Highlights
-
•
In Titule, onchocerciasis suspected skin lesions were associated with epilepsy.
-
•
Frequent activities at rapid flowing rivers were associated with epilepsy.
-
•
A historical lack of Ivermectin treatment was a risk factors for epilepsy.
Keywords: Onchocerciasis, Nodding syndrome, Epilepsy, Risk factors, Case-control study, Democratic Republic of the Congo
Summary
Background
The reason for the high prevalence of epilepsy in onchocerciasis endemic areas remains unknown. The aim of this study was to detect risk factors associated with epilepsy in a region endemic for onchocerciasis.
Methods
In June 2014, a case–control study was performed in Titule, Bas-Uélé Province in the Democratic Republic of the Congo. Individuals with unprovoked convulsive epilepsy of unknown aetiology were enrolled as cases (n = 59). Healthy members of families without cases of epilepsy in the same village were recruited as controls (n = 61). A multivariate binomial logistic regression analysis was performed to identify potential risk factors associated with epilepsy. To evaluate the potential protective effect of ivermectin treatment on the development of epilepsy, a nested age-matched case–control study was performed including only those who were eligible for ivermectin treatment in the year before they developed epilepsy.
Results
Suspected onchocerciasis skin lesions were more often present in cases than in controls: 12/41 (29%) vs. 1/56 (2%), respectively (odds ratio (OR) 20.26, 95% confidence interval (CI) 2.42–170; p < 0.01). Ivermectin had been taken 7 months earlier in 29/59 (49%) cases and 29/61 (48%) controls. Onchocerca volvulus (OV) DNA was detected by PCR in skin snips in 26/34 cases (76%) and 10/14 controls (71%) (p = 0.7), and there was presence of OV IgG4 antibodies in 35/48 (73%) cases and 15/18 (83%) controls (p = 0.5). OV DNA was not detected in the cerebrospinal fluid of cases (controls not tested). Both cases and controls reported frequent bites by blackflies (Diptera, Simuliidae). Bathing daily as opposed to less often (OR 16.7, 95% CI 2.2–125.8; p < 0.01), bathing between 11 a.m. and 4 p.m. (OR 12.7, 95% CI 1.6–103.7; p = 0.02), and washing clothes between 11 a.m. and 4 p.m. (OR 10.9, 95% CI 1.5–77.3; p = 0.02) were all independently associated with epilepsy. Blood screening by specific PCR tests for Toxoplasma and Wuchereria bancrofti was negative in all cases and controls. A Loa loa infestation was found in only one case and one control by PCR and Giemsa smear. Antibodies to Taenia solium, Toxocara, and Trypanosoma sp were not detected in any of the participants. In an age-matched case–control analysis, 16/18 (89%) cases had not taken ivermectin the year before they developed epilepsy, compared to 7/18 (39%) controls that same year (p = 0.002).
Conclusions
These data suggest that frequent activities at rivers known to be blackfly breeding sites and a historical lack of ivermectin treatment were risk factors for epilepsy in this onchocerciasis endemic area.
1. Introduction
The prevalence of convulsive epilepsy is greater in Sub-Saharan African countries than in high-income countries, and parasitic infestations are thought to contribute to this increased burden.1, 2 A high prevalence of epilepsy has been reported in many onchocerciasis endemic areas,3, 4 and is particularly high in certain localities in South Sudan, Tanzania, and northern Uganda, where a special form of atonic epilepsy called ‘nodding syndrome’ (NS) has been described.5, 6, 7, 8 The cause of NS remains unclear,5, 9 but case–control studies have regularly found an association between onchocerciasis and NS.5, 7, 10, 11 In a study in Cameroon, the intensity of infestation with Onchocerca volvulus (OV) was found to be higher in people with epilepsy than in controls.3
The Democratic Republic of the Congo (DRC) is a country with large areas where onchocerciasis is still hyperendemic.12 Between April 2014 and June 2014, several epilepsy prevalence studies were performed in villages in the Bas-Uélé Province, namely Liguga,13 Dingila, and Titule14 (Figure 1). Among the 12 776 people in Dingila, 373 (2.9%) individuals with epilepsy were identified. In a door-to-door survey in Titule, 68 (2.3%) of the 2908 people who participated in the survey were found to present episodes of epilepsy.14 In Titule, epilepsy showed a marked spatial pattern, with clustering of cases occurring within and between adjacent households. The individual risk of epilepsy was found to be associated with living close to the Bima River, a fast flowing river where blackflies (Diptera: Simuliidae) – the vector of OV – oviposit and breed.14 The degree of onchocerciasis endemicity in Titule was not estimated in 2014, but in 1999, during a rapid epidemiological mapping of onchocerciasis (REMO) survey, all of the 33 persons examined in the village had nodules. As a consequence, ivermectin has been distributed in Titule once a year to persons over 5 years of age, but not to pregnant or breastfeeding women, since 2000. The coverage initially was reported to be 23%, but the coverage has increased over the years to 73–81% of the eligible individuals (F. Tepage, personal communication).
Figure 1.
Location of Titule in the Bas-Uele district, Democratic Republic of the Congo.
During the epilepsy prevalence survey in Titule, a case–control study was also performed to investigate whether exposure to Simulium spp blackflies is indeed a risk factor for developing epilepsy and to determine whether treatment with ivermectin may protect against developing epilepsy.
2. Materials and methods
2.1. Setting
Titule, a locality crossed by the Bima River, has a population of 11 882 inhabitants. Our visit to Titule was announced by the local doctor and the volunteers of the ‘relais communautaire’. These are villagers making up the community surveillance network, who are involved in the community-directed treatment with ivermectin campaigns (CDTI), vaccination campaigns, and mosquito net distribution; therefore they know the members of their community well. On arrival, at least 50 patients were waiting for us. During our stay in Titule at least 100 additional patients wanted to be seen by us. Two patients with epilepsy did not want to participate in the study because they did not want to undergo a lumbar puncture, a procedure included in the study protocol. No incentives were given to the patients to participate.
2.2. Design
The first 59 patients with confirmed active epilepsy after examination by one of the study doctors and who agreed to participate in the study were enrolled in a case–control study. Active epilepsy was defined as a patient who had presented at least two unprovoked seizures of unknown origin in the last 12 months. With regard to the seizures, mainly tonic–clonic generalized seizures and episodes of absence of sudden onset and of brief duration were considered.
Controls were healthy volunteers from families without any cases of epilepsy, chosen from the same village among individuals of the same age and sex groups.
2.3. Procedures
After written informed consent was obtained, the person with epilepsy or the healthy control, or their parent/guardian, was interviewed in their native language by Congolese physicians (MM, GMa, GMu) and local nurses (FM, BY) using a standardized questionnaire. This questionnaire included questions on ethnicity of the mother and the father, movement of the family in the past, time spent at the major river (bathing, fishing, fetching water, washing clothes), episodes of stay in temporary settlements along rivers or in the forest, frequency of insect bites, exposure to domestic animals, the consumption of insects and larvae, year of onset of the epilepsy, years of ivermectin intake, and history of febrile convulsions (defined as seizures in children <5 years old, associated with fever, without an underlying cause). For cases, exposure to the potential risk factors in the period before they developed epilepsy was assessed, while for controls, potential risk factors immediately prior to the interview were assessed.
After the interview, cases and controls were examined by a physician (GM, MM, GMa, RC). On physical examination, cases and controls were assessed for onchocerciasis nodules, skin abnormalities, vision, and mental status. Height and weight were measured using a stadiometer and a digital scale and these measurements were used to calculate the body mass index (BMI, kg/m2). Visual acuity was mainly assessed by history taking and not by a formal ophthalmological examination. A blind person was considered a person who had no light perception.
Blood samples were collected from all cases and controls in heparinized collection vials and adsorbed on Serobuvard. A malaria antigen test (SD BIOLINE Malaria Ag P.f) was only performed in the cases, and Giemsa smears for the presence of Loa loa were performed in a batch of cases and controls. Because of time restrictions, physical examinations and laboratory tests could not be performed for all patients.
A skin snip was taken from the left and right iliac crests of all subjects with a Holtz corneoscleral punch (2 mm) and stored in 90% ethanol to be tested for OV using an in-house PCR method (Supplementary Material, supplemental methods). Cases were examined neurologically, and if there were no contraindications (no focal neurological deficit and no clinical signs of increased intracranial hypertension), a cerebrospinal tap was performed and cerebrospinal fluid (CSF) obtained. All lumbar punctures were performed by a single physician (GMu), who had received special training in neurology while working as a medical doctor in a trypanosomiasis treatment programme. After the procedure, patients were able to rest and received paracetamol.
Blood specimens were screened using an in-house PCR for molecular evidence of OV, Onchocerca ochengi, L. loa, Mansonella spp, Wuchereria bancrofti, and Toxoplasma gondii (see Supplementary Material, supplemental methods). Serological tests were also performed targeting OV IgG4 antibodies (Ov16 Standard Diagnostics, Inc., Alere SD BIOLINE, Gyeonggi-do, Republic of Korea), Taenia solium antibodies by immunoblot assay (LDBIO Diagnostics, Lyon, France), Trypanosoma brucei antibodies by immunofluorescence test, and Toxocara canis antibodies by enzyme immunoassay (Bordier Affinity products SA, Crissier, Switzerland). Skin snips and CSF were also screened for OV DNA by in-house PCR assay. In skin snips of 19 cases and 35 controls, the Wolbachia ftsZ gene was quantified from purified DNA by real-time PCR (qPCR) (see Supplementary Material, supplemental methods).
Participants with a positive malaria antigen test received anti-malaria treatment (artemether and lumefantrine). All patients who had not received ivermectin the previous year were offered a dose of ivermectin. A stock of anti-epileptic drugs (phenobarbital) was handed over to the local health centre.
2.4. Statistical analyses
Factors describing the properties of the case and control populations (weight, height, BMI, clinical symptoms, and potential risk factors such as contact with insects, domestic animals, and rivers) were assessed by age-adjusted univariate models (linear regression for factors measured on a continuous scale, or else binomial logistic regression) to identify significant differences in the properties of the two groups.
Participants were not asked about their contact with insects and domestic animals before epilepsy appeared, but whether or not they currently experienced exposure. However for factors concerning exposure to rivers (e.g., through washing clothes and bathing), participants were specifically asked about exposure before the onset of the epilepsy. Therefore, in a multivariate binomial logistic regression analysis, only variables assessing exposure to rivers were included to identify significant associations with epilepsy status. Factors with a p-value of 0.5 or more from the univariate analysis were discarded from this multivariate analysis, as this suggested they were highly likely not to be significantly associated. A minimum adequate model was obtained using backwards stepwise selection to identify which of the factors describing river exposure conferred the greatest explanatory power. Chi-square statistics and associated p-values were calculated for each nested version of the model arising from the deletion of each individual term, and the term missing from the model with the highest p-value was deleted from the model. This was repeated until no further factors could be removed that would not cause a significant decrease in the explanatory power of the model.15 The age adjustment was maintained through this reduction process. For each risk factor, age-adjusted odds ratios (OR) and respective 95% confidence intervals (CI) were estimated. Finally, it was attempted to minimize the possibility that any factor associated with an increased risk of epilepsy was actually associated with an increased risk of onchocerciasis of which epilepsy was just one possible symptom. Hence, the stepwise selection procedure was run again and a new minimum adequate model generated, adjusted for onchocerciasis, with individuals (both cases and controls) classified as onchocerciasis-positive if they showed any evidence of onchocerciasis, either clinical or laboratory-based.
In each year from 2000 to 2013, the proportion of cases and controls eligible to receive ivermectin treatment (i.e., 5 years old or more) who received treatment was calculated. In order to identify differences in treatment history in cases and controls, these time-series were compared using a univariate binary logistic regression model adjusted by year. To minimize the chances that any difference detected was actually attributable simply to a difference in prevalence of onchocerciasis, this model was run a second time including only those individuals (both cases and controls) who could be classified as onchocerciasis-positive by laboratory-based criteria (OV PCR skin snip positivity and/or OV IgG4 antibody positivity).
The relationship between infection status with parasites and epilepsy status was assessed using univariate binary logistic regression models.
Analyses were performed using the R statistical computing package.16
3. Results
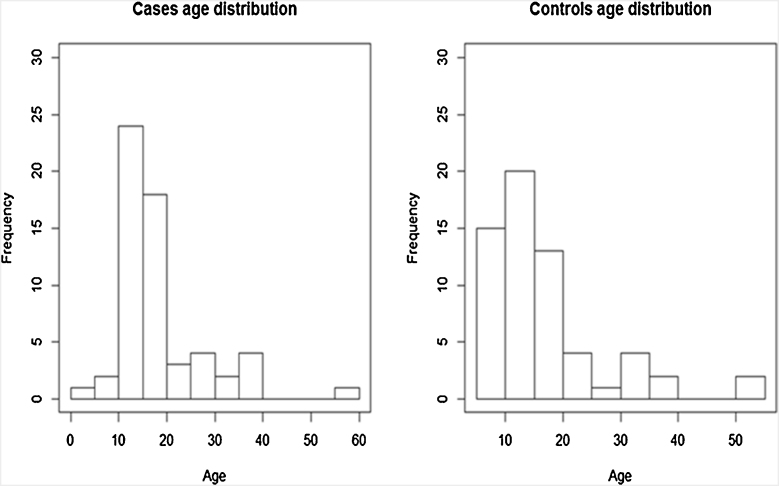
Fifty-nine cases with epilepsy and 61 controls were enrolled in the study. The sex distribution was similar in the cases and controls: males made up 47% of the case population and 46% of the control population. The control population had an older median age of 16 years in comparison to 15 years for the cases. The age distributions are shown in Figure 2.
Figure 2.
Histograms showing age distribution of cases and controls.
Cases and controls were similar concerning ethnicity of the parents (Boa and Zande were the most frequent ethnicities, represented by 56% and 28% of cases and 49% and 30% of controls, respectively) and place of residence and birth (mainly Titule, >97% of cases and controls being residents, 71% of cases and 57% of controls having been born there). More parents of cases were born in Titule compared with controls (39% in cases, 22% in controls; univariate binomial logistic regression, OR 2.30, p = 0.04). Cases and controls did not differ in the distribution of the main money-generating activities of their family (mainly agriculture). Cases had typically spent 1 year less in primary school (only 3 years compared to 4 years for controls).
3.1. Clinical characteristics
There was a history of tonic–clonic seizures in 58 (98%) cases, with drooling in 41 (70%) and urinary incontinence in 34 (57%). Absence seizures were reported in 18 (31%), in all but one of whom there was also a history of tonic–clonic seizures. Neuropsychiatric abnormalities were present in 18 (31%), with severe cognitive impairment. Spatial disorientation was noted in 12 (20%) and temporal disorientation in 13 (22%); 14 (24%) regularly got lost in the village. Nineteen (33%) had problems in speaking, and episodes of hallucinations were reported in 19 (32%). Three (5%) showed stunted growth and a lack of secondary sexual characteristics (one of them was a boy, 16 years old, 21 kg, 128 cm, without pubic hair). The mean age at onset of epilepsy was 12 years (interquartile range 9–14 years, range 2–37 years). Mean body weight, height, and BMI were significantly lower in cases than in controls (Table 1). Cases were more likely to present with onchocerciasis-associated symptoms, to have burn scars, and to have a history of febrile convulsions.
Table 1.
Clinical characteristics of cases and controls
| Clinical characteristics | Cases (n = 59) | Controls (n = 61) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Body weight (kg), mean (SD) | 38.9 (11.21) | 46.7 (15.27) | <0.001a | |
| Height (cm), mean (SD) | 148.4 (15.03) | 154.0 (17.8) | <0.001a | |
| BMI (kg/m2), mean (SD) | 17.83 (2.86) | 18.43 (2.69) | 0.001a | |
| Itching | 65% (26/40) | 16% (8/51) | 13.31 (4.34–40.8) | <0.01b |
| Onchocerciasis suspected skin lesions | 29% (12/41) | 2% (1/56) | 20.26 (2.42–170) | <0.01b |
| Abnormal vision | 8% (4/52) | 0% (0/52) | -c | -c |
| Blindness | 2% (1/59) | 0% (0/61) | -c | -c |
| Nodules | 8% (3/40) | 4% (2/46) | 1.13 (0.14–9.3) | 0.9b |
| Burn scars | 18% (10/57) | 0% (0/61) | -c | <0.01 |
| History of febrile convulsions | 21% (12/56) | 3% (2/61) | 8.23 (1.74–38.8) | <0.01b |
OR, odds ratio; CI, confidence interval; SD, standard deviation; BMI, body mass index.
Age-adjusted linear regression model.
Age-adjusted binomial logistic regression model.
OR not calculated due to null value in one or more classes.
3.2. Potential risk factors associated with epilepsy
Nearly all cases and controls reported exposure to Simuliidae (called ‘Mbitiri’ in the local language) (Table 2). Exposure to Culicoides spp (called ‘invisibles’) and eating insects was more often reported by controls. Contact with chickens, pigs, and goats was more often reported by controls. On the other hand, daily bathing was more frequently reported by cases. In particular, day time bathing and washing (11 a.m.–4 p.m.) were more frequently reported by cases.
Table 2.
Potential risk factors associated with epilepsya
| Factor | Cases | Controls | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Contact with insects | ||||
| Contact with Simuliidae | 96% (55/57) | 98% (60/61) | 0.46 (0.04–5.20) | 0.5 |
| Contact with Culicoides | 71% (41/57) | 86% (53/61) | 0.39 (0.15–1.00) | 0.05 |
| Contact with Chrysops | 82% (47/57) | 72% (44/61) | 1.84 (0.15–1.00) | 0.18 |
| Contact with tsetse | 19% (11/57) | 21% (13/61) | 0.88 (0.36–2.19) | 0.80 |
| Eating insects | 75% (43/57) | 98% (59/60) | 0.05 (0.01–0.42) | <0.01 |
| Eating larvae | 82% (47/57) | 100% (60/60) | 0.00 (0.00–Inf) | 0.99 |
| Contact with domestic animals | ||||
| Cat | 4/57 (7%) | 5/41 (12%) | 0.55 (0.14–2.20) | 0.4 |
| Dog | 4/57 (7%) | 2/41 (5%) | 1.48 (0.26–8.52) | 0.7 |
| Chicken | 21/57 (37%) | 24/41 (59%) | 0.42 (0.18–0.95) | 0.04 |
| Cow | 0/57 (0%) | 2/41 (5%) | -b | 0.17c |
| Pig | 0/57 (0%) | 4/41 (10%) | -b | 0.03c |
| Goat | 6/57 (11%) | 13/41 (32) | 0.25 (0.09–0.73) | 0.01 |
| Contact with rivers | ||||
| Bathing: never (reference value) | 33% (17/51) | 40% (22/55) | ||
| Bathing: less than daily | 20% (10/51) | 38% (21/55) | 0.62 (0.23–1.65) | 0.34 |
| Bathing: daily | 47% (24/51) | 22% (12/55) | 2.59 (1.01–6.62) | 0.05 |
| Washing clothes: never (reference value) | 25% (13/55) | 22% (12/55) | ||
| Washing clothes: less than daily | 62% (34/55) | 76% (42/55) | 0.75 (0.3–1.85) | 0.53 |
| Washing clothes: daily | 15% (8/55) | 2% (1/55) | 7.31 (0.8–68.1) | 0.08 |
| Bathing 5 a.m.–10 a.m. | 40% (23/58) | 22% (13/58) | 2.25 (0.98–5.01) | 0.06 |
| Bathing 11 a.m.–4 p.m. | 58% (34/58) | 28% (16/58) | 3.86 (1.76–8.47) | <0.01 |
| Bathing 5 p.m.–10 p.m. | 36% (21/58) | 36% (21/58) | 0.94 (0.44–2.04) | 0.90 |
| Washing clothes 5 a.m.–10 a.m. | 38% (21/56) | 52% (29/56) | 0.55 (0.26–1.17) | 0.12 |
| Washing clothes 11 a.m.–4 p.m. | 63% (35/56) | 38% (21/56) | 2.71 (1.24–5.89) | 0.01 |
| Washing clothes 5 p.m.–10 p.m. | 23% (13/56) | 13% (7/56) | 2.01 (0.73–5.58) | 0.18 |
OR, odds ratio; CI, confidence interval.
ORs and p-values estimated from age-adjusted univariate binomial logistic regression, except for those labelled ‘c’, where a Fisher's exact test was performed due to null values in one of the groups. In the case of bathing and washing times, the comparison is with those who bathed or washed but at other times.
OR not computed as there were no records in the case group.
An age-adjusted multivariate binomial logistic regression analysis was performed on a subset of individuals who reported washing and bathing at any time (Table 3). This reduced the dataset to 54 individuals, but maintained a balance of 54% controls and 46% cases. This analysis showed that bathing daily and bathing between 11 a.m. and 4 p.m. were independently associated with epilepsy.
Table 3.
Age-adjusted multivariate binomial logistic regression analysis of contact with the river as a potential risk factor for epilepsy
| Factor | Cases | Controls | aOR (95% CI) | p-Value |
|---|---|---|---|---|
| Bathing daily (reference less than daily) | 47% (24/51) | 22% (12/55) | 16.33 (1.99–134.2) | 0.009 |
| Washing clothes daily (reference less than daily) | 15% (8/55) | 2% (1/55) | 1.07 (0.07–16.6) | 0.96 |
| Bathing 5 a.m.–10 a.m. | 40% (23/58) | 22% (13/58) | 3.28 (0.49–22.1) | 0.22 |
| Bathing 11 a.m.–4 p.m. | 58% (34/58) | 28% (16/58) | 22.65 (1.77–289.3) | 0.016 |
| Washing clothes 5 a.m.–10 a.m. | 38% (21/56) | 52% (29/56) | 1.67 (0.27–10.5) | 0.58 |
| Washing clothes 11 a.m.–4 p.m. | 63% (35/56) | 38% (21/56) | 9.78 (0.93–103.1) | 0.058 |
aOR, age-adjusted odds ratio; CI, confidence interval.
Whilst maintaining the age adjustment, a minimal model was obtained containing daily bathing (OR 16.7, 95% CI 2.2–125.8; p < 0.01) and bathing in the daytime (OR 12.7, 95% CI 1.6–103.7; p = 0.02), both previously significant in the full multivariate model, and also washing clothes in the daytime (OR 10.9, 95% CI 1.5–77.3; p = 0.02), previously marginally non-significant. All three may be seen as proxies for the frequency and intensity of contact with blackflies.
3.3. Ivermectin treatment history
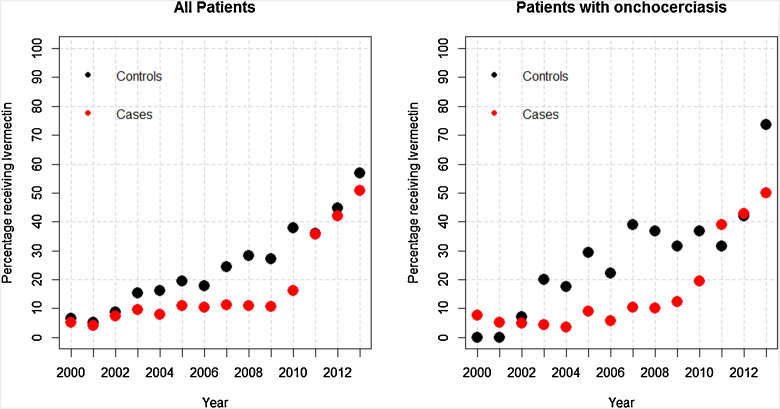
The percentage of patients receiving ivermectin was found to be significantly lower in cases than in controls (univariate binomial generalized linear model (GLM) adjusted by year, p = 0.03), and this relationship was maintained when considering only patients who had positive onchocerciasis laboratory test results (univariate binomial GLM adjusted by year, p = 0.01) (Figure 3).
Figure 3.
Percentage of patients who were eligible to receive Ivermectin, that were recorded to have taken it in each year 2000-2013. This is shown for all patients (left) and only for patients with documented OV infestation (right).
Eighteen age-matched case–control pairs were available in the dataset. Sixteen (98%) cases did not take ivermectin the year before they developed epilepsy, compared to seven (39%) controls that same year (p = 0.002).
3.4. Laboratory results
OV DNA was detected by PCR in skin snips with similar frequency in both cases (76%) and controls (71%); in 73% of cases and 83% of controls there was also presence of OV IgG4 antibodies (Table 4). In none of the cases was OV DNA detected in the CSF. Mansonella spp were frequently found in cases and controls, while a L. loa infestation was found in only one case and one control.
Table 4.
Laboratory test results for cases and controls
| Test | Cases | Controls | p-Value |
|---|---|---|---|
| Skin OV PCR-positive | 26/34 (76%) | 10/14 (71%) | 0.7 |
| OV IgG4 antibodies | 39/49 (78%) | 15/18 (83%) | 0.5 |
| Mansonella spp (PCR on buffy coat) | 12/49 (78%) | 13/20 (65%) | 0.91 |
| Loa loa (PCR blood and Giemsa smear) | 5% (1/20) | 4% (1/26) | 0.9 |
OV, Onchocerca volvulus.
There was no difference in the quantity of Wolbachia genes per microfilaria between cases and controls. PCR screening of blood for O. ochengi, T. solium, T. gondii, and W. bancrofti were all negative. Toxocara and T. solium antibodies were absent in all participants; T. brucei antibodies were slightly increased in one case and one control. A malaria test was positive in 33/44 (77%) cases, but was not performed in controls.
4. Discussion
This is the first case–control study in the DRC with the aim of detecting risk factors for the development of epilepsy in an onchocerciasis endemic region. The most important finding of this study is that cases reported a more frequent contact with the river. Two proxies for the frequency and intensity of exposure to blackflies were risk factors for developing epilepsy. The reason why being at the riverside between 11 a.m. and 4 p.m. was a risk factor for epilepsy is less clear. Indeed most members of the Simulium damnosum complex have a bimodal daily biting pattern, with a peak in the morning (7 a.m. to 9 a.m.) and another peak in the afternoon (4 p.m. to 6 p.m.).17 In the present study, bathing between 5 a.m. and 10 a.m. was slightly more often reported in cases than controls (p = 0.06). Respondents were not asked about exposure to the river between 4 p.m. and 6 p.m. Biting patterns can also vary with cytospecies, and a unimodal (middle-of-the-day) pattern is known within the S. damnosum complex, and is more likely to be found in forest areas than in savannah areas.
Given that exposure to blackfly bites is very frequent in Titule, and greatest for those spending the most time at the riverside, this suggests a potential role for blackflies either in transmitting an agent or triggering an immune response that may cause epilepsy.
A case–control study performed at five Health and Demographic Surveillance System centres in Sub-Saharan Africa demonstrated that exposure to multiple parasites was associated with active convulsive epilepsy. Particularly, individuals with antibodies to O. volvulus (OR 1.98, 95% CI 1.52–2.58; p < 0.001), T. canis (OR 1.52, 95% CI 1.23–1.87; p < 0.001), and T. gondii (OR 1.28, 95% CI 1.04–1.56; p = 0.018) were more likely to have active convulsive epilepsy.18 In the present study, it was found that cysticercosis, Toxocara infestation, and trypanosomiasis cannot explain the high prevalence of epilepsy in the region.
Skin lesions suggesting an onchocerciasis infestation were more often present in cases than in controls. Five patients with epilepsy presented with a leopard type of skin. However, OV DNA was detected in skin snips in an equal percentage of cases and controls (in 76% of cases and in 71% of controls). OV DNA was not detected in the CSF of cases (controls not tested). The latter is similar to the finding of a study performed in Tanzania.19, 20 The former is in contrast with other case–control studies that have found OV microfilaria to be present more often in cases compared to controls.4, 7, 21 However, previous case–control studies investigating the association between epilepsy and onchocerciasis were performed in populations prior to the introduction of ivermectin. In the present study, nearly 50% of cases and controls received ivermectin 7 months before the skin snip examination. The equal percentage of cases and controls with OV IgG4 antibodies is also in contrast with other case–control studies, which have shown a higher percentage of OV IgG4 antibodies in cases.10 The explanation for the discrepancy between clinical and laboratory onchocerciasis findings could be that cases became infected earlier and/or acquired a higher degree of infestation than controls, particularly in the period before they developed epilepsy. This is suggested by the presence of leopard-type skin lesions only in the cases. Such lesions are sequelae of a severe cumulative OV infestation in the past.22, 23 In a case–control study performed in December 2015 in Drazu, a village in Ituri Province, where ivermectin had never been distributed, persons with epilepsy were significantly more often infested with OV and had a higher microfilariae load than random selected age-matched village controls without epilepsy (data not yet published).
Mansonella spp infestation was very frequent in cases and controls. Exposure to Ceratopogonidae (Culicoides spp, vector of Mansonella spp) was also very frequently reported by cases and controls, but slightly more often by controls than cases. A higher prevalence of Mansonella perstans infestation has been found in a NS case–control study performed in South Sudan.7 However, M. perstans infestations have so far been considered not to cause any serious illness.24
Cases had a lower body weight, height, and BMI than controls. Moreover, three cases presented with important stunted growth and with a lack of secondary sexual characteristics. This has also been reported in case–control studies on NS.25 However the lower body weight and BMI and the clinical signs of onchocerciasis can also be explained by a selection bias, because controls were required to be healthy.
A differential in ivermectin use was identified between cases and controls, even if comparisons are made only between individuals with evidence of having onchocerciasis. This finding is an additional argument that part of the epilepsy in onchocerciasis endemic regions is caused directly or indirectly by the OV infestation. It further suggests that the use of ivermectin may protect against the development of epilepsy as a comorbidity of onchocerciasis.
How an OV infestation could lead to epilepsy remains unclear. Indeed, OV microfilariae are only rarely found in the nervous system.20 In Tanzania, similar to this study, PCR tests on the CSF of patients with NS and epilepsy with other seizure types failed to identify OV DNA.20 A recent study showed that 11/19 (58%) patients with NS had detectable serum autoantibodies to leiomodin 1 (a protein present in certain neurons) compared to 5/19 (26%) controls (matched OR 7.0, 95% CI 0.9–11.1).26 These antibodies, also present in the CSF of patients with NS, were found to be neurotoxic in vitro and cross-reacting with OV-specific proteins.26 In a recent study in Uganda, serum antibodies against voltage-gated potassium channel (VGKC) complex proteins were detected in 15 (48%) of 31 patients with NS compared to only one (9%) of 11 sibling controls.27 However, whether these autoantibodies are the cause of the NS or the consequence of damage to the neurons caused by another mechanism28 remains to be investigated.
NS, and convulsive epilepsy without NS, often occur in the same families in onchocerciasis endemic areas and are therefore probably caused by the same pathological process.29 If convulsive epilepsy without NS is also caused by an antibody response to OV-specific proteins, one would then expect a high prevalence of epilepsy in all onchocerciasis hyperendemic regions.
Cases more often reported a history of febrile convulsions. This confirms the observation of others that febrile convulsions may be associated with epilepsy later in life.30 Families of cases were less likely to consume fried insects and larvae and were less likely to have domestic animals such as chickens, pigs, and goats, compared with controls. This finding may be the consequence of the epilepsy. Indeed, in Titule it is believed that persons with epilepsy should not eat termites, caterpillars, chicken, pork, or goat (F. Tepage, personal communication). It has also been suggested that if humans are bitten by blackflies infected by O. ochengi (the cause of onchocerciasis in cattle, but non-pathogenic for humans), they may develop O. ochengi antibodies that provide a degree of immunity against OV.31 Whether a similar phenomenon may occur with goats infested by Onchocerca is unknown.31 It also may be that families of persons with epilepsy own fewer animals and are poorer than families without persons with epilepsy.
This study has several limitations. First the team did not include a neurologist, and a validated neuropsychological instrument was not used to assess patients with epilepsy. Therefore it was not possible to provide a detailed description of the different forms of epilepsy and the cognitive abnormalities. Second, although cases and controls were chosen among the same age and sex groups, only 18 cases and controls were individually matched for age. Third, cases were asked about their activities before they developed epilepsy and controls about their current activities. Fourth, interviewers were aware of the epileptic status of the participants. However, all questions were asked with great caution so as not to elicit certain answers. Finally, because of time constraints, physical examinations and laboratory tests were not performed for all cases and controls and less frequently for healthy controls. Moreover, certain examinations such as parasitological stool examinations were not performed at all.
In conclusion, despite these limitations, this case–control study shows that frequent activities at rivers known to harbour blackflies and a historical lack of ivermectin treatment were associated with the presence of epilepsy. The study results indicate that future studies looking at the aetiology of epilepsy need to focus on questions around exposure to rivers and blackflies, the degree of onchocerciasis endemicity, and the intake of ivermectin.
Acknowledgements
We thank the following for support: Esther Sterk and Belle Asani Yokana, Doctors without Borders Switzerland for logistical support, Faustin Monga nurse supervisor Monga Health Zone for assisting with interviewing the participants, Madeleine Mangemitata and Bennet Isangi Yokana for performing the field laboratory test, and Robert O. Cannon for reviewing the paper. The University of Kisangani (and the Centre de Surveillance de la Biodiversité (CSB; Biodiversity Monitoring Centre)), PNLO director Dr Naomie Awaca, and the ERAIFT, Kinshasa (DRC), in particular Prof. Baudouin Michel and Bruno Muyaya, for providing scientific logistical support and shape files, and bassinducongo.reddspot.org for access to SPOT images of the study area. The technical assistance of Christine Lämmer, Helene Neufeld, and Sabine Nachtsheim, University Hospital of Bonn, is greatly appreciated.
Ethics: The study was approved by the Institutional Review Board of the University of Kisangani and the provincial division of the Ministry of Health, DRC. The purpose and the nature of the investigation were explained to participants or parents/guardians, including risks and benefits of each of the procedures. A lumbar puncture was proposed to the participants with epilepsy because it was felt that this procedure was important to identify the cause and pathogenesis of the epilepsy. All participants or parents/guardians provided written informed consent (either by signature or by finger printing if the participant was unable to sign). The ethics committee of the University of Kisangani had approved the use of thumbprint consent. Authorization to collect, transport, and ship biological material were granted by the Faculty of Sciences of the University of Kisangani, DRC.
Funding: We received a small grant from the Mectizan donation programme. The funder was not involved in study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Conflict of interest: The authors do not have any conflict of interest.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijid.2016.05.018.
Appendix A. Supplementary data
References
- 1.Ngugi A.K., Bottomley C., Kleinschmidt I., Sander J.W., Newton C.R. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51:883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul A., Adeloye D., George-Carey R., Kolcic I., Grant L., Chan K.Y. An estimate of the prevalence of epilepsy in Sub-Saharan Africa: a systematic analysis. J Glob Health. 2012;2:020405. doi: 10.7189/jogh.02.020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pion S.D., Kaiser C., Boutros-Toni F., Cournil A., Taylor M.M., Meredith S.E. Epilepsy in onchocerciasis endemic areas: systematic review and meta-analysis of population-based surveys. PLoS Negl Trop Dis. 2009;3:e461. doi: 10.1371/journal.pntd.0000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boussinesq M., Pion S.D., Demanga N., Kamgno J. Relationship between onchocerciasis and epilepsy: a matched case–control study in the Mbam Valley, Republic of Cameroon. Trans R Soc Trop Med Hyg. 2002;96:537–541. doi: 10.1016/s0035-9203(02)90433-5. [DOI] [PubMed] [Google Scholar]
- 5.Dowell S.F., Sejvar J.J., Riek L., Vandemaele K.A., Lamunu M., Kuesel A.C. Nodding syndrome. Emerg Infect Dis. 2013;19:1374–1384. doi: 10.3201/eid1909.130401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sejvar J.J., Kakooza A.M., Foltz J.L., Makumbi I., Atai-Omoruto A.D., Malimbo M. Clinical, neurological, and electrophysiological features of nodding syndrome in Kitgum, Uganda: an observational case series. Lancet Neurol. 2013;12:166–174. doi: 10.1016/S1474-4422(12)70321-6. [DOI] [PubMed] [Google Scholar]
- 7.Tumwine J.K., Vandemaele K., Chungong S., Richer M., Anker M., Ayana Y. Clinical and epidemiologic characteristics of nodding syndrome in Mundri County, southern Sudan. Afr Health Sci. 2012;12:242–248. doi: 10.4314/ahs.v12i3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkler A.S., Friedrich K., Konig R., Meindl M., Helbok R., Unterberger I. The head nodding syndrome—clinical classification and possible causes. Epilepsia. 2008;49:2008–2015. doi: 10.1111/j.1528-1167.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 9.Vogel G. Tropical diseases. Mystery disease haunts region. Science. 2012;336:144–146. doi: 10.1126/science.336.6078.144. [DOI] [PubMed] [Google Scholar]
- 10.Foltz J.L., Makumbi I., Sejvar J.J., Malimbo M., Ndyomugyenyi R., Atai-Omoruto A.D. An epidemiologic investigation of potential risk factors for nodding syndrome in Kitgum District, Uganda. PLoS One. 2013;8:e66419. doi: 10.1371/journal.pone.0066419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser C., Rubaale T., Tukesiga E., Kipp W., Asaba G. Nodding syndrome, Western Uganda, 1994. Am J Trop Med Hyg. 2015;93(1):198–202. doi: 10.4269/ajtmh.14-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makenga Bof J.C., Maketa V., Bakajika D.K., Ntumba F., Mpunga D., Murdoch M.E. Onchocerciasis control in the Democratic Republic of Congo (DRC): challenges in a post-war environment. Trop Med Int Health. 2015;20:48–62. doi: 10.1111/tmi.12397. [DOI] [PubMed] [Google Scholar]
- 13.Colebunders R., Hendy A., Mokili J.L., Wamala J.F., Kaducu J., Kur L. Nodding syndrome and epilepsy in onchocerciasis endemic regions: comparing preliminary observations from South Sudan and the Democratic Republic of the Congo with data from Uganda. BMC Res Notes. 2016;9:182. doi: 10.1186/s13104-016-1993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colebunders R., Tepage F., Rood E., Mandro M., Abatih E.N., Musinya G. Prevalence of river epilepsy in the Orientale Province in the Democratic Republic of the Congo. PLoS Negl Trop Dis. 2016;10:e0004478. doi: 10.1371/journal.pntd.0004478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers J.M. Linear models. In: Chambers J.M., Hastie T.J., editors. Statistical models. Wadsworth and Brooks/Cole; 1992. [Google Scholar]
- 16.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2015. R: a language and environment for statistical computing. [Google Scholar]
- 17.Crosskey . John Wiley; Chichester, UK: 1990. The natural history of blackflies. [Google Scholar]
- 18.Kamuyu G., Bottomley C., Mageto J., Lowe B., Wilkins P.P., Noh J.C. Exposure to multiple parasites is associated with the prevalence of active convulsive epilepsy in Sub-Saharan Africa. PLoS Negl Trop Dis. 2014;8:e2908. doi: 10.1371/journal.pntd.0002908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkler A.S., Friedrich K., Velicheti S., Dharsee J., Konig R., Nassri A. MRI findings in people with epilepsy and nodding syndrome in an area endemic for onchocerciasis: an observational study. Afr Health Sci. 2013;13:529–540. doi: 10.4314/ahs.v13i2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konig R., Nassri A., Meindl M., Matuja W., Kidunda A.R., Siegmund V. The role of Onchocerca volvulus in the development of epilepsy in a rural area of Tanzania. Parasitology. 2010;137:1559–1568. doi: 10.1017/S0031182010000338. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser C., Pion S.D., Boussinesq M. Case–control studies on the relationship between onchocerciasis and epilepsy: systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7:e2147. doi: 10.1371/journal.pntd.0002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emukah E.C., Osuoha E., Miri E.S., Onyenama J., Amazigo U., Obijuru C. A longitudinal study of impact of repeated mass ivermectin treatment on clinical manifestations of onchocerciasis in Imo State, Nigeria. Am J Trop Med Hyg. 2004;70:556–561. [PubMed] [Google Scholar]
- 23.Edungbola L.D., Watts S.J., Kayode O.O. Endemicity and striking manifestations of onchocerciasis in Shao, Kwara State, Nigeria. Afr J Med Sci. 1987;16:147–156. [PubMed] [Google Scholar]
- 24.Asio S.M., Simonsen P.E., Onapa A.W. Mansonella perstans filariasis in Uganda: patterns of microfilaraemia and clinical manifestations in two endemic communities. Trans R Soc Trop Med Hyg. 2009;103:266–273. doi: 10.1016/j.trstmh.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Wadman M. African outbreak stumps experts. Nature. 2011;475:148–149. doi: 10.1038/475148a. [DOI] [PubMed] [Google Scholar]
- 26.Johnson T, Tyagi R, Lee PR, Lee M, Johnson KR, Kowalak J, et al. Detection of auto-antibodies to leiomodin-1 in patients with nodding syndrome [abstract]. Neuroimmunology. http://dx.doi.org/10.1016/j.jneuroim.2014.08.275.
- 27.Idro R., Opar B., Wamala J., Abbo C., Onzivua S., Mwaka D.A. Is nodding syndrome an Onchocerca volvulus induced neuro-inflammatory disorder? Uganda's story of research in understanding the disease. Int J Infect Dis. 2016;45:112–117. doi: 10.1016/j.ijid.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Colebunders R, Hendy A, Nanyunja M, Wamala JF, van Oijen M. Nodding syndrome—a new hypothesis and new direction for research. Int J Infect Dis 2014;27:74-7. http://dx.doi.org/10.1016/j.ijid.2014.08.001. [DOI] [PubMed]
- 29.Wamala J.F., Malimbo M., Tepage F., Lukwago L., Okot C.L., Cannon R.O. Nodding syndrome may be only the ears of the hippo. PLoS Negl Trop Dis. 2015;9:e0003880. doi: 10.1371/journal.pntd.0003880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubé C.M., McClelland S., Choy M.K., Brewster A.L., Noam Y., Baram T.Z. Fever, febrile seizures and epileptogenesis. In: Noebels J.L., Avoli M., Rogawski M.A., Olsen R.W., Delgado-Escueta A.V., editors. Jasper's Basic Mechanisms of the Epilepsies [Internet] 4th edition. National Center for Biotechnology Information (US); Bethesda (MD): 2012. [PubMed] [Google Scholar]
- 31.Trees A.J., Graham S.P., Renz A., Bianco A.E., Tanya V. Onchocerca ochengi infections in cattle as a model for human onchocerciasis: recent developments. Parasitology. 2000;120(Suppl):S133–S142. doi: 10.1017/s0031182099005788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.