Abstract
Recurrent urinary tract infections (RUTI) are prevalent and pose significant clinical challenges. Although the term RUTI has long been vaguely defined, a consensus definition has emerged in recent years. The exact etiology behind RUTI remains under debate, with valid arguments for both ascending reinfections as well as persistent infection inside the bladder. These persistent infections exist in the form of quiescent intracellular reservoirs in the mouse model and may represent a novel concept to explain UTI recurrence in humans. Manageable risk factors such as behavioral patterns alongside nonmanageable risk factors including genetic susceptibility are growing fields of investigation. Acute UTI have been studied through two model bacterial strains: Escherichia coli UTI89 and CFT073. However, the clinical relevance to RUTI of these two strains has not been firmly established. Current treatment strategies for RUTI are limited and remain dominated by antibiotic usage despite variable efficacy. The majority of studies in humans have focused on younger groups of women with little information available about the postmenopausal population despite a heightened risk of RUTI in this age group.
Keywords: intracellular bacterial communities, postmenopausal women, quiescent intracellular reservoir, recurrent urinary tract infections, reinfection, risk factors for urinary tract infections
1. Introduction
Urinary tract infections (UTI) are the most common adult bacterial infection in the world.1 In 2000, an estimated $2.5 billion was spent on UTI treatment excluding outpatient prescriptions.2 Considering that the majority of women presenting with UTI have a history of more than two previous infections, recurrence represents a substantial social cost.3 Recurrence also poses significant clinical challenges and has a major impact on quality of life. Unfortunately, few long-term or follow-up studies of recurrent UTI (RUTI) have been published to guide in evaluation and treatment. Current evidence indicates that the rate of recurrence following an initial UTI is high. A 1990 study at the University of Michigan involving female students aged 17–39 years showed that after a single UTI event, 27% of women will experience a second recurrence in the following 6 months with a further 3% experiencing a third UTI within the same time period.4 An older study from Denmark showed that for women aged 16–65 years, rate of recurrence is highest during the first 2 months post-treatment and between 25% and 35% of women will have recurrence within 3–6 months.5 Studies of UTI recurrence that include older women are rarer still. The results of a single study on recurrence in Finnish women indicated that 44% of women aged 17–82 years will experience recurrence within 12 months.6 Rates of infection have been found to be lowest in the winter months.7 Interestingly, the majority of women experiencing recurrence do so despite culture directed antibiotic treatment, having no anatomical abnormalities in the lower and upper urinary tracts, and being otherwise healthy individuals.8
In this review, we have considered only women suffering from RUTI who are otherwise normal with no obvious causal factor. Subpopulations excluded include pregnant women, infants, diabetics, patients with neurogenic bladder-like multiple sclerosis or AIDS, and individuals with abnormal genitourinary anatomy. Current guidelines for the diagnosis and management of RUTI in women along with indications for specialist referrals are beyond the scope of this review, but can be found elsewhere.9 Following a systematic review of RUTI literature, we present a current update on how UTIs are defined, animal models and strains to study RUTI, risk factors for recurrence among different age groups, relevant clinical studies, and therapeutic options.
2. Definition
Until 2000, there was no generally accepted and broadly used definition for RUTI in women. Since then, the majority of publications on RUTI define the condition as “at least three episodes of urinary tract infections during the previous 12 months”.8,10–8 Additionally, six publications included a non-mutually exclusive alternative of “at least two episodes of UTI during the previous 6 months”.10,11,13,14,16,18 Proof of a positive urine culture was also frequently incorporated in the definition8,11,15 with a specific concentration of > 103 colony forming units/mL mentioned in one instance.15 Therefore, to define RUTI as “three episodes of urine culture positive UTI in the previous 12 months or two episodes within the 6 months” represents an acceptable compromise in contemporary studies.
3. Etiology
There is evidence to support that RUTIs may be caused by one of two mechanisms: repeated ascending infections or chronic/persistent infection in the bladder. Repeated ascending infections are thought to occur by the endogenous rectal flora via a fecal–perineal–urethral route. Bacteria migrate from the gastrointestinal tract into the periurethral area, ultimately ascending the urethra into the bladder. The finding that causative Escherichia coli strains are often detectable in a women's endogenous rectal flora during active UTIs as well as the fact that sexual intercourse is known to be a definitive risk factor for RUTI support this hypothesis.19,20 In addition, women who suffer from RUTI have been found to have a higher frequency of infection with endogenous rectal flora, specifically E. coli and Enterococcus faecalis.21
However, several observations have countered this acceptable theory of ascending infections. First, following initial treatment with commonly used antibiotics, 77% of UTIs observed were due to a relapse with an E. coli strain identical to the primary infecting strain.8 Second, antibiotics applied to the perineal area have been shown to be ineffective in reducing the risk of RUTI.22 Finally, it has been noted that following antibiotic treatment, E. coli in the fecal flora are reduced in number.23 That is to say, the fecal flora represents an unstable reservoir for recurrence. This evidence argues for an alternative source of infection.
One such plausible alternative is the survival of bacteria in the urinary bladder through the progression of transient intracellular bacterial communities (IBC) into persistent quiescent intracellular reservoirs (QIR). IBC formation and its relationship to bacterial colonization was first studied in the late 1970s with the support of data gathered from aquatic ecosystems. This theory was then applied to industrial water systems where bacterial biofilms represent a major concern.24 To date, the majority of studies on RUTI-related IBC creation and subsequent QIR formation have been performed in murine models with E. coli strains UTI89 and CFT073.25–28 Through the work of Hultgren et al,29–33 the murine IBC/QIR pathogenic cycle has been gradually elucidated and is now largely understood.
IBCs are initially created when bacteria ascend the urethra and attach onto the bladder urothelium. In E. coli, luminal attachment is mediated via type 1 pili and results in urothelial envelopment.30 Initial IBC formation is rapid and can be seen as early as 3 hours postinoculation.31 By 12 hours postinfection, over half of all bacteria are intracellular.34 This biofilm allows bacteria to replicate while protected from innate immune defenses such as neutrophil phagocytosis.33 However, the majority of the bacteria are expelled from the bladder through TLR-4 dependent urothelial hyperplasia. The bacteria that escape this expulsion remain to create clonal recurrent IBCs as this process repeats itself.35
Acute IBC formation can result in a simple resolution, but the infection may also persist in the form of either chronic cystitis or QIRs. Chronic cystitis can be defined as persistent bacteriuria, which differs from recurrence. Recurrence implies that there is a period of time without bacteriuria or signs of infection. Through fluorescent tagging studies, it has been shown that during the initial acute infection, higher numbers of IBCs are more predictive of chronic cystitis than QIR formation.35 Although IBC expulsion and reformation may undergo many cycles and be called chronic, this process is limited by the fact that in response to infection, the bladder urothelium becomes hyperplastic. The underlying smaller maturing urothelial cells left after exfoliation cannot support large IBCs.32 As a result, each round of IBC formation is associated with slower bacterial replication and smaller IBCs with eventual resolution of infection.
Although the IBC cycle is self-limited, invasion of urothelial cells can result in recurrence through the creation of quiescent intracellular reservoirs. Uropathogenic E. coli (UPEC) have been shown to remain in the urothelium inside LAMP-1 positive vesicles (Fig. 1).36 In contrast to IBCs, these quiescent bacteria are non-replicating and do not elicit an immune response.33 As the epithelium turns over, these quiescent bacteria are released and emerge to create new acute infections. These recurrences may occur for months after the initial acute infection. In this fashion, as shown in animal models, some species of E. coli possess the ability to create a state of quiescent infection in the bladder that may be responsible for multiple recurrences.36
Fig. 1.
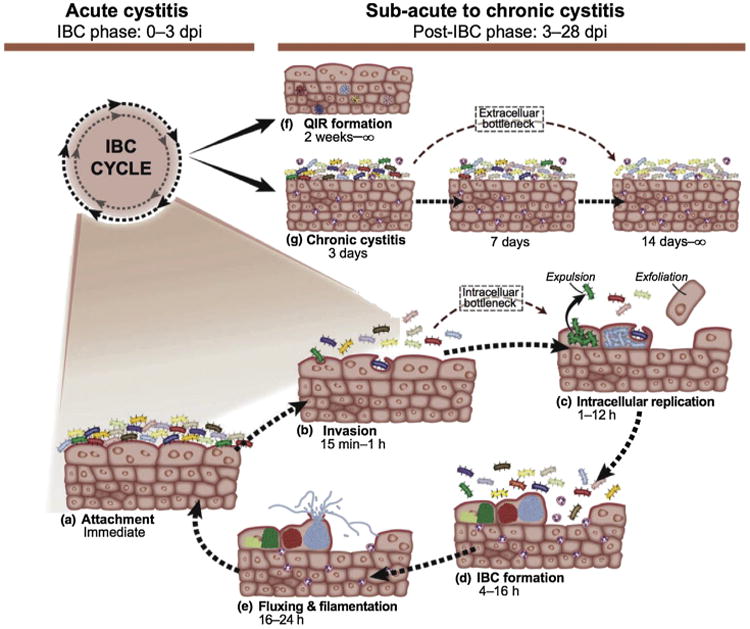
Uropathogenic Escherichia coli model for acute cystitis, chronic cystitis, and quiescent intracellular reservoir formation: intracellular bacterial community (IBC) formation starts when bacteria attach onto the apical transitional epithelium of the bladder via type 1 pili. These bacteria are then enveloped and invade the epithelium – replicating and forming IBCs. As a host response to infection, the urothelium typically exfoliates, resulting in IBC liberation and IBC recreation in a clonal fashion. IBCs may also progress to quiescent intracellular reservoirs, which are not metabolically active and do not produce a measurable inflammatory response.
Note. From “Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection,” by T.J. Hannan, M. Totsika, K.J. Mansfield, K.H. Moore, M.A. Schembri, and S.J. Hultgren, 2012, FEMS Microbiol Rev, 3, p. 616–48. Copyright 2012, Nature Publishing Group. Reprinted with permission.
So far, the model for UPEC persistence in the urinary bladder has not been confirmed in humans. Nevertheless, the presence of IBC exfoliates and filamentous bacteria in the urine of women with RUTI compared to controls suggests that IBC formation with progression to QIR may be a reality in humans.30 Evidence supports the validity of murine model IBC formation as an accurate representation of human IBC formation. In 2007, Rosen et al30 found human and murine urine cytologies to be identical. Moreover, E. coli have been found to have the ability to invade and colonize human urothelium.37
As in animal models, human urothelium typically undergoes apoptosis-induced exfoliation upon infection.31 This exfoliation may be observed by cystoscopy as particulates floating in the bladder lumen. Additionally, IBCs may be detectable in human urine samples. Urine samples have long been considered the established norm for UTI diagnosis. However, urine cultures that are found to be culture-negative can contain various bacterial communities and factors that may not be detectable through the standard cultivation techniques used for routine urine cultures.38 One such example is bacterial filaments in the urine. Observation of bacterial filaments in the urine through light microscopy and immunofluorescence is thought to either represent bacteria that have recently emerged from IBCs or is a result of induction of the SOS response.39 The presence of these filaments has been associated with increased longevity of RUTI symptoms.30 In Rossen et al's30 2007 study at the University of Washington involving 100 women aged 18–41 years, 100% of the women with IBCs had filamentous bacteria in their urine compared to only 29% for controls. At present, the standard for detecting the presence of IBC filaments is screening by light microscopy and confirmation via immunofluorescence30 making IBC filament examination of each urine sample impractical due to both time and financial limitations.
Current evidence supports both ascending reinfection (Fig. 2) and QIR recurrence as models for RUTI. In six studies of women experiencing RUTI following a primary infection, the rate reinfection by primary strain varied from 47% to 81%.40–45 Although five of the six studies propose a slightly higher rate of reinfection by QIR recurrence (> 50% reinfection by primary strain),41–45 the aggregated results suggest that both ascending reinfection and QIR recurrence are significant causes of RUTI in women (Table 1).
Fig. 2.
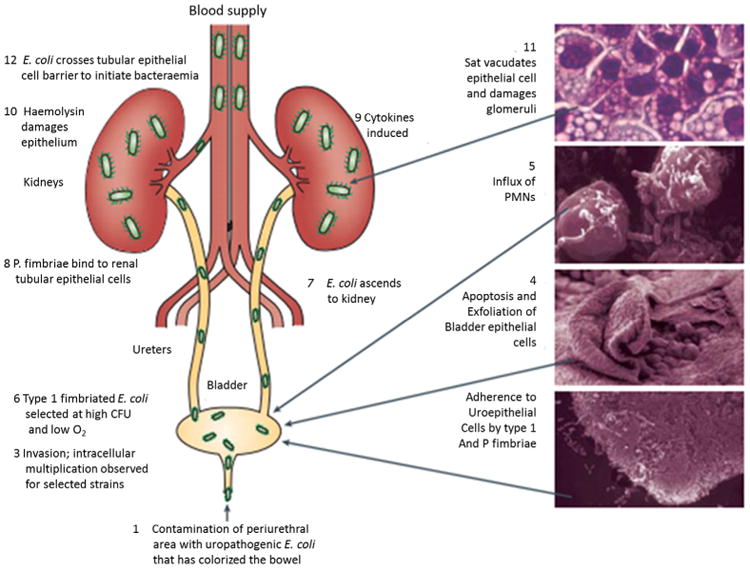
Ascending reinfection. Uropathogens originate from the rectal flora and colonize the periurethral area leading to ascension through the urethra into the bladder.
Note. From “Pathogenic Escherichia coli” by J.B. Kaper, J.P. Nataro, and H.L. Mobley, 2004. Nat Rev Microbiol, 2, p. 123–40. Copyright 2004. Nature Publishing Group. Reprinted with permission.
Table 1.
Recurrent urinary tract infection strain analysis.a
| Authors (y)ref | No. with reinfection/total no. of women analyzed | Technique | % Reinfection by primary infecting Escherichia coli strain (recurrence) |
|---|---|---|---|
| Jacobson et al (1992)40 | 19/19 | PhP-EC system (BioSys Inova) | 47% |
| Russo et al (1995)41 | 23/23 | Chromosomal restriction fragment length polymorphism | 68% |
| Ejrnaes et al (2006)42 | 60/156 | Pulsed-field gel electrophoresis | 77% |
| Vosti (2007)43 | 148/203 | O serotype grouping by antisera | 80.6%, 62.1%, and 68.8% for each of the first 3 mo, respectively |
| Skjøt-Rasmussen et al (2011)44 | 10/13 | Pulsed-field gel electrophoresis | 77% |
| Beerepoot et al (2012)45 | 35/50 | Pulsed-field gel electrophoresis | 70% |
Using a PubMed search, six studies were found in which the reinfecting strain of E. coli was compared with the primary infecting strain. The aggregate results are conclusive for neither ascending reinfection (0% reinfection by primary strain) nor quiescent intracellular reservoirs (QIR) recurrence (100% reinfection by primary strain). There is slight bias towards QIR recurrence with five of the six studies showing a reinfection rate by primary strain > 50%. Overall, the results suggest that both ascending reinfections as well as QIR recurrence are likely causes of recurrent urinary tract infection in women.
4. Risk factors
Risk factors for RUTI are less well defined than for UTI alone, and can be divided between pre- and postmenopausal groups. In premenopausal women, sexual intercourse and a prior history of UTI are the factors most associated with recurrence. Diaphragm use and exposure to oral contraceptives and spermicides have also been independently shown to be important risk factors.10 Although it is accepted that the majority of RUTI occur in women with anatomically normal urinary tracts, a study involving a total of 213 college age women indicated a significantly shorter anal–urethral distance of < 4.5 cm [95% confidence interval (CI), 1.2–4.8; p = 0.013] in those suffering from RUTI compared to controls suggesting a role for variations in pelvic anatomy.46 Many women believe that certain behavior patterns such as pre- and post-coital voiding, frequency of urination, delayed voiding habits, wiping patterns, douching, hot tub usage, choice of clothing, and body mass index may have an association with risk of RUTI. However, in a single study involving 229 women with a history of RUTI and 253 controls (no UTI in the preceding 12 months and no period of fewer than 1 UTI in any 12 month period), none of these plausible behavioral factors were shown to significantly increase the risk of RUTI (p < 0.05).10
Risk factors for RUTI in postmenopausal women have been less well described. A case–control study by Raz et al47 comparing 149 postmenopausal women with a history of RUTI to 53 age-matched controls reported that a history of RUTI and nonsecretor status were strongly associated with RUTI. Likewise, in the same study, urodynamic factors of incontinence (41% vs. 9% control, p < 0.001), residual urine volume (28% vs. 2% control, p < 0.001), and presence of a cystocele (19% vs. 0% control, p < 0.001) were shown to predispose these women to recurrence.47 Estrogen levels also seem to play an important role. Reduced levels of estrogen result in an increased vaginal pH, decreased endogenous vaginal microflora, and increased incidence of prolapse due to muscle weakness.48 Each of these factors may predispose women to pathogenic E. coli colonization. Furthermore, in murine models, decreases in estrogen have been found to be related to delayed development of the protective bladder glycosaminoglycan layer, increasing susceptibility to bacterial colonization and IBC formation.49 Postmenopausal women aged ≥ 70 years face unique challenges with regard to RUTI due to institutionalization and decreased functional status. Increased rates of catheterization, incontinence, and prolapse surgery lead to a substantial increase in risk of RUTI in this specific population.
The fact that the vast majority of RUTI are clustered in healthy women who have anatomically and functionally normal urinary tracts suggests the presence of underlying genetic factors related to decreased bladder defenses. These genetic differences among individuals may be characterized at a superficial level through family history. Predisposition to RUTI has been shown to have strong familial linkage. A case–control study of 1278 women aged 18–49 years found the risk of RUTI and pyelonephritis to be increased for women who had at least one female relative with a reported UTI.50 This genetic increased risk of RUTI was found to be similar in magnitude to that of four to eight episodes of intercourse/month, odds ratio (OR) 5.0 (95% CI, 3.1–8.1) versus OR 5.8 (95% CI, 3.1–10.6), respectively.10,50 Among all female relatives, a history of RUTI in the mother has been shown to most strongly predispose to RUTI in the daughter.10 The increased risk of RUTI among relatives may also be attributed in part to common environmental factors or behaviors.
More specifically, polymorphisms between individuals may include differences in blood groups and cell-mediated immunity. That bacteria can cross-react with ABO blood groups has been known since the 1960s. As a result, women who are nonsecretors of blood group antigens B or AB are up to four times more susceptible to RUTI.51 It is hypothesized that anti-B isohemagglutinins may interact with specific blood group like antigen binding sites on the bacterial cell wall, thereby inhibiting uroepithelial attachment.52 Given that neutrophil-defense is essential for cell-mediated UTI resistance, cytokine polymorphisms or deficiencies may alter neutrophil recruitment and predispose to RUTI. A 2005 study by Smithson et al53 of 20 premenopausal women aged 21–36 years noted that decreased levels of the CXCR2 IL-8 receptor could lead to RUTI susceptibility in women with a history of childhood UTIs. Presumably this decrease of CXCR2 leads to poor neutrophil chemotaxis. Knockout mice for the murine IL-8 receptor homologue show findings similar to humans with decreased CXCR2 expression: the mice were unable to clear bacterial infections from their kidney and bladder and developed systemic disease.54 Tolllike receptors (TLR) are a family of receptors that become activated upon recognizing pathogen associated molecules, resulting in leukocyte recruitment and host-defense gene transcription. A 2009 case–control study by Hawn et al55 investigated TLR genes in more than 430 adult women aged 18–49 years. Polymorphisms of the TLR5 receptor, responsible for recognizing the flagellin of mobile bacteria, were found to result in increased susceptibility to RUTI but not pyelonephritis (OR, 1.81; 95% CI, 1.00–3.08). Additionally, reduced levels of TLR4 were found in women with recurrent asymptomatic bacteriuria compared with controls.55 TLR4 is responsible for detecting the lipopolysaccharide from Gram-negative bacteria.33 The genetic background of susceptibility to RUTI is not fully understood, but remains an important area of investigation. Further investigation in this area may help lead to early identification of adults predisposed to RUTI and prediction of recurrence rates in offspring.
4.1. RUTI strains and models
The causative agent in the majority of RUTI is UPEC.33 Unlike UTIs, studies specifically genotyping all bacteria that can cause RUTI are scarce. An older study by Stamey and Sexton21 indicated that women suffering from RUTI had a higher percentage of colonization with E. coli, E. faecalis, Proteus mirabilis, and Klebsiella than did women without a history of RUTI. Colonization with Gram-negative bacilli was found to be more prolific and longer lasting in women with RUTI.21 Evidence suggests that these bacterial species, UPEC in particular, may be more common RUTI causative agents due to their greater propensity for making IBCs with subsequent QIR formation.30 A 2006 RUTI species genotyping study found that 77% of RUTI are caused by relapse with a UPEC strain identical to the primary infecting strain.42 Additionally, a 2000 study of college age women found the risk of a secondary infection within a 6-month time frame for E. coli to be 23% versus 7% for non-E. coli infections.56
Because of its prevalence and responsiveness to manipulation, E. coli is fittingly the most common model for RUTI study. Two strains in particular, CFT073 and UTI89, have been thoroughly investigated. Neither CFT073 nor UTI89 was isolated from patients suffering from RUTI. CFT073 was originally isolated from the blood and urine of a woman with acute pyelonephritis whereas UTI89 was isolated from a patient suffering from acute cystitis.57,58 The UPEC genome is a mosaic continuum such that no single feature can be used to differentiate strains. Similarly, no virulence factor alone is sufficient to cause disease.59 However, through observation, some generalities can be made distinguishing CFT073 from UTI89: CFT073 has the ability to form IBCs, but is also extremely toxic, causing severe damage to the urothelium. UTI89 is likewise highly toxic, but has a higher propensity for intracellular invasion with IBC formation and progression to QIRs.60
Common among all UPECs, including CFT073 and UTI89, are virulence factors of various types that together allow for the establishment of urinary tract infections. One such group of virulence factors is adhesion molecules, which mediate urothelial attachment and bladder invasion. Of the adhesions molecules, type 1 fimbriae and P fimbriae have been best characterized.61,62 Type 1 fimbriae are more associated with IBC formation whereas P fimbriae have a close association with pyelonephritis.8 UPEC also must overcome the challenge of obtaining iron from their environment. Iron is the limiting nutrient for UPEC in the urinary tract.63 UPEC accomplishes this feat through the use of iron binding siderophores and siderophore receptors, effectively creating a siderophore–iron receptor that is taken up by the bacteria with iron being released into the cytosol.33,63,64 UPEC creates many toxins that have the ability to not only mediate cell lysis causing tissue damage and exfoliation, but also to modulate host-signaling pathways. One such example of host modulation is the disruption of phagocyte function.65 Along with IBC formation, UPEC have intrinsic protective factors that can confer resistance to phagocytosis, opsonization, and lysis (Table 2).65
Table 2.
Sample of uropathogenic Escherichia coli virulence factors.a
| Type | Name | Function | Gene |
|---|---|---|---|
| Adhesion62,84 | Type 1 fimbriae | Adhesion, urothelial binding and invasion, IBC formation | fimH |
| Adhesion62 | P fimbriae | Adhesion, urothelial binding, associated with pyelonephritis | PapG |
| Iron metabolism63,84,85 | Aerobactin siderophore receptor | Ferric iron uptake | iutA |
| Toxin/nitrogen metabolism84,86,87 | Urease | Cleaves urea to NH3 and CO2 | Ure |
| Toxin65 | α-hemolysin | Erythrocyte lysis, tissue injury, urothelial exfoliation, host signal modulation | hlyA |
| Protection65 | Serum survival protein | Impairs complement activity at outer membrane | iss |
| Protection65,84,88 | K1 capsule | Shields bacteria from phagocytosis, blocks alternative complement | kpsMT |
Uropathogenic E. coli possess a diverse array of virulence factors, each of which contributes to infection of the urinary tract. No single virulence factor is sufficient for infection. Rather, it is the cumulative effect of multiple factors (protective, toxigenic, metabolism, adhesion) that allows for infection of the urinary tract.59
4.2. RUTIs in postmenopausal women
It is accepted that older women are more likely to get a UTI and suffer from recurrence. Overall, approximately 10–15% of women over 60 years have had at least one incidence of RUTI.66 A 1996 prospective study by Ikäheimo et al6 followed 179 adult women for 12 months after a UTI episode caused by E. coli. They found a 55% recurrence rate in women over 55 years compared to 36% in younger women. This increased susceptibility with age may be due in part to a natural decrease in inflammatory and immune functions as well as a decrease in postmenopausal estrogen. Bacteriuria can also be found in up to 40% of elderly, noninstitutionalized women.66
Despite the fact that recurrence occurs more frequently in the elderly than in any other age group, the vast majority of RUTI research has been performed in younger women. As a result, there is a surprising absence of literature about RUTI in postmenopausal women. Many RUTI studies include postmenopausal women in their cohorts, but few studies have focused specifically on this population. In reviewing literature since 2000, we were able to find only three studies directly involving both postmenopausal women and RUTI. Of these three studies, two involved treatment options whereas one focused on risk factors. Lacking are studies on the mechanism behind recurrence in the elderly, what species may be causing these infections, and treatment options specific for postmenopausal women (Table 3).
Table 3.
Recurrent urinary tract infection (RUTI) studies: postmenopausal women versus controls.a
| Authors (y)ref | Total no. of women (patients) | Total no. of women (controls) | Average age (y; patients) | Average age (y; controls) | Result |
|---|---|---|---|---|---|
| Raz et al (2000)47 | 149 | 53 | 65.7 ± 7.2 | 66.6 ± 6.6 | Incontinence, cystocele, and post voiding residual urine are strongly associated with RUTI |
| Raz et al (2003)89 | 86 (estriol pessary) | 85 (nitrofurantoin) | 68 ± 7.2 | 66.9 ± 7.9 | Estriol vaginal pessaries are less effective than oral nitrofurantoin in RUTI prevention |
| Zhong et al (2011)16 | 37 (continuous) | 31 (intermittent) | 62.3 ± 7 | 62.8 ± 7.3 | Single-dose patient-initiated antibiotic prophylaxis is as effective as low-dose daily antibiotic prophylaxis for preventing RUTI |
Using a PubMed search, only three studies since 2000 were found to include both exclusively postmenopausal women and RUTI. Of these three studies, two were authored by Raz et al,47,89 one focusing on risk factors for RUTI and the other on comparative treatment of RUTI by estriol vaginal ring versus oral nitrofurantoin. Most recently, a 2011 study by Zhong et al16 on prophylaxis dosing regimens found single-dose patient-initiated antibiotic prophylaxis to be as effective as low-dose daily antibiotic prophylaxis for preventing RUTI.
4.3. Current treatment strategies
Treatments for UTI and RUTI are similar in that the first line of defense involves antibiotic therapy. Trimethoprim/sulfamethoxazole (TMP-SMX), fluoroquinolones (Ciprofloxacin), β-lactams, and nitrofurantoin (Macrobid) are the most common antimicrobial agents used in daily practice.8 However, dosing regimens may differ in women with frequent RUTI, favoring patient-initiated treatment when symptoms start, postcoital therapy, and long-term daily prophylaxis.66 In a Cochrane meta-analysis, 10 out of 19 trials evaluated explored antibiotic prophylaxis versus placebo. During prophylaxis, the rates of recurrence were found to be 0–0.9/person/year compared to 0.8–3.6 in the placebo group. The relative risk of at least one recurrence was found to be 0.21 (95% CI, 0.13–0.33) in support of antibiotic prophylaxis.67 The determining factor in whether antimicrobial prophylaxis should be tried is typically the degree of discomfort felt upon an infection.20 Empiric evidence suggests a 6-month prophylaxis period may decrease rate of recurrence.7,68 Nevertheless, there remains no decisive conclusion on the optimal duration of prophylaxis and in some cases simple postcoital prophylaxis may be as effective as long-term therapy.67 Unless behavioral patterns such as sexual activity or diaphragm and spermicide use are changed, women tend to relapse back to their previous pattern of recurrent infections once prophylaxis is stopped. An estimated 40–60% of women suffer from recurrence within 6 months of ending prophylaxis.69
One advantage of single dose postcoital antibiotics over prophylaxis is a lower net dose of antibiotics.70 Many uncontrolled studies have been published comparing postcoital efficacy for decreasing rate of reinfection for various antibiotics versus continuous prophylaxis. Each of these studies supports a similar magnitude of reduction of reinfection with postcoital TMP-SMX, nitrofurantoin, cephalexin, fluoroquinolones, and ciprofloxacin when compared to prophylaxis.71–76 To our knowledge, only one placebo controlled trial for postcoital prophylaxis has shown a decrease in cystitis recurrence rate with postcoital TMP-SMX of 40/200 mg (0.3/patient–year) compared to placebo (3.6/patient–year).77
Treatment for RUTI is generally the same for postmenopausal as for premenopausal women. It is accepted that if complicating factors can be excluded, antibiotic treatment should be carried out as for premenopausal women.48 One additional treatment option is available for postmenopausal women in the form of estrogen therapy. An older double-blind, placebo-controlled study of 93 postmenopausal women by Raz and Stamm78 indicated that vaginal estriol treatment dramatically decreased rates of recurrence (0.5 episodes/year compared with 5.9 for placebo). More recently, Eriksen79 obtained similar results with an estradiol vaginal ring with 45% of women receiving estradiol being free of recurrence versus only 20% for placebo. Studies involving oral estrogens have been less conclusive. A Cochrane review analyzed four studies involving a total of 2798 women treated with oral estrogen versus placebo. They found no statistically significant difference in rates of recurrence (RR, 1.08, 95% CI, 0.88–1.33).80 Clinically, estrogen therapy is currently recommended for patients that are either infected with antibiotic-resistant uropathogens or in patients whose recurrence may be related to atrophic vaginitis.48
Treatment of recurrence due to QIRs is difficult. Due to the rapid kinetics of IBC formation, there exists only a small window in which to treat prior to when antibiotics are unable to penetrate the biofilm. Once biofilm formation is complete, few treatment options remain and progression to QIR is likely. One possible method for dealing with established QIRs involves the physical destruction of the involved urothelium. The tissue can be destroyed through a short outpatient endoscopic fulguration of the trigonal areas involved with chronic infection (trigonitis). Our group has reported preliminary encouraging data on this approach, with long-term follow-up still lacking.81
Anecdotal evidence has long supported cranberry juice as a treatment option for RUTI in women. However, recent studies have failed to find any such protective value. A 2011 study by Barbosa-Cesnik et al82 of 319 college women was unable to find any decrease in the 6-month incidence of UTI recurrence among those drinking 225 g of 27% cranberry twice daily compared to placebo. Likewise, Stapleton et al83 could not find any significant (p < 0.05) reduction in risk of RUTI between 120 women aged 18–45 years taking 225 g of cranberry daily versus 56 women receiving placebo. Interestingly, cranberry juice was found to reduce the rate of symptomatic UTI caused by P-fimbriated E. coli strains. Although not statistically significant, this observation lends support to the hypothesis that cranberry or a breakdown metabolite may inhibit P-fimbriated E. coli attachment to the bladder epithelium, giving a plausible biological mechanism explanation for the proposed efficacy of cranberry juice.83 To our knowledge, no studies exploring the efficacy of cranberry prophylaxis exclusively in postmenopausal women have been performed.
5. Conclusion
RUTI is a prevalent and challenging condition among women of all ages. At present, there are two models to explain RUTI: repeated reinfection from the fecal–perineal–urethral route and IBC progression leading to QIR recurrence. Among UPEC, both CFT073 and UTI89 have been extensively studied, including their propensity for biofilm production. However, it is not known if these strains represent the dominant forms of E. coli encountered in women with RUTI. RUTI management remains difficult with treatment largely based on antibiotic regimens of various durations. RUTI has been studied more extensively in younger women than in postmenopausal women. Given the aging of our population, and the growing issues of antibiotic allergies and bacterial resistance, this gap in knowledge must be bridged in the future.
Acknowledgments
Sources of funding: None.
Footnotes
There are 3 CME questions based on this article.
Conflicts of interest statement: The authors declare that they have no financial or non-financial conflicts of interest related to the subject matter or materials discussed in the manuscript.
References
- 1.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl. 1A):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 2.Griebling TL. Urologic diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173:1281–7. doi: 10.1097/01.ju.0000155596.98780.82. [DOI] [PubMed] [Google Scholar]
- 3.Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10:509–15. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 4.Foxman B. Recurring urinary tract infection: incidence and risk factors. Am J Public Health. 1990;80:331–3. doi: 10.2105/ajph.80.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mabeck CE. Treatment of uncomplicated urinary tract infection in non-pregnant women. Postgrad Med J. 1972;48:69–75. [PMC free article] [PubMed] [Google Scholar]
- 6.Ikäheimo R, Siitonen A, Heiskanen T, Kärkkäinen U, Kuosmanen P, Lipponen P, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;22:91–9. doi: 10.1093/clinids/22.1.91. [DOI] [PubMed] [Google Scholar]
- 7.Stamm WE, McKevitt M, Roberts PL, White NJ. Natural history of recurrent urinary tract infections in women. Rev Infect Dis. 1991;13:77–84. doi: 10.1093/clinids/13.1.77. [DOI] [PubMed] [Google Scholar]
- 8.Ejrnæs K. Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli. Dan Med Bull. 2011;58:B4187. [PubMed] [Google Scholar]
- 9.Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5:316–22. doi: 10.5489/cuaj.11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scholes D, Hooton TM, Roberts PL, Stapleton AE, Gupta K, Stamm WE. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177–82. doi: 10.1086/315827. [DOI] [PubMed] [Google Scholar]
- 11.Raz R. Postmenopausal women with recurrent UTI. Int J Antimicrob Agents. 2001;17:269–71. doi: 10.1016/s0924-8579(00)00355-1. [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz A, Bahat E, Yilmaz GG, Hasanoglu A, Akman S, Guven AG. Adjuvant effect of vitamin A on recurrent lower urinary tract infections. Pediatr Int. 2007;49:310–3. doi: 10.1111/j.1442-200X.2007.02370.x. [DOI] [PubMed] [Google Scholar]
- 13.Grabe M, Bishop MC, Bjerklund-Johansen TE, Botto H, Çek M, Lobel B, et al. Guidelines on the management of urinary and male genital tract infections. Arnhem: European Association of Urology. 2008 doi: 10.1159/000049840. [DOI] [PubMed] [Google Scholar]
- 14.Grimes CL, Lukacz ES. Urinary tract infections. Female Pelvic Med Reconstr Surg. 2011;17:272–8. doi: 10.1097/SPV.0b013e318237b99d. [DOI] [PubMed] [Google Scholar]
- 15.Damiano R, Quarto G, Bava I, Ucciero G, De Domenico R, Palumbo MI, et al. Prevention of recurrent urinary tract infections by intravesical administration of hyaluronic acid and chondroitin sulphate: a placebo-controlled randomised trial. Eur Urol. 2011;59:645–51. doi: 10.1016/j.eururo.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Zhong YH, Fang Y, Zhou JZ, Tang Y, Gong SM, Ding XQ. Effectiveness and safety of patient-initiated single-dose versus continuous low-dose antibiotic prophylaxis for recurrent urinary tract infections in postmenopausal women: a randomized controlled study. J Int Med Res. 2011;39:2335–43. doi: 10.1177/147323001103900633. [DOI] [PubMed] [Google Scholar]
- 17.Nygaard I, Brubaker L, Chai TC, Markland AD, Menefee SA, Sirls L, et al. Risk factors for urinary tract infection following incontinence surgery. Int Urogynecol J. 2011;22:1255–65. doi: 10.1007/s00192-011-1429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Starre WE, van Nieuwkoop C, Paltansing S, van't Wout JW, Groeneveld GH, Becker MJ, et al. Risk factors for fluoroquinolone-resistant Escherichia coli in adults with community-onset febrile urinary tract infection. J Antimicrob Chemother. 2011;66:650–6. doi: 10.1093/jac/dkq465. [DOI] [PubMed] [Google Scholar]
- 19.Moreno E, Andreu A, Pérez T, Sabaté M, Johnson JR, Prats G. Relationship between Escherichia coli strains causing urinary tract infection in women and the dominant faecal flora of the same hosts. Epidemiol Infect. 2006;134:1015–23. doi: 10.1017/S0950268806005917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents. 2001;17:259–68. doi: 10.1016/s0924-8579(00)00350-2. [DOI] [PubMed] [Google Scholar]
- 21.Stamey TA, Sexton CC. The role of vaginal colonization with Enterobacteriaceae in recurrent urinary infections. J Urol. 1975;2:214–7. doi: 10.1016/s0022-5347(17)59447-1. [DOI] [PubMed] [Google Scholar]
- 22.Cass AS, Ireland GW. Antibacterial perineal washing for prevention of recurrent urinary tract infections. Urology. 1985;25:492–4. doi: 10.1016/0090-4295(85)90458-3. [DOI] [PubMed] [Google Scholar]
- 23.Nord CE, Kager L, Heimdahl A. Impact of antimicrobial agents on the gastrointestinal microflora and the risk of infections. Am J Med. 1984;76:99–106. doi: 10.1016/0002-9343(84)90250-x. [DOI] [PubMed] [Google Scholar]
- 24.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reigstad CS, Hultgren SJ, Gordon JI. Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J Biol Chem. 2007;282:21259–67. doi: 10.1074/jbc.M611502200. [DOI] [PubMed] [Google Scholar]
- 26.Garofalo CK, Hooton TM, Martin SM, Stamm WE, Palermo JJ, Gordon JI, et al. Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infect Immun. 2007;75:52–60. doi: 10.1128/IAI.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Justice SS, Lauer SR, Hultgren SJ, Hunstad DA. Maturation of intracellular Escherichia coli communities requires SurA. Infect Immun. 2006;74:4793–800. doi: 10.1128/IAI.00355-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson GG, Goller CC, Justice S, Hultgren SJ, Seed PC. Polysaccharide capsule and sialic acid-mediated regulation promote biofilm-like intracellular bacterial communities during cystitis. Infect Immun. 2010;78:963–75. doi: 10.1128/IAI.00925-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tenke P, Koves B, Nagy K, Hultgren SJ, Mendling W, Wullt B, et al. Update on biofilm infections in the urinary tract. World J Urol. 2012;30:51–7. doi: 10.1007/s00345-011-0689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson GG, Martin SM, Hultgren SJ. Host subversion by formation of intracellular bacterial communities in the urinary tract. Microbes Infect. 2004;6:1094–101. doi: 10.1016/j.micinf.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A. 2004;101:1333–8. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hannan TJ, Totsika M, Mansfield KJ, Moore KH, Schembri MA, Hultgren SJ. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev. 2012;36:616–48. doi: 10.1111/j.1574-6976.2012.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, et al. Induction and Evasion of Host Defenses by Type 1-Piliated Uropathogenic Escherichia coli. Science. 1998;282:1494–7. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz DJ, Chen SL, Hultgren SJ, Seed PC. Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infect Immun. 2011;79:4250–9. doi: 10.1128/IAI.05339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A. 2006;103:14170–5. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez JJ, Muvley MA, Schilling JD, Pinkner JS, Hultgren SJ. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000;19:2803–12. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolfe AJ, Toh E, Shibata N, Rong R, Kenton K, Fitzgerald M, et al. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. 2012;50:1376–83. doi: 10.1128/JCM.05852-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li B, Smith P, Horvath DJ, Jr, Romesberg FE, Justice SS. SOS regulatory elements are essential for UPEC pathogenesis. Microbes Infect. 2010;12:662–8. doi: 10.1016/j.micinf.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Jacobson SH, Kühn I, Brauner A. Biochemical fingerprinting of urinary Escherichia coli causing recurrent infections in women with pyelonephritic renal scarring. Scand J Urol Nephrol. 1992;26:373–7. doi: 10.3109/00365599209181229. [DOI] [PubMed] [Google Scholar]
- 41.Russo TA, Stapleton A, Wenderoth S, Hooton TM, Stamm WE. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J Infect Dis. 1995;2:440–5. doi: 10.1093/infdis/172.2.440. [DOI] [PubMed] [Google Scholar]
- 42.Ejrnaes K, Sandvang D, Lundgren B, Ferry S, Holm S, Monsen T, et al. Pulsed-field gel electrophoresis typing of Escherichia coli strains from samples collected before and after pivmecillinam or placebo treatment of uncomplicated community-acquired urinary tract infection in women. J Clin Microbiol. 2006;44:1776–81. doi: 10.1128/JCM.44.5.1776-1781.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vosti KL. A prospective, longitudinal study of the behavior of serologically classified isolates of Escherichia coli in women with recurrent urinary tract infections. J Infect. 2007;55:8–18. doi: 10.1016/j.jinf.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Skjøt-Rasmussen L, Hammerum AM, Jakobsen L, Lester CH, Larsen P, Frimodt-Møller N. Persisting clones of Escherichia coli isolates from recurrent urinary tract infection in men and women. J Med Microbiol. 2011;60:550–4. doi: 10.1099/jmm.0.026963-0. [DOI] [PubMed] [Google Scholar]
- 45.Beerepoot MA, den Heijer CD, Penders J, Prins JM, Stobberingh EE, Geerlings SE. Predictive value of Escherichia coli susceptibility in strains causing asymptomatic bacteriuria for women with recurrent symptomatic urinary tract infections receiving prophylaxis. Clin Microbiol Infect. 2012;18:E84–90. doi: 10.1111/j.1469-0691.2012.03773.x. [DOI] [PubMed] [Google Scholar]
- 46.Hooton TM, Stapleton AE, Roberts PL, Winter C, Scholes D, Bavendam T, et al. Perineal anatomy and urine-voiding characteristics of young women with and without recurrent urinary tract infections. Clin Infect Dis. 1999;29:1600–1. doi: 10.1086/313528. [DOI] [PubMed] [Google Scholar]
- 47.Raz R, Gennesin Y, Wasser J, Stoler Z, Rosenfeld S, Rottensterich E, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000;30:152–6. doi: 10.1086/313596. [DOI] [PubMed] [Google Scholar]
- 48.Raz R. Urinary tract infection in postmenopausal women. Korean J Urol. 2011;52:801–8. doi: 10.4111/kju.2011.52.12.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anand M, Wang C, French J, Isaacson-Schmid M, Wall LL, Mysorekar IU. Estrogen affects the glycosaminoglycan layer of the murine bladder. Female Pelvic Med Reconstr Surg. 2012;18:148–52. doi: 10.1097/SPV.0b013e31824b76bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scholes D, Hawn TR, Roberts PL, Li SS, Stapleton AE, Zhao LP, et al. Family history and risk of recurrent cystitis and pyelonephritis in women. J Urol. 2010;184:564–9. doi: 10.1016/j.juro.2010.03.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, Rogatko A, Fair WR. Association of the Lewis blood-group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773–7. doi: 10.1056/NEJM198903233201205. [DOI] [PubMed] [Google Scholar]
- 52.Kinane DF, Blackwell CC, Brettle RP, Weir DM, Winstanley FP, Elton RA. ABO blood group, secretor state, and susceptibility to recurrent urinary tract infection in women. Br Med J (Clin Res Ed) 1982;285:7–9. doi: 10.1136/bmj.285.6334.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smithson A, Sarrias MR, Barcelo J, Suarez B, Horcajada JP, Soto SM, et al. Expression of interleukin-8 receptors (CXCR1 and CXCR2) in premenopausal women with recurrent urinary tract infections. Clin Diagn Lab Immunol. 2005;12:1358–63. doi: 10.1128/CDLI.12.12.1358-1363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frendéus B, Godaly G, Hang L, Karpman D, Lundstedt AC, Svanborg C. Interleukin 8 receptor deficiency confers susceptibility to acute experimental pyelonephritisand may have a human counterpart. J Exp Med. 2000;192:881–90. doi: 10.1084/jem.192.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hawn TR, Scholes D, Li SS, Wang H, Yang Y, Roberts PL, et al. Toll-like receptor polymorphisms and susceptibility to urinary tract infections in adult women. PLoS One. 2009;4:e5990. doi: 10.1371/journal.pone.0005990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foxman B, Gillespie B, Koopman J, Zhang L, Palin K, Tallman P, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194–205. doi: 10.1093/oxfordjournals.aje.a010170. [DOI] [PubMed] [Google Scholar]
- 57.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–9. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mobley HL, Green DM, Trifillis AL, Johnson DE, Chippendale GR, Lockatell CV, et al. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990;58:1281–9. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson JR. Microbial virulence determinants and the pathogenesis of urinary tract infection. Infect Dis Clin North Am. 2003;17:261–78. doi: 10.1016/s0891-5520(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 60.Ulett GC, Totsika M, Schaale K, Carey AJ, Sweet MJ, Schembri MA. Uropathogenic Escherichia coli virulence and innate immune responses during urinary tract infection. Curr Opin Microbiol. 2013;16:1–8. doi: 10.1016/j.mib.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol. 2007;9:2230–41. doi: 10.1111/j.1462-5822.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- 62.Mulvey MA. Adhesion and entry of uropathogenic Escherichia coli. Cell Microbiol. 2002;4:257–71. doi: 10.1046/j.1462-5822.2002.00193.x. [DOI] [PubMed] [Google Scholar]
- 63.Andrews SC, Robinson AK, Rodríguez-Quinones F. Bacterial iron hemostasis. FEMS Microbiol Rev. 2003;27:215–37. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 64.Henderson JP, Crowley JR, Pinkner JS, Walker JN, Tsukayama P, Stamm WE, et al. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathogens. 2009;5:1–11. doi: 10.1371/journal.ppat.1000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dwyer PL, O'Reilly M. Recurrent urinary tract infection in the female. Curr Opin Obstet Gynecol. 2002;14:537–43. doi: 10.1097/00001703-200210000-00016. [DOI] [PubMed] [Google Scholar]
- 67.Albert X, Huertas I, Pereiró I, Sanfélix J, Gosalbes V, Perrota C. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women (Review) Cochrane Database Syst Rev. 2004;3:CD001209. doi: 10.1002/14651858.CD001209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kraft JK, Stamey TA. The natural history of symptomatic recurrent bacteriuria in women. Medicine (Baltimore) 1977;56:55–60. [PubMed] [Google Scholar]
- 69.Stapleton A, Stamm WE. Prevention of urinary tract infection. Infect Clin Dis North Am. 1997;11:719–33. doi: 10.1016/s0891-5520(05)70382-2. [DOI] [PubMed] [Google Scholar]
- 70.Lichtenberger P, Hooton TM. Antimicrobial prophylaxis in women with recurrent urinary tract infections. Int J Antimicrob Agents. 2011;38:36–41. doi: 10.1016/j.ijantimicag.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 71.Pfau A, Sacks TG. Effective prophylaxis for recurrent urinary tract infections during pregnancy. Clin Infect Dis. 1992;14:810–4. doi: 10.1093/clinids/14.4.810. [DOI] [PubMed] [Google Scholar]
- 72.Pfau A, Sacks TG. Effective postcoital quinolone prophylaxis of recurrent urinary tract infections in women. J Urol. 1994;152:136–8. doi: 10.1016/s0022-5347(17)32837-9. [DOI] [PubMed] [Google Scholar]
- 73.Pfau A, Sacks TG. Effective prophylaxis of recurrent urinary tract infections in premenopausal women by postcoital administration of cephalexin. J Urol. 1989;142:1276–8. doi: 10.1016/s0022-5347(17)39055-9. [DOI] [PubMed] [Google Scholar]
- 74.Nicolle LE, Harding GK, Thompson M, Kennedy J, Urias B, Ronald AR. Prospective, randomized, placebo-controlled trial of norfloxacin for the prophylaxis of recurrent urinary tract infection in women. Antimicrob Agents Chemother. 1989;33:1032–5. doi: 10.1128/aac.33.7.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chew LD, Fihn SD. Recurrent cystitis in nonpregnant women. West J Med. 1999;170:274–7. [PMC free article] [PubMed] [Google Scholar]
- 76.Melekos MD, Asbach HW, Gerharz E, Zarakovitis IE, Weingaertner K, Naber KG. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157:935–9. [PubMed] [Google Scholar]
- 77.Stapleton A, Latham RH, Johnson C, Stamm WE. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection. A randomized, double-blind, placebo-controlled trial. JAMA. 1990;264:703–6. [PubMed] [Google Scholar]
- 78.Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329:753–6. doi: 10.1056/NEJM199309093291102. [DOI] [PubMed] [Google Scholar]
- 79.Eriksen B. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999;5:1072–9. doi: 10.1016/s0002-9378(99)70597-1. [DOI] [PubMed] [Google Scholar]
- 80.Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women (Review) Cochrane Database Syst Rev. 2008;2:CD005131. doi: 10.1002/14651858.CD005131.pub2. [DOI] [PubMed] [Google Scholar]
- 81.Mierzwiak J, Murray S, Zimmern P. Favorable role of trigone fulguration in the management of recurrent urinary tract infections. Neurourol Urodynamics. 2010;29:304. [Google Scholar]
- 82.Barbosa-Cesnik C, Brown MB, Buxton M, Zhang L, DeBusscher J, Foxman B. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011;52:23–30. doi: 10.1093/cid/ciq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stapleton AE, Dziura J, Hooton TM, Cox ME, Yarova-Yarovaya Y, Chen S, et al. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial. Mayo Clin Proc. 2012;87:143–50. doi: 10.1016/j.mayocp.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–40. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 85.Watts RE, Totsika M, Challinor VL, Mabbett AN, Ulett GC, De Voss JJ, Schembri MA. Contribution of siderophore systems to growth and urinary tract colonization of asymptomatic bacteriuria. Escherichia coli Infect Immun. 2012;80:333–44. doi: 10.1128/IAI.05594-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oelschlaeger TA, Dobrindt U, Hacker J. Pathogenicity islands of uropathogenic E. coli and the evolution of virulence. Int J Antimicrob Agents. 2002;19:517–21. doi: 10.1016/s0924-8579(02)00092-4. [DOI] [PubMed] [Google Scholar]
- 87.Friedrich AW, Köck R, Bielaszewska M, Zhang W, Karch H, Mathys W. Distribution of the urease gene cluster among and urease activities of enter-ohemorrhagic Escherichia coli O157 isolates from humans. J Clin Microbiol. 2005;43:546–50. doi: 10.1128/JCM.43.2.546-550.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao L, Gao S, Huan H, Xu X, Zhu X, Yang W, et al. Comparison of virulence factors and expression of specific genes between uropathogenic Escherichia coli and avian pathogenic E. coli in a murine urinary tract infection model and a chicken challenge model. Microbiology. 2009;155:1634–44. doi: 10.1099/mic.0.024869-0. [DOI] [PubMed] [Google Scholar]
- 89.Raz R, Colodner R, Rohana Y, Battino S, Rottensterich E, Wasser I, et al. Effectiveness of estriol-containing vaginal pessaries and nitrofurantoin macrocrystal therapy in the prevention of recurrent urinary tract infection in postmenopausal women. Clin Infect Dis. 2003;36:1362–8. doi: 10.1086/374341. [DOI] [PubMed] [Google Scholar]


