Abstract
As with many processes in nature, appropriate timing in biological systems is of paramount importance. In the neuroendocrine system, the efficacy of hormonal influence on major bodily functions, such as reproduction, metabolism and growth, relies on timely communication within and across many of the brain's homeostatic systems. The activity of these circuits is tightly orchestrated with the animal's internal physiological demands and external solar cycle by a master circadian clock. In mammals, this master clock is located in the hypothalamic suprachiasmatic nucleus (SCN), where the ensemble activity of thousands of clock neurones generates and communicates circadian time cues to the rest of the brain and body. Many regions of the brain, including areas with neuroendocrine function, also contain local daily clocks that can provide feedback signals to the SCN. Although much is known about the molecular processes underpinning endogenous circadian rhythm generation in SCN neurones and, to a lesser extent, extra‐SCN cells, the electrical membrane clock that acts in partnership with the molecular clockwork to communicate circadian timing across the brain is poorly understood. The present review focuses on some circadian aspects of reproductive neuroendocrinology and processes involved in circadian rhythm communication in the SCN, aiming to identify key gaps in our knowledge of cross‐talk between our daily master clock and neuroendocrine function. The intention is to highlight our surprisingly limited understanding of their interaction in the hope that this will stimulate future work in these areas.
Keywords: suprachiasmatic nuclei, circadian rhythm, clock genes, neuroendocrine system, reproduction, electrical activity, ion channels
The developmental onset of some fundamental neuroendocrine processes of the body, such as those involved in reproductive maturity, ranges from months to years depending on the species in question, and relies on constellations of tightly regulated hormonal signalling processes within the brain and body. Once established, this timely coordinated balance in hormonal activity and reproduction is temporally regulated by our master circadian clock in the suprachiasmatic nuclei (SCN). Indeed, work spanning over 50 years is unravelling the important and intimate link between SCN activity and appropriate timing in neuroendocrine function.
Circadian rhythms are daily timing in behaviour and physiology that persist (free run) in the absence of external time cues, such as light. In mammals, the master circadian clock resides in the SCN, where the activity of thousands of cell‐autonomous clock neurones is synchronised each day to output ensemble circadian time cues to the rest of the brain and body. At its most basic level, the circadian clockwork is conceptualised as an intrinsic intracellular process involving a dynamic interplay between the protein products of core clock genes such as Period1/2 (Per1/2), Cryptochrome 1/2 (Cry 1/2), Clock and Bmal1. In the past three decades, significant progress has been made to dissect and understand the intricate inner workings of this molecular timing machine that operates as a near 24‐h transcription–translation molecular feedback loop (TTFL) 1, 2.
Our current understanding of circadian rhythm generation in the SCN is that the TTFL activity within single SCN neurones drives daily changes in membrane excitability 3, 4 (although see also the later section on the membrane clock). Indeed, neurones of the central nervous system can alter their intrinsic excitability by modulating the activity of their ion channels that provide excitatory or inhibitory drive to the membrane 5. How the TTFL achieves this, however, is largely unknown. Broadly speaking, activation of sodium and some calcium channels and/or reduction in potassium channel activity depolarise the resting membrane potential (RMP) of the neurones, causing action potential (AP) discharge. By contrast, activation of potassium and/or chloride channels provides hyperpolarising forces that suppress electrical activity. In SCN neurones, both the conductivity and transcript activity of several ion channels are under circadian control 3, 4. This allows SCN neurones to generate daily rhythms in electrical outputs, a hallmark feature of SCN cells that is paramount to the functioning of the circadian timing system. Measurements of AP firing rate in unidentified SCN neurones both in vivo and in vitro SCN brain slice preparations show that, at the population level, SCN neurones are significantly more active during the day (an up state: discharging APs at 4–6 Hz) than at night (a down state: generating APs at 0.1–2 Hz) (Fig. 1. Even when isolated from the SCN tissue, single SCN neurones retain the intrinsic ability to generate daily rhythm in AP activity 6, 7 that can be maintained for days. It is considered that this day–night alteration in electrical states synchronises the activity of SCN neurones and communicates circadian information to the brain and body 3. Evidence from imaging and electrophysiological studies, however, suggests that the manner through which SCN neurones encode and communicate circadian information is more complex than first assumed and may not solely rely on day–night variation in AP firing rate.
Figure 1.
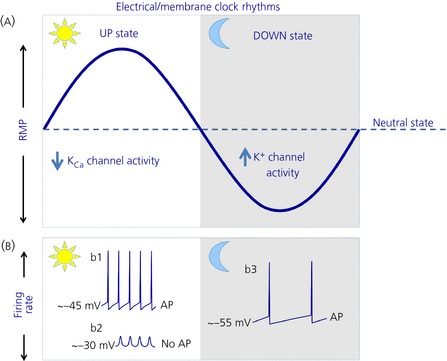
A schematic view of the daily electrical profiles of suprachiasmatic nucleus (SCN) neurones. (a) Over the day–night cycle, SCN neurones show overt changes in their resting membrane potential (RMP), and traverse through several points of neutral rest state (indicated by where the solid line crosses the dashed line). During the day, the RMP of SCN neurones is depolarised. In some cells, reduced activity of L‐type calcium and calcium activated potassium (KC a) channels partly underpins this up state 128. At night, increased conductivity of multiple potassium channels hyperpolarises the neurones, placing them into a down state 3, 4. (b) In some SCN neurones, this daytime up state causes action potential (AP) discharge (b1). In others, however, the RMP becomes too positive (~ −30 mV) to sustain AP production (b2). Instead, these neurones display depolarised low‐amplitude membrane oscillation (b2). At night, during the RMP down state, SCN neurones generate action potentials at a significantly reduced rate (b3). This shows the complexity, richness and diversity of electrical communication in SCN neurones. The light area indicates the day, whereas the shaded region shows the night. The increase or decrease underlying ion channel activity is indicated by upward‐ and downward‐pointing blue arrows, respectively.
Under natural conditions, SCN clock neurones are entrained/synchronised to the light and dark cycle by responding to the daily glutamatergic activity of the retino‐hypothalamic tract (Fig. 2). This excitatory drive is conveyed to the SCN by specialised melanopsin‐containing retinal ganglion cells that communicate ambient light levels directly to SCN neurones 8, causing Per1 gene activation in these cells 9. This allows environmental light cues to reset SCN clock neurones and coordinate their activity to generate a stable internal representation of solar time.
Figure 2.
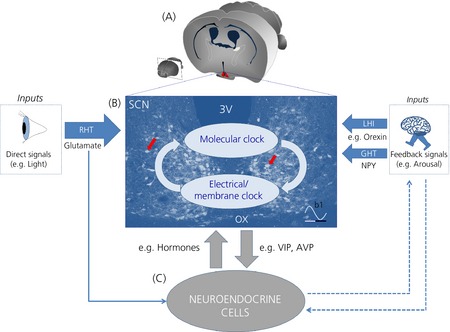
Cross‐talk between the suprachiasmatic nucleus (SCN) and neuroendocrine cells. (a) Cross‐section of the mouse brain at the anatomical level of the SCN (in red). (b) Bidirectional communication between the molecular clock (transcription–translation molecular feedback loop; TTFL) and electrical/membrane clock. In this model, the TTFL clock drives day–night rhythms in the electrical activity of SCN neurones, and electrical activity feedback onto the TTFL clock through unknown mechanisms (light blue arrows). This may underlie how the TTFL outputs signals to neuroendocrine cells (c) and how neuroendocrine processes feedback to adjust circadian timing in the SCN (grey arrows). This drawing is superimposed on top of a modified image taken at the SCN mid‐coronal section showing Per1‐EGFP neurones (red arrows). Darker blue arrows represent inputs to the SCN, with their sizes denoting feedback magnitude. Solid arrows indicate an established link, whereas broken arrows show tentative interactions. (b1) Stylised waveform showing daily variation in SCN Per1 and electrical activity. White and black bars underneath represent day and night, respectively. RHT, retino‐hypothalamic tract; LHI, lateral hypothalamic input; GHT, geniculo‐hypothalamic tract; OX, optic chiasm; 3V, third ventricle; NPY, neuropeptide Y; VIP, vasoactive intestinal polypeptide; AVP, arginine vasopressin.
Several internal physiological signals also feedback and fine tune timing precision in the SCN. Such intrinsic feedback cues [referred to as Zeitnehmer (‘time‐taker’), a term applied to input pathways that are rhythmically regulated by feedback from an oscillator] 10, 11 emerge mainly from the body's homeostatic systems and are communicated to the SCN using a variety of signalling neuropeptides to mainly suppress Per1/2 gene expression and electrical activity. These feedback signals include neuropeptide Y (NPY) neurones of the thalamic intergeniculate leaflet, which send monosynaptic projections to the SCN via the geniculo‐hypothalamic tract 12, as well as arousal‐promoting orexinergic neurones of the lateral hypothalamus 13, which also send axonal contacts to SCN neurones 14. In the SCN, these neuropeptidergic signals can converge onto single clock neurones to modulate electrical activity and circadian timing in this brain structure 14, 15. As such, SCN neurones integrate both intrinsic and extrinsic signals and send collective circadian time cues by neural and paracrine pathways to the rest of the brain, including many neuroendocrine hypothalamic structures (Fig. 2. Thus, the physiology and behaviour of the organism are tuned to anticipate and adapt to the solar day–night cycle, a key physiological prerequisite for survival and reproductive success. The SCN therefore represents a good example of localised autonomous function in the brain, and provides a unique opportunity in neuroscience to link highly organised behaviours (e.g. sleep–wake cycle, daily feeding drive and neuroendocrine functions) to the activities of a known population of neurones in the brain.
Circadian regulation of neuroendocrine functions
The realisation of the importance of circadian timing in hormone function started with the work of Everett and Sawyer in the 1950s. In a pioneering study, it was discovered that, in nocturnal rodents, a stimulatory drive occurring at a narrow temporal window in the pro‐oestrus afternoon is necessary for the induction of ovulation later at night 16. Subsequently, a number of studies both in primates (including humans) and rodents have reported circadian rhythms in gonadotrophin‐releasing hormone (GnRH) gene expression 17 and in the levels of many of the body's endocrine hormones, such as luteinising hormone (LH), testosterone, prolactin (PRL), cortisol and gonadotrophins after the onset of puberty 18, 19, 20, 21, 22. A more in‐depth description of hormonal secretion profiles is provided elsewhere 23.
Indeed, when placed in constant conditions, the oestrus cycle in rodents free‐runs 24, 25; a description of circadian terms is provided in Liu et al. 26. This supports its reliance on a master timing process governing daily cycles in mammalian endocrine physiology. Furthermore, ablation of the SCN or its output pathways abolishes the night‐time LH surge and circadian rhythms in the release of a number of endocrine hormones, such as PRL and gonadotrophin 27, 28, 29.
Perhaps the most direct evidence of an SCN‐dependent driven rhythm in hormonal secretion comes from elegant studies in female hamsters. Under normal conditions, both halves of the bilateral SCN in hamsters operate in synchrony. When these animals are housed in constant light, however, the SCN activity and behavioural rhythms ‘split’ in some hamsters, giving rise to two activity bouts in a 24‐h period 30. Remarkably, these ‘split’ females display two daily LH surges, each in anti‐phase and of half the concentration seen in ‘nonsplit’ controls 31. More recently, work in rodents has definitively linked circadian clock gene activity in the SCN with circadian timing in hormone synthesis and secretion 32.
Taken together, these studies demonstrate that important aspects of neuroendocrine physiology exhibit circadian variation, and that the master circadian clock is central to the timing of endocrine processes. This provides an excellent model system in which to investigate circadian and endocrine interactions.
Circadian signalling to neuroendocrine targets
Neurones of the SCN are neurochemically and functionally heterogeneous and form distinct anatomical clusters within this brain structure. Ventral SCN neurones synthesise vasoactive intestinal polypeptide (VIP), whereas the dorsal neurones contain arginine vasopressin (AVP) 33, two neuropeptides that are rhythmically produced in the SCN and are critically involved in appropriate circadian function 6, 34, 35, 36. Although the VPAC2 receptor, the preferred receptor for VIP in the SCN, is expressed throughout this nucleus 37, VIP neurones generally project dorsally to the vicinity of AVP cells 33. Here, they form an ensemble bundle with AVP‐axons that project away from the SCN.
In addition to forming conventional cell‐to‐cell synaptic contacts, SCN neurones can also signal circadian timing to the body in a paracrine fashion. Indeed, the timing of behavioural rhythms in rodents, such as locomotor activity, drinking and gnawing, are under the control of the SCN paracrine tone 38, 39, 40, 41. An ingenious in vitro study has also recently shown that some intrinsic SCN clock function relies on paracrine communication amongst its neurones (mainly through diffusible neuropeptides such as VIP) 42, although further in vivo studies are needed to establish the role of paracrine signalling in SCN function. When sending circadian signals to the neuroendocrine system, however, the SCN does so via direct synaptic contacts onto endocrine cells or through populations of neurones that contact endocrine cells. This suggests that, unlike daily timing in behaviour (neuromodulation) 43, SCN communication to neuroendocrine cells requires the precision and speed of discrete synaptic transmission.
In the hypothalamic preoptic area (POA) of many species, VIP terminals originating from the SCN form direct contacts onto GnRH neurones, and indirectly connect to oestrodiol‐concentrating interneurones that communicate to GnRH cells 30, 44, 45, 46, 47. The VPAC2 receptor is expressed by a subset of GnRH neurones 48, 49 and VIP can directly modulate the activity of GnRH cells in a time‐of‐day related manner, therefore controlling the timing of LH release 50, 51, 52, 53. Alteration of VIP signalling both in vivo and in vitro causes marked changes in GnRH, LH and gonadotrophin release 54, 55, 56, as well as in the magnitude and timing of the LH surge 55, 57. Evidence also suggests that SCN VIP signalling to neuroendocrine dopaminergic neurones is involved in modulating pituitary PRL release 58. Indeed, paraventricular nucleus (PVN) dopaminergic neurones express the VPAC2 receptor, hypothalamic VIP expression is in phase with PRL release, and work in rodents indicates that the activity of PVN dopaminergic cells suppresses pituitary PRL secretion 59, 60, 61.
There is also strong evidence in rodents suggesting that AVP signalling in the POA modulates the activity of GnRH neurones and is involved in PRL and LH secretion. AVP fibres contact GnRH neurones, and AVP secretion occurs in phase with GnRH release 45, 62. Furthermore, work in female rats shows that mRNA for the AVP receptor (V1aR) is expressed by a small population of POA GnRH neurones 63 and blockade of AVP receptors attenuates LH and PRL release 64. In SCN‐lesioned animals, time‐of‐day‐dependent AVP administration in the medial preoptic area rescues the LH surge 65, 66, 67. In the CLOCK‐Δ19 mutant mouse, where the activity of the circadian clock is severely disrupted, and the LH surge is dampened, the expression of AVP and V1aR in the SCN is also significantly reduced 68. Administration of AVP into the POA of these animals rescues the LH surge 68.
In the past few decades, it has also become increasingly clear that electrical and TTFL activity of SCN neurones contributes to the regulation of seasonal rhythms 69. Day‐length encoding by the SCN relies strongly on the plasticity of its neuronal network 70, which allows this brain structure to shorten and lengthen the phase relationship among its neurones, coding for the short winter and long summer days, respectively. This regulates the activity of key regions of the body that are important for seasonal changes in reproductive function, such as the seasonal release profile of the pineal gland neurohormone melatonin 69.
Taken together, these observations link the activity of the SCN, the plasticity of its neural circuits and the rhythmic release of some of its neuropeptides with the fundamental control of neurohormone secretion.
Extra‐SCN endocrine circadian clocks
The discovery and understanding of the inner workings of the TTFL come with the realisation that, although the SCN harbours the principal daily clock, many areas of the brain and body also have local circadian clockworks 71, 72. These act in concert with the SCN to give rise to the extended circadian system. Indeed, core circadian clock genes are active in several neuroendocrine regions of the brain, pituitary and pineal glands 73, 74, 75, 76, as well as in a number of peripheral endocrine tissues, such as the adrenal gland, testis and ovary 77, 78. At the single cell level, clock gene expression is detected in GnRH and neuroendocrine dopaminergic neurones 79, 80.
Furthermore, the daily expression profile of key clock genes, such as Per1, reveals rhythmic activity in several neuroendocrine brain regions and individual neurones, such as in dopaminergic cells that control PRL secretion 79, 81, 82, 83. Studies using pituitary explants from transgenic animals bearing bioluminescent reporters of circadian clock activity have conclusively shown that pituicytes can sustain intrinsic circadian rhythms in clock gene expression persisting over several days 84, 85. Rhythmic expression of core clock genes is also reported in the pineal gland 86, in hypothalamic GnRH neurones 87, 88, 89 and in immortalised GnRH cell lines 80, 87, where they serve to regulate GnRH cell activity and gate the sensitivity of these cells to daily hormonal signals 90, 91. Disruption of clock gene activity in GnRH cell lines interrupts daily GnRH release in these cells 91 and, in preoptic‐explants from Bmal1 knockout mice, the stimulating drive in GnRH release is significantly compromised 88. Clock gene mutant rodent models are now confirming that disruption of appropriate circadian timing signals impairs hypothalamic and peripheral hormone secretion and interferes with reproductive success 92, 93.
Taken together, these observations demonstrate that the functioning of local circadian clocks in neuroendocrine tissues and at the level of the SCN is important for normal neuroendocrine function. These neuroendocrine clock circuits may not only serve as gating mechanisms that determine whether and how neuroendocrine cells respond to periodic SCN time cues, but also provide tissue‐specific local circadian time signals. This may influence output feedback signals from these tissues to the SCN 94. As such, the extended circadian system interacts to maintain optimal daily temporal alignment in physiology across the many tissues and organ systems of the body, although how SCN output molecules affect circadian gene expression (e.g. Per1) in neuroendocrine cells remains unknown.
Neuroendocrine feedback to the SCN: neuroendocrine neurones talking back
Although there is limited evidence of direct neuroendocrine cell projections to the SCN 95, 96, 97, 98, several rhythmic hormonal output signals originating from neuroendocrine cell populations are known to act in the SCN. For example, in all animal species studied thus far, including humans, high‐affinity receptors for melatonin, oestrogen, androgen and progesterone are present in the SCN 99, 100, 101, 102, 103, 104. These hormones can act to modulate the electrical activity of SCN neurones and adjust the phase of the SCN clockworks 105, 106, 107, 108, 109. Furthermore, enzymatic conversion of these hormones, such as the aromatisation of testosterone to oestrogen, provides additional complexity to hormonal signals conveyed to the SCN 110. However, unlike the well‐studied actions of feedback signals (e.g. NPY, serotonin and melatonin) on SCN clock functions, it remains unknown whether the phase of the molecular clockwork and the resulting electrical state of SCN neurones determines whether and how sex hormones act in the SCN. Moreover, how neuroendocrine feedback signals affect SCN circadian gene expression is also not known. Thus, more work is needed before the effects of these hormones on the SCN clockwork can be fully determined.
Although significant recent progress in circadian neuroendocrine studies now places the activity of the newly‐identified kisspeptin and RFamide‐related peptide at the core of the communication conduits interfacing SCN signals with neuroendocrine activity 111, 112, many key questions still remain. For example, how does circadian information encoded by the hands of the clock (TTFL) translate into meaningful outputs that can be processed by the rest of the brain, including neuroendocrine cells, and how does the brain talk back to sculpt circadian timing in SCN clock neurones? Furthermore, retinal melanopsin neurones that project to the SCN via the RHT also form functional connectivity with a number of neuroendocrine hypothalamic regions 113, 114. Therefore, can environmental light directly modulate the activity of hypothalamic neuroendocrine clock neurones and influence circadian and seasonal timing in these cells? To achieve such communication, signals travelling to and from the TTFL both in the SCN and neuroendocrine clock neurones must flow through the cell membrane (Fig. 3). However, the mechanistic nature of these signals that interweave the molecular clockwork and membrane activity to produce physiological circadian rhythms is largely unknown. This is a dilemma that is now hampering major progress in circadian biology research, although some progress has been made in the SCN. Below, reference is made to the membrane clock as a model for addressing the mechanisms of information flow between the membrane and TTFLs: the all‐important electrical–genetic interplay.
Figure 3.
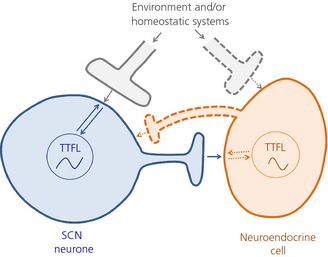
A schematic view of communication between the transcription–translation molecular feedback loop (TTFL) in the suprachiasmatic nucleus (SCN) and neuroendocrine cells through the membrane clock. In this model, signals travelling to and from the TTFL both in the SCN and neuroendocrine clock neurones must flow through the cell membrane (bidirectional arrows) by unknown mechanisms. This process may underlie how the SCN TTFL outputs circadian signals to neuroendocrine cells (blue solid axon and arrow) and how neuroendocrine TTFL feedback to adjust circadian timing in SCN neurones (orange dotted axon and arrow). Through similar unknown processes, environmental and/or homeostatic cues can sculpt the activity of the TTFL in SCN and endocrine neurones (grey solid and dotted axon terminals, and arrows). Solid and broken axons/arrows show known and tentative links, respectively.
The membrane clock
Over the past 10 years, pioneering studies have revealed that the membrane properties, activity and excitability of SCN neurones are not only the proximal target of the TTFL, but also act to transmit information from the external world and body to the circadian molecular clockwork (Figs 2 and 3). Indeed, work on mammalian and Drosophila clock neurones strongly supports the concept that the electrical activity of clock neurones is integral to the functioning of the intracellular molecular clockwork 115, 116, 117, 118, 119. Indeed, the electrical state of clock neurones can impose time‐of‐day stamps onto its transcriptional programmes, thereby acting as an intrinsic zeitgeber (time‐giver) 120. Studies performed in cultured neonatal SCN slices have also established a tight link between the TTFL activity, as measured by bioluminescence imaging, and membrane excitability in clock neurones. Here, abolishing AP discharge by tetrodotoxin (TTX) blockade of sodium channel activity, which also stops AP‐dependent neurone‐to‐neurone communication, desynchronised the SCN clock and dampened TTFL rhythm amplitude within individual SCN neurones 121, 122. Interestingly, however, similar analysis using SCN brain slices from young adults showed that TTX administration had much less dramatic effects (123; A. T. Hughes and H. D. Piggins, unpublished observations). In support, in vivo chronic TTX infusion in adult animals also failed to interfere with SCN function 124. This highlights the complex relationship between the TTFL and membrane clock and/or suggests developmental issues within the SCN neuronal network that require careful consideration 125. Therefore, from the paracrine influence to the electrical–genetic interplay in single SCN neurones, the SCN neural network plays a critical role in adult SCN function 70.
Nevertheless, growing evidence supports the idea that information flow between the membrane and TTFL may well underlie the processes by which neurotransmitters that are released from retinal cells and the body's homeostatic systems sculpt the timing precision in the SCN. TTFL–membrane communication might also underpin how the SCN network organises excitability of its neurones to accommodate extrinsic cues to communicate circadian and seasonal signals to extra‐SCN TTFLs in the body (Fig. 3). Our limited mechanistic understanding of how this is achieved, however, may be inherently linked with the way we measure and report excitability in clock neurones, primarily in the SCN.
Further considerations: measuring electrical excitability in SCN neurones
Although the rate at which SCN neurones discharge APs is routinely used to report clock phase and functionality, measurement of AP firing frequency alone is not providing us with the complete picture. This is because SCN neurones also show overt day–night differences in their RMP and input resistance (Fig. 1 126, which are other important modes of excitability in SCN neurones. Relying on AP firing rate measurements alone means that excitability in SCN neurones cannot be assessed when they are not producing APs; even in the up state during the day, single SCN neurones generate APs for 4–6 h only and not for the duration of the light phase. Further complexity arises when considering that not all SCN neurones contain a functional molecular clock 70.
Indeed, targeted recording of fluorescence tagged SCN neurones [from mice in which enhanced green fluorescent protein (EGFP) indicates Per1 promoter activity (Per1‐EGFP+ve cells)] 127 demonstrates that the electrical activity of SCN neurones is more complex and richer than was first assumed 128. It was revealed that the well‐described day–night difference in AP generation in the SCN mostly comprises the activity of neurones in which the EGFP construct cannot be detected (EGFP‐ve cells: presumed nonclock neurones). Per1‐EGFP+ve SCN neurones stop discharging APs in the middle of the day as their RMP becomes too positive (~ −30 mV) to sustain AP generation. Instead, through reducing the activity of their calcium‐activated potassium channels, these neurones enter a state of intrinsic depolarisation silencing (depolarisation blockade), producing L‐type calcium channel dependent depolarised low‐amplitude membrane oscillations (DLAMOs) instead of APs 128 (Fig. 1 b). These modes of excitability may be important for the normal functioning of the circadian clock, and may be necessary to modulate intracellular calcium levels in SCN neurones, a key signalling molecule in SCN circadian rhythm generation processes 3, 4. Indeed, the relative inability of TTX to suppress TTFL rhythms in young adult SCN neurones 123 suggests that continuous AP production may not be the sole mechanism for intercellular communication and calcium signalling in this brain structure.
In recent years, several studies using genetically encoded calcium sensors (e.g. GCaMP3) or synthetic fluorescent probes (e.g. Fura‐2) have documented the steady‐state intracellular calcium [Ca2+]i concentration in SCN neurones. Most 129, 130, 131, 132, but not all 133, reported higher [Ca2+]i levels during the day than at night. Furthermore, the concentration of [Ca2+]i reported by these studies across the day and at night is not consistent. This ranges from 191 to 440 nm during the day and from 50 to 119 nm at night. Moreover, although, under some experimental conditions, TTX abolished the day–night difference in [Ca2+]i levels 134, in others, TTX had no effect 131. Although, across experiments, animal age (neonate versus young adult tissues), species differences (rat versus mouse) and [Ca2+]i detection methods (variety of genetically encoded calcium sensors used and Fura‐2) may account for some of these discrepancies, there is no doubt that heterogeneity in SCN neurones and as yet unconfirmed excitability states of SCN cells (AP versus TTX insensitive depolarised DLAMOs) contribute significantly to these apparent inconsistencies. In support, from a single study by Hong et al. 135, TTX suppression of day–night [Ca2+]i rhythms was seen in only half of SCN neurones, leaving daily cycles of this neuronal excitability measure unaffected in others.
Although the steady‐state [Ca2+]i concentration induced by DLAMOs is yet to be described, this electrical state may be crucial for optimal daytime calcium signalling and circadian rhythm generation by clock genes 115, 119. Indeed, the predicted [Ca2+]i concentration during DLAMOs 115 is consistent with the higher [Ca2+]i concentration and calcium channel conductance values [440 nm 131 and > 40 pA 136, respectively] measured experimentally in SCN neurones. An extreme DLAMO‐like depolarised state (~ −30 mV) is observed in cerebellar and habenular neurones 137, 138, 139 and can be induced in central amygdala neurones 140. Although we have yet to determine whether single neurones in these brain areas are cell‐autonomous circadian oscillators, clock genes are expressed in these brain regions 71, 81, 141.
Taken together, these observations show that electrical signalling in the SCN is complex and that extreme depolarisation in central neurones extends beyond the borders of the SCN. This raises the possibility that these severe depolarised electrical states are more widespread than previously assumed and may be necessary for normal brain function. Studying these electrical states may reconcile some of the apparent inconsistencies seen in SCN neurophysiology and may extend our understanding of the all‐important electrical–genetic interactions in this brain structure, which may also highlight functionality in other brain circuits containing circadian clock genes. Indeed, to date, we have no records of day–night electrical excitability and [Ca2+]i levels in neuroendocrine neurones. We also have little understanding of whether and how neuroendocrine cells change their excitability to SCN and other hormonal cues over the circadian day, and whether changes in excitability of these cells can influence their genetic programmes (Fig. 3).
Conclusions and perspectives
Despite our growing understanding of the cell‐autonomous processes that drive circadian rhythms in clock gene expression, our knowledge of how the molecular clockwork interacts with the membrane to drive daily changes in excitability of SCN neurones is limited. Furthermore, the nature of membrane feedback signals that convey external information to the SCN molecular clockwork is also unknown. Clock genes are found in many of the body's tissue and organ systems, and these extra‐SCN clocks are considered to be vital in providing local circadian timing in a tissue‐specific manner 142. Understanding the nature of the signals responsible for reciprocal connectivity between the molecular clockwork and membrane activity will unravel how circadian rhythms are generated and communicated in SCN and endocrine neurones. Furthermore, this will also reveal how circadian gene activity in neuroendocrine cells gates excitability in these cells to hormones, SCN signals and other physiological demands. This is important if we are to understand how circadian signals influence reproductive neuroendocrinology.
The observation that SCN neurones can convey circadian time cues to each other and to the body both by neural and paracrine signals demonstrates the diversity and richness of communication ‘modes’ in the SCN. Targeted recording of SCN neurones is also adding to our understanding that the electrical repertoire of SCN neurones is much broader than first appreciated. This highlights the challenges facing chronobiologists, physiologists, endocrinologists and neuroscientists in understanding circadian rhythm generation and communication in both the SCN and its targets. It is therefore prudent that, in addition to AP frequency measurements, multiple parameters of membrane excitability are assessed, preferably simultaneously, when interrogating the electrical state of SCN and extra‐SCN neurones. This will greatly increase our understanding of the processes governing cross‐talk between the master circadian clock and the neuroendocrine system, and how these signalling conduits are influenced by external cues.
Acknowledgements
I thank Professor Hugh Piggins for his critical reading of the manuscript and for his continuing support. I also would like to thank Drs N. Glossop, B. Bano‐Otalora, A. Hughes and N. Nunn for their constructive comments and discussions. This work is for the Michael Harbuz Young Investigator Perspective Prize, awarded by the British Society for Neuroendocrinology, and is supported by a BBSRC grant to Hugh Piggins and David Bechtold with MDCB as co‐investigator. The author has no conflicts of interest to declare.
References
- 1. Glossop NR. Circadian timekeeping in Drosophila melanogaster and Mus musculus. Essays Biochem 2011; 49: 19–35. [DOI] [PubMed] [Google Scholar]
- 2. Mohawk JA, Takahashi JS. Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci 2011; 34: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci 2011; 12: 553–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown TM, Piggins HD. Electrophysiology of the suprachiasmatic circadian clock. Prog Neurobiol 2007; 82: 229–255. [DOI] [PubMed] [Google Scholar]
- 5. Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates Inc., 2001. [Google Scholar]
- 6. Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci 2005; 8: 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 1995; 14: 697–706. [DOI] [PubMed] [Google Scholar]
- 8. Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW, O'Hagan JB, Price LL, Provencio I, Skene DJ, Brainard GC. Measuring and using light in the melanopsin age. Trends Neurosci 2014; 37: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lowrey PL, Takahashi JS. Genetics of the mammalian circadian system: photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annu Rev Genet 2000; 34: 533–562. [DOI] [PubMed] [Google Scholar]
- 10. Hughes AT, Piggins HD. Feedback actions of locomotor activity to the circadian clock. Prog Brain Res 2012; 199: 305–336. [DOI] [PubMed] [Google Scholar]
- 11. Roenneberg T, Kantermann T, Juda M, Vetter C, Allebrandt KV. Light and the human circadian clock. Handb Exp Pharmacol 2013; 217: 311–331. [DOI] [PubMed] [Google Scholar]
- 12. Morin LP. Neuroanatomy of the extended circadian rhythm system. Exp Neurol 2013; 243: 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mieda M, Sakurai T. Overview of orexin/hypocretin system. Prog Brain Res 2012; 198: 5–14. [DOI] [PubMed] [Google Scholar]
- 14. Belle MD, Hughes AT, Bechtold DA, Cunningham P, Pierucci M, Burdakov D, Piggins HD. Acute suppressive and long‐term phase modulation actions of orexin on the mammalian circadian clock. J Neurosci 2014; 34: 3607–3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Besing RC, Hablitz LM, Paul JR, Johnson RL, Prosser RA, Gamble KL. Neuropeptide Y‐induced phase shifts of PER2:LUC rhythms are mediated by long‐term suppression of neuronal excitability in a phase‐specific manner. Chronobiol Int 2012; 29: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Everett JW, Sawyer CH. A 24‐hour periodicity in the ‘LH‐release apparatus’ of female rats, disclosed by barbiturate sedation. Endocrinology 1950; 47: 198–218. [DOI] [PubMed] [Google Scholar]
- 17. Gore AC. Diurnal rhythmicity of gonadotropin‐releasing hormone gene expression in the rat. Neuroendocrinology 1998; 68: 257–263. [DOI] [PubMed] [Google Scholar]
- 18. Plant TM. Time courses of concentrations of circulating gonadotropin, prolactin, testosterone, and cortisol in adult male rhesus monkeys (Macaca mulatta) throughout the 24 h light–dark cycle. Biol Reprod 1981; 25: 244–252. [DOI] [PubMed] [Google Scholar]
- 19. Andrews WW, Ojeda SR. A detailed analysis of the serum luteinizing hormone secretory profile in conscious, free‐moving female rats during the time of puberty. Endocrinology 1981; 109: 2032–2039. [DOI] [PubMed] [Google Scholar]
- 20. Boyar RM, Wu RH, Roffwarg H, Kapen S, Weitzman ED, Hellman L, Finkelstein JW. Human puberty: 24‐hour estradiol in pubertal girls. J Clin Endocrinol Metab 1976; 43: 1418–1421. [DOI] [PubMed] [Google Scholar]
- 21. Jakacki RI, Kelch RP, Sauder SE, Lloyd JS, Hopwood NJ, Marshall JC. Pulsatile secretion of luteinizing hormone in children. J Clin Endocrinol Metab 1982; 55: 453–458. [DOI] [PubMed] [Google Scholar]
- 22. Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology 1975; 96: 57–62. [DOI] [PubMed] [Google Scholar]
- 23. Hastings MH. Neuroendocrine rhythms. Pharmacol Ther 1991; 50: 35–71. [DOI] [PubMed] [Google Scholar]
- 24. Alleva JJ, Waleski MV, Alleva FR. A biological clock controlling the estrous cycle of the hamster. Endocrinology 1971; 88: 1368–1379. [DOI] [PubMed] [Google Scholar]
- 25. Fitzgerald K, Zucker I. Circadian organization of the estrous cycle of the golden hamster. Proc Natl Acad Sci USA 1976; 73: 2923–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu AC, Lewis WG, Kay SA. Mammalian circadian signaling networks and therapeutic targets. Nat Chem Biol 2007; 3: 630–639. [DOI] [PubMed] [Google Scholar]
- 27. Antunes‐Rodrigues J, McCann SM. Effect of suprachiasmatic lesions on the regulation of luteinizing hormone secretion in the female rat. Endocrinology 1967; 81: 666–670. [DOI] [PubMed] [Google Scholar]
- 28. Pan JT, Gala RR. Central nervous system regions involved in the estrogen‐induced afternoon prolactin surge. I. Lesion studies. Endocrinology 1985; 117: 382–387. [DOI] [PubMed] [Google Scholar]
- 29. Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology 1982; 34: 395–404. [DOI] [PubMed] [Google Scholar]
- 30. de la Iglesia HO, Meyer J, Schwartz WJ. Lateralization of circadian pacemaker output: activation of left‐ and right‐sided luteinizing hormone‐releasing hormone neurons involves a neural rather than a humoral pathway. J Neurosci 2003; 23: 7412–7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Swann JM, Turek FW. Multiple circadian oscillators regulate the timing of behavioral and endocrine rhythms in female golden hamsters. Science 1985; 228: 898–900. [DOI] [PubMed] [Google Scholar]
- 32. Poletini MO, McKee DT, Kennett JE, Doster J, Freeman ME. Knockdown of clock genes in the suprachiasmatic nucleus blocks prolactin surges and alters FRA expression in the locus coeruleus of female rats. Am J Physiol Endocrinol Metab 2007; 293: E1325–E1334. [DOI] [PubMed] [Google Scholar]
- 33. Morin LP, Allen CN. The circadian visual system, 2005. Brain Res Rev 2006; 51: 1–60. [DOI] [PubMed] [Google Scholar]
- 34. Hughes AT, Guilding C, Lennox L, Samuels RE, McMahon DG, Piggins HD. Live imaging of altered period1 expression in the suprachiasmatic nuclei of Vipr2–/– mice. J Neurochem 2008; 106: 1646–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maywood ES, Reddy AB, Wong GK, O'Neill JS, O'Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol 2006; 16: 599–605. [DOI] [PubMed] [Google Scholar]
- 36. Kalsbeek A, Fliers E, Hofman MA, Swaab DF, Buijs RM. Vasopressin and the output of the hypothalamic biological clock. J Neuroendocrinol 2010; 22: 362–372. [DOI] [PubMed] [Google Scholar]
- 37. Usdin TB, Bonner TI, Mezey E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology 1994; 135: 2662–2680. [DOI] [PubMed] [Google Scholar]
- 38. Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci 1987; 7: 1626–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science 1990; 247: 975–978. [DOI] [PubMed] [Google Scholar]
- 40. Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 1996; 382: 810–813. [DOI] [PubMed] [Google Scholar]
- 41. Silver R, Lehman MN, Gibson M, Gladstone WR, Bittman EL. Dispersed cell suspensions of fetal SCN restore circadian rhythmicity in SCN‐lesioned adult hamsters. Brain Res 1990; 525: 45–58. [DOI] [PubMed] [Google Scholar]
- 42. Hastings MH, Brancaccio M, Maywood ES. Circadian pacemaking in cells and circuits of the suprachiasmatic nucleus. J Neuroendocrinol 2014; 26: 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bucher D, Marder E. SnapShot: neuromodulation. Cell 2013; 155: 482. [DOI] [PubMed] [Google Scholar]
- 44. Van Der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin‐releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol 1997; 384: 569–579. [DOI] [PubMed] [Google Scholar]
- 45. Mahoney MM, Smale L. Arginine vasopressin and vasoactive intestinal polypeptide fibers make appositions with gonadotropin‐releasing hormone and estrogen receptor cells in the diurnal rodent Arvicanthis niloticus. Brain Res 2005; 1049: 156–164. [DOI] [PubMed] [Google Scholar]
- 46. Van Der Beek EM, Wiegant VM, van der Donk HA, Van den Hurk R, Buijs RM. Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide‐containing projection to gonadotrophin‐releasing hormone neurons in the female rat. J Neuroendocrinol 1993; 5: 137–144. [DOI] [PubMed] [Google Scholar]
- 47. Van Der Beek EM, van Oudheusden HJ, Buijs RM, van der Donk HA, Van den Hurk R, Wiegant VM. Preferential induction of c‐fos immunoreactivity in vasoactive intestinal polypeptide‐innervated gonadotropin‐releasing hormone neurons during a steroid‐induced luteinizing hormone surge in the female rat. Endocrinology 1994; 134: 2636–2644. [DOI] [PubMed] [Google Scholar]
- 48. Smith MJ, Jiennes L, Wise PM. Localization of the VIP2 receptor protein on GnRH neurons in the female rat. Endocrinology 2000; 141: 4317–4320. [DOI] [PubMed] [Google Scholar]
- 49. Olcese J, McArdle CA, Middendorff R, Greenland K. Pituitary adenylate cyclase‐activating peptide and vasoactive intestinal peptide receptor expression in immortalized LHRH neurons. J Neuroendocrinol 1997; 9: 937–943. [DOI] [PubMed] [Google Scholar]
- 50. Christian CA, Moenter SM. Vasoactive intestinal polypeptide can excite gonadotropin‐releasing hormone neurons in a manner dependent on estradiol and gated by time of day. Endocrinology 2008; 149: 3130–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alexander MJ, Clifton DK, Steiner RA. Vasoactive intestinal polypeptide effects a central inhibition of pulsatile luteinizing hormone secretion in ovariectomized rats. Endocrinology 1985; 117: 2134–2139. [DOI] [PubMed] [Google Scholar]
- 52. Stobie KM, Weick RF. Vasoactive intestinal peptide inhibits luteinizing hormone secretion: the inhibition is not mediated by dopamine. Neuroendocrinology 1989; 49: 597–603. [DOI] [PubMed] [Google Scholar]
- 53. Weick RF, Stobie KM. Vasoactive intestinal peptide inhibits the steroid‐induced LH surge in the ovariectomized rat. J Endocrinol 1992; 133: 433–437. [DOI] [PubMed] [Google Scholar]
- 54. Ohtsuka S, Miyake A, Nishizaki T, Tasaka K, Tanizawa O. Vasoactive intestinal peptide stimulates gonadotropin‐releasing hormone release from rat hypothalamus in vitro . Acta Endocrinol (Copenh) 1988; 117: 399–402. [DOI] [PubMed] [Google Scholar]
- 55. Van Der Beek EM, Swarts HJ, Wiegant VM. Central administration of antiserum to vasoactive intestinal peptide delays and reduces luteinizing hormone and prolactin surges in ovariectomized, estrogen‐treated rats. Neuroendocrinology 1999; 69: 227–237. [DOI] [PubMed] [Google Scholar]
- 56. Kimura F, Mitsugi N, Arita J, Akema T, Yoshida K. Effects of preoptic injections of gastrin, cholecystokinin, secretin, vasoactive intestinal peptide and PHI on the secretion of luteinizing hormone and prolactin in ovariectomized estrogen‐primed rats. Brain Res 1987; 410: 315–322. [DOI] [PubMed] [Google Scholar]
- 57. Harney JP, Scarbrough K, Rosewell KL, Wise PM. In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging‐like changes in the estradiol‐induced luteinizing hormone and prolactin surges. Endocrinology 1996; 137: 3696–3701. [DOI] [PubMed] [Google Scholar]
- 58. Gerhold LM, Horvath TL, Freeman ME. Vasoactive intestinal peptide fibers innervate neuroendocrine dopaminergic neurons. Brain Res 2001; 919: 48–56. [DOI] [PubMed] [Google Scholar]
- 59. Mai LM, Shieh KR, Pan JT. Circadian changes of serum prolactin levels and tuberoinfundibular dopaminergic neuron activities in ovariectomized rats treated with or without estrogen: the role of the suprachiasmatic nuclei. Neuroendocrinology 1994; 60: 520–526. [DOI] [PubMed] [Google Scholar]
- 60. Poletini MO, Kennett JE, McKee DT, Freeman ME. Central clock regulates the cervically stimulated prolactin surges by modulation of dopamine and vasoactive intestinal polypeptide release in ovariectomized rats. Neuroendocrinology 2010; 91: 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Egli M, Bertram R, Sellix MT, Freeman ME. Rhythmic secretion of prolactin in rats: action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology 2004; 145: 3386–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Funabashi T, Shinohara K, Mitsushima D, Kimura F. Gonadotropin‐releasing hormone exhibits circadian rhythm in phase with arginine‐vasopressin in co‐cultures of the female rat preoptic area and suprachiasmatic nucleus. J Neuroendocrinol 2000; 12: 521–528. [DOI] [PubMed] [Google Scholar]
- 63. Kalamatianos T, Kallo I, Goubillon ML, Coen CW. Cellular expression of V1a vasopressin receptor mRNA in the female rat preoptic area: effects of oestrogen. J Neuroendocrinol 2004; 16: 525–533. [DOI] [PubMed] [Google Scholar]
- 64. Funabashi T, Aiba S, Sano A, Shinohara K, Kimura F. Intracerebroventricular injection of arginine‐vasopressin V1 receptor antagonist attenuates the surge of luteinizing hormone and prolactin secretion in proestrous rats. Neurosci Lett 1999; 260: 37–40. [DOI] [PubMed] [Google Scholar]
- 65. Palm IF, Van Der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol‐treated rats with lesions of the suprachiasmatic nucleus. Neuroscience 1999; 93: 659–666. [DOI] [PubMed] [Google Scholar]
- 66. Kalsbeek A, Van Heerikhuize JJ, Wortel J, Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamo‐pituitary‐adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J Neurosci 1996; 16: 5555–5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Palm IF, Van Der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. The stimulatory effect of vasopressin on the luteinizing hormone surge in ovariectomized, estradiol‐treated rats is time‐dependent. Brain Res 2001; 901: 109–116. [DOI] [PubMed] [Google Scholar]
- 68. Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS. Vasopressin regulation of the proestrous luteinizing hormone surge in wild‐type and Clock mutant mice. Biol Reprod 2006; 75: 778–784. [DOI] [PubMed] [Google Scholar]
- 69. Coomans CP, Ramkisoensing A, Meijer JH. The suprachiasmatic nuclei as a seasonal clock. Front Neuroendocrinol 2014; doi:10.1016/j.yfrne.2014.11.002 . [DOI] [PubMed] [Google Scholar]
- 70. Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol 2010; 72: 551–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur J Neurosci 2007; 25: 3195–3216. [DOI] [PubMed] [Google Scholar]
- 72. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 2012; 35: 445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shieh KR. Distribution of the rhythm‐related genes rPERIOD1, rPERIOD2, and rCLOCK, in the rat brain. Neuroscience 2003; 118: 831–843. [DOI] [PubMed] [Google Scholar]
- 74. von GC, Garabette ML, Kell CA, Frenzel S, Dehghani F, Schumm‐Draeger PM, Weaver DR, Korf HW, Hastings MH, Stehle JH. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat Neurosci 2002; 5: 234–238. [DOI] [PubMed] [Google Scholar]
- 75. Sitzmann BD, Lemos DR, Ottinger MA, Urbanski HF. Effects of age on clock gene expression in the rhesus macaque pituitary gland. Neurobiol Aging 2010; 31: 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fukuhara C, Yamazaki S, Liang J. Pineal circadian clocks gate arylalkylamine N‐acetyltransferase gene expression in the mouse pineal gland. J Neurochem 2005; 93: 156–162. [DOI] [PubMed] [Google Scholar]
- 77. Bittman EL, Doherty L, Huang L, Paroskie A. Period gene expression in mouse endocrine tissues. Am J Physiol Regul Integr Comp Physiol 2003; 285: R561–R569. [DOI] [PubMed] [Google Scholar]
- 78. Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron 1998; 20: 1103–1110. [DOI] [PubMed] [Google Scholar]
- 79. Kriegsfeld LJ, Korets R, Silver R. Expression of the circadian clock gene Period 1 in neuroendocrine cells: an investigation using mice with a Per1:GFP transgene. Eur J Neurosci 2003; 17: 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Olcese J, Domagalski R, Bednorz A, Weaver DR, Urbanski HF, Reuss S, Middendorff R. Expression and regulation of mPer1 in immortalized GnRH neurons. NeuroReport 2003; 14: 613–618. [DOI] [PubMed] [Google Scholar]
- 81. Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci 2002; 22: 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Guilding C, Hughes AT, Brown TM, Namvar S, Piggins HD. A riot of rhythms: neuronal and glial circadian oscillators in the mediobasal hypothalamus. Mol Brain 2009; 2: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sellix MT, Egli M, Poletini MO, McKee DT, Bosworth MD, Fitch CA, Freeman ME. Anatomical and functional characterization of clock gene expression in neuroendocrine dopaminergic neurons. Am J Physiol Regul Integr Comp Physiol 2006; 290: R1309–R1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2:LUCIFERASE real‐time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 2004; 101: 5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hughes AT, Guilding C, Piggins HD. Neuropeptide signaling differentially affects phase maintenance and rhythm generation in SCN and extra‐SCN circadian oscillators. PLoS ONE 2011; 6: e18926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Andrade‐Silva J, Cipolla‐Neto J, Peliciari‐Garcia RA. The in vitro maintenance of clock genes expression within the rat pineal gland under standard and norepinephrine‐synchronized stimulation. Neurosci Res 2014; 81–82: 1–10. [DOI] [PubMed] [Google Scholar]
- 87. Gillespie JM, Chan BP, Roy D, Cai F, Belsham DD. Expression of circadian rhythm genes in gonadotropin‐releasing hormone‐secreting GT1‐7 neurons. Endocrinology 2003; 144: 5285–5292. [DOI] [PubMed] [Google Scholar]
- 88. Choe HK, Kim HD, Park SH, Lee HW, Park JY, Seong JY, Lightman SL, Son GH, Kim K. Synchronous activation of gonadotropin‐releasing hormone gene transcription and secretion by pulsatile kisspeptin stimulation. Proc Natl Acad Sci USA 2013; 110: 5677–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hickok JR, Tischkau SA. In vivo circadian rhythms in gonadotropin‐releasing hormone neurons. Neuroendocrinology 2010; 91: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhao S, Kriegsfeld LJ. Daily changes in GT1‐7 cell sensitivity to GnRH secretagogues that trigger ovulation. Neuroendocrinology 2009; 89: 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotropin‐releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH‐secreting GT1‐7 cell line. J Neurosci 2003; 23: 11202–11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol 2004; 14: 1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Miller BH, Takahashi JS. Central circadian control of female reproductive function. Front Endocrinol (Lausanne) 2013; 4: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Duguay D, Cermakian N. The crosstalk between physiology and circadian clock proteins. Chronobiol Int 2009; 26: 1479–1513. [DOI] [PubMed] [Google Scholar]
- 95. de la Iglesia HO, Blaustein JD, Bittman EL. Oestrogen receptor‐alpha‐immunoreactive neurones project to the suprachiasmatic nucleus of the female Syrian hamster. J Neuroendocrinol 1999; 11: 481–490. [DOI] [PubMed] [Google Scholar]
- 96. Van Der Beek EM, Wiegant VM, van Oudheusden HJ, van der Donk HA, Van den Hurk R, Buijs RM. Synaptic contacts between gonadotropin‐releasing hormone‐containing fibers and neurons in the suprachiasmatic nucleus and perichiasmatic area: an anatomical substrate for feedback regulation? Brain Res 1997; 755: 101–111. [DOI] [PubMed] [Google Scholar]
- 97. Merchenthaler I, Gorcs T, Setalo G, Petrusz P, Flerko B. Gonadotropin‐releasing hormone (GnRH) neurons and pathways in the rat brain. Cell Tissue Res 1984; 237: 15–29. [DOI] [PubMed] [Google Scholar]
- 98. Jennes L, Stumpf WE. LHRH‐systems in the brain of the golden hamster. Cell Tissue Res 1980; 209: 239–256. [DOI] [PubMed] [Google Scholar]
- 99. Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res 2000; 76: 191–204. [DOI] [PubMed] [Google Scholar]
- 100. Kruijver FP, Swaab DF. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology 2002; 75: 296–305. [DOI] [PubMed] [Google Scholar]
- 101. Su JD, Qiu J, Zhong YP, Chen YZ. Expression of estrogen receptor ‐alpha and ‐beta immunoreactivity in the cultured neonatal suprachiasmatic nucleus: with special attention to GABAergic neurons. NeuroReport 2001; 12: 1955–1959. [DOI] [PubMed] [Google Scholar]
- 102. Clancy AN, Whitman C, Michael RP, Albers HE. Distribution of androgen receptor‐like immunoreactivity in the brains of intact and castrated male hamsters. Brain Res Bull 1994; 33: 325–332. [DOI] [PubMed] [Google Scholar]
- 103. Fernandez‐Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol 2000; 425: 422–435. [DOI] [PubMed] [Google Scholar]
- 104. Dubocovich ML, Benloucif S, Masana MI. Melatonin receptors in the mammalian suprachiasmatic nucleus. Behav Brain Res 1996; 73: 141–147. [DOI] [PubMed] [Google Scholar]
- 105. Scott FF, Belle MD, Delagrange P, Piggins HD. Electrophysiological effects of melatonin on mouse Per1 and non‐Per1 suprachiasmatic nuclei neurones in vitro . J Neuroendocrinol 2010; 22: 1148–1156. [DOI] [PubMed] [Google Scholar]
- 106. Butler MP, Karatsoreos IN, LeSauter J, Silver R. Dose‐dependent effects of androgens on the circadian timing system and its response to light. Endocrinology 2012; 153: 2344–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kow LM, Pfaff DW. Suprachiasmatic neurons in tissue slices from ovariectomized rats: electrophysiological and neuropharmacological characterization and the effects of estrogen treatment. Brain Res 1984; 297: 275–286. [DOI] [PubMed] [Google Scholar]
- 108. Pevet P, Challet E. Melatonin: both master clock output and internal time‐giver in the circadian clocks network. J Physiol Paris 2011; 105: 170–182. [DOI] [PubMed] [Google Scholar]
- 109. Morin LP, Cummings LA. Effect of surgical or photoperiodic castration, testosterone replacement or pinealectomy on male hamster running rhythmicity. Physiol Behav 1981; 26: 825–838. [DOI] [PubMed] [Google Scholar]
- 110. Roy EJ, Wade GN. Role of estrogens in androgen‐induced spontaneous activity in male rats. J Comp Physiol Psychol 1975; 89: 573–579. [DOI] [PubMed] [Google Scholar]
- 111. Smarr BL, Morris E, de la Iglesia HO. The dorsomedial suprachiasmatic nucleus times circadian expression of Kiss1 and the luteinizing hormone surge. Endocrinology 2012; 153: 2839–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Khan AR, Kauffman AS. The role of kisspeptin and RFamide‐related peptide‐3 neurones in the circadian‐timed preovulatory luteinising hormone surge. J Neuroendocrinol 2012; 24: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin‐expressing retinal ganglion cells in the mouse. J Comp Neurol 2006; 497: 326–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Brown TM, Wynne J, Piggins HD, Lucas RJ. Multiple hypothalamic cell populations encoding distinct visual information. J Physiol 2011; 589: 1173–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Diekman CO, Belle MD, Irwin RP, Allen CN, Piggins HD, Forger DB. Causes and consequences of hyperexcitation in central clock neurons. PLoS Comput Biol 2013; 9: e1003196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Nitabach MN, Wu Y, Sheeba V, Lemon WC, Strumbos J, Zelensky PK, White BH, Holmes TC. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci 2006; 26: 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free‐running circadian clock. Cell 2002; 109: 485–495. [DOI] [PubMed] [Google Scholar]
- 118. Wu Y, Cao G, Pavlicek B, Luo X, Nitabach MN. Phase coupling of a circadian neuropeptide with rest/activity rhythms detected using a membrane‐tethered spider toxin. PLoS Biol 2008; 6: e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lundkvist GB, Kwak Y, Davis EK, Tei H, Block GD. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J Neurosci 2005; 25: 7682–7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mizrak D, Ruben M, Myers GN, Rhrissorrakrai K, Gunsalus KC, Blau J. Electrical activity can impose time of day on the circadian transcriptome of pacemaker neurons. Curr Biol 2012; 22: 1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 2003; 302: 1408–1412. [DOI] [PubMed] [Google Scholar]
- 122. Maywood ES, O'Neill JS, Chesham JE, Hastings MH. Minireview: The circadian clockwork of the suprachiasmatic nuclei–analysis of a cellular oscillator that drives endocrine rhythms. Endocrinology 2007; 148: 5624–5634. [DOI] [PubMed] [Google Scholar]
- 123. Myung J, Hong S, Hatanaka F, Nakajima Y, De SE, Takumi T. Period coding of Bmal1 oscillators in the suprachiasmatic nucleus. J Neurosci 2012; 32: 8900–8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Schwartz WJ, Gross RA, Morton MT. The suprachiasmatic nuclei contain a tetrodotoxin‐resistant circadian pacemaker. Proc Natl Acad Sci USA 1987; 84: 1694–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ono D, Honma S, Honma K. Cryptochromes are critical for the development of coherent circadian rhythms in the mouse suprachiasmatic nucleus. Nat Commun 2013; 4: 1666. [DOI] [PubMed] [Google Scholar]
- 126. Kuhlman SJ, McMahon DG. Rhythmic regulation of membrane potential and potassium current persists in SCN neurons in the absence of environmental input. Eur J Neurosci 2004; 20: 1113–1117. [DOI] [PubMed] [Google Scholar]
- 127. Kuhlman SJ, Quintero JE, McMahon DG. GFP fluorescence reports Period 1 circadian gene regulation in the mammalian biological clock. NeuroReport 2000; 11: 1479–1482. [PubMed] [Google Scholar]
- 128. Belle MD, Diekman CO, Forger DB, Piggins HD. Daily electrical silencing in the mammalian circadian clock. Science 2009; 326: 281–284. [DOI] [PubMed] [Google Scholar]
- 129. Brancaccio M, Maywood ES, Chesham JE, Loudon AS, Hastings MH. A Gq‐Ca2+ axis controls circuit‐level encoding of circadian time in the suprachiasmatic nucleus. Neuron 2013; 78: 714–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Enoki R, Ono D, Hasan MT, Honma S, Honma K. Single‐cell resolution fluorescence imaging of circadian rhythms detected with a Nipkow spinning disk confocal system. J Neurosci Methods 2012; 207: 72–79. [DOI] [PubMed] [Google Scholar]
- 131. Ikeda M, Sugiyama T, Wallace CS, Gompf HS, Yoshioka T, Miyawaki A, Allen CN. Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons. Neuron 2003; 38: 253–263. [DOI] [PubMed] [Google Scholar]
- 132. Irwin RP, Allen CN. Neuropeptide‐mediated calcium signaling in the suprachiasmatic nucleus network. Eur J Neurosci 2010; 32: 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ikeda M, Yoshioka T, Allen CN. Developmental and circadian changes in Ca2+ mobilization mediated by GABAA and NMDA receptors in the suprachiasmatic nucleus. Eur J Neurosci 2003; 17: 58–70. [DOI] [PubMed] [Google Scholar]
- 134. Colwell CS. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur J Neurosci 2000; 12: 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hong JH, Jeong B, Min CH, Lee KJ. Circadian waves of cytosolic calcium concentration and long‐range network connections in rat suprachiasmatic nucleus. Eur J Neurosci 2012; 35: 1417–1425. [DOI] [PubMed] [Google Scholar]
- 136. Pennartz CM, de Jeu MT, Bos NP, Schaap J, Geurtsen AM. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature 2002; 416: 286–290. [DOI] [PubMed] [Google Scholar]
- 137. Sakhi K, Belle MD, Gossan N, Delagrange P, Piggins HD. Daily variation in the electrophysiological activity of mouse medial habenula neurones. J Physiol 2014; 592: 587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Pugh JR, Raman IM. Nothing can be coincidence: synaptic inhibition and plasticity in the cerebellar nuclei. Trends Neurosci 2009; 32: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Raman IM, Gustafson AE, Padgett D. Ionic currents and spontaneous firing in neurons isolated from the cerebellar nuclei. J Neurosci 2000; 20: 9004–9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Pape HC, Driesang RB. Ionic mechanisms of intrinsic oscillations in neurons of the basolateral amygdaloid complex. J Neurophysiol 1998; 79: 217–226. [DOI] [PubMed] [Google Scholar]
- 141. Guilding C, Hughes AT, Piggins HD. Circadian oscillators in the epithalamus. Neuroscience 2010; 169: 1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell 2008; 134: 728–742. [DOI] [PMC free article] [PubMed] [Google Scholar]


