Abstract
Five nonionic surfactants (Brij™ 30, Span® 20, Ecosurf™ EH-3, polyoxyethylene sorbitol hexaoleate, and R-95™ rhamnolipid) were evaluated for their ability to enhance PAH desorption and biodegradation in contaminated soil after treatment in an aerobic bioreactor. Surfactant doses corresponded to aqueous-phase concentrations below the critical micelle concentration in the soil-slurry system. The effect of surfactant amendment on soil (geno)toxicity was also evaluated for Brij 30, Span 20, and POESH using the DT40 B-lymphocyte cell line and two of its DNA-repair-deficient mutants. Compared to no-surfactant controls, incubation of the bioreactor-treated soil with all surfactants increased PAH desorption and all except R-95 substantially increased PAH biodegradation. POESH had the greatest effect, removing 50% of total measured PAHs. Brij 30, Span 20, and POESH were particularly effective at enhancing biodegradation of four- and five-ring PAHs, including five of the seven carcinogenic PAHs, with removals up to 80%. Surfactant amendment also significantly enhanced the removal of alkyl-PAHs. Most treatments significantly increased soil toxicity. Only the no-surfactant control and Brij 30 at the optimum dose significantly decreased soil genotoxicity as evaluated with either mutant cell line. Overall, these findings have implications for the feasibility of bioremediation to achieve cleanup levels for PAHs in soil.
Graphical abstract
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are a class of compounds that are of environmental and public health concern because of their known or suspected toxicity and genotoxicity and their frequent occurrence at contaminated sites.1 Bioremediation is one option for the treatment of PAH-contaminated systems such as soil and sediment, but its efficacy may be limited by incomplete removal of the target PAHs.2
Due to their hydrophobicity, PAHs are often strongly associated with non-polar soil domains such as soil organic matter, combustion residue, and non-aqueous-phase liquids, and therefore may be unavailable to degrading microorganisms. Studies measuring PAH desorption from soil into the aqueous phase suggest that the fast-desorbing or bioaccessible fraction of a PAH can serve as an estimate of the biodegradable fraction in field-contaminated soil.3, 4 The addition of surfactants has been proposed as a means of enhancing the bioavailability of PAHs to degrading microorganisms,5–7 but previous studies have led to conflicting conclusions on the effects of surfactants on the biodegradation of PAHs in field-contaminated soils or in spiked soils.
Surfactants can increase the rate of PAH desorption from a geosorbent through two mechanisms: micellar solubilization and direct modification of the contaminant matrix. Micellar solubilization involves partitioning of PAHs into surfactant micelles at aqueous-phase surfactant concentrations above the critical micelle concentration (CMC), increasing the rate of desorption by maximizing the concentration gradient between the geosorbent and aqueous phase.8 Significant sorption of surfactant to soil, however, necessitates larger surfactant doses to reach the CMC in the aqueous phase of soil/water systems.9 As opposed to solubilization, modification of the contaminant matrix can occur at concentrations above and below the CMC. Surfactants have been shown to increase desorption of PAHs from contaminated field soil at doses corresponding to aqueous-phase surfactant concentrations less than the CMC (sub-CMC doses) in the soil/water system.10, 11 Hypothesized effects of surfactants on the contaminant matrix include increased PAH diffusivity and increased geosorbent interfacial surface area caused by wetting and dispersion of non-polar matrices.12–16 Additionally, sorption of surfactant to bacteria can increase the adherence of bacteria to a geosorbent, potentially increasing the rate of PAH desorption directly into biofilms or adherent cells.17–19
Previous research on field-contaminated soil suggests that surfactant addition is most beneficial for systems in which PAH biodegradation is limited by low bioaccessibility. This would be the case, for example, with soil treated in a conventional bioremediation system for which residual PAH concentrations might exceed cleanup targets.10 Studies in which surfactant-free controls achieve substantial PAH removal tend to demonstrate no improvement or even inhibition of PAH removal as a result of surfactant addition.10, 20–23 Studies in which surfactant-free controls exhibit negligible PAH removal, however, tend to demonstrate positive effects of surfactant addition.10, 23–25 If these surfactant-free controls perform poorly due to limited PAH bioaccessibility, then surfactant-enhanced desorption may explain improved biodegradation. Although there is a cost savings associated with using less surfactant, there has been limited work on surfactant-amended bioremediation of PAH-contaminated field soil at sub-CMC doses. 10, 20, 22
The objective of the present study was to investigate the effect of sub-CMC surfactant doses on the bioremediation of PAH-contaminated soil from a former manufactured-gas plant (MGP) site which had already undergone biological treatment in a slurry-phase bioreactor. We evaluated five relatively hydrophobic nonionic surfactants (hydrophile-lipophile balance [HLB] between 8 and 10) based on our previous study in which the hydrophobic surfactant, Brij 30, enhanced desorption and biodegradation more than the hydrophilic surfactant, C12E8.10 In this study, we hypothesized that surfactants of similar hydrophobicity but having a variety of hydrophilic moieties might influence the microbial community differently and, therefore, have a different impact on PAH removal as well as the toxicity of the soil. We evaluated the effect of surfactant amendment on desorption and biodegradation of residual PAHs, and for the three most effective surfactants we also evaluated the effect of treatment on soil (geno)toxicity.
MATERIALS AND METHODS
PAH standards for high performance liquid chromatography (HPLC) analysis (EPA 610 PAH mixture and individual PAHs), Brij 30, Span 20, polyoxyethylene sorbitol hexaoleate (POESH), Tenax TA beads (60/80 mesh), and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.). Ecosurf™ EH-3 (EH-3) was obtained from Chemical Marketing Concepts (New Milford, CT, U.S.A.). R-95 rhamnolipid biosurfactant (R-95) was obtained from AGAE Technologies (Corvallis, OR, U.S.A.). Properties of the surfactants are summarized in Table S1 and molecular structures are provided in Figure S1. SnakeSkin™ Dialysis Tubing (10,000 MWCO, 22-mm diameter) was obtained from Thermo Scientific (Rockford, IL, U.S.A.). PAH standards for gas chromatography-mass spectrometry (GC-MS) were obtained from Accustandard Inc. (New Haven, CT, U.S.A.). All solvents were HPLC grade and were obtained from either Fisher Scientific (Pittsburgh, PA, U.S.A.) or VWR International (Radnor, PA, U.S.A.).
Bioreactor Operation
Contaminated soil used in this study was collected from a former manufactured-gas plant site in Salisbury, North Carolina, processed, and characterized as described elsewhere.26, 27 The soil was treated in a continuously stirred, semi-continuous, slurry-phase (15% w/w) laboratory-scale aerobic bioreactor. Every seven days, 20% of the treated slurry was replaced with a slurry of untreated (feed) soil in a pH 7.5 buffer containing 5-mM phosphate and 2.5-mM ammonium nitrate. Treated slurry was centrifuged at 3900 RPM for 20 minutes, the supernatant discarded, and the centrifuged bioreactor-treated soil used in the experiments described below. Moisture content of the centrifuged soil was determined in triplicate by heating 1-g wet-weight aliquots of soil to dryness in preweighed ceramic crucibles over a Bunsen burner until a stable value of dry mass could be obtained. Typical soil moisture content was approximately 45% (w/w).
Surfactant Dose Selection
The CMC of each surfactant in phosphate buffer (5 mM, pH 7.5) was measured by following surface tension as a function of surfactant concentration. The nominal CMC’s of each surfactant are shown in Table S1. Because surfactants sorb to soil, it was necessary to evaluate surfactant doses (mass surfactant per mass dry weight soil) required to achieve the CMC in soil/buffer slurries as a basis for selecting sub-CMC doses for each surfactant. The surfactant was added to bioreactor-treated soil in a 15% (w/w) slurry in phosphate buffer (5 mM, pH 7.5). The aqueous-phase surfactant concentration and percent total pyrene solubilized as a function of dose were determined for each surfactant as described in Supporting Information and are reported in Figures S2 and S3. Pyrene was chosen as a representative PAH because of its presence at liquid-phase concentrations above the lower limit of quantification (LLOQ) for a wide range of surfactant doses. At aqueous-phase surfactant concentrations below the apparent CMC, liquid-phase PAH concentrations are low because solubilization is negligible. Based on these results, two doses (referred to as higher and lower) below the apparent CMC in the soil-slurry system were selected for each surfactant. The higher dose of R-95, however, was slightly above the nominal CMC. The higher doses for Brij 30, Span 20, EH-3, POESH, and R-95 were 12, 15, 60, 24, and 9-mg-surfactant/g-dry-soil respectively. Lower doses were equal to 1/3 the higher doses. Doses are shown in Table S1. For Brij 30, Span 20, EH-3, and R-95 the selected doses corresponded to less than 1% of total pyrene solubilized in the liquid-phase of the slurry (Figures S2 and S3). For POESH the selected doses corresponded to less than 6% of total initial pyrene solubilized in the liquid-phase of the slurry.
PAH Desorption
Desorption of PAHs from bioreactor-treated soil during surfactant-amended treatment was evaluated using Tenax beads as an infinite sink. Incubations with each surfactant were prepared in triplicate for the lower and higher doses and in quadruplicate for no-surfactant controls. Briefly, bioreactor-treated soil resuspended in fresh phosphate buffer (15% w/w) was incubated with or without surfactant on a rotary shaker for 48 hours, then Tenax contained in dialysis tubing was added to each incubation.28 After an additional 7 days, a period of time sufficient to reach an apparent maximum desorption of PAHs in our previous work,28 the incubations were sacrificed. The recovered Tenax was extracted with methanol and analyzed by HPLC with fluorescence detection to determine the masses of desorbed PAHs. More details on the procedure are in the Supporting Information.
PAH Biodegradation
A preliminary biodegradation experiment was conducted by setting up incubations over multiple weeks (one surfactant per week) using separate batches of bioreactor-treated soil, each analyzed for PAH concentrations in six replicates; three of the replicates were spiked with a known amount of anthracene-D10 as a recovery surrogate while the other three were used for toxicity testing as described below. For each surfactant, incubations were prepared at both the lower and higher doses. A no-surfactant control and an azide-inhibited control with surfactant at the higher dose were prepared in parallel for each surfactant. Incubations under each condition were prepared by adding 1.6-g dry weight bioreactor-treated soil to each of five 30-mL glass centrifuge tubes with PTFE-lined septa and screw caps. Surfactant stock solution in bioreactor buffer was added to deliver the target surfactant dose. The inhibited controls were spiked with 1 mL of 50-g/L sodium azide solution for a final nominal sodium azide concentration of 4.2 g/L. Bioreactor buffer was then added to give a final solids content of 15% (w/w).
All incubations were purged with nitrogen and put on an orbital shaker at 275 RPM for 48 hours in the dark to allow sorption of the surfactant to the soil with minimal aerobic biodegradation of surfactant. After 48 hours, incubations were kept on the orbital shaker for an additional 14 days and uncapped daily for five minutes to allow air into the incubation vessel. All incubations were then centrifuged and the soil pellets extracted and analyzed for PAHs. The supernatants from each higher-dose surfactant incubation were syringe-filtered through 0.8-μm polycarbonate membrane filters and analyzed for PAHs.
Results from the preliminary biodegradation experiment were used to select the best-performing surfactants and their doses for direct comparison with a single batch of soil removed from the bioreactor (referred to below as the followup biodegradation experiment). Incubations were prepared and analyzed as described above for the preliminary experiment, except the liquid phase of the incubation at the lower dose of Brij 30 was also analyzed for PAHs.
Soil Extraction and PAH Analysis
Bioreactor-treated soil (~3g wet weight per replicate) and incubation soil pellets were extracted in their centrifuge tubes by mixing with 10-g sodium sulfate and extracting overnight twice, each time with 10-mL acetone and 10-mL dichloromethane as described elsewhere.29 Soil solvent-extracts, Tenax solvent-extracts, and incubation supernatants (liquid-phase) were analyzed for the concentrations of 14 PAHs denoted in the caption of Table S2 by HPLC with fluorescence detection as described elsewhere.29 Unless noted otherwise, “total PAH” refers to the sum of these 14 PAHs. Soil solvent-extracts of selected conditions (no-surfactant control, lower dose of Brij 30, and higher doses of POESH and Span 20) from the followup biodegradation experiment were analyzed for additional PAHs and alky-PAHs by GC-MS as described in Supporting Information. Extraction and analysis of the feed soil for the bioreactor is also described in Supporting Information. Concentrations of PAHs in the feed soil are provided in Tables S2–S3 and concentrations of PAHs in the bioreactor-treated soil for the preliminary biodegradation experiments are provided in Table S4.
(Geno)toxicity
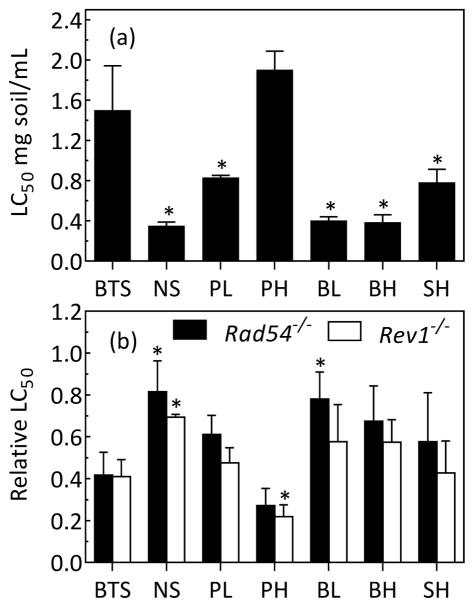
The effects of surfactant amendment on soil toxicity and genotoxicity were evaluated using solvent extracts from the followup biodegradation experiment. For each condition, 8-mL aliquots from each of the five replicate extracts for a given condition were combined in a preweighed vial and evaporated to dryness under a gentle stream of nitrogen. For bioreactor-treated soil prior to surfactant addition, triplicate aliquots (not spiked with anthracene-D10) were extracted and 12.5 mL of each extract were combined and evaporated to dryness under a gentle stream of nitrogen. Residue mass was then determined gravimetrically. Toxicities of the residues reconstituted in DMSO were evaluated in triplicate using a 96-well plate-based DT40 chicken lymphocyte DNA-damage response assay adapted from Ridpath, et al.30 and Hu, et al.27 Dose ranges were sufficient to bracket the LC50 of the residue. The Rad54−/− and Rev1−/− DNA-repair deficient mutants were tested alongside the isogenic DT40 parental cell line because of their reported sensitivity to soil residue in previous experiments.27 The Rad54−/− knock-out is deficient in the homologous recombination DNA repair pathway, while the Rev1−/− knock-out is deficient in the in translesion synthesis pathway. LC50 values (mg residue/mL media) were calculated by fitting the log concentration vs % survival in GraphPad Prism version 6.05 for Windows. The LC50 values measured for residue mass were converted to an equivalent soil LC50 (mg soil/mL). Relative LC50’s for each mutant cell line (LC50 of the mutant divided by the LC50 of the parental cell line) were calculated as a measure of genotoxicity, as described elsewhere.27
A followup experiment was conducted to assess the effect of POESH at the higher dose on the (geno)toxicity of bioreactor-treated soil independent of PAH biodegradation or potential surfactant biodegradation. Biodegradation was minimized by incubating bioreactor-treated soil anaerobically and by omitting ammonium nitrate in the buffer; a nitrogen headspace was maintained for the duration of the incubation. In parallel, bioreactor-treated soil was also incubated with POESH at the higher dose under aerobic conditions. No-surfactant controls were also incubated under both aerobic and anaerobic conditions. Five replicates were prepared for each condition as described above for the preliminary biodegradation experiment and evaluated for (geno)toxicity.
Data Analysis
Statistical analysis was conducted with SAS Enterprise Guide 6.1 (SAS Institute, Cary, NC, U.S.A.). For each PAH, a comparison of mass desorbed (μg) in the no-surfactant control with each surfactant-containing condition was conducted with two-sample t-tests (two-tail, homoscedastic, α = 0.05). To identify enhanced PAH removal, multiple comparisons (one-way ANOVA followed by Tukey’s Studentized range test, α=0.05) among all treatments were performed using the final soil concentrations of each PAH. Comparisons were made between the LC50 and relative LC50’s of bioreactor-treated soil with those of each treatment using two-sample t-tests (two-tail, homoscedastic, α = 0.05). The standard deviations of percent desorbed and percent removal were calculated by propagation of error using the means and standard deviations of the data used in the calculations.
RESULTS
PAH Desorption
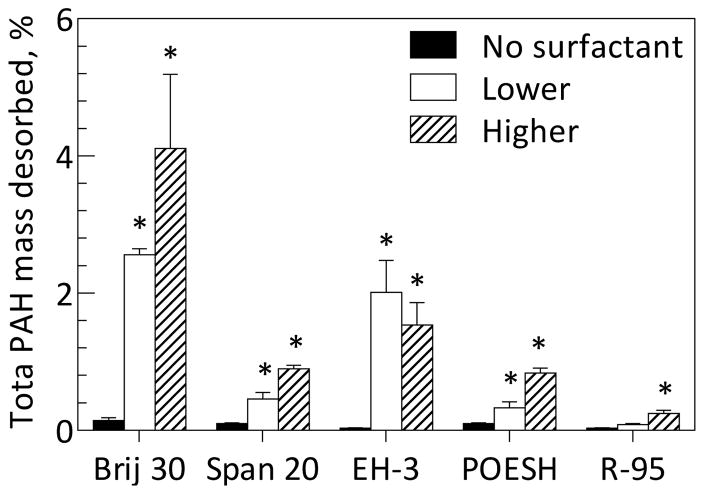
The effect of two surfactant doses below the CMC on desorption of residual PAHs from soil previously treated in a slurry-phase bioreactor was evaluated. At these doses the majority of surfactant is sorbed to the soil and solubilization is not expected to be a major mechanism of improving PAH bioaccessibility. Incubation of the bioreactor-treated soil with all surfactants resulted in modest increases in total PAH desorption compared to no-surfactant controls (Figure 1). Brij 30 at the higher dose was most effective; percent masses desorbed for individual PAHs are shown in Figure S4 for Brij 30 and in Tables S5–S7 for all surfactants
Figure 1.
Cumulative desorption of total PAH mass from bioreactor-treated soil after seven days in the absence of surfactant or in the presence of five different surfactants, each added at two doses designated “lower” and “higher” as defined in Materials and Methods and shown in Table S1. Bars represent means and standard deviations of three replicates for surfactant conditions and four replicates for no-surfactant controls. An asterisk indicates a significant difference (α=0.05) between the total mass of PAH desorbed in a treatment and no-surfactant control.
PAH Biodegradation
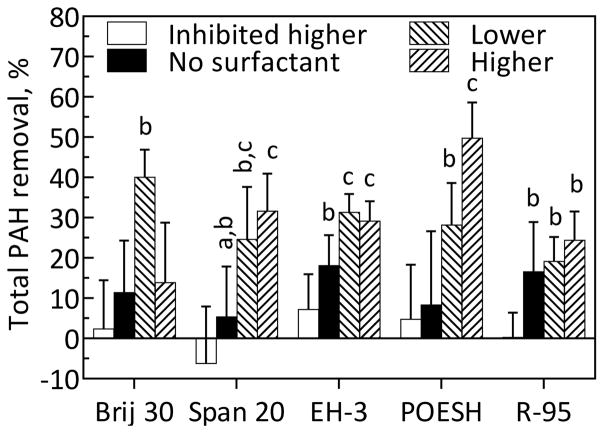
Biodegradation of residual PAHs in the treated soil from a slurry-phase bioreactor was evaluated at the two selected doses for each surfactant. All surfactants except R-95 rhamnolipid significantly increased total PAH removal from the bioreactor-treated soil relative to the no-surfactant control (Figure 2). POESH had the greatest effect, resulting in removal of 50% of total PAH. Significant dose-dependent effects were observed for both Brij 30 and POESH. While the lower dose of Brij 30 enhanced total PAH removal relative to the controls, the higher dose did not. Brij 30, Span 20, and POESH were particularly effective at enhancing the removal of 4- and 5-ring PAHs (Figures S5–S7) and therefore were chosen for further evaluation in a followup experiment. Individual PAH removals for the remaining surfactants can be found in Figures S8–S9.
Figure 2.
Effect of surfactants on residual total PAH from bioreactor-treated soil after 16 days. “Lower” and “Higher” refer to surfactant doses defined in Materials and Methods and shown in Table S1. “Inhibited” refers to controls to which sodium azide was added. Bars represent means and standard deviations of five replicates for all surfactants except Span 20 (four replicates). Conditions for which there was not a significant difference (α=0.05) in final total PAH concentration detected by Tukey’s method are assigned the same letter. Bars for which no letters are shown are implicitly designated “a”.
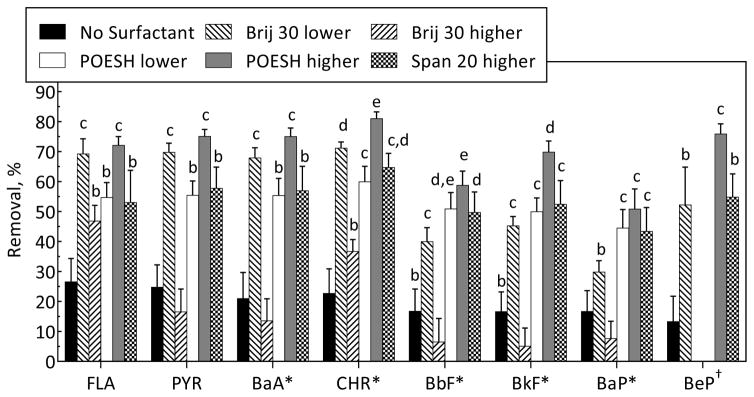
Both doses of Brij 30 and POESH, and the higher dose of Span 20, were evaluated in the followup experiment. Compared to no-surfactant controls, surfactant addition did not significantly improve removal of 2-ring PAHs. Of the 3-ring PAHs, only phenanthrene biodegradation was significantly improved with surfactant addition for both doses of Brij 30 and the higher dose of POESH. All three surfactants enhanced the removal of 4-ring PAHs, although the higher dose of Brij 30 enhanced the removal of only fluoranthene and chrysene (Figure 3). All three surfactants enhanced the removal of 5-ring PAHs except dibenzo[a,h]anthracene. Brij 30 at the higher dose, however, either had no significant effect or a significantly negative effect on the removal of 5-ring PAHs. At the end of the incubations, individual concentrations of the 14 PAHs measured by HPLC in the liquid phase were below LLOQ’s, corresponding to no more than 5% of the initial mass of any individual PAH.
Figure 3.
Effect of Brij 30, POESH, and Span 20 on residual 4- and 5-ring PAHs from bioreactor-treated soil after 16 days. Abbreviations are defined in Table S2. Bars represent means and standard deviations of five replicates. Asterisks indicate PAHs designated by EPA as probable human carcinogens. PAHs for which there were no significant differences between no-surfactant controls and all surfactant-amended samples are not shown. †BeP was measured by GC-MS and not measured for Brij 30 higher or POESH lower. Other notes as in Figure 2.
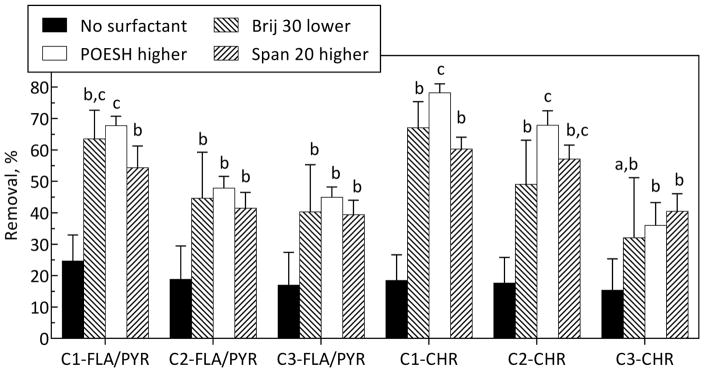
GC-MS analysis of samples from selected conditions from the followup biodegradation experiment revealed increased removal of additional PAHs upon surfactant addition. Removals of benzo[e]pyrene (Figure 3), alkylated 2- and 3-ring PAHs (Figure S10), and alkylated 4-ring PAHs (Figure 4) were significantly enhanced with surfactant addition. Concentrations of individual PAHs in the followup experiment for the bioreactor-treated soil and soils treated further with or without surfactant are provided in Table S9. Overall PAH removals relative to the feed soil for the bioreactor and followup experiments are provided in Table S10 to illustrate the combined impact of bioreactor treatment plus surfactant amendment as a secondary treatment step.
Figure 4.
Effect of Brij 30, POESH, and Span 20 on biodegradation of residual alkylated 4-ring PAHs from bioreactor-treated soil after 16 days. Abbreviations are defined in Table S3. Other notes as in Figure 3.
(Geno)toxicity
The effects of surfactant amendment on bioreactor-treated soil toxicity (LC50) and genotoxicity (relative LC50) were assessed. Solvent extracts from the followup biodegradation experiment were evaluated using the DT40 DNA-damage response assay. All treatments except POESH at the higher dose significantly increased soil toxicity to the parental cell line (Figure 5a). Treatment without surfactant significantly decreased soil genotoxicity as evaluated with both mutant cell lines (Figure 5b). Brij 30 at the lower dose significantly reduced the genotoxicity as measured using the Rad54−/− mutant. Treatment with POESH at the higher dose significantly increased soil genotoxicity as evaluated with the Rev1−/− mutant.
Figure 5.
Effect of incubation of bioreactor-treated soil with selected surfactants in the followup PAH biodegradation experiment on (a) toxicity to the parental DT40 cell line, and (b) genotoxicity as determined by relative LC50 values (mutant LC50/parental LC50) using the DNA repair-deficient mutants Rad54−/− and Rev1−/−. Bars represent means and standard deviations of three experiments. An asterisk indicates a significant difference (α=0.05) for a given cell line between a treatment and the initial bioreactor-treated soil. BTS, bioreactor-treated soil; NS, no-surfactant control; P, POESH, B, Brij 30; S, Span 20; L, lower surfactant dose; H, higher surfactant dose.
To assess whether POESH could have a significant effect on observed soil genotoxicity in the absence of biodegradation, we evaluated the effect of incubating bioreactor-treated soil with POESH at the higher dose under conditions intended to minimize biodegradation of the PAHs and/or the surfactant (anaerobic incubation without inorganic nitrogen amendment). No more than 20% of any individual PAH was removed for the POESH anaerobic condition (Figure S11) and no significant increase in toxicity or genotoxicity was observed (Figure S12). In pairwise comparisons with the bioreactor-treated soil before POESH addition, however, neither treatment without surfactant nor treatment with POESH aerobically had a significant effect on soil toxicity or genotoxicity. Since this was contrary to the results of the initial (geno)toxicity experiment, we conducted the DT40 bioassay side-by-side with solvent extracts of bioreactor-treated soil, no-surfactant controls, and higher-dose POESH amendment (aerobic) from both experiments. In this reevaluation the increase in toxicity to the parental DT40 cell line for the no-surfactant controls was observed for both experiments (Figure S13a). The reduction of genotoxicity associated with the no-surfactant controls was observed in the Rad54−/− mutant for both experiments but was observed in Rev1−/− only for the initial (geno)toxicity experiment (Figure S13a,b). Higher-dose POESH treatment was associated with a slight, but statistically significant, increase in genotoxicity to Rad54−/− only in the followup (geno)toxicity experiment.
Taken together, the initial and followup (geno)toxicity experiments suggest that further treatment without surfactant increased soil toxicity, but generally reduced genotoxicity. Treatment with POESH at the higher dose was associated either with no significant change or a slight increase in soil genotoxicity.
DISCUSSION
Although surfactant addition to contaminated soil has been suggested as a means of enhancing the biodegradation of hydrophobic contaminants such as PAHs, most studies do not articulate that the concept is most relevant to the fraction of a given compound that is relatively non-bioavailable or –bioaccessible. We previously reported that the nonionic surfactant Brij 30 substantially improved the desorption and biodegradation of residual PAHs from contaminated soil that had already undergone aerobic treatment in a lab-scale bioreactor.10 In this study, we extended the concept by comparing five nonionic surfactants of similar hydrophobicities but with different hydrophilic moieties on the removal of residual PAHs from a different contaminated soil after bioreactor treatment.
Incubation of the bioreactor-treated soil with all surfactants at sub-CMC doses resulted in modest increases in PAH desorption but substantial increases in total PAH biodegradation for all surfactants except the R-95 rhamnolipid biosurfactant. The limited PAH removal of R-95 may be related to its having the least effect on PAH desorption of any tested surfactant. The surfactants Brij 30, Span 20, and POESH were particularly effective at enhancing removal of 4- and 5-ring PAHs, including five of the seven PAHs designated by EPA as human carcinogens.
We specifically evaluated sub-CMC doses of each surfactant, at which micellar solubilization of PAHs would be negligible. Enhanced desorption of PAHs at surfactant doses below the apparent CMC in the soil slurry system is consistent with other studies treating field-contaminated soil.10, 11, 16 Luthy, et al. describe the three major components in PAH-contaminated soil as soil organic matter (SOM), combustion residue, and non-aqueous-phase liquids (NAPLs).31 Common NAPLs found at PAH-contaminated sites include coal tar, creosote, and petroleum products such as oil or diesel fuel.32 We assume that relatively hydrophobic nonionic surfactants sorb to these domains in contaminated soil and alter the contaminant matrix in a way that favors desorption or other means of increasing microbial access to the PAHs. Yeom, et al. treated coal tar-contaminated soil with several surfactants, including Brij 30, and found substantial increases in phenanthrene desorption under conditions corresponding to aqueous-phase surfactant concentrations below the CMC.16 The authors attributed this to increased PAH diffusivity within the coal-tar matrix.
During further treatment with or without surfactant, total PAH removal far exceeded the amount desorbed; (compare Figures 1 and 2). The difference between desorption and removal was particularly striking for the 4- and 5-ring PAHs; (compare Figures S4 and S5 for Brij 30 and Tables S6–S7 with Figures S6–S9 for other surfactants). Large differences between measured PAH desorption and removal have been observed in our lab in previous studies of bioremediation26, 28 and during surfactant-enhanced bioremediation specifically.10 While the impact of surfactant on the functionality of Tenax as an infinite sink was not investigated in this study and the incubations used to measure biodegradation were carried out an additional 7 days longer than those used to measure desorption, an infinite-sink method at best can measure only abiotic desorption into the aqueous phase. Evidence suggests that bacteria can enhance PAH desorption by adhering to hydrophobic contaminant matrices.33 The rate of PAH mass transfer from geosorbent to adherent cells or biofilm may be faster than the rate of PAH mass transfer into a bulk aqueous phase. It is also possible that the smaller distances between bacteria and geosorbent or the ability of bacteria to enter small soil pores may cause a steeper concentration gradient than can be created with solid resins.
The surfactants we evaluated may have enhanced the rate of PAH biodegradation by increasing the interaction of bacteria with PAH-containing soil compartments. This could occur through increased geosorbent interfacial surface area onto which bacteria may adhere or through modification of cell- or soil-surface properties to favor adhesion. Surfactants can alter cell surface hydrophobicity in ways that can either promote or inhibit bacterial adhesion.18 Rhamnolipids in particular have a concentration-dependent effect on cell attachment to both hydrophilic and hydrophobic surfaces and have been suggested as a method of inhibiting biofilm formation.34 It is possible that the limited PAH removal in the presence of the rhamnolipid used in this study was due to inhibited bacterial adhesion to PAH-containing soil domains. While the two doses of Brij 30 led to comparable PAH desorption, the higher dose led to significantly less PAH removal. This suggests that factors other than abiotic PAH desorption affected biodegradation. In previous work, addition of Brij 30 at doses well above the CMC enhanced only the removal of 3-ring PAHs, while lower doses also enhanced removal of 4- and 5-ring PAHs.10 It is possible that differences in PAH removal between Brij 30 doses reflect differences in the effects on the microbial community from the bioreactor. In the earlier study,35 there was a reduction in relative abundance of known pyrene degraders at the supra-CMC dose of Brij 30 compared to incubations without surfactant and incubations at sub-CMC doses. In work to be published elsewhere, we observed substantial effects of surfactant addition on the bacterial community under conditions similar to the followup experiment in this study. Although we hypothesized that differences in surfactant structure could also influence the microbial community and, therefore, PAH biodegradation, it is not possible to infer from the results of this work if the surfactant structure was the primary factor responsible for observed differences among the surfactants.
Overall, our results confirm the hypothesis that sub-CMC surfactant addition can enhance PAH desorption and biodegradation in soils in which PAH bioaccessibility is limited. While we did not evaluate any surfactant with an HLB value greater than 10, sub-CMC doses of more-hydrophilic surfactants tested in previous studies did not enhance biodegradation.10, 20, 22 More-hydrophilic surfactants may have a lower affinity for the hydrophobic PAH-containing soil compartments, so that the CMC may be reached before a substantial amount of surfactant interacts with these compartments.
The genotoxicity of the bioreactor-treated soil determined in this study is consistent with our previous studies treating the same contaminated source soil.27, 28 The Rad54−/− and Rev−/− mutants used in this study are deficient in the Rad54 and Rev1 proteins, respectively. These two proteins are implicated in the repair or tolerance of damage caused by the major types of PAH-induced genotoxicity: strand breaks caused by oxidative stress (Rad54), and adduction of DNA by stable or depurinating adducts (Rev1).36, 37 Additionally, the Rad54−/− mutant is sensitive to replication fork blockage,38 a common result of DNA damage caused by a range of genotoxic chemicals.39–44 This sensitivity makes the Rad54−/− mutant a broadly applicable detector of genotoxicity. In general, however, increased PAH removal from the bioreactor-treated soil did not correspond to a reduction in soil toxicity or genotoxicity. Amendment with POESH at the higher dose removed substantial amounts of 4- and 5-ring PAHs, including some considered to be human carcinogens, yet had either no effect or caused a slight increase in genotoxicity. Meanwhile, further treatment of the bioreactor-treated soil without surfactant removed less than 30% of any 4- or 5-ring PAH, but resulted in a significant reduction in soil genotoxicity. This finding may imply that an increased residence time (batch incubation of 14 days following treatment in the bioreactor) led to the removal of genotoxic constituents present in the treated soil obtained from the bioreactor. Overall, the (geno)toxicity of remediated soil will depend both on the remaining parent compounds and the formation or removal of any products of incomplete metabolism. The bioavailability of any genotoxic metabolites formed as a result of biological treatment of contaminated soil must also be taken into account when evaluating the efficacy of bioremediation.28 However, the bioavailability of residual contaminants at the end of incubations with surfactants, was not evaluated in the present study.
This work demonstrated the effectiveness of surfactant-amended treatment for enhanced biodegradation of the residual, less bioaccessible fraction of PAHs in soil after primary treatment in a conventional bioreactor. Employing this two-stage treatment process could increase the likelihood of meeting site cleanup goals, which are typically based on the concentrations of PAHs in the soil independent of their bioavailability or bioaccessibility. Substantial amounts of PAHs remained in the soil even after surfactant treatment, suggesting that there is a fraction of any given PAH resistant to further desorption. The observation that parent PAH removal did not necessarily correspond to a reduction in genotoxicity, however, highlights the need for further research to identify genotoxic products to improve risk assessment and remediation strategies.
Supplementary Material
Acknowledgments
Analyses of PAHs by GCMS were performed by Peter Lazaro and Xin-Rui Xia at North Carolina State University. This work was supported by the U.S. National Institute of Environmental Health Sciences under the Superfund Research Program (grant 5 P42ES005948).
Footnotes
Details of experimental procedures described in summary form in Materials and Methods; PAH concentrations in feed soil, bioreactor-treated soil samples, and samples from biodegradation experiments; masses of individual PAHs desorbed in desorption experiment; results for surfactant sorption and pyrene solubilization as a function of surfactant dose; supporting data for design of desorption experiment; supporting data for genotoxicity evaluation.
References
- 1.ATSDR. Toxicological profile for polycyclic aromatic hydrocarbons (PAHs) U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; Atlanta, GA: 1995. p. xvii.p. 458. [PubMed] [Google Scholar]
- 2.Vila J, Tauler M, Grifoll M. Bacterial PAH degradation in marine and terrestrial habitats. Curr Opin Biotechnol. 2015;33:95–102. doi: 10.1016/j.copbio.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Lei L, Suidan MT, Khodadoust AP, Tabak HH. Assessing the Bioavailability of PAHs in Field-Contaminated Sediment Using XAD-2 Assisted Desorption. Environ Sci Technol. 2004;38(6):1786–1793. doi: 10.1021/es030643p. [DOI] [PubMed] [Google Scholar]
- 4.Cornelissen G, Rigterink H, Ferdinandy MMA, van Noort PCM. Rapidly Desorbing Fractions of PAHs in Contaminated Sediments as a Predictor of the Extent of Bioremediation. Environ Sci Technol. 1998;32(7):966–970. [Google Scholar]
- 5.Li JL, Chen BH. Surfactant-mediated Biodegradation of Polycyclic Aromatic Hydrocarbons. Materials. 2009;2(1):76–94. [Google Scholar]
- 6.Makkar RS, Rockne KJ. Comparison of synthetic surfactants and biosurfactants in enhancing biodegradation of polycyclic aromatic hydrocarbons. Environ Toxicol Chem. 2003;22(10):2280–2292. doi: 10.1897/02-472. [DOI] [PubMed] [Google Scholar]
- 7.Elliot R, Singhal N, Swift S. Surfactants and Bacterial Bioremediation of Polycyclic Aromatic Hydrocarbon Contaminated Soil-Unlocking the Targets. Crit Rev Environ Sci Technol. 2011;41(1):78–124. [Google Scholar]
- 8.Grasso D, Subramaniam K, Pignatello JJ, Yang Y, Ratté D. Micellar desorption of polynuclear aromatic hydrocarbons from contaminated soil. Colloids Surf Physicochem Eng Aspects. 2001;194(1–3):65–74. [Google Scholar]
- 9.Liu Z, Edwards DA, Luthy RG. Sorption of non-ionic surfactants onto soil. Water Res. 1992;26(10):1337–1345. [Google Scholar]
- 10.Zhu H, Aitken MD. Surfactant-Enhanced Desorption and Biodegradation of Polycyclic Aromatic Hydrocarbons in Contaminated Soil. Environ Sci Technol. 2010;44(19):7260–7265. doi: 10.1021/es100112a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frutos FJG, Escolano O, Garcia S, Ivey GA. Mobilization Assessment and Possibility of Increased Availability of PAHs in Contaminated Soil Using Column Tests. Soil Sediment Contam. 2011;20(5):581–591. [Google Scholar]
- 12.Churchill SA, Griffin RA, Jones LP, Churchill PF. Biodegradation Rate Enhancement of Hydrocarbons by an Oleophilic Fertilizer and a Rhamnolipid Biosurfactant. J Environ Qual. 1995;24(1):19–28. [Google Scholar]
- 13.Kile DE, Chiou CT. Water Solubility Enhancements of Ddt and Trichlorobenzene by Some Surfactants Below and above the Critical Micelle Concentration. Environ Sci Technol. 1989;23(7):832–838. [Google Scholar]
- 14.Zhang YM, Miller RM. Enhanced Octadecane Dispersion and Biodegradation by a Pseudomonas Rhamnolipid Surfactant (Biosurfactant) Appl Environ Microbiol. 1992;58(10):3276–3282. doi: 10.1128/aem.58.10.3276-3282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong J, Chowdhry B, Leharne S. Investigation of the Wetting Behavior of Coal Tar in Three Phase Systems and Its Modification by Poloxamine Block Copolymeric Surfactants. Environ Sci Technol. 2003;38(2):594–602. doi: 10.1021/es026426q. [DOI] [PubMed] [Google Scholar]
- 16.Yeom IT, Ghosh MM, Cox CD. Kinetic aspects of surfactant solubilization of soil-bound polycyclic aromatic hydrocarbons. Environ Sci Technol. 1996;30(5):1589–1595. [Google Scholar]
- 17.Mohanty S, Mukherji S. Alteration in cell surface properties of Burkholderia spp. during surfactant-aided biodegradation of petroleum hydrocarbons. Appl Microbiol Biotechnol. 2012;94(1):193–204. doi: 10.1007/s00253-011-3703-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhang D, Zhu L. Controlling microbiological interfacial behaviors of hydrophobic organic compounds by surfactants in biodegradation process. Front Environ Sci Eng. 2014;8(3):305–315. [Google Scholar]
- 19.Mohanty G, Mukherji S. Enhancement of NAPL bioavailability by induction of cell-surface hydrophobicity in Exiguobacterium aurantiacum and Burkholderia cepacia. Indian J Biotechnol. 2008;7(3):295–306. [Google Scholar]
- 20.Lei L, Khodadoust AP, Suidan MT, Tabak HH. Biodegradation of sediment-bound PAHs in field-contaminated sediment. Water Res. 2005;39(2–3):349–361. doi: 10.1016/j.watres.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Deschenes L, Lafrance P, Villeneuve JP, Samson R. Adding sodium dodecyl sulfate and Pseudomonas aeruginosa UG2 biosurfactants inhibits polycyclic aromatic hydrocarbon biodegradation in a weathered creosote-contaminated soil. Appl Microbiol Biotechnol. 1996;46(5–6):638–646. doi: 10.1007/s002530050874. [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, Weber WJ., Jr Polycyclic aromatic hydrocarbon behavior in bioactive soil slurry reactors amended with a nonionic surfactant. Environ Toxicol Chem. 2005;24(2):268–76. doi: 10.1897/04-219r.1. [DOI] [PubMed] [Google Scholar]
- 23.Bueno-Montes M, Springael D, Ortega-Calvo J-J. Effect of a Nonionic Surfactant on Biodegradation of Slowly Desorbing PAHs in Contaminated Soils. Environ Sci Technol. 2011;45(7):3019–3026. doi: 10.1021/es1035706. [DOI] [PubMed] [Google Scholar]
- 24.Di Gennaro P, Franzetti A, Bestetti G, Lasagni M, Pitea D, Collina E. Slurry phase bioremediation of PAHs in industrial landfill samples at laboratory scale. Waste Manage (Oxford) 2008;28(8):1338–1345. doi: 10.1016/j.wasman.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Tiehm A, Stieber M, Werner P, Frimmel FH. Surfactant-Enhanced Mobilization and Biodegradation of Polycyclic Aromatic Hydrocarbons in Manufactured Gas Plant Soil. Environ Sci Technol. 1997;31(9):2570–2576. [Google Scholar]
- 26.Richardson SD, Aitken MD. Desorption and bioavailability of polycyclic aromatic hydrocarbons in contaminated soil subjected to long-term in situ biostimulation. Environ Toxicol Chem. 2011;30(12):2674–2681. doi: 10.1002/etc.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu J, Nakamura J, Richardson SD, Aitken MD. Evaluating the Effects of Bioremediation on Genotoxicity of Polycyclic Aromatic Hydrocarbon-Contaminated Soil Using Genetically Engineered, Higher Eukaryotic Cell Lines. Environ Sci Technol. 2012;46(8):4607–4613. doi: 10.1021/es300020e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu J, Adrion AC, Nakamura J, Shea D, Aitken MD. Bioavailability of (Geno)toxic Contaminants in Polycyclic Aromatic Hydrocarbon-Contaminated Soil Before and After Biological Treatment. Environ Eng Sci. 2014;31(4):176–182. doi: 10.1089/ees.2013.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson SD, Lebron BL, Miller CT, Aitken MD. Recovery of Phenanthrene-Degrading Bacteria after Simulated in Situ Persulfate Oxidation in Contaminated Soil. Environ Sci Technol. 2011;45(2):719–725. doi: 10.1021/es102420r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridpath JR, Takeda S, Swenberg JA, Nakamura J. Convenient, multi-well plate-based DNA damage response analysis using DT40 mutants is applicable to a high-throughput genotoxicity assay with characterization of modes of action. Environ Mol Mutagen. 2011;52(2):153–60. doi: 10.1002/em.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luthy RG, Aiken GR, Brusseau ML, Cunningham SD, Gschwend PM, Pignatello JJ, Reinhard M, Traina SJ, Weber WJ, Westall JC. Sequestration of hydrophobic organic contaminants by geosorbents. Environ Sci Technol. 1997;31(12):3341–3347. [Google Scholar]
- 32.Mueller JG, Cerniglia Carl E, Pritchard P Hap. Bioremediation: Principles and Applications. Cambridge University Press; 1996. [Google Scholar]
- 33.Mukherji S, Ghosh I. Bacterial Degradation of High Molecular Weight Polynuclear Aromatic Hydrocarbons. In: Singh SN, editor. Microbial Degradation of Xenobiotics. Springer; Berlin Heidelberg: 2012. pp. 189–211. [Google Scholar]
- 34.Nickzad A, Déziel E. The involvement of rhamnolipids in microbial cell adhesion and biofilm development – an approach for control? Lett Appl Microbiol. 2014;58(5):447–453. doi: 10.1111/lam.12211. [DOI] [PubMed] [Google Scholar]
- 35.Zhu H, Singleton DR, Aitken MD. Effects of nonionic surfactant addition on populations of polycyclic aromatic hydrocarbon-degrading bacteria in a bioreactor treating contaminated soil. Environ Sci Technol. 2010;44(19):7266–71. doi: 10.1021/es100114g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedberg EC. DNA repair and mutagenesis. ASM Press; Washington, D.C: 2006. [Google Scholar]
- 37.Penning TM. Chemical carcinogenesis. Springer Verlag; 2011. [Google Scholar]
- 38.Sonoda E, Hochegger H, Saberi A, Taniguchi Y, Takeda S. Differential usage of non-homologous end-joining and homologous recombination in double strand break repair. DNA Repair. 2006;5(9–10):1021–1029. doi: 10.1016/j.dnarep.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki MS, Takata M, Sonoda E, Tachibana A, Takeda S. Recombination repair pathway in the maintenance of chromosomal integrity against DNA interstrand crosslinks. Cytogenet Genome Res. 2004;104(1–4):28–34. doi: 10.1159/000077463. [DOI] [PubMed] [Google Scholar]
- 40.Ridpath JR, Nakamura A, Tano K, Luke AM, Sonoda E, Arakawa H, Buerstedde J-M, Gillespie DAF, Sale JE, Yamazoe M, Bishop DK, Takata M, Takeda S, Watanabe M, Swenberg JA, Nakamura J. Cells Deficient in the FANC/BRCA Pathway Are Hypersensitive to Plasma Levels of Formaldehyde. Cancer Res. 2007;67(23):11117–11122. doi: 10.1158/0008-5472.CAN-07-3028. [DOI] [PubMed] [Google Scholar]
- 41.Minca EC, Kowalski D. Replication fork stalling by bulky DNA damage: localization at active origins and checkpoint modulation. Nucleic Acids Res. 2011;39(7):2610–23. doi: 10.1093/nar/gkq1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lundin C, Erixon K, Arnaudeau C, Schultz N, Jenssen D, Meuth M, Helleday T. Different Roles for Nonhomologous End Joining and Homologous Recombination following Replication Arrest in Mammalian Cells. Mol Cell Biol. 2002;22(16):5869–5878. doi: 10.1128/MCB.22.16.5869-5878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szyjka SJ, Aparicio JG, Viggiani CJ, Knott S, Xu W, Tavare S, Aparicio OM. Rad53 regulates replication fork restart after DNA damage in Saccharomyces cerevisiae. Genes Dev. 2008;22(14):1906–20. doi: 10.1101/gad.1660408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berniak K, Rybak P, Bernas T, Zarebski M, Biela E, Zhao H, Darzynkiewicz Z, Dobrucki JW. Relationship between DNA damage response, initiated by camptothecin or oxidative stress, and DNA replication, analyzed by quantitative 3D image analysis. Cytometry A. 2013;83(10):913–24. doi: 10.1002/cyto.a.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.