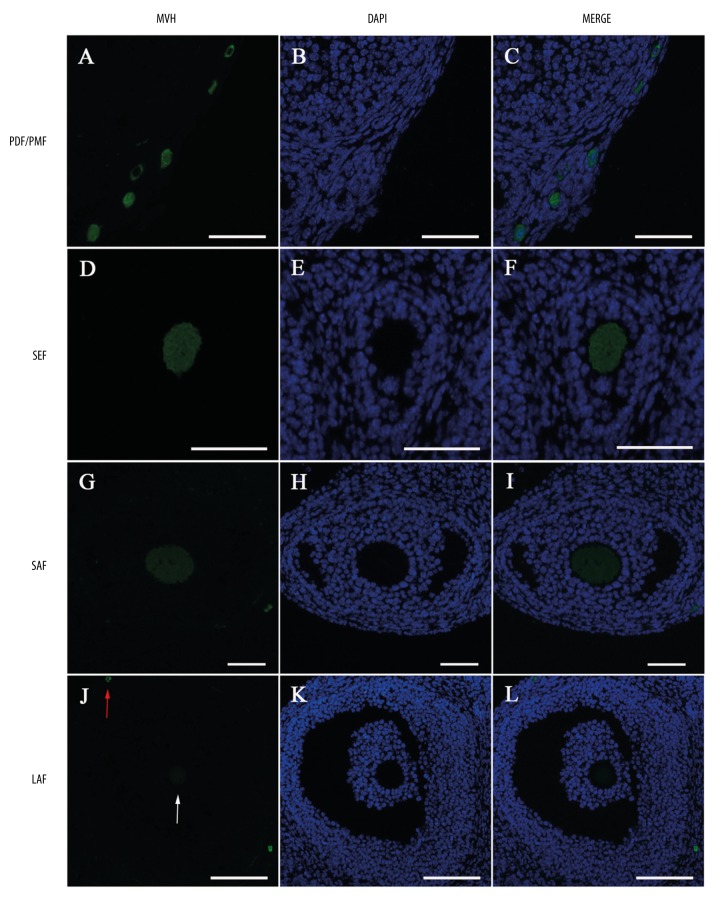
MVH was detected in the cytoplasm of oocytes in all non-apoptotic follicles: primordial and primary follicles (Figure 1A–1C), secondary follicles (Figure 1D–1F), small antral follicles (Figure 1G–1I), and large antral follicles (Figure 1J–1L). The expression intensity of MVH in the ovary appeared to decrease with follicular development. A relatively strong expression was detected in primordial, primary, and secondary follicles (Figure 1A–1F), while in antral follicles, the fluorescent intensity obviously undergone progressive decline (Figire 1G–1L). An extremely weak MVH expression was detected in large antral follicles (Figure 1J–1L). A weak fluorescent intensity in the cytoplasm of the oocyte in a large antral follicle compared with a strong one in a primordial follicle.
Figure 1.
Localization of MVH protein in the ovary by immunofluorescence. Green: Anti-MVH staining. Blue: DAPI-stained nuclei. MVH is expressed in the cytoplasm of oocytes in (A–C) primordial follicles (PDF) and primary follicles (PMF), (D–F) secondary follicles (SEF), (G–I) small antral follicles (SAF), and (J–L) large antral follicles (LAF). Arrows in (J) display a very weak MVH expression in large antral follicles (white arrow) compared with a strong one in primordial follicles (red arrow). Scale bars in (A–I): 50 μm, in (J–L): 100 μm.
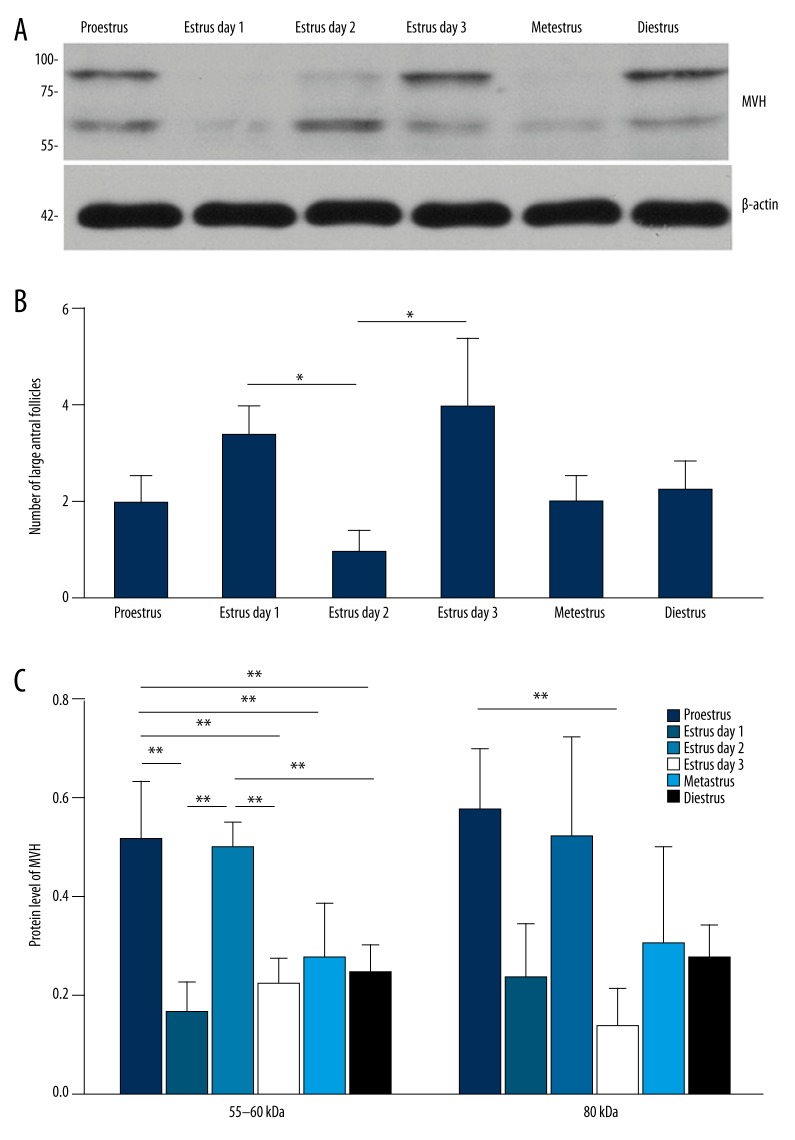
The ovarian expression pattern was also analyzed to make a comparison. Its expression in the ovary exhibited a pattern, especially the ~55–60 kDa isoform: MVH expression level was higher in proestrus and on the 2nd day of estrus than in metestrus, diestrus, and on the 1st and 3rd day of estrus (Figure 2A). Although the ~80 kDa isoform did not show such an obvious expression pattern, there was still a significant difference between its expression in proestrus and on the 3rd day of estrus (Figure 2B).
Figure 2.
Expression pattern of MVH protein in different stages of estrous cycle. (A) Western blot for MVH in the bone marrow in different stages of estrous cycle. N=4 each. (B) Western blot for MVH in the ovary in different stages of estrous cycle. N=6 each. The numbers on the left in (A, B) are the size standards of molecular weight (in kDa). The expression level of MVH in (A-B) was normalized to that of β-actin. (C) Analysis of follicular counts of large antral follicles. Data in (A–C) are shown in mean values ±SEM. The statistical significance is shown as * P<0.05; ** P<0.01.
The higher expression levels of ~55–60 kDa MVH isoform in the ovary were followed by the accumulations of preovulatory follicles
We analyzed the number of developing follicles of each stage, and found that compared with the 1st day of estrus, the number of large antral follicles (preovulatory follicles) decreased on the 2nd day, and increased again on the 3rd day (Figure 2C), indicating 2 concentrated ovulatory activities on the 1st and 3rd day of estrus in female C57BL/6 mice. Notably, ~55–60 kDa MVH protein level was higher in proestrus and on the 2nd day of estrus (Figure 2B), followed by the accumulations of preovulatory follicles on the 1st and 3rd day of estrus.
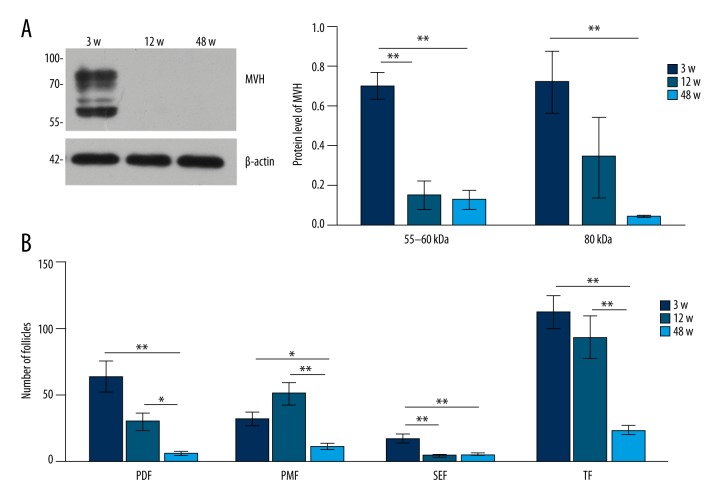
Compared with 3-week-old mice (Figure 3A), the expression level of MVH in the ovary decreased in 12- and 48-week-old mice (Figure 3B). The expression level of MVH in (Figure 4A, 4B) was normalized to that of β-actin. Analysis of follicular counts in different stages (Figure 4C).
Figure 3.
Expression pattern of MVH protein in 3-, 12-, and 48-week-old female mice. Western blot for MVH in the bone marrow in 3-, 12-, and 48-week-old female mice. N=10 each. (B) Western blot for MVH in the ovary in 3-, 12-, and 48-week-old female mice. N=4 each. The numbers on the left in (A, B) are the size standards of molecular weight (in kDa).
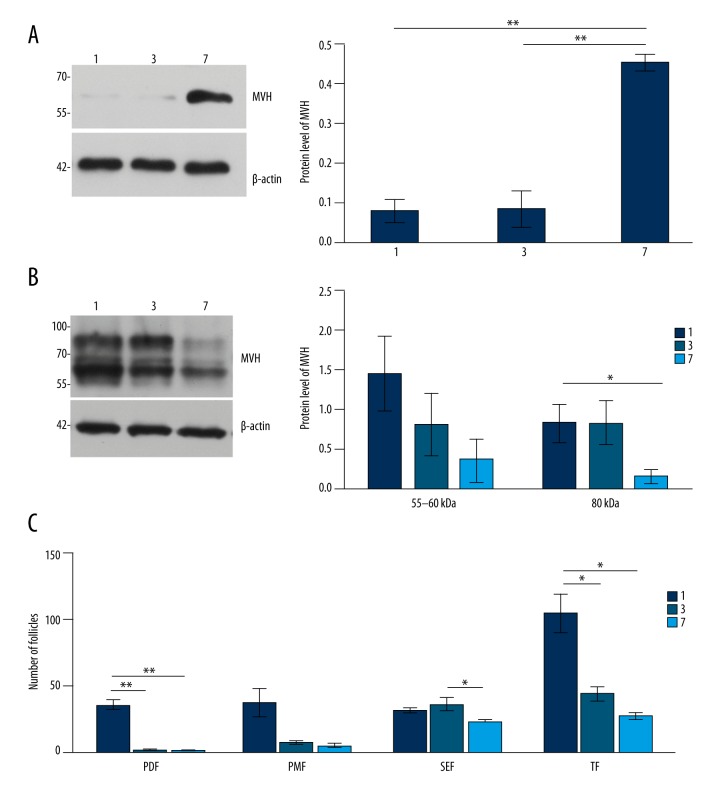
Figure 4.
The expression level of MVH in (A, B) was normalized to that of β-actin. The expression level of MVH in (A, B) was normalized to that of β-actin. (C) Analysis of follicular counts in different stages. PDF – primordial follicles; PMF – primary follicles; SEF – secondary follicles; TF – total follicles. Data in (A–C) are shown in mean values ±SEM. The statistical significance is shown as * P<0.05; ** P<0.01.
Discussion
Higher levels of MVH protein in the ovary might be a preparation of oocytes for the competence to resume meiosis
In the present study, we found that the higher expression levels of ~55–60 kDa MVH isoform in the ovary were followed by the accumulations of preovulatory follicles (Figure 2B, 2C). So is it related to follicular development or ovulation? Ai Khim Lim et al. [12] observed the tissue sections of MVH mutant mice ovaries, and found normal folliculogenesis and corpora lutea, while other researchers found that follicular development and ovulation were unaffected [13]. VASA is known as a component of the nuage, which is an electron-dense structure in germ cells implicated in retrotransposon repression in meiosis [14]. In the MVH homozygous mutant male mice, spermatogenesis is blocked at the stage from leptotene to zygotene, showing an essential role in meiosis of spermatogenesis [10]. In female mice, MVH protein shows a low expression level in oogonia at the onset of mitosis and a high expression level in oocytes by the onset of meiosis [15]. Unlike continuous meiotic events in spermatogenesis, meiosis in oocytes is arrested at meiotic prophase I from E14.5 to pre-ovulation and reentered by the induction of LH surge. Therefore, ovulatory activity in females is accompanied by an important event: the resumption of meiosis in the oocyte. The evidence that the higher levels of MVH expression in the ovary were followed by the accumulations of preovulatory follicles suggests a role of MVH in this process.
In our study, we found that a relatively strong fluorescent intensity of MVH was detected in primordial, primary, and secondary follicles (Figure 1A–1F). In antral follicles, the expression obviously underwent a progressive decline (Figure 1G–1L), suggesting its role before the antrum formation, while the oocyte acquires the competence to resume meiosis at about the time that an antrum is formed [16]. It is possible that the higher levels of MVH protein might be a preparation of oocytes for the competence to resume meiosis.
It is still a puzzle why MVH mutant female mice did not display any obvious reproductive defects and the offspring showed no difference in litter size or litter frequency vs. wild types [13]. Similarly, mutations in other genes involved in meiosis, such as Rad21L and Pms2, have been found to cause male infertility in mice but females are fertile [17,18]. The female meiotic regulatory mechanism is more complicated than in males in that oocytes undergo meiotic arrest twice. Additional signaling pathways regulate the processes of meiotic arrest and meiotic reentry in females [16]. It is possible that MVH is involved in meiotic process, but not as the only essential regulatory gene.
Conclusions
Higher levels of MVH protein in the ovary might be a preparation of oocytes for the competence to resume meiosis.
Footnotes
Disclosure of conflict of interest
None.
Source of support: The present study was supported by the National Natural Science Foundation of China (grant no. 81173396)
References
- 1.Hay B, Jan LY, Jan YN. A protein component of Drosophila polar granules is encoded by vasa and has extensive sequence similarity to ATP-dependent helicases. Cell. 1988;55:577–87. doi: 10.1016/0092-8674(88)90216-4. [DOI] [PubMed] [Google Scholar]
- 2.Hay B, Ackerman L, Barbel S, et al. Identification of a component of Drosophila polar granules. Development. 1988;103:625–40. doi: 10.1242/dev.103.4.625. [DOI] [PubMed] [Google Scholar]
- 3.Lasko PF, Ashburner M. Posterior localization of vasa protein correlates with, but is not sufficient for, pole cell development. Genes Dev. 1990;4:905–21. doi: 10.1101/gad.4.6.905. [DOI] [PubMed] [Google Scholar]
- 4.Rosner A, Moiseeva E, Rinkevich Y, et al. Vasa and the germ line lineage in a colonial urochordate. Dev Biol. 2009;331:113–28. doi: 10.1016/j.ydbio.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 5.Raz E. The function and regulation of vasa-like genes in germ-cell development. Genome Biol. 2000;1(3):REVIEWS1017. doi: 10.1186/gb-2000-1-3-reviews1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustafson EA, Wessel GM. Vasa genes: Emerging roles in the germ line and in multipotent cells. Bioessays. 2010;32:626–37. doi: 10.1002/bies.201000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouhana L, Shibata N, Nishimura O, et al. Different requirements for conserved post-transcriptional regulators in planarian regeneration and stem cell maintenance. Dev Biol. 2010;341:429–43. doi: 10.1016/j.ydbio.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 8.Wagner DE, Ho JJ, Reddien PW. Genetic regulators of a pluripotent adult stem cell system in planarians identified by RNAi and clonal analysis. Cell Stem Cell. 2012;10:299–311. doi: 10.1016/j.stem.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rebscher N, Zelada-Gonzalez F, Banisch TU, et al. Vasa unveils a common origin of germ cells and of somatic stem cells from the posterior growth zone in the polychaete Platynereis dumerilii. Dev Biol. 2007;306:599–611. doi: 10.1016/j.ydbio.2007.03.521. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka SS, Toyooka Y, Akasu R, et al. The mouse homolog of Drosophila vasa is required for the development of male germ cells. Genes Dev. 2000;14:841–53. [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson J, Bagley J, Skaznik-Wikiel M, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–15. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim AK, Knowles BB. Controlling endogenous retroviruses and their chimeric transcripts during natural reprogramming in the oocyte. J Infect Dis. 2015;212(Suppl 1):S47–51. doi: 10.1093/infdis/jiu567. [DOI] [PubMed] [Google Scholar]
- 13.Lim AK, Lorthongpanich C, Chew TG, et al. The nuage mediates retrotransposon silencing in mouse primordial ovarian follicles. Development. 2013;140:3819–25. doi: 10.1242/dev.099184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aravin AA, van der Heijden GW, Castaneda J, et al. Cytoplasmic compartmentalization of the fetal piRNA pathway in mice. PLoS Genet. 2009;5:e1000764. doi: 10.1371/journal.pgen.1000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickford DE, Frankenberg S, Pask AJ, et al. DDX4 (VASA) is conserved in germ cell development in marsupials and monotremes. Biol Reprod. 2011;85:733–43. doi: 10.1095/biolreprod.111.091629. [DOI] [PubMed] [Google Scholar]
- 16.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herran Y, Gutierrez-Caballero C, Sanchez-Martin M, et al. The cohesin subunit RAD21L functions in meiotic synapsis and exhibits sexual dimorphism in fertility. EMBO J. 2011;30:3091–105. doi: 10.1038/emboj.2011.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker SM, Bronner CE, Zhang L, et al. Male mice defective in the DNA mismatch repair gene PMS2 exhibit abnormal chromosome synapsis in meiosis. Cell. 1995;82:309–19. doi: 10.1016/0092-8674(95)90318-6. [DOI] [PubMed] [Google Scholar]