Summary
Background
Understanding the extent to which HIV burden differs across communities and the drivers of local disparities is critical for an effective and targeted HIV response. We assessed community-level variations in HIV prevalence, risk factors, and treatment and prevention service uptake in Rakai, Uganda.
Methods
The Rakai Community Cohort Study (RCCS) is an open, population-based cohort surveying persons aged 15–49 in 40 communities. Participants are HIV tested and interviewed to obtain sociodemographic, behavioral, and health information. RCCS data from August 2011 to May 2013 were used to classify communities as agrarian (n=27), trading (n=9), or lakeside fishing sites (n=4). HIV prevalence was mapped using Bayesian methods, and variability across and within community classifications was characterized. Differences in HIV risk factors and uptake of antiretroviral therapy and male circumcision between community types were assessed.
Findings
17,119 individuals were included; 9215 (54%) were female. 9931 participants resided in agrarian, 3318 in trading, and 3870 in fishing communities. There was large variation in HIV prevalence, ranging from 9% to 43% across communities. Fishing communities had a higher median HIV prevalence (41%, range: 37–43%) compared to trading (17%, range: 11–22%) and agrarian communities (14%, range: 9–26%); ART and male circumcision coverage were significantly lower in fishing communities. Self-reported risk behaviors were significantly higher in men compared to women and in fishing communities compared to other community types.
Interpretation
There is substantial heterogeneity in HIV prevalence, risk factors, and service uptake across communities within one region of Uganda. These findings underscore the need for local surveillance and have important implications for the design of targeted HIV responses. In particular, the extremely high HIV burden and risk behaviors, and low use of combination HIV prevention in fishing communities make these areas a priority for intervention.
Introduction
Increasingly, funders of HIV treatment and prevention programs are calling for targeted approaches which focus on geographic areas and populations at highest risk so that limited resources may have greatest impact.1 Correspondingly, attention has focused on the utility of HIV epidemiologic data at finer levels of scale to inform targeted responses, with granularity becoming a watchword within the field of HIV.2,3 However, most population-based studies in sub-Saharan Africa utilize sparsely collected HIV surveillance data at national administrative unit scales, limiting the reliability and depth of inferences which can be made regarding local HIV epidemics.3–5
Finer resolution, population-based epidemiologic data may provide a more nuanced understanding of sub-national HIV epidemics. For example, community-level surveillance may reveal “hotspots” (i.e. geographic areas with significantly higher HIV prevalence), important population-level differences in sexual behaviors, or critical gaps in HIV service coverage. Such data may explain why HIV epidemics in some regions have not substantially declined despite significant scale-up of HIV services nationally, as may be the case in Uganda.6
The Rakai Community Cohort Study (RCCS), a population-based cohort of agrarian, trading, and fishing communities in and around Rakai District, Uganda, offers a rare opportunity to empirically study heterogeneities in HIV disease burden, sexual behaviors, and treatment and prevention service coverage in sub-Saharan Africa. The first AIDS cases in East Africa were identified in Rakai District,7 and Rakai continues to have among the highest HIV prevalence levels in Uganda.8 Here, we report on community-level variations in HIV prevalence, risk factors, and treatment and prevention service uptake in the RCCS, and discuss study implications for targeting the HIV response.
Methods
Study Design
Study Setting
Rakai District (area ~2200 km2, population ~518,000) is mostly rural and located in south-central Uganda.9 It is bordered to the south by Tanzania and to the east by Lake Victoria (Figure 1A). Kampala, the capital of Uganda, is ~150km northeast of the Rakai District. The Rakai region has two primary highways, one connecting Kampala to Tanzania and the Trans-African Highway which connects Kampala to the Congo.
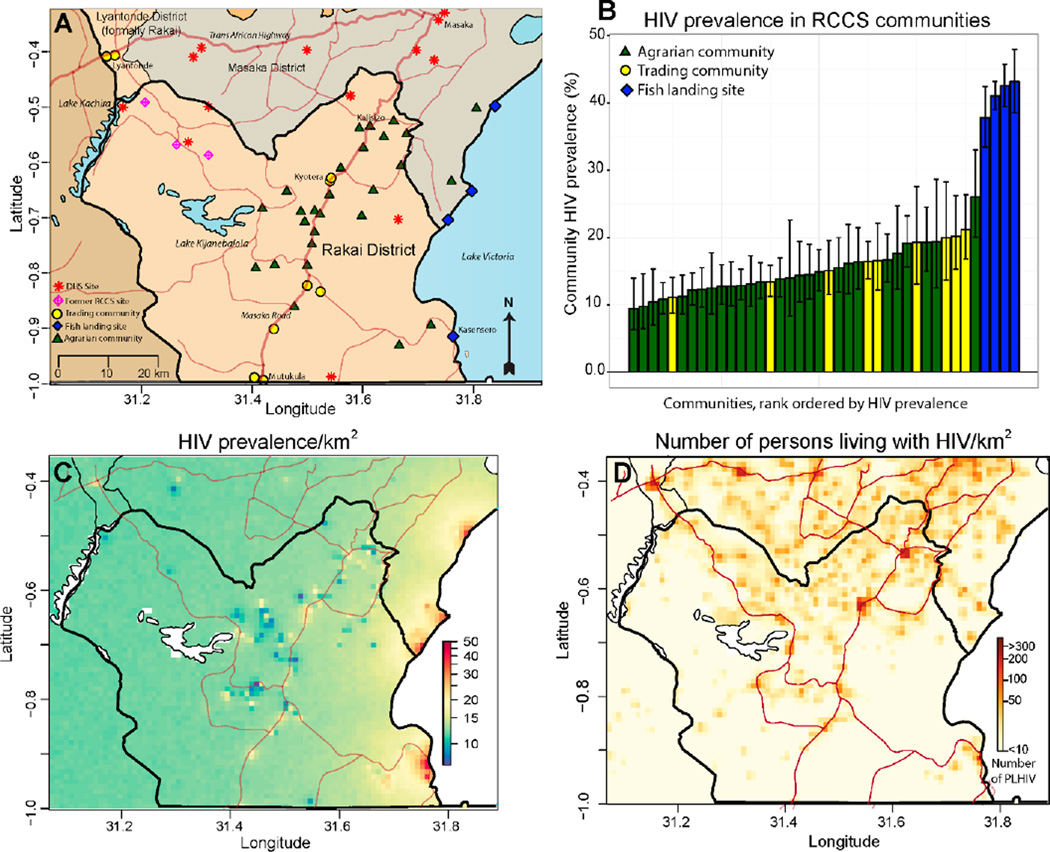
Figure 1. HIV disease burden in the Rakai region.
A) Map of the Rakai region with RCCS study communities (agrarian=green triangle, trading community=yellow circle, fishing landing site=blue diamond) and Demographic and Health survey sites (red star) shown. B) Barplot of community HIV prevalence in 40 RCCS study communities. C) Interpolated map of HIV prevalence (1 km2 resolution). D) Interpolated map of the number of persons living with HIV (1 km2 resolution).
The Rakai Community Cohort Study (RCCS)
Conducted by the Rakai Health Sciences Program (RHSP), the RCCS is an open, population-based cohort established in 1994.10 The RCCS currently surveys individuals aged 15–49 in 40 community clusters in and near Rakai District. Agrarian and trading community boundaries were established in 1994 as part of a community-randomized trial on treatment of sexually transmitted infections for HIV prevention.10 In 2011, the four largest Lake Victoria fishing communities in the Rakai region were added to the RCCS based upon their proximity and access to Rakai District’s non-fishing community populations.
To identify eligible cohort participants in these study communities, the RCCS first conducts an informational community mobilization event. A census is then performed as follows: All households within the community clusters are systematically approached, household GPS coordinates are recorded, and all resident household members are enumerated by gender, age, and duration of residence, regardless of whether they are present or absent at the time of the census. RCCS eligibility requires residency for at least six months in agrarian and trading communities and 1 month with intention to stay longer in fishing communities. After the census, the RCCS enrolls eligible participants in central community locations (“hubs”). Eligible participants who are not captured at the hubs are then approached at their household to request their participation. If needed, up to two return visits are made to the household to enroll eligible participants.
For all participants who provide written informed consent, the RCCS conducts interviews to assess demographics, sexual and health-seeking behaviors, and HIV service uptake. Following the interview, free HIV testing is performed using a three rapid test algorithm with participants offered results and post-test counseling by on-site counselors. For most of these analyses, unless otherwise noted, RCCS data from a single survey conducted between August 10, 2011 and May 30, 2013 was used. This was the first RCCS survey to include the four fishing communities, thus representing their baseline assessment. This RCCS survey also included 27 agrarian communities and 9 trading center communities located on or near the main highways. Communities were classified as trading centers if the proportion of their population reporting trading as their primary occupation was in the top quartile among all RCCS communities: within this community stratum, 16–36% of the surveyed population listed trading as their main occupation and all known major trading centers within Rakai District were included.
Antiretroviral therapy (ART) and male circumcision services have been provided since 2004 and 2007, respectively, to RCCS trading and agrarian communities by the RHSP through support from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). Beginning in 2011, the RHSP provided combination HIV prevention and treatment services in the four fishing communities in conjunction with their initial RCCS survey. Previously, some service coverage in these fishing communities had been provided by other non-government organizations.
Statistical Analysis
Mapping HIV prevalence and case counts in the Rakai region
A hierarchical Bayesian modeling framework was used to estimate and map (1) HIV prevalence and (2) the number of persons living with HIV throughout the Rakai District and neighboring areas (Fig.1A). Maps were generated using three data sources: the RCCS, the Ugandan Demographic Health Survey (DHS), and the WorldPop database.11 Specifically, we used RCCS household GPS and HIV serostatus information from RCCS participants residing in the 40 communities included in the 2011–2013 RCCS survey period. Data from three additional RCCS communities (all agrarian) included in a prior RCCS survey period (September 21, 2010 to November 26, 2010) but subsequently dropped from the RCCS due to financial constraints were included in the mapping analysis in order to improve estimates of HIV prevalence and case counts in the western region of the Rakai District where data were more sparse (Fig. 1A). Cluster-level GPS and weighted HIV serostatus data from the DHS conducted in Uganda in 2011 were also included. DHS clusters in the Rakai region did not geographically overlap RCCS communities. RCCS and DHS data were used in combination to estimate HIV prevalence and then were combined with WorldPop data, which provides high spatial resolution population density estimates, to estimate the number of people living with HIV.
To examine fine-scale spatial variation in prevalence and the number of people living with HIV within the region, the region was divided into 1km2 grid cells, excluding grid cells entirely covered by water or with a population density of 0 in the Worldpop dataset. HIV prevalence in each grid cell was modeled using a logistic regression with log10 distance to the Lake Victoria shoreline used as a covariate. The logistic regression included a spatially autocorrelated random effects term modeled by a conditional autoregressive (CAR) distribution with a flexible spatial dependence parameter to account for overdispersion and spatial autocorrelation in HIV infection status.12–14 This binomial-logistic hierarchical model was implemented using Stan 2.8.0.15
Comparing HIV burden, risk factors, and service uptake in the RCCS
We also compared HIV burden (i.e., the estimated numbers of people living with HIV), risk factors, and HIV service utilization using data at the individual and community levels from the 2011–2013 RCCS survey from the three community strata (agrarian, trading, and fishing). We first obtained estimates of gender and age-specific HIV prevalence in five-year age groups using Poisson regression models separately for each of the three community types. Next we compared the prevalence of high risk sexual behaviors, genital ulcer disease, HIV, self-reported ART use in people living with HIV, male circumcision status in non-Muslim males to assess uptake of circumcision for HIV prevention services between individuals residing in the three strata separately by gender, using age-adjusted modified Poisson regression models with the relative prevalence and 95% confidence intervals (CI) reported as adjusted prevalence risk ratios (PRR). In a sensitivity analysis, we used inverse probability weighting to account for potential differences associated with study participation. Inverse probability weights were constructed using a logistic regression model and RCCS census data on the age, gender, and community-stratum (e.g., agrarian, fishing, and trading) of participants and non-participants. Lastly, we measured community-level coverage of ART use among people living with HIV and male circumcision in non-Muslim males using aggregated self-reported data for each of the 40 communities, and compared the median community coverage estimates between the three community strata using a non-parametric Wilcoxon rank sum test.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The co-first authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Study population and demographics
The RCCS surveyed 17,119 individuals (54% female) in 27 agrarian communities (n=9931), 9 trading communities (n=3318), and 4 Lake Victoria fishing communities (n=3870) between August 10, 2011 and May 30, 2013. The median community sizes were 311 participants (IQR: 213–480) in agrarian, 309 participants (IQR: 173–502) in trading, and 899 participants (IQR: 694–1227) in fishing communities. Average community-level participation was 70% (standard deviation ±9.0%) of the censused eligible population. At time of survey, 99% of eligible individuals present agreed to participate. Of those individuals who were eligible by census but who did not participate in the survey (n=8,090), 97% were away for work or school, 59% of whom were men. These missing individuals were significantly more likely to be younger (PRR=0.98, 95%CI: 0.97–0.98, per year of age), male (PRR=1.41; 95%CI: 1.35–1.48), and residents of trading communities (reference: agrarian residents; PRR=1.49, 95%CI: 1.42–1.57). The likelihood of study participation was marginally higher in fishing communities compared to agrarian communities (PRR=1.11; 95%CI: 1.04–1.18).
The demographic composition of agrarian, trading and fishing communities was substantially different (Table 1). Individuals in fishing communities were more likely to be males (52%), of prime working age (20–39 years), previously married, and less formally educated compared to persons residing in either agrarian or trading communities. In fishing communities compared to other community types, women were more likely to be involved in bar and restaurant work, and men in fishing-related occupations.
Table 1.
Individual demographic characteristics of 17,119 RCCS participants living in 40 agrarian, trading, and Lake Victoria fishing communities in Uganda, 2011–2013.
| Women (N=9215) | Men (N=7904) | |||||
|---|---|---|---|---|---|---|
| Agrarian | Trading | Fishing | Agrarian | Trading | Fishing | |
| Total | 5416 | 1940 | 1859 | 4515 | 1378 | 2011 |
| Age (years) | ||||||
| 15–19 | 1001 (19) | 288 (15) | 201 (11) | 1047 (23) | 247 (18) | 147 (7) |
| 20–29 | 1992 (37) | 820 (42) | 823 (44) | 1459 (43) | 546 (40) | 803 (40) |
| 30–39 | 1695 (31) | 613 (31) | 653 (35) | 1315 (29) | 387 (28) | 777 (39) |
| 40–49 | 728 (13) | 219 (11) | 182 (10) | 694 (15) | 198 (14) | 286 (14) |
| Marital Status† | ||||||
| Never married | 1277 (24) | 396 (20) | 145 (8) | 1784 (40) | 517 (38) | 403 (20) |
| Married, monogamous union | 2556 (47) | 807 (42) | 914 (49) | 2969 (46) | 639 (46) | 1060 (53) |
| Married, polygamous union | 667 (12) | 291 (15) | 262 (14) | 294 (7) | 121 (9) | 181 (9) |
| Previously married | 912 (17) | 445 (23) | 538 (29) | 365 (8) | 101 (7) | 367 (18) |
| Educational status† | ||||||
| None | 242 (4) | 100 (5) | 213 (11) | 116 (3) | 53 (4) | 164 (8) |
| Primary | 3180 (59) | 1027(53) | 1287 (69) | 2830 (63) | 755 (55) | 1475(73) |
| Secondary | 1568 (29) | 639 (33) | 318 (17) | 1143 (25) | 414 (30) | 294 (15) |
| Tertiary | 364 (7) | 168 (9) | 33 (8) | 306 (7) | 141 (10) | 40 (2) |
| Religion† | ||||||
| Catholic | 3644 (67) | 1062(55) | 1038 (56) | 3046 (67) | 761 (55) | 1179(59) |
| Muslim | 601 (11) | 390 (20) | 340 (18) | 452 (10) | 270 (20) | 346 (17) |
| Protestant | 885 (16) | 318 (16) | 283 (15) | 709 (16) | 221 (16) | 299 (15) |
| Saved/Pentecostal | 190 (4) | 146 (8) | 171 (9) | 152 (3) | 94 (7) | 123 (6) |
| Other | 34 (1) | 18 (1) | 19 (1) | 36 (1) | 17 (1) | 26 (1) |
| Primary occupation | ||||||
| Agricultural/housework | 2855 (53) | 33 (33) | 569 (31) | 1270 (28) | 144 (10) | 104 (5) |
| Bar/Restaurant work | 172 (3) | 157 (8) | 344 (19) | 13 (0) | 17 (1) | 7 (0) |
| Boda boda/Trucking | 0 (0) | 0 (0) | 0 (0) | 80 (2) | 41 (3) | 33 (1.6) |
| Fishing | 1 (0) | 0 (0) | 8 (0) | 52 (1) | 1 (0) | 1100(55) |
| Student | 872 (16) | 174 (9) | 40 (2) | 1266 (28) | 229 (17) | 56 (3) |
| Trader/Shop keeper | 541 (10) | 529 (27) | 526 (28) | 578 (12) | 336 (24) | 339 (17) |
| Other | 975 (18) | 447 (23) | 372 (20) | 1246 (28) | 610 (44) | 372 (18) |
There was missing data for 8 (<0.1%) individuals on marital status (5 women, 3 men) and 249 (1.4%) individuals on religion and educational status (173 men, 76 women).
Community and spatial variation in HIV burden
There was large variation in community HIV prevalence, ranging from 9% to 43%, across RCCS communities. Fishing communities had a significantly higher median HIV prevalence (41%, range: 37–43%) compared to trading (17%, range: 11–22%) and agrarian communities (14%, range: 9–26%) (Fig. 1B). In a sensitivity analysis using inverse probability weighting to account for differences in study participation, weighted community HIV-prevalence estimates were not statistically different from the observed estimates shown in Figure 1B (Supplementary Fig. 1). An interpolated map of HIV prevalence throughout the Rakai region revealed very high HIV prevalence along Lake Victoria and patchy HIV prevalence in the interior of the district with areas of intermediate to high HIV prevalence frequently adjacent to areas with relatively low HIV prevalence (Fig 1C). While the highest HIV prevalence areas were found along the Lake Victoria coast, the preponderance of people living with HIV were concentrated inland within major trading centers on the international highways where population density is greatest (Fig 1D).
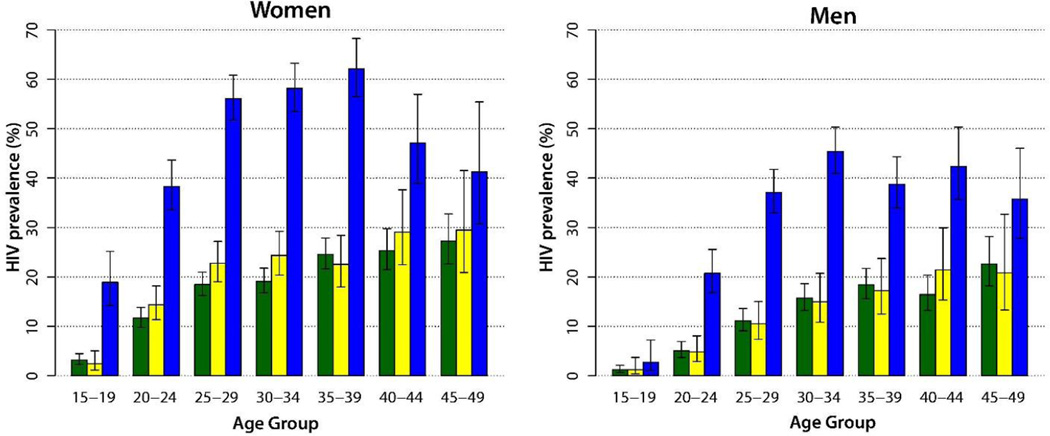
HIV prevalence in young women (15–24 years) and men (20–29 years) in fishing communities was high relative to those residing in other Rakai communities (Figure 2). In fishing communities, the peak HIV prevalence among women was 62% (95%CI: 56–68%) aged 35–39 years and among men 45% (95%CI: 41–50%) aged 30–34 years.
Figure 2.
Age-specific HIV prevalence in men and women in agrarian communities (green), trading communities (yellow), and fish landing sites (blue).
Prevalence of HIV-related risk factors
Higher levels of HIV risk behaviors were observed in communities with higher HIV prevalence (Tables 2A–B). The proportion of sexually active adults and the prevalence of high risk sexual behaviors in both men and women were significantly higher in fishing communities, intermediate in trading communities, and lowest in agrarian communities. For example, 22% of all sexually active women in the fishing communities reported multiple sexual partnerships in the last year compared to 12% (adjPRR=2.12; 95%CI: 1.65–2.75) of women in trading communities and 7% (adjPRR=3.15, 95%CI: 2.71–3.68) of women in agrarian communities. Findings were similar when analyses were restricted to married women only (Table 2A). Overall, sexually active men (39%; n=2532/6437) were more likely to report multiple sexual partnerships in the last year than were women (11%; n=855/7718). More men in fishing communities (61%) had multiple sexual partnerships than men in trading communities (45%; adjPRR=1.36, 95%CI: 1.22–1.52) and men in agrarian communities (33%; adjPRR=1.85, 95%CI: 1.67–2.06). The proportion of married men reporting non-marital partnerships was also higher in fishing and trading communities than agrarian communities. Male and female self-reported consistent condom use with non-marital partners was low in all communities (Tables 2A and B), and significantly lower among men in fishing communities compared to either trading or agrarian communities. Findings did not change substantially when analyses were stratified by HIV serostatus: men and women in fishing communities had elevated risk behaviors regardless of whether they were HIV-infected or not (Supplemental Tables 1A–B, 2A–B).
Table 2.
| A. Sexual behaviors, STI symptoms, HIV
prevalence, and HIV treatment and prevention uptake in 9,215 female RCCS participants residing in agrarian, trading, and fishing communities in Uganda, 2011–2013. | ||||||
|---|---|---|---|---|---|---|
| Agrarian (N=5416) |
Trading (N=1940) |
Fishing (N=1859) |
Trading vs. Agrarian |
Fishing vs. Agrarian |
Fishing vs. Trading |
|
| N (%) | Age-adjusted PRR (95%CI) | |||||
| Sexually active in the past year |
4383 (81) | 1633 (84) | 1702 (92) | 1.04 (0.98–1.10) | 1.13 (1.07–1.09) | 1.09 (1.02–1.16) |
| Multiple partners in the past year† |
295 (7) | 193 (12) | 367 (22) | 1.73 (1.44–2.07) | 3.15 (2.71–3.68) | 2.12 (1.65–2.75) |
| Non-marital partners in the past year among married persons‡ |
158 (5) | 90 (8) | 193 (16) | 1.66 (1.27–2.14) | 3.21 (2.60–3.96) | 1.97(1.54–2.55) |
| Used alcohol before sex† |
789 (18) | 307 (19) | 574 (34) | 1.08 (0.94–1.23) | 1.93 (1.74–2.15) | 1.80 (1.57–2.07) |
| Sex with partner outside community in the past year† |
961 (22) | 450 (28) | 350 (21) | 1.25 (1.12–1.40) | 0.93 (0.82–1.05) | 0.75 (0.65–0.86) |
| Symptoms of genital ulcer disease in the past 12 months |
602 (11) | 214 (11) | 416 (22) | 0.99 (0.85–1.16) | 2.02 (1.78–2.28) | 2.03 (1.72–2.40) |
| HIV seropositive | 888 (16) | 368 (19) | 907 (49) | 1.18 (1.05–1.33) | 2.99 (2.73–3.28) | 2.55 (2.26–2.88) |
| Consistent condom use with non-marital partners ¥ |
299 (22) | 116(19) | 133 (19) | 0.84 (0.68–1.04) | 0.87 (0.71–1.06) | 1.03 (0.81–1.33) |
| ART use among HIV seropositive persons* |
294 (33) | 139 (38) | 164 (18) | 1.22 (0.99–1.49) | 0.65 (0.54–0.79) | 0.53 (0.42–0.66) |
| B. Sexual behaviors, STI symptoms, HIV
prevalence, and HIV treatment and prevention uptake among 7,904 male RCCS participants residing in agrarian, trading, and fishing communities in Rakai, Uganda, 2011–2013. | ||||||
|---|---|---|---|---|---|---|
| Agrarian (N=4515) |
Trading (N=1378) |
Fishing (N=2011) |
Trading vs. Agrarian |
Fishing vs. Agrarian |
Fishing vs. Trading |
|
| N (%) | Age-adjusted PRR (95%CI) | |||||
| Sexually active in the past year |
3436 (76) | 1152 (84) | 1849 (92) | 1.10 (1.03–1.18) | 1.16 (1.10–1.23) | 1.07 (1.01–1.02) |
| Multiple partners in the past year† |
1043 (33) | 466 (45) | 1023 (61) | 1.35 (1.21–1.51) | 1.85 (1.70–2.02) | 1.36 (1.22–1.52) |
| Non-marital partners in the past year among married persons‡ |
694 (29) | 321 (42) | 697 (56) | 1.42 (1.24–1.62) | 1.85 (1.67–2.06) | 1.31 (1.15–1.50) |
| Used alcohol before sex† |
1343 (39) | 390 (34) | 950 (51) | 0.90 (0.8–1.01) | 1.33 (1.22–1.44) | 1.49 (1.33–1.68) |
| Sex with partner outside community in the past year† |
1295 (38) | 548 (48) | 892 (48) | 1.24 (1.12–1.37) | 1.28 (1.18–1.39) | 1.01 (0.91–1.13) |
| Symptoms of genital ulcer disease in the past 12 months |
335 (7) | 115 (8) | 397 (20) | 1.12 (0.91–1.38) | 2.62 (2.27–3.04) | 2.37 (1.93–2.93) |
| HIV seropositive | 486 (11) | 148 (11) | 691 (34) | 1.01 (0.84–1.21) | 2.99 (2.66–3.36) | 3.01 (2.53–3.61) |
| Consistent condom use with non- marital partners ¥ |
614 (35) | 220 (31) | 352 (27) | 0.88 (0.76–1.03) | 0.77 (0.68–0.88) | 0.86 (0.73–1.02) |
| ART use among HIV seropositive persons* |
131 (27) | 35 (24) | 90 (13) | 0.89 (0.60–1.27) | 0.55 (0.42–0.72) | 0.64 (0.44–0.97) |
| Circumcised** | 1250 (32) | 466 (43) | 334 (21) | 1.35 (1.21–1.50) | 0.64 (0.56–0.72) | 0.48 (0.42–0.55) |
Analysis restricted to sexually active women;
Analysis restricted to married women (agrarian, N=3223; trading, N=1098; fishing, N=1176).
Analysis restricted to women who reported non-marital sexual partners (agrarian, N=1457; trading, N=685; fishing, N=750).
Analysis restricted to HIV seropositive women (agrarian, N=888; trading, N=368; fishing, N=907).
Analysis restricted to sexually active men.
Analysis restricted to married men (agrarian, N=2363; trading, N=760; fishing, N=1241)
Analysis restricted to men who reported non-marital sexual partners (agrarian, N=1894; trading, N=773; fishing, N=1361).
Analysis restricted to HIV seropositive men (agrarian, N=486; trading, N=148; fishing, N=691);
Analysis restricted to non-Muslim men (agrarian, N=3943, trading, N=1093; fishing, N=1627)
Individual and community-level coverage of ART and male circumcision
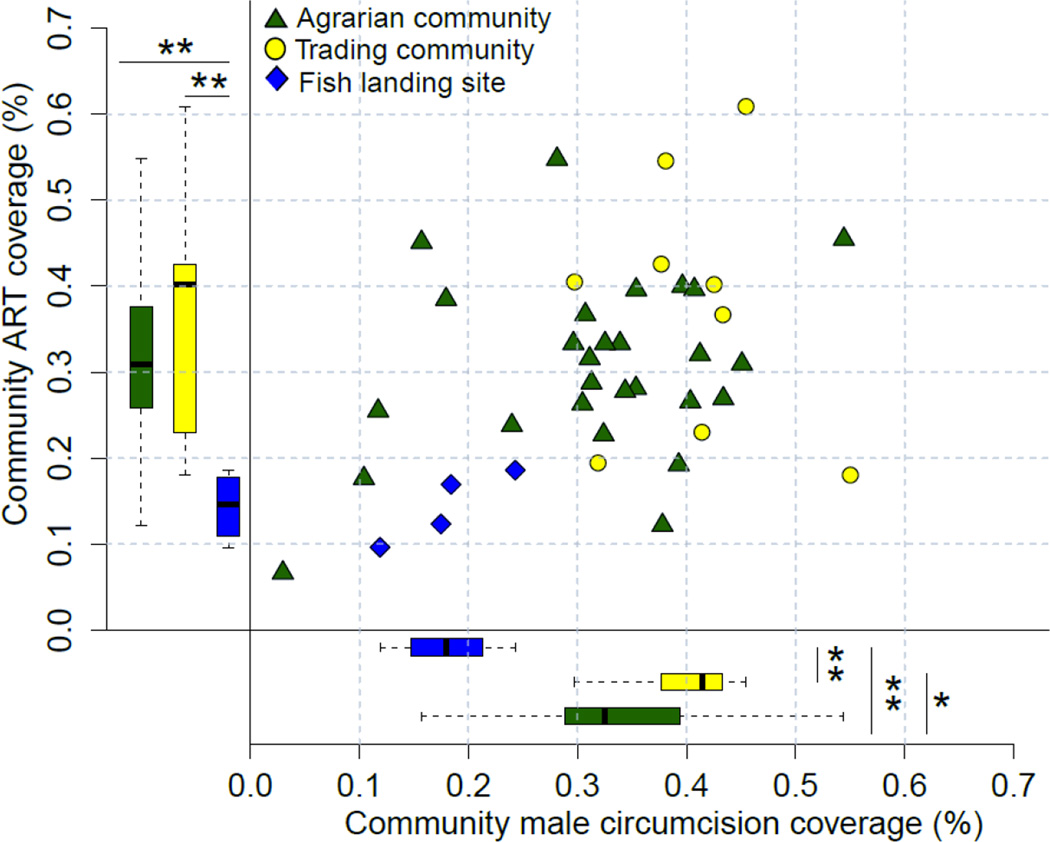
At the individual-level, fishing community residents were significantly less likely to report ART use and non-Muslim men in these communities were less likely to report being circumcised compared to individuals in agrarian or trading communities (Tables 2A–B). At the community-level, ART coverage among people living with HIV ranged from 7 to 61% and male circumcision coverage ranged from 3 to 55%. Community ART coverage was highest in trading communities (median=40%, IQR=23–43%), compared to 31% in agrarian communities (IQR=26–38%) and 15% in fishing communities (IQR=12–17%). Community male circumcision coverage was significantly higher in trading (median=41%; IQR=38–43%) and agrarian communities (median=33%; IQR=29–39%) compared to fishing communities (median=18%; IQR=16–20%). Overall, communities with higher ART coverage also tended to have higher male circumcision coverage (Spearman correlation coefficient=0.337; p=0.010, Figure 3).
Figure 3.
RCCS community ART and male circumcision coverage in non-Muslim men. *p<0.05; **p<0.01
Discussion
An analysis of the HIV epidemic in Rakai, Uganda demonstrated substantial heterogeneity in HIV prevalence, risk factors, and service coverage. In particular, Lake Victoria fishing communities were notable for their high HIV burden, high risk behaviors, and low use of combination HIV prevention services. This is the first study, to our knowledge, which directly compares HIV epidemiology and service uptake between fishing and inland communities using population-based data.
The median HIV prevalence in the four RCCS fishing communities (41%) is higher than prior reports from similar communities on Lake Victoria (range: 20–37%).16–20 The HIV burden in many agrarian and trading communities in this study was also substantial, and these communities, which had larger populations, had the greatest numbers of people living with HIV. Notably, there was also marked variation in HIV prevalence within agrarian and trading community types, an indication of the difficulty in defining characteristics which can clearly identify higher risk communities.
While high levels of HIV behavioral risk factors were seen in all study community strata, consistent with previous reports,21–23 fishing communities were notable for their significantly higher rates of risk behaviors.16,24 With complex structural factors affecting fishing communities (e.g. a recently built fish factory and improved road infrastructure likely attracted a large influx of migrants) and the recent scale-up of HIV prevention and treatment services, ongoing research will be needed to understand the evolving HIV epidemic in these local settings.
UNAIDS, WHO, PEPFAR, and Global Fund have called for targeting resources to populations with the greatest need, i.e. a population-location approach, based in part on the assumption that high prevalence hotspots disseminate infection into lower-risk populations.1,3,25 This study found that fishing communities were HIV hotspots compared to most inland communities. However, it is unclear how many infections are transmitted from these hotspots to lower-risk populations. Importantly, we found that the highest burden of HIV in terms of case counts for people living with HIV were located in the larger, lower-risk populations. Where need is greatest could be defined by high HIV seroprevalence (i.e., a hotspot focus) or by high HIV case counts (i.e., the largest populations of people living with HIV). How best to target limited resources remains unclear and model-based and empiric impact evaluations are needed. We believe there is a clear HIV public health emergency in fishing communities which demands urgent action, but the HIV service needs of relatively lower prevalence populations with numerically larger caseloads should not be neglected.
ART and male circumcision coverage were significantly lower in fishing communities, likely in part because these populations had poor access to HIV services prior to 2011. In contrast to the fishing communities, the highest HIV service coverage was in trading centers, which likely had greater access to HIV services. In 2011, Uganda changed its national ART guidelines to include fisherfolk as a key population eligible to receive ART at time of diagnosis irrespective of CD4 count, i.e., Test and Treat.26 The effectiveness of this targeted approach in increasing ART coverage and its impact on HIV transmission needs empirical evaluation.
In this study, detailed epidemiologic data were available to provide an in-depth understanding of a multifaceted HIV epidemic, an understanding which would have been obscured by district- or national-level aggregate statistics. While requests for granular HIV data have been made,2 it remains unclear how finely resolved such data should be, what resources are required to implement detailed HIV surveillance at scale, and the impact of targeting interventions based upon these detailed data.25 For example, this study provides more granular data than the recent “location-population” UNAIDS report which used first and second subnational administrative data only.3 As a consequence, we believe that our inferences regarding the HIV epidemic in this part of south-central Uganda are more detailed and may help programs develop focused, community-specific responses. Given that many other regions of sub-Saharan Africa also contain diverse community types in close proximity, these findings also have broader relevance. Further research on HIV heterogeneity in other settings and how these differences impact HIV epidemic dynamics would be informative.
This study has a number of limitations. First, communities and participants in the cohort may not be fully representative of the regional HIV epidemic and treatment and prevention estimates may not accurately reflect levels of heterogeneity in other national epidemics. However, this study’s participation rates are comparable or higher than in similar community cohort studies in Africa,27,28 cohort findings are largely consistent with Uganda DHS data on HIV prevalence and risk factors for the larger south-central region of Uganda,29 and other countries appear to have similarly complex HIV epidemics.30 This study also utilized self-reports of behaviors, ART, and male circumcision which may be subject to respondent desirability bias. Additionally, for this analysis, we used cross-sectional survey data collected over a two year period and results may have been subject to secular changes. Finally, interpolation of HIV prevalence and case counts required assumptions and inferences on missing data.
In conclusion, this detailed epidemiologic study of an HIV epidemic in the Rakai region highlights the marked diversity in HIV disease burden, sexual behaviors, and treatment and prevention service coverage existing across communities in relatively close proximity. The extensive heterogeneity in the HIV epidemic likely has critical implications for targeted treatment and prevention programs in sub-Saharan Africa. Most notably, our analysis showed that Lake Victoria fishing communities have significantly higher HIV prevalence and risky sexual behaviors compared to agrarian and trading communities but have had limited coverage of the necessary health services required to reduce their high HIV burden. Our findings indicate the need for strong local HIV surveillance programs, a better understanding of the HIV transmission links between high-risk and lower-risk populations, and evaluation of targeted HIV interventions.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for studies on longitudinal HIV cohort studies which included fishing communities in sub-Saharan Africa published up to April 21, 2016. Key search terms included “HIV or AIDS”, “COHORT or OBSERVATIONAL”, “FISHING”, and “AFRICA”. No language limitation was placed. A small number of studies were found reporting high levels of HIV seroprevalence and risk factors in fishing communities, but none used population-level data to compare findings directly with inland communities. Given study heterogeneity, no meta-analyses was performed. No comparable analysis from this particular cohort has been reported since the early 1990’s.
Added value of this study
This study provides an update on HIV epidemiology in the Rakai Community Cohort Study (RCCS), one of few large population-based studies of HIV in sub-Saharan Africa. The RCCS has been ongoing since 1994, and today surveys ~17,000 individuals in 40 communities. Community-level HIV burden and risk factor distributions in the RCCS were first reported at its inception. Since that time, the RCCS has expanded its surveillance to include Lake Victoria fishing communities, a key population. In this study, we reexamine HIV epidemiology in the RCCS more than 20 years after its founding using highly granular data obtained at the community and household-levels. We show that within a single region in Uganda there exists extensive heterogeneity in HIV disease burden, behavioral risk factors, and service coverage. In addition, our analyses of the HIV epidemiology in Lake Victoria fishing communities, for the first time, uses population-level data to directly compare these key populations with inland communities in sub-Saharan Africa.
Implications of all the available evidence
The available evidence indicates the need for strong local HIV surveillance programs, a better understanding of the HIV transmission links between high-risk and lower-risk populations, and evaluation of targeted HIV interventions.
Acknowledgments
We thank the participants of the Rakai Health Sciences Program Rakai Community Cohort Study (RCCS). This study was supported by the National Institute of Mental Health (K23MH086338, R01MH107275), the National Institute of Allergy and Infectious Diseases (R01AI110324, U01AI100031, R01AI110324, R01AI102939), the National Institute of Child Health and Development (RO1HD070769, R01HD050180), and the National Institute for Allergy and Infectious Diseases Division of Intramural Research, National Institutes of Health, the Bill & Melinda Gates Foundation (22006.02), and the Johns Hopkins University Center for AIDS Research (P30AI094189).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors participated equally in revising and the final approval of this manuscript. L.C. and M.K.G conceptualized and designed the study. R.S., F.N., G.K., B.N., T.C.Q., S.J.R., R.H.G., D.S., and M.J.W. oversaw data collection and laboratory testing. M.K.G., S.M., and J.L. conducted the statistical analysis.
Conflicts of interest
We declare that we have no conflicts of interest.
Contributor Information
Larry W Chang, Division of Infectious Diseases, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland, USA; Rakai Health Sciences Program, Entebbe, Uganda; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Social and Behavioral Interventions Program, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Mary K Grabowski, Rakai Health Sciences Program, Entebbe, Uganda; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Robert Ssekubugu, Rakai Health Sciences Program, Entebbe, Uganda.
Fred Nalugoda, Rakai Health Sciences Program, Entebbe, Uganda.
Godfrey Kigozi, Rakai Health Sciences Program, Entebbe, Uganda.
Betty Nantume, Rakai Health Sciences Program, Entebbe, Uganda.
Justin Lessler, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Sean M Moore, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Thomas C Quinn, Division of Infectious Diseases, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland, USA; Laboratory of Immunoregulation, Division of Intramural Research, National Institute for Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Steven J Reynolds, Division of Infectious Diseases, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland, USA; Laboratory of Immunoregulation, Division of Intramural Research, National Institute for Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Ronald H Gray, Rakai Health Sciences Program, Entebbe, Uganda; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
David Serwadda, Makerere University School of Public Health, Kampala, Uganda; Rakai Health Sciences Program, Entebbe, Uganda.
Maria J Wawer, Rakai Health Sciences Program, Entebbe, Uganda; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
References
- 1.PEPFAR 3.0. [accessed August 18, 2015]; http://www.pepfar.gov/documents/organization/234744.pdf.
- 2.Puskas C, Hogg RS, Ferrand R, et al. Fast track to 2030: granularity at a global scale. The Lancet. 2015;2(1):e1–e32. doi: 10.1016/S2352-3018(14)00040-X. [DOI] [PubMed] [Google Scholar]
- 3.World AIDS Day 2015 Report. Focus on location and population. UNAIDS. 2015 [Google Scholar]
- 4.Anderson SJ, Cherutich P, Kilonzo N, et al. Maximising the effect of combination HIV prevention through prioritisation of the people and places in greatest need: a modelling study. Lancet. 2014;384(9939):249–256. doi: 10.1016/S0140-6736(14)61053-9. [DOI] [PubMed] [Google Scholar]
- 5.Kerr CC, Stuart RM, Gray RT, et al. Optima: A Model for HIV Epidemic Analysis, Program Prioritization, and Resource Optimization. J Acquir Immune Defic Syndr. 2015;69(3):365–376. doi: 10.1097/QAI.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 6.Global report: UNAIDS report on the global AIDS epidemic. 2013
- 7.Serwadda D, Mugerwa RD, Sewankambo NK, et al. Slim disease: a new disease in Uganda and its association with HTLV-III infection. Lancet. 1985;2(8460):849–852. doi: 10.1016/S0140-6736(85)90122-9. [DOI] [PubMed] [Google Scholar]
- 8.Grabowski MK, Lessler J, Redd AD, et al. The role of viral introductions in sustaining community-based HIV epidemics in rural Uganda: evidence from spatial clustering, phylogenetics, and egocentric transmission models. PLoS Med. 2014;11(3):e1001610. doi: 10.1371/journal.pmed.1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uganda Bureau of Statistics. National Population and Housing Census 2014. 2014 Nov [Google Scholar]
- 10.Wawer MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group. Lancet. 1999;353(9152):525–535. doi: 10.1016/s0140-6736(98)06439-3. [DOI] [PubMed] [Google Scholar]
- 11.WorldPop Database. [accessed November 1, 2015]; http://www.worldpop.org.uk/ [Google Scholar]
- 12.Besag J, York J, Mollié A. Bayesian image restoration, with two applications in spatial statistics. Ann Inst Stat Math. 1991;43(1):1–20. [Google Scholar]
- 13.Stern HS, Cressie N. Posterior predictive model checks for disease mapping models. Stat Med. 2000;19(17–18):2377–2397. doi: 10.1002/1097-0258(20000915/30)19:17/18<2377::aid-sim576>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Lee D. A comparison of conditional autoregressive models used in Bayesian disease mapping. Spat Spatiotemporal Epidemiol. 2011;2(2):79–89. doi: 10.1016/j.sste.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Stan Development Team. Stan: A C++ Library for Probability and Sampling, Version 2.8.0. 2015 [Google Scholar]
- 16.Kissling E, Allison EH, Seeley JA, et al. Fisherfolk are among groups most at risk of HIV: cross-country analysis of prevalence and numbers infected. AIDS. 2005;19(17):1939–1946. doi: 10.1097/01.aids.0000191925.54679.94. [DOI] [PubMed] [Google Scholar]
- 17.Kiwanuka N, Ssetaala A, Mpendo J, et al. High HIV-1 prevalence, risk behaviours, and willingness to participate in HIV vaccine trials in fishing communities on Lake Victoria, Uganda. J Int AIDS Soc. 2013;16(1):18621. doi: 10.7448/IAS.16.1.18621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opio A, Muyonga M, Mulumba N. HIV infection in fishing communities of Lake Victoria Basin of Uganda--a cross-sectional sero-behavioral survey. PLoS One. 2013;8(8):e70770. doi: 10.1371/journal.pone.0070770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asiki G, Mpendo J, Abaasa A, et al. HIV and syphilis prevalence and associated risk factors among fishing communities of Lake Victoria, Uganda. Sexually transmitted infections. 2011;87(6):511–515. doi: 10.1136/sti.2010.046805. [DOI] [PubMed] [Google Scholar]
- 20.Uganda AIDS Commission. HIV and AIDS Uganda Country Progress Report. 2013 [Google Scholar]
- 21.Kagaayi J, Gray RH, Whalen C, et al. Indices to measure risk of HIV acquisition in Rakai, Uganda. PLoS One. 2014;9(4):e92015. doi: 10.1371/journal.pone.0092015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serwadda D, Wawer MJ, Musgrave SD, Sewankambo NK, Kaplan JE, Gray RH. HIV risk factors in three geographic strata of rural Rakai District, Uganda. AIDS. 1992;6(9):983–989. doi: 10.1097/00002030-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Wawer MJ, Serwadda D, Musgrave SD, Konde-Lule JK, Musagara M, Sewankambo NK. Dynamics of spread of HIV-I infection in a rural district of Uganda. BMJ. 1991;303(6813):1303–1306. doi: 10.1136/bmj.303.6813.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwena ZA, Bukusi E, Omondi E, Ng'ayo M, Holmes KK. Transactional sex in the fishing communities along Lake Victoria, Kenya: a catalyst for the spread of HIV. Afr J AIDS Res. 2012;11(1):9–15. doi: 10.2989/16085906.2012.671267. [DOI] [PubMed] [Google Scholar]
- 25.Lyerla R, Murrill CS, Ghys PD, Calleja-Garcia JM, DeCock KM. The use of epidemiological data to inform the PEPFAR response. J Acquir Immune Defic Syndr. 2012;60(Suppl 3):S57–S62. doi: 10.1097/QAI.0b013e31825d279a. [DOI] [PubMed] [Google Scholar]
- 26.Kampala: Uganda AIDS Commission; 2011. National HIV Prevention Strategy 2011–2015. [Google Scholar]
- 27.Asiki G, Murphy G, Nakiyingi-Miiro J, et al. The general population cohort in rural south-western Uganda: a platform for communicable and non-communicable disease studies. Int J Epidemiol. 2013;42(1):129–141. doi: 10.1093/ije/dys234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larmarange J, Mossong J, Barnighausen T, Newell ML. Participation dynamics in population-based longitudinal HIV surveillance in rural South Africa. PLoS One. 2015;10(4):e0123345. doi: 10.1371/journal.pone.0123345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uganda Bureau of Statistics (UBOS) and ICF International Inc. Uganda demographic and Health survey 2011. 2012 [Google Scholar]
- 30.Mee P, Collinson MA, Madhavan S, et al. Evidence for localised HIV related micro–epidemics associated with the decentralised provision of antiretroviral treatment in rural South Africa: a spatio–temporal analysis of changing mortality patterns (2007–2010) Journal of Global Health. 2014;4(1):010403. doi: 10.7189/jogh.04.010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.