Two decades ago, a postmortem eye donor study identified a unique autosomal dominant disease and named it late-onset retinal degeneration (L-ORD).1 The distinguishing feature of L-ORD was a retina-wide thick layer of extracellular deposit between the retinal pigment epithelium (RPE) and Bruch membrane (BrM). A founder mutation, p.Ser163Arg, in the C1QTN5 gene was causative.2 Recent study of the crystal structure of C1QTN5 suggested L-ORD may be due to reduced adhesion of the RPE.3
Has there been further clinical progress? High resolution cross-sectional imaging should allow in vivo detection of the sub-RPE deposit. Reports have illustrated focal sub-RPE material associated with drusen-like lesions in L-ORD.4,5 We studied members of the original family with L-ORD, proven to have the p.Ser163Arg mutation in C1QTNF5, and asked whether retina-wide sub-RPE deposit was detectable and quantifiable. The relationship of deposit to photoreceptor structure and co-localized rod and cone vision was determined.
Report of Cases
Approval for these studies was obtained from the University of Pennsylvania Institutional Review Board. Written informed consent was obtained from the participants prior to beginning the study procedures.
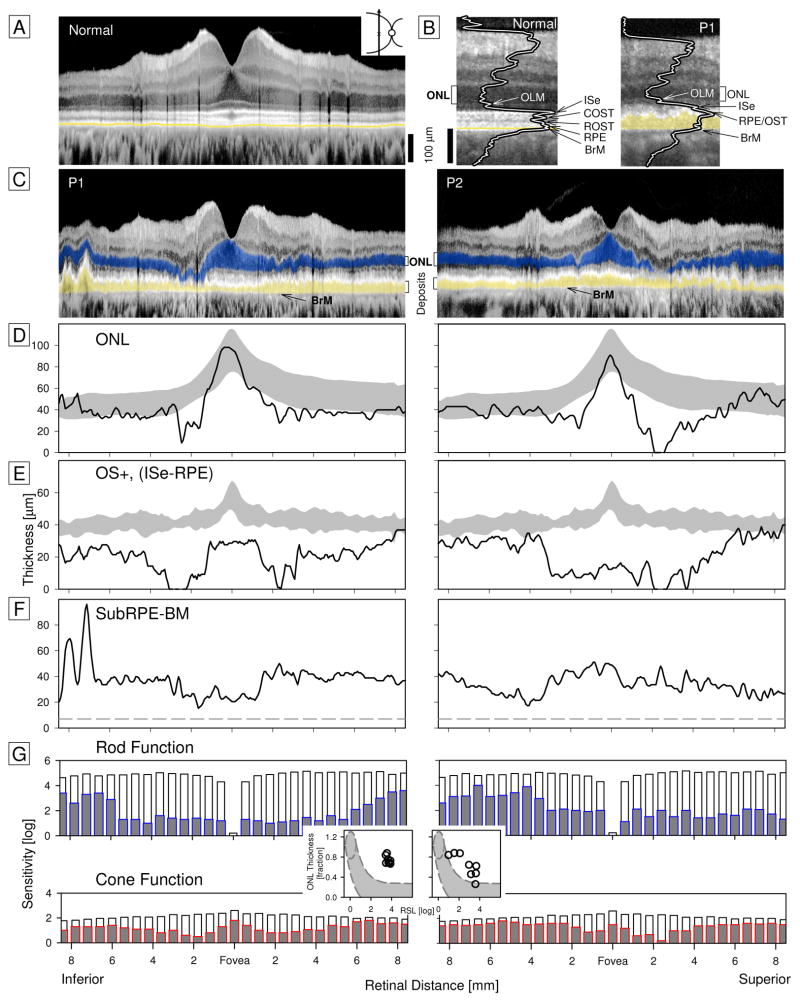
P1 (age 58) and P2 (age 66) complained of night blindness beginning 5–10 years earlier. P1 had visual acuities of 20/20; P2 had 20/80 and 20/25. OCTs in a normal subject, P1 and P2 were compared (Figure 1A–C). Magnified images with overlaid reflectivity profiles and labeled laminae indicated that RPE and BrM were separated in P1, unlike normal, by a thick (~40μm) hyperreflective layer. This abnormal layer was present across the scans in P1 and P2. Outer retinal laminae were quantified and compared to normal limits (Figure 1D,E). Outer nuclear layer (ONL) was normal at fixation, reduced paracentrally and borderline normal at greater eccentricities. Laminae that include the outer segments were abnormally reduced across the scans with better preservation in P1 centrally than in P2. The sub-RPE to BrM lamina was ~30–40μm thick (Figure 1F).
Figure 1. Outer Retinal Structure and Visual Function in Patients with C1QTNF5 disease.
A, Vertical OCT scan (57° span) in a normal subject. B, Magnified images at 11–13° superior retina (white bracket above full scans) comparing normal laminar architecture with that in P1. Longitudinal reflectivity profiles are overlaid on scans to show basis of labeling. Yellow highlights abnormal hyperreflectivity in subRPE-BrM space. C, Scans across P1 (OD) and P2 (OS) with highlighted subRPE-BrM abnormality (yellow) and ONL (ONL thickness includes anatomical layers of photoreceptor nuclei and Henle’s fiber layer; blue). D, ONL thickness in patients (lines) compared with normal limits (±2SD; gray). E, Outer segment lamination (OS+) plotted across eccentricity and compared with normal. F, Measurement of the thickness of the subRPE-BrM lamina in P1 and P2. Dashed line is maximum intervening space in normals. Instrument used: RTVue-100, Optovue Inc. G, Rod sensitivity with a 500nm stimulus (rod-mediated by dark-adapted two-color chromatic perimetry) and cone sensitivity with a 600nm stimulus (light-adapted), in log units. Patient data are gray bars with blue outline (rod) or red outline (cone) superimposed on normal mean for each locus (white bars extending from 0 to top of bars). Insets between rod and cone function are graphs of ONL thickness (expressed as a fraction of normal at 10–16.5° eccentricity vertically) versus rod sensitivity loss (RSL) at the same eccentricities. Gray region outlined by dashes shows the predicted relationship in an idealized photoreceptor degeneration.
Co-localized rod sensitivity was ≥1 log unit reduced at all loci. Cone sensitivities were reduced paracentrally and approached normal with increasing eccentricity (Figure 1G). Rod sensitivity loss and ONL thickness were compared in a region of rod-dominant retina. P1 showed greater dysfunction than could be accounted for by a model of photoreceptor loss alone. P2 had this relationship for most loci but some were close to the predictive model (Figure 1G, insets). Rod dysfunction greater than predicted by photoreceptor structure has been previously described in early systemic vitamin A deficiency.6
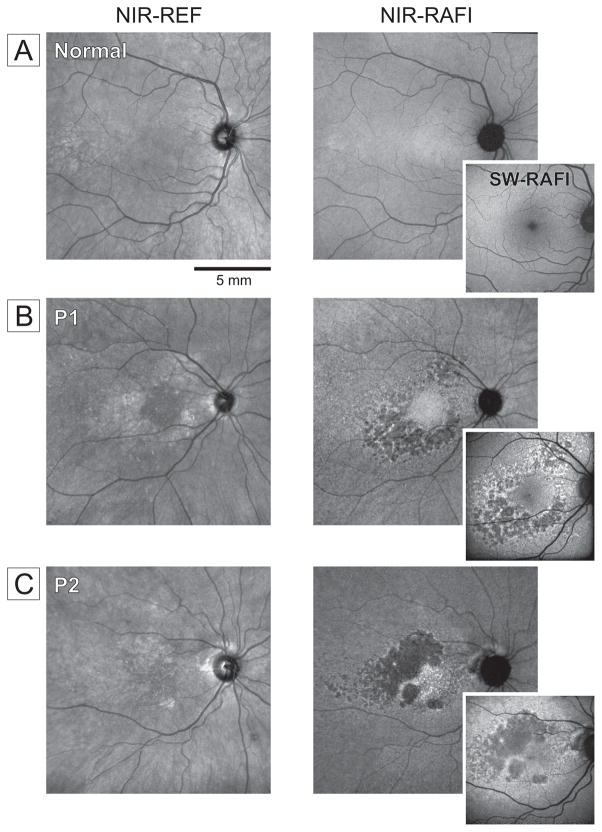
Near-infrared (NIR) and short-wavelength (SW) reduced-illuminance autofluorescence imaging (RAFI) was also performed. P1 and P2 showed paracentral regions of heterogeneous autofluorescence (Figure 2B,C). In both patients, there were also larger regions of hypo-autofluorescence on NIR-RAFI; unexpectedly, the same regions retained low but detectable SW-RAFI signal (insets).
Figure 2. Autofluorescence Imaging in Patients with C1QTNF5 disease.
A, Representative normal subject (59 yrs). B, Right eye of P1. C, Left eye of P2 (displayed as right eye). Larger panels are digital mosaics obtained from multiple overlapping 30° views. Images are shown contrast stretched and gamma corrected for visibility of features in brighter and dimmer parts of the images. NIR-REF, near infrared reflectance; NIR-RAFI, near infrared-reduced autofluorescence imaging; SW-RAFI, short wavelength-reduced autofluorescence imaging. Instrument used: HRA2, Heidelberg Engineering.
Discussion
The retina-wide thick sub-RPE deposit, previously quantified only by histopathology,1 is detectable and quantifiable in vivo; and, in rod-dominated retinal regions with deposit, the degree of rod visual dysfunction compared to photoreceptor structure is consistent with the well-known pattern in vitamin A deficiency.6 Autofluorescence imaging results may indicate: a) existence of a stage of RPE disease with complete loss of melanin fluorophores while partially retaining lipofuscin, or b) unmasking of a fluorophore within the sub-RPE deposits excited by SW lights upon RPE atrophy. Long-term follow-up of patients should allow differentiation between the alternatives.
The results form the basis for identification in the modern clinic of this rare disease masquerading as RP and AMD, and lead to suggestions for a clinical trial. L-ORD should enter the differential diagnosis when there are late-onset symptoms, dominant inheritance, OCTs showing sub-RPE deposit, and night blindness. Previous demonstration of improved vision with a short trial of high dose vitamin A, taken together with the present findings, suggests potential value of a clinical trial of modest doses of vitamin A supplementation before severe degeneration ensues.
References
- 1.Kuntz CA, Jacobson SG, Cideciyan AV, Li ZY, Stone EM, Possin D, Milam AH. Sub-retinal pigment epithelial deposits in a dominant late-onset retinal degeneration. Invest Ophthalmol Vis Sci. 1996;37(9):1772–82. [PubMed] [Google Scholar]
- 2.Hayward C, Shu X, Cideciyan AV, Lennon A, Barran P, Zareparsi S, Sawyer L, Hendry G, Dhillon B, Milam AH, Luthert PJ, Swaroop A, Hastie ND, Jacobson SG, Wright AF. Mutation in a short-chain collagen gene, CTRP5, results in extracellular deposit formation in late-onset retinal degeneration: a genetic model for age-related macular degeneration. Hum Mol Genet. 2003;12(20):2657–67. doi: 10.1093/hmg/ddg289. [DOI] [PubMed] [Google Scholar]
- 3.Tu X, Palczewski K. Crystal structure of the globular domain of C1QTNF5: Implications for late-onset retinal macular degeneration. J Struct Biol. 2012;180(3):439–46. doi: 10.1016/j.jsb.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent A, Munier FL, Vandenhoven CC, Wright T, Westall CA, Héon E. The characterization of retinal phenotype in a family with C1QTNF5-related late-onset retinal degeneration. Retina. 2012;32(8):1643–51. doi: 10.1097/IAE.0b013e318240a574. [DOI] [PubMed] [Google Scholar]
- 5.Soumplis V, Sergouniotis PI, Robson AG, Michaelides M, Moore AT, Holder GE, Webster AR. Phenotypic findings in C1QTNF5 retinopathy (late-onset retinal degeneration) Acta Ophthalmol. 2013;91(3):e191–5. doi: 10.1111/aos.12010. [DOI] [PubMed] [Google Scholar]
- 6.Dowling JE, Wald G. Vitamin A deficiency and night blindness. Proc Natl Acad Sci USA. 1958;44(7):648–61. doi: 10.1073/pnas.44.7.648. [DOI] [PMC free article] [PubMed] [Google Scholar]