Immune thrombocytopenia purpura (ITP) is an autoimmune disorder whose pathophysiology is characterized by immune mediated platelet destruction and decreased platelet production (Johnsen 2012). Platelet destruction in ITP occurs by a variety of immune mediated mechanisms, including humoral and cell mediated immunity.
Increasing evidence suggests a role for complement activation in ITP (Peerschke, et al 2009, Najaoui, et al 2011). Although early work by Frank et al (1975) demonstrated that an intact classical complement pathway was required for damage of antibody sensitized mammalian cell membranes and the development of thrombocytopenia in a guinea pig model, the role for classical pathway (CP) complement activation in human ITP has not been definitively established. The lectin pathway has been reported also to be activated by antibodies and autoantibodies, generating the same activated complement proteins downstream of C1, as the classical pathway (Malhotra et al, 1995).
In the present study, we performed an in vitro trial of a novel classical pathway complement inhibitor (TNT003), a murine monoclonal antibody directed against C1s, to evaluate the role of CP activation in ITP, and provide in vitro proof of principle that CP inhibition prevents complement activation in ITP patient plasma. TNT003 is a novel C1s inhibitor that has been shown to inhibit cold agglutinin mediated complement deposition on the surface of red blood cells in vitro (Shi, et al 2014).
Patients (n=55) consisted of males (n=21), age 39 ± 24 years (mean ± S.D.) (range 8–87 years) and females (n=34), age 48 ± 23 years (range 17–86 years), with a median ITP duration of 106 months and 99.5 months, respectively. At the time of blood collection, patients were undergoing treatment with a variety of modalities including Rituximab, IVIG, Eltrombopag, Romiplostin,, Veltuzumab, Cyclopsorin, Danazol, Azathioprine, Prednisone, Dexamethasone, and Mycophenolate mofetil, either alone or in combination. Fifteen patients had undergone splenectomy.
Results of CP activation were compared to platelet count, obtained as part of the patient’s clinical laboratory assessment, and the presence of antiplatelet antibodies (IgG, IgM, IgA) directed against major platelet membrane glycoprotein antigens, IIb/IIIa, Ia/IIa, and Ib/IX, using the Lifecodes Pak12 assay (Immucor GTI Diagnostics, Inc. Waukesha, WI). This study was approved by the Institutional Review Boards of Weill Cornell Medical School and Memorial Sloan Kettering Cancer Center.
CP activation was evaluated using a previously described assay (Peerschke, et al 2009). Since complement activation occurs spontaneously on activated or immobilized platelets (Peerschke, et al 2010), CP activation by patient plasma was expressed as a ratio relative to pooled normal control plasma, in order to detect enhanced complement activation. Enhanced complement activation was defined as a ratio of ≥ 1.5, representing values greater than 3 S.D. above the reference interval (97.5% confidence limit).
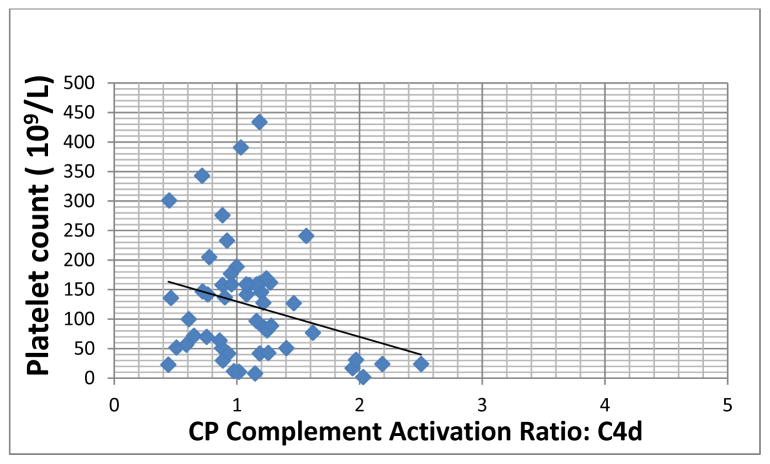
Increased complement activation was noted in 26/55 patients with ITP (~47%). Elevated C1q deposition was found in 42% of patients (23/55 patient plasma samples). Enhanced C4d deposition was demonstrated in ~13% of patients (7/55 plasma samples). The latter was associated with a statistically significant inverse correlation (p=0.042) with platelet count (Figure). In 6 of 7 patients with heightened C4d deposition, the circulating whole blood platelet count was below 100K/μl, including 5 patients with platelet counts below 50K/μl.
Figure.
Correlation between classical pathway (CP) complement activation, represented here by C4d deposition, and platelet count (Advia 2120, Siemens Healthcare Systems, Tarrytown, NY) in patients with chronic ITP. CP activation is expressed as a ratio relative to baseline complement activation observed using normal donor plasma. A ratio of >1.5, representing >3 S.D. from the reference range, was considered positive. (Correlation coefficient, r2= −0.414, p= 0.042).
Blood was obtained during patient clinic visits, using vacutainer tubes containing 3.2% sodium citrate (blood: anticoagulant ratio 1:10) (Becton Dickinson, Franklin Lakes, NJ). Platelet free plasma was prepared within 60 min of blood collection by centrifugation (1000g, 20 min, room temperature), aliquoted, and frozen at −80C until testing. CP activation was evaluated using a previously described assay (Peerschke, et al 2009) in which immobilized heterologous platelets are exposed to patient plasma or normal pooled control plasma (George King Bio-Medical, Inc, Overland Park, KS), and complement deposition is measured using monoclonal antibodies to C1q, C4d, C3b and C5b-9 (Quidel Corp., Santa Clara, CA).
Increased C4d deposition was associated with the presence of autoantibodies directed against major platelet antigens in all 5 patients with platelet counts below 50K/μl. In these patients (5/5), antibodies directed against GPIIb-IIIa were identified. Three patients additionally demonstrated antibodies against GPIa/IIa. A single patient exhibited detectable autoantibodies also against GPIb/IX. These findings are consistent with previous reports summarized by McMillan (2009), who described anti platelet antibodies in approximately 58% of patients, with reactivity to GPIIb-IIIa being the most common.
CP activation was inhibited by TNT003, as demonstrated by reduced C4d deposition, and markedly reduced downstream C3b and C5b-9 deposition from patient plasma (n=55)(Table). More complete complement inhibition was achieved by chelation of divalent cations with 10 mM EDTA, confirming the participation also of the alternative pathway in complement activation on platelets (Peerschke et al, 2009). TNT003 appears to impact CP activation predominantly downstream of C1 binding, and supports the notion that CP activation plays a major role in terminal complement pathway activation in ITP plasma. Indeed, in vitro platelet lysis has been described following normal platelet exposure to autoantibodies from ITP patient sera (McMillan, et al 1981).
Table.
Inhibition of classical pathway (CP) complement activation in plasma from patients with chronic ITP (n=55)
| Complement Deposition | C1s Inhibition by TNT003 | Complement Inhibition by EDTA | Difference between Complement inhibition by EDTA and TNT003 |
|---|---|---|---|
| % Inhibition* | % Inhibition | ||
| C1q | 12.81 ± 13.23 (p<0.001) | −7.53 ± −23.75 (Not Significant) | p<0.001 |
| C4d | 44 ± 43% (p<0.001) | 58.4 ± 22.2% (p<0.001) | p=0.08 |
| C3b | 72 ± 17% (p<0.001) | 99.7 ± 10.1% (p<0.001) | p<0.001 |
| C5b-9 | 82 ± 14% (p<0.001) | 96.4 ± 12.5% (p<0.001) | p<0.001 |
Patient and control plasma were preincubated with TNT003 (100 μg/ml ) or its isotype control antibody, for 5 min at 37°C, before analysis of CP activation. TNT003 inhibition was calculated relative to results obtained in the presence of isotype control antibody. For comparison, maximum complement inhibition was induced by addition of EDTA (10mM) to patient plasma, and calculated relative to a buffer control.
Statistical significance was assessed using the Student t-test. Significance was defined as p<0.05.
In addition to direct cellular damage via C5b-9 lytic complexes, autoantibody mediated complement deposition promotes platelet clearance by the reticuloendothelial system (Johnsen, 2012). Interestingly, ITP patients with a high degree of platelet associated complement deposition/fixation have been reported to benefit significantly from splenectomy (Bell, 2002). In the present study, increased plasma CP activation was noted in 10 of 15 patients who had undergone splenectomy (p= 0.032, Fisher’s Exact Test). These preliminary in vitro findings may suggest that inhibition of CP activation could be a potential alternative to splenectomy for select patients with ITP.
Taken together, the present study provides direct evidence of increased CP activation in ITP and proof of principle that CP inhibition may effectively target this process. CP blockade using C1 esterase inhibitor (C1 INH) has been used clinically, predominantly in patients with hereditary angioedema. Although C1 INH therapy is well tolerated in humans, it exerts effects beyond regulation of the classical complement pathway, including modulation of the lectin pathway and kinin, coagulation, and fibrinolytic systems. Targeted inhibition of C1s, therefore, may represent more specific inhibition of CP activation. Indeed, TNT003, when tested in vitro against C1 INH was found to be >3 orders of magnitude more potent for inhibiting antibody dependent complement activation in hemolysis based assays (data not shown). Evaluation of CP complement inhibition in ITP and the potential risk for infection by pyogenic bacteria with chronic use awaits clinical trails.
Acknowledgments
This work was supported in part by TrueNorth Therapeutics, Inc., South San Francisco, CA. The authors are grateful to Nenita Francisco and Kajal Kothadia for expert technical assistance.
Footnotes
Authors’ contributions
EP, SP and JB designed the study; EP performed the research; SP and JB contributed essential reagents; EP, SP and JB analyzed the data; EP, SP, and JB wrote the paper.
Conflict of interest
Sandip Panicker is an employee of TrueNorth Therapeutics, Inc.
References
- 1.Bell WR., Jr Role of splenectomy in immune (idiopathic) thrombocytopenic purpura. Blood Review. 2002;16:39–41. doi: 10.1054/blre.2001.0180. [DOI] [PubMed] [Google Scholar]
- 2.Frank MM, May JE, Kane MA. Studies on the biologic effects of selective C4 deficiency. Birth Defects Original Article Series. 1975;11:568–570. [PubMed] [Google Scholar]
- 3.Johnsen J. Pathogenesis in immune thromboycytopenia: new insights. Hematology. 2012;2012:306–312. doi: 10.1182/asheducation-2012.1.306. [DOI] [PubMed] [Google Scholar]
- 4.Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nature Medicine. 1995;1:237–243. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- 5.McMillan R. Antiplatelet antibodies in chronic immune thrombocytopenia and their role in platelet destruction and defective platelet production. Hematology Oncology Clinics North America. 2009;23:1163–1175. doi: 10.1016/j.hoc.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 6.McMillan R, Martin M. Fixation of the third component of complement (C3) to platelets in vitro by antiplatelet antibody for patients with ITP. British Journal of Haematology. 1981;47:251–256. doi: 10.1111/j.1365-2141.1981.tb02786.x. [DOI] [PubMed] [Google Scholar]
- 7.Najaoui A, Bakchoul T, Stoy J, Bein G, Rummel MJ, Santosos S, Sachs UJ. Autoantibody-mediated complement activation on platelets is a common finding in patients with immune thrombocytopenic purpura (ITP) European Journal of Haematology. 2011;88:167–174. doi: 10.1111/j.1600-0609.2011.01718.x. [DOI] [PubMed] [Google Scholar]
- 8.Peerschke EIB, Andemariam B, Yin WQ, Bussel JB. Complement activation on platelets correlates with a decrease in circulating immature platelets in patients with immune thrombocytopenic purpura. British Journal of Haematology. 2009;148:638–645. doi: 10.1111/j.1365-2141.2009.07995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peerschke EI, Yin W, Ghebrehiwet B. Complement activation on platelets: implications for vascular inflammation and thrombosis. Molecular Immunology. 2010;47:2170–2175. doi: 10.1016/j.molimm.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi J, Rose EL, Singh A, Hussain S, Stagliano NE, Parry GC, Panicker S. TNT003, an inhibitor of the serine protease C1s, prevents complement activation induced by cold agglutinins. Blood. 2014;123:2015–2022. doi: 10.1182/blood-2014-02-556027. [DOI] [PubMed] [Google Scholar]