Abstract
Although they are classically viewed as continuously recirculating through the lymphoid organs and blood, lymphocytes also establish residency in non-lymphoid tissues, most prominently at barrier sites, including the mucosal surfaces and skin. These specialized tissue-resident lymphocyte subsets span the innate-adaptive continuum and include innate lymphoid cells (ILCs), unconventional T cells (e.g., NKT, MAIT, γδ T cells, and CD8αα+IELs), and tissue-resident memory T (TRM) cells. Although these diverse cell types differ in the particulars of their biology, they nonetheless exhibit important shared features, including a role in the preservation of tissue integrity and function during homeostasis, infection, and non-infectious perturbations. In this Review, we discuss the hallmarks of tissue-resident innate, innate-like, and adaptive lymphocytes, as well as their potential functions in non-lymphoid organs.
Recirculating and Tissue-Resident Lymphocyte Subsets
From an evolutionary perspective, the mammalian adaptive immune system is the pinnacle of metazoan immune defenses in terms of its complexity and potential for molecular specificity. In contrast to innate immune systems, which rely on germline-encoded receptors to recognize stereotypic motifs associated with broad classes of pathogens, the hallmark of adaptive immunity is the generation of near-limitless antigen receptor diversity through somatic recombination, which in turn provides the foundation for immunological memory through the differentiation, expansion, and persistence of long-lived antigen-specific lymphocytes (Janeway, 1989; Medzhitov and Janeway, 2000; Medzhitov, 2009). Although they serve as direct effectors of immunity by elaborating cytotoxic function and antibody production, cells of the adaptive immune system act foremost as principal controllers, amplifying or limiting the responses of diverse cell types through positive and negative feedback loops.
At a basic level, the mammalian adaptive immune response is initiated by antigen-presenting cells (APCs) migrating from the site of infection to the draining lymph node to present captured microbial antigens to naive T cells, which constitutively recirculate between lymph nodes to survey presented antigens. When a naive T cell encounters its cognate antigen, it undergoes clonal expansion, a process that takes several days and results in the differentiation of both effector and memory T cells. While effector T cells home to the site of the primary infection and contribute to pathogen clearance, circulating memory T cells persist and are poised to mount a superior response to secondary infection. Teleologically, this efficient system of naive lymphocyte recirculation is necessitated by the minute frequencies at which individual lymphocyte clones are present, such that a given clone, incapable of being in all anatomical locations at once, instead patrols strategically positioned lymph nodes, which collect information on the statuses of tissues and organs—i.e., the antigenic landscape (Jenkins et al., 2010; Maryanski et al., 1996; von Andrian and Mackay, 2000). In contrast to this classical view of adaptive lymphocytes, studies in the last 10 years have led to a characterization of lymphocyte populations that are non-recirculating residents of non-lymphoid tissues and organs. These populations include tissue-resident memory T cells (TRM); “unconventional” T cells such as invariant natural killer T (iNKT) cells, mucosal-associated invariant T (MAIT) cells, γδ T cells, and intestinal intraepithelial lymphocytes (IELs); and the emerging family of innate lymphoid cells (ILCs) (Artis and Spits, 2015; Clark, 2015; Eberl et al., 2015; Godfrey et al., 2015; Schenkel and Masopust, 2014). These tissue-resident lymphocytes span the innate-adaptive continuum but nonetheless share a number of particular features pertaining to their tissue-resident functions. In this Review, we will discuss the properties and functions of lymphocytes residing in non-lymphoid tissues. Just as manipulation of lymphocyte recirculation has resulted in effective therapies for autoimmune diseases such as multiple sclerosis (Pelletier and Hafler, 2012; Ransohoff, 2007; von Andrian and Engelhardt, 2003), a better understanding of tissue-resident lymphocytes may reveal new cellular mechanisms of organ dysfunction in a multitude of inflammatory, infectious, and neoplastic processes and suggest novel approaches for their treatment.
Definition of Tissue-Resident Populations
The discovery of tissue-resident lymphocytes owes to experimental approaches that allow for discrimination of circulating and tissue-resident populations. One of the most commonly used means of assessing tissue residency is parabiosis, whereby two congenic mice expressing distinct allelic markers of hematopoietic cells are surgically conjoined through adjacent skin, such that they develop a shared anastomotic circulation (Wright et al., 2001). This approach pinpoints tissue-resident cell populations by their exclusive expression of the host congenic marker, in contrast to actively recirculating cells and their progeny, which exhibit both host and donor markers in equal proportion. It must be noted that the extent and kinetics of equilibration between circulating cells originating from the two parabionts is dependent on the turnover rate of a given cell subset. While two populations may be similarly replaced by circulating precursors, the longer-lived population is replaced more slowly and, thus, appears to be tissue-resident to a greater extent. Furthermore, failure of a population to exchange in parabiosis does not imply that cells of that population are sessile and static. For example, in the absence of inflammation, Langerhans cells remain overwhelmingly host-derived in parabiotic mice (Merad et al., 2002), although they migrate continuously and unidirectionally from the epidermis to skin-draining lymph nodes (Bajaña et al., 2012; Ohl et al., 2004; Tomura et al., 2014). Additionally, imaging studies have shown that liver-resident iNKT cells, epidermis-resident CD8+ TRM cells, and intestine-resident ILCs exhibit dynamic behavior within their respective tissues (Gebhardt et al., 2011; Geissmann et al., 2005; Mackley et al., 2015; Pearson et al., 2016; Zaid et al., 2014) but self-renew locally and are not replaced by circulating precursors (Gasteiger et al., 2015; Jiang et al., 2012; Thomas et al., 2011). Thus, cell tracking and imaging approaches offer an essential complement to the “10,000-foot view” provided by parabiosis. The mobile or sessile “styles” of tissue residency, and signal-dependent waves of migration to and from the tissue, have been elucidated through methodologies such as photo-switchable or constitutive cell tagging, direct visualization of cellular behavior using intravital imaging, and analysis of tissue explants or transplants into congenically marked mice.
The Spectrum of Tissue-Resident Lymphocytes
A combination of these approaches has revealed that a variety of tissue-resident lymphocytes, representing the innate and adaptive branches of immunity, differ in their distribution in non-lymphoid tissues yet exhibit common “innate”-like properties. These tissue-resident lymphocytes represent an integral part of a network of cells whose connections and hierarchy are poorly understood. However, it is reasonable to assume that they act as sensors of perturbed tissue integrity stemming from infection, injury, and potentially other forms of deviation from the homeostatic norm. In parallel to the role of their circulating counterparts in amplifying or suppressing innate immunity, tissue-resident lymphocytes likely support the functioning of non-lymphoid tissues by serving as sentinels of tissue integrity, recruiters of bloodborne reinforcements, and amplifiers of homeostatic mechanisms through feedback on parenchymal cells and non-lymphoid accessory cells (e.g., macrophages, fibroblasts, and endothelial cells) (Figure 1) (Medzhitov, 2008). Below, we will briefly review features of innate and adaptive tissue-resident lymphocytes and discuss experimental observations supporting this hypothetical model.
Figure 1. Tissue-Resident versus Recirculating Lymphocytes and Their Functions.
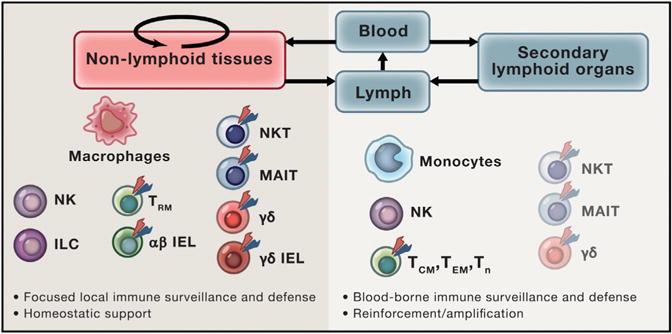
Tissue-resident lymphocytes are principally found in barrier tissues, where they serve as sentinels and frontline defenders of tissue integrity in response to infection and non-infectious insults. Recirculating lymphocyte subsets actively survey the body for similar perturbations of homeostasis by patrolling the lymphatic and blood circulatory systems and associated secondary lymphoid organs (lymph nodes and spleen). While some lymphocyte subsets, most prominently ILCs, display almost exclusively tissue-resident behavior, circulating counterparts to other adaptive lymphocyte subsets, including NK, NKT, MAIT, γδ T, and αβ T cells can also be found in peripheral blood, albeit at lower frequencies.
Innate Lymphoid Cells
Toward the “innate”-most end of the innate-adaptive spectrum are ILCs, a diverse family of lymphocytes, including natural killer (NK) cells, lymphoid tissue inducer (LTi) cells, and the “helper-like” ILCs. Like other lymphocytes, all ILCs develop from the common lymphoid progenitor. Helper-like ILCs develop through a common helper-like ILC precursor (ChILP) shared with LTi but not NK cells and, subsequently, through an intermediate expressing transcription factor PLZF with only helper-like ILC potential (Constantinides et al., 2015, 2014; Klose et al., 2014b; Wong et al., 2012). Long-term parabiosis experiments suggested that ILC and NK subsets residing in the non-lymphoid tissues and secondary lymphoid organs of adult mice are likely maintained through self-renewal with minimal contribution from hematogenous precursors under physiologic and inflammatory conditions (Gasteiger et al., 2015; Peng et al., 2013; Sojka et al., 2014). Consistent with these findings, distinct features have been reported for salivary-gland-resident NK cells and ILCs with the key properties of the latter coordinately evolving with the development of their “home” organ (i.e., salivary gland) (Cortez et al., 2014; M. Colonna, personal communication). Likewise, mature ILCs appear in the lung as early as day 8 of postnatal life, and intestinal ILCs appear to derive from a precursor present early in life (Bando et al., 2015; Nussbaum et al., 2013). Lending further support to the notion that ILCs serve important homeostatic functions in support of organs and tissues, the first characterized subset of ILC with a defined function were RORγt and lymphotoxin-expressing LTi cells essential for the organogenesis of lymph nodes and Peyer’s patches (Adachi et al., 1997; Eberl et al., 2004; Kelly and Scollay, 1992; Mebius et al., 1997; Sun et al., 2000).
Lacking classical antigen receptors, ILCs are instead activated by cytokines, whereas NK cells are additionally stimulated through activating receptors such as NKG2D and Ly49H, or through a receptor for the immunoglobulin constant region (FcγRIII/CD16). Mirroring the T-helper polarization of conventional CD4+ T cells, helper-like ILCs can be subdivided into types 1, 2, and 3 (ILC1, ILC2, and ILC3) based on differential expression of the lineage-specifying transcription factors T-bet, GATA-3, and RORγt (respectively) and production of the corresponding effector cytokines interferon-γ(ILC1), IL-5 and IL-13 (ILC2), and IL-17 (ILC3) (Artis and Spits, 2015; Eberl et al., 2015). Besides producing cytokines that orchestrate and amplify antimicrobial defenses, ILCs also elaborate soluble factors that promote tissue maintenance. Different ILC subsets respond to the pro-inflammatory cytokine IL-23 or the alarmin IL-33 by producing tissue-protective factors IL-22 or amphiregulin, respectively (Cella et al., 2009; Monticelli et al., 2015, 2011; Sanos et al., 2009).
“Innate-like” T Cells
In contrast to ILCs, “unconventional” or “innate-like” T cells express T cell receptors (TCRs) of limited diversity, which recognize antigens in the context of non-classical, non-polymorphic MHC-like molecules, or independently of MHC-related presenting molecules altogether (Godfrey et al., 2015). Lymphocytes belonging to this group include αβTCR-expressing iNKT, MAIT, and γδ T cells.
iNKT cells express an invariant TCRα chain (Vα14-Jα18 in mice, Vα24-Jα18 in humans) paired with a TCRβ chain of limited diversity (Brennan et al., 2013; Salio et al., 2014). Unlike most αβTCRs, iNKT TCRs recognize glycolipid antigens presented by the MHC class I-like molecule CD1d (Bendelac et al., 1994, 1995). Functionally, iNKT cells can be activated by potent bacterial ligands, such as cell wall sphingolipids from Sphingomonas, Borrelia, or Streptococcus (Kinjo et al., 2006, 2011; Sriram et al., 2005). They can also sense changes in host lipid metabolism through recognition of transiently expressed, less potent, rare, or unstable endogenous lipids, a number of which have been proposed (Brennan et al., 2011, 2014; Facciotti et al., 2012; Kain et al., 2015; Zhou et al., 2004). This endogenous lipid sensing, which may in fact be the dominant mode of iNKT cell activation in vivo, allows iNKT cells to indirectly detect the breach of tissue integrity resulting from infection with viruses, fungi, and bacteria like Salmonella typhimurium, which lack potent agonist ligands (Cohen et al., 2011; Mattner et al., 2005). As with ILCs, NKT cell subsets analogous to TH1, TH2, and TH17 conventional T cells have been described. These subsets express the corresponding cytokines and transcriptional regulators of their T-helper counterparts and have been shown to localize to different tissues (Constantinides and Bendelac, 2013; Coquet et al., 2008; Lee et al., 2013; Lee et al., 2015b; Michel et al., 2007; Watarai et al., 2012).
Like iNKT cells, MAIT cells express a semi-invariant TCR combining a unique TCRα chain (Vα19-Jα33 in mice, Vα7.2-Jα33 in humans) with a restricted set of TCRβ chains and reside in the liver but also in the intestine (Dusseaux et al., 2011; Le Bourhis et al., 2011; Salio et al., 2014). MAIT TCRs, however, are activated by bacterial riboflavin biosynthesis intermediates presented by the MHC class I-like molecule MR1 (Corbett et al., 2014; Kjer-Nielsen et al., 2012; Treiner et al., 2003). Additionally, it seems likely from the MR1 crystal structure that its ligand-binding groove can accommodate other ligands. In fact, it has been shown that pterin derivatives can bind MR1 but fail to stimulate MAIT cells (Kjer-Nielsen et al., 2012). Distinct subsets of MAIT cells can produce IFN-γ and IL-17 (Dusseaux et al., 2011; Rahimpour et al., 2015). Although MAIT cells have been suggested to play a role in antibacterial immunity through sensing of MR1-bound microbial products (Chua et al., 2012; Georgel et al., 2011; Kjer-Nielsen et al., 2012; Le Bourhis et al., 2010; Meierovics et al., 2013), it is tempting to speculate that these cells may also be involved in mediating beneficial host-commensal interactions in the intestine and, potentially, the skin or lung. Such interactions could be reminiscent of the previously proposed roles of commensal-derived short-chain fatty acids in maintaining colonic immune homeostasis through the de novo differentiation of Treg cells and the tolerogenic imprinting of APCs (Arpaia et al., 2013; Chang et al., 2014; Furusawa et al., 2013; Smith et al., 2013).
T cells expressing the γδTCR represent another prominent innate-like T cell subset that resides in barrier tissues and is especially enriched among IELs. In mice, specific Vγ and Vδ segment rearrangements within the γδTCR locus occur in a highly ordered fashion during embryonic development (Chien et al., 1987; Ito et al., 1989; Itohara et al., 1989). This results in the sequential appearance of distinct waves of γδ T cells bearing oligoclonal or monoclonal TCRs that populate different epithelial tissues (Havran and Allison, 1988; Itohara et al., 1990). For example, dendritic epidermal T cells (DETCs), which are the only lymphocytes to reside in the epidermis of naive mice, are a monoclonal population of intraepithelial γδ T cells that arise from fetal thymic precursors between embryonic days 14 and 16 and exclusively express the Vγ3Vδ1 TCR (Asarnow et al., 1988; Havran and Allison, 1990; Havran et al., 1989). Unlike the αβTCR, the γδTCR exhibits a longer, immunoglobulin-like CDR3 structure, and several modes of antigen recognition by γδ T cells have been elucidated, in contrast to the strict MHC restriction of conventional αβ T cells (Rock et al., 1994). Different subsets of γδ T cells have been shown to recognize ligands as diverse as lipids presented on CD1 family members; a conformational change in butyrophilin-3A induced by prenyl pyrophosphate derivatives; MHC class I-like molecules (e.g., MICA, ULBP4, T10, T22), which may be induced by cellular stress; conventional MHC molecules irrespective of loaded peptide; and even some soluble ligands in the absence of presenting molecules (Chien et al., 2014; Sandstrom et al., 2014). As with MAIT cells, IFN-γ and IL-17 are produced by different γδ T cell subsets (Jensen et al., 2008).
It must be noted that intestinal IELs, unlike their epidermal counterparts in mice—monoclonal Vγ3Vδ1+ DETCs—consist of a heterogeneous assortment of αβ T cells, γδ T cells, and ILCs united by their localization (Cheroutre et al., 2011; Fuchs et al., 2013). A distinguishing feature of many αβ and γδ IELs is their expression of the CD8αα homodimer, as opposed to the CD8αβ heterodimer expressed by conventional cytotoxic T cells. In addition to recognizing MHC class I, CD8αα also binds the non-classical MHC class I molecule, thymus leukemia antigen (TLA), which is expressed by intestinal epithelial cells (Leishman et al., 2001). Thymocytes can differentiate into CD4− CD8αβ− “natural” IELs by escaping from negative selection after experiencing agonist signaling in the thymus (Gangadharan et al., 2006; Leishman et al., 2002; McDonald et al., 2014, 2015; Pobezinsky et al., 2012; Rocha et al., 1992; Yamagata et al., 2004). Alternatively, mature T cells can be induced to become IELs in the periphery, in which case they may retain expression of CD4 or CD8αβ. The strong self-reactivity of TCRs from natural IELs thus explains the likely function of CD8αα as a repressor of activation and the concomitant expression of a variety of inhibitory receptors (e.g., LAG-3, PD-1, and Ly49 family NK inhibitory receptors) on natural IELs (Cheroutre et al., 2011; Denning et al., 2007; Mayans et al., 2014; Shires et al., 2001). After exiting the thymus, natural IEL precursors mature upon IL-15-induced upregulation of T-bet in the periphery, and a similar differentiation pathway has been demonstrated for induced IELs, which can develop from CD4+ T cells after upregulation of T-bet and Runx3, and loss of ThPOK (Klose et al., 2014a; Mucida et al., 2013; Reis et al., 2014). However, induced IEL differentiation appears to be driven by exogenous antigen rather than self-antigen, and these cells may therefore be the antigen-specific “adaptive” counterparts of innate-like natural IELs (Mucida et al., 2013). Consistent with a role of antigen in this process, germ-free mice and mice fed an elementary diet free of protein antigens show decreased intestinal IEL numbers (Bandeira et al., 1990; Menezes et al., 2003; Helgeland et al., 1996; Umesaki et al., 1993).
Hallmarks of Tissue-Resident Lymphocytes
Besides their ability to self-renew in tissues independently of circulating precursors, there are other important properties shared by tissue-resident lymphocytes (Box 1). Importantly, far from being rare, tissue-resident lymphocytes are among the most abundant lymphocyte populations. First, taking into consideration the large surface areas of barrier tissues, a careful accounting of the absolute number of epidermal and intestinal IELs shows that each population comprises a substantial fraction of the total lymphocyte pool (Beagley et al., 1995; Clark et al., 2006). However, common methods of isolation are inefficient for lymphocytes residing in non-lymphoid tissues, causing their numerical importance to be underappreciated (Steinert et al., 2015). Second, tissue-resident lymphocytes often comprise a large percentage of lymphocytes in the tissue compartments that they inhabit and may localize to specific niches that are relatively inaccessible to recirculating cells that enter the same tissue. For example, in the absence of challenge, DETCs are the only lymphocytes in mouse epidermis, and resident γδ T cells also comprise a large fraction of lymphocytes in the mouse dermis (Gray et al., 2011; Sumaria et al., 2011). Intestinal IELs are mostly resident lymphocytes, and iNKT or MAIT cells are abundant among liver lymphocytes in mice and humans, respectively (Dusseaux et al., 2011; Poussier et al., 1992; Rahimpour et al., 2015; Sugahara et al., 1999; Suzuki et al., 1998). Importantly, the antigen specificities of semi-invariant tissue-resident T cells are abundant relative to the individual clonal frequencies of naive T cells. While roughly one in a million naive T cells possesses the antigen specificity to respond to a given pathogen-derived peptide-MHC complex (Jenkins et al., 2010), innate and innate-like lymphocytes are present in populations in which nearly all cells can respond in the presence of signal. This means that the appropriate stimulus can activate large numbers of innate lymphocytes to produce significant effector function during the time in which the few reactive clones of adaptive lymphocytes must be expanded.
Box 1. Hallmarks of Tissue-Resident Lymphocytes.
Long-term maintenance and self-renewal
Abundance at barrier tissues
Sensing of microbial products, cytokines, alarmins, and stress ligands
Rapid provision of antimicrobial and tissue-protective factors
The second hallmark of innate tissue lymphocytes is their recognition of a wide variety of microbial ligands and host-derived signals that collectively signify infection, inflammation, and tissue injury (Figure 2). These signals activate tissue lymphocytes through the aforementioned semi-invariant antigen receptors, NK activating receptors, and alarmin and cytokine receptors to elicit the rapid production of effector cytokines. The semi-invariant TCRs of iNKT cells recognize glycolipids, the most potent of which possess an α-anomeric glycosidic linkage and can be produced by pathogenic bacteria (Brennan et al., 2013; Salio et al., 2014). αβ T cells restricted for other CD1 molecules (CD1a, CD1b, CD1c) have been described in humans, and some of these cells respond most strongly to lipids from mycobacteria (Godfrey et al., 2015). As noted above, the most potent activating antigens for MAIT cells (riboflavin derivatives) and for human Vγ9Vδ2+ T cells (“phosphoantigens”) are also bacterial products (Corbett et al., 2014; Hintz et al., 2001; Kjer-Nielsen et al., 2012; Le Bourhis et al., 2010). Thus, because of their direct activation by microbial products, the invariant TCRs of unconventional T cells functionally resemble pattern recognition receptors. However, in the absence of potent microbial antigens, these cells can also be activated by lower-affinity endogenous ligands with concurrent cytokine stimulation, most notably from IL-12 and IL-18 (Brigl et al., 2003; Nagarajan and Kronenberg, 2007; Tyznik et al., 2008). This may be a major mode of iNKT activation even for pathogens that make suitable TCR ligands (Brigl et al., 2011). It is appealing to think that glycolipid-sensing iNKT cells, with their prominent enrichment in the liver and adipose tissue, may also be triggered in response to sterile metabolic stresses, which are accompanied by altered glycolipid synthesis and turnover. Indeed, iNKT cells decrease in the adipose tissue, liver, and omenta of obese humans or mice (Ji et al., 2012; Kotas et al., 2011; Lynch et al., 2009, 2012; Schipper et al., 2012). CD1d-deficient mice on a high-fat diet show exacerbated metabolic abnormalities (Kotas et al., 2011; Lynch et al., 2012; Schipper et al., 2012), though reports conflict as to whether this phenotype is recapitulated in mice specifically deficient in Jα18-expressing iNKT cells. Besides iNKT cells, recognition of self-ligands is also a feature of other unconventional T cell types: Vγ9Vδ2+ T cells can be activated by endogenous prenyl intermediates that accumulate in cells upon inhibition of the isoprenoid biosynthesis pathway (Gober et al., 2003), and various IEL subsets are also thought to primarily recognize self-ligands. For example, DETCs recognize a self-ligand whose precise molecular identity is unknown but is expressed transiently by injured keratinocytes within an hour of cutaneous wounding (Havran et al., 1991; Komori et al., 2012). Natural IELs exhibit self-reactive TCRs that result from thymic agonist selection and may be kept in a unique “activated yet resting” state by the regulated expression of co-stimulatory and co-inhibitory molecules (Shires et al., 2001). Accordingly, IELs, along with iNKT cells, MAIT cells, γδ T cells, and several other tissue-resident subsets, express NK activating receptors such as NKG2D, which detect molecules present on stressed epithelial cells. Some γδ T cells may additionally recognize these or other stress ligands through their TCR (Kong et al., 2009; Wu et al., 2002). Thus, besides reacting to pathogens, tissue-resident lymphocytes also directly monitor the condition of tissue parenchymal cells.
Figure 2. Modes of Sensing and Provision of Effector Function by Tissue-Resident Innate, Innate-like and Adaptive Lymphocytes.
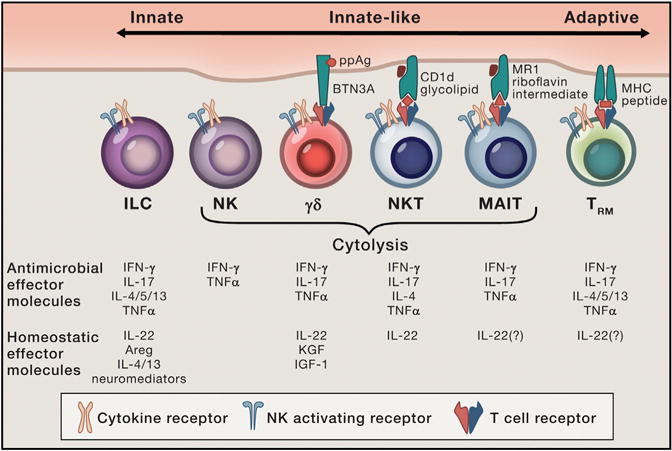
Note that γδTCRs can be activated by a variety of antigens with or without presenting molecules; only one mode of γδTCR activation is shown. Also, both CD4+ and CD8+ TRM cells have been described, although only the former is depicted.
Another hallmark of tissue-resident lymphocytes is their “memory”-like phenotype—specifically, their ability to rapidly produce effector cytokines, cytolytic molecules, and growth factors upon activation at early time points during infection (Figure 2). Innate lymphocytes can respond on the order of hours, which is in contrast to the days that are required for the activation and clonal expansion of naive conventional T cells during a primary immune response. For example, vaccinia virus infection induces IFN-γ production by γδ T cells as early as day 2 post-infection (Selin et al., 2001), NKT cells produce IFN-γ at day 2 after BCG infection (Chiba et al., 2008), and ILC2s are early producers of IL-13 after infection with the helminth Nippostrongylus brasiliensis (Fallon et al., 2006; Moro et al., 2010; Neill et al., 2010; Price et al., 2010; Saenz et al., 2010; Voehringer et al., 2006). After intraperitoneal injection of E. coli, γδ T cells serve as the major producers of IL-17, peaking at 6 hr post-infection; antibody-mediated depletion of γδ T cells or IL-17 impairs neutrophil recruitment to the peritoneal cavity (Shibata et al., 2007). The importance of this early cytokine production is further borne out by the phenotypes of mice that lack certain innate lymphocyte populations. For example, mice lacking γδ T cells show increased bacterial burden in the spleen at 3–4 hr (but not beyond 24 hr) after oral Salmonella infection, as well as increased viral load and mortality early after vaccinia infection (Ismail et al., 2011; Selin et al., 2001). The memory-like properties common to innate tissue-resident lymphocytes may have their basis in the shared expression of the transcription factor PLZF by many of these cell types, including iNKT cells, MAIT cells, some γδ T cells, and ILC precursors (Alonzo et al., 2010; Constantinides et al., 2014; Kovalovsky et al., 2008; Lu et al., 2015; Rahimpour et al., 2015; Savage et al., 2008). Indeed, PLZF-deficient mice show a profound deficiency of iNKT cells and MAIT cells, and the few iNKT cells remaining exhibit aberrant function and localization (Kovalovsky et al., 2008; Savage et al., 2008; Rahimpour et al., 2015). Conversely, forced expression of PLZF is sufficient to confer certain features of iNKT cells on conventional T cells (Kovalovsky et al., 2010; Raberger et al., 2008; Savage et al., 2011; Thomas et al., 2011). A history of PLZF expression may therefore explain the superficial similarities between ILCs lacking antigen receptors and unconventional T cells that arise from agonist TCR selection. In accordance with these shared functional features, the cell-surface phenotype of tissue-resident lymphocytes also resembles that of antigen-experienced memory T cells. For example, many tissue-resident lymphocyte subsets express CD44, a receptor for hyaluronan; integrin αE (CD103), which recognizes E-cadherin; and integrin α1 (CD49a), which binds type IV collagen. These markers mediate adhesion to extracellular matrix, reflecting their probable function in controlling cellular localization and motility in non-lymphoid tissues.
Given their close association with parenchymal cells, often beginning early in development, tissue-resident lymphocytes are well poised to sense dysfunction of parenchymal cells and to produce factors that contribute to tissue protection and maintenance. Cytokines and alarmins released by myeloid, stromal, and parenchymal cells are important signals that convey the presence of infection and the perturbation of tissue homeostasis to resident lymphocytes. Myeloid cells integrate inputs received through various pattern recognition receptors into a distinct combination of cytokine outputs (e.g., IL-12 or IL-23), thereby activating specific subsets of innate lymphocytes while also shaping the polarization of the adaptive response. Collectively, cytokine and alarmin signals appear to be the most important stimuli for the activation of ILCs, which lack antigen receptors. Epithelial cells appear to be the major source of the cytokines that initiate type 2 immune responses—namely, IL-33, IL-25, and thymic stromal lymphopoietin (TSLP). By producing their own cytokines in turn, innate lymphocytes comprise an important amplification loop in this process (Figure 3). For example, IL-33 released from dying epithelial cells, and IL-25 from chemosensory tuft cells, are both potent activators of ILC2s during helminth infection (Gerbe et al., 2016; Howitt et al., 2016; Moro et al., 2010; Neill et al., 2010; Price et al., 2010; Saenz et al., 2010; von Moltke et al., 2016). Activated ILC2s produce IL-13, which acts on the intestinal stem cell compartment to facilitate goblet cell and tuft cell hyperplasia, two functional adaptations that promote worm expulsion and additional IL-25 production, respectively. Mice deficient in IL-25, IL-33, or tuft cells (Pou2f3−/−) are unable to sustain this feed-forward loop and are less able to expel Nippostrongylus brasiliensis (Fallon et al., 2006; Hung et al., 2013; Gerbe et al., 2016; Neill et al., 2010; von Moltke et al., 2016). Notably, Rag1-deficient mice, which lack adaptive lymphocytes, are less efficient at expelling worms, but mice, in which T cells are unable to produce IL-4 and IL-13, show no such defect (Voehringer et al., 2006; von Moltke et al., 2016). These findings illustrate the role of the adaptive immune system in amplifying and reinforcing the responses of innate tissue-resident lymphocytes to clear an infection.
Figure 3. Amplification of Immune Responses by Tissue-Resident Lymphocytes.
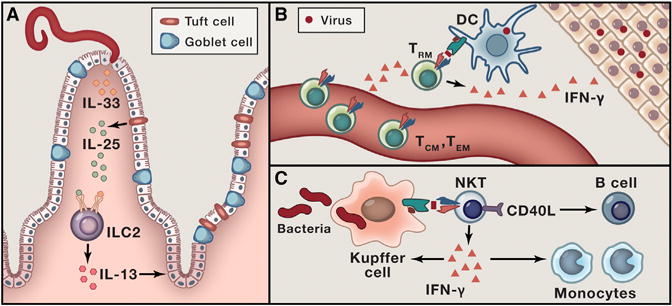
(A) Feed-forward loop in the early type 2 response to intestinal helminth infection. Helminth infection triggers release of IL-33 from dying epithelial cells and IL-25 from chemosensory tuft cells. These cytokines activate ILC2s to produce IL-13, which acts on the stem cell compartment to induce goblet and tuft cell hyperplasia.
(B) Triggered by cognate antigen, CD8+ TRM cells release interferon-gamma, which initiates a tissue-wide state of alarm and recruits circulating memory cells.
(C) NKT cells rapidly produce cytokines and upregulate CD40L after being activated by Kupffer cells presenting CD1d-bound glycolipids derived from bloodborne bacterial pathogens.
Notably, tissue repair is a prominent feature of type 2 immunity, which may have evolved to defend mammalian hosts from the widespread tissue damage associated with migrating helminth larvae (Allen and Sutherland, 2014; Gause et al., 2013). In further support of this notion, the major type 2 cytokines IL-4 and IL-13 activate collagen deposition by fibroblasts, as well as epithelial remodeling of the intestinal mucosa, skin, and airways. IL-33 stimulation of tissue-resident TH2 cells, ILC2 cells, and regulatory T (Treg) cells also results in the production of the EGFR ligand amphiregulin (Areg), which promotes epithelial cell proliferation. Tissue Treg cells are an important source of Areg (Burzyn et al., 2013), and mice in which Treg cells lack Areg exhibit impaired pulmonary function and exacerbated tissue pathology during influenza infection (Arpaia et al., 2015). However, production of trophic factors is not restricted to type 2 immune responses; the pro-inflammatory cytokine IL-18 can also stimulate Areg production from Treg cells (Arpaia et al., 2015). In addition, IL-22 produced by ILC3s and TH17 cells has been demonstrated to act as a major tissue-protective factor in a number of settings, including thymic radiation damage and intestinal inflammation or infection (Aujla et al., 2008; Dudakov et al., 2012; Hanash et al., 2012; Lindemans et al., 2015; Zenewicz et al., 2008; Zheng et al., 2008). IELs also produce a number of trophic or regulatory factors, including keratinocyte growth factor, IGF-1, and TGF-β, and mice lacking γδ T cells experience delayed epithelial repair (Boismenu and Havran, 1994; Chen et al., 2002; Jameson et al., 2002; Sharp et al., 2005; Shires et al., 2001). Finally, recent studies have suggested that ILC2 cells regulate the conversion of white adipose tissue into thermogenic beige adipose tissue, possibly through the production of neuropeptides (Brestoff et al., 2015; Lee et al., 2015a). Resident T cells that synthesize acetylcholine as part of a neuroimmune circuit have also been identified (Rosas-Ballina et al., 2011). These diverse observations suggest the centrality of tissue repair and maintenance functions to tissue-resident lymphocyte biology. Collectively, these hallmarks of innate and innate-like tissue-resident lymphocytes—abundance, especially at barrier tissues and seeding early in development; sensing of microbial products, cytokines, and stress-induced self-ligands; and rapid “memory”-like provision of antimicrobial and tissue reparative effector function—endow these cells with the ability to detect infection or injury and to coordinate protective responses. Furthermore, restoring and safeguarding the integrity of barrier tissues is a critical function that goes hand in hand with the sensing and clearance of pathogens.
Redundancy and Unique Functions of Tissue-Resident Lymphocytes
Given the functional properties that tissue-resident lymphocyte subsets share, to what extent are these subsets redundant or essential? For example, in the mucosal tissues of healthy uninfected mice, αβ and γδ IELs, MAIT cells, and ILC1s are all innate tissue-resident cells potentially capable of producing IFN-γ or inducing cytolysis. Such a redundancy in innate and innate-like tissue-resident lymphocytes likely allows for the robustness of defense against infection and tissue protection. Therefore, tissue immune responses can be seen as multilayered, with an ordered involvement of different cell types with partially overlapping functions in a relay-like manner (“passing the baton”). For example, early during influenza virus infection, lung Treg cells represent the numerically most significant source of amphiregulin and are essential for protection against lung injury, whereas at later times, expanded pools of ILCs, myeloid cells, and effector T cells may provide sufficient protection (Arpaia et al., 2015). Yet, it is possible that such a “relay” of different cell types allows for non-redundant, still-to-be-recognized functions. Consistent with this idea, the different types of tissue-resident lymphocytes are present at different frequencies and may occupy distinct locations within the same non-lymphoid organ (e.g., intra- versus extravascular or connective tissue versus intraepithelial localization). Independently of their frequency and distribution, tissue-resident lymphocytes may also exhibit unique and non-redundant functions by being activated by an overlapping but distinct array of stimuli, presented on different molecules, potentially by different APCs. For example, iNKT cells are abundant in the mouse liver. Borrelia burgdorferi, the causal agent of Lyme disease, produces an α-linked glycolipid presented to liver-resident iNKT cells by CD1d expressed on Kupffer cells (Kinjo et al., 2006; Lee et al., 2010). Upon blood-borne dissemination of Borrelia, activated iNKT cells cease patrolling liver sinusoids, cluster on Kupffer cells, and produce IFN-γ. Importantly, Jα18- or CD1d-deficient mice lacking iNKT or all NKT cells, respectively, exhibit sharply increased microbial load in their joints upon Borrelia infection (Kumar et al., 2000; Lee et al., 2010; Tupin et al., 2008). Thus, in this infection, iNKT cells serve an early, non-redundant protective function, presumably due to their anatomical location within the sinusoids of the liver, their sensing of CD1d-presented antigen through their semi-invariant TCR, and their rapid production of a key protective cytokine.
The fact that several of the innate and innate-like lymphocyte subsets exhibit considerable species-specific variation in their abundance suggests that their functions are conserved, but not the particulars—i.e., the specific cell type responsible for these functions. As an illustration, iNKT cells, a major liver lymphocyte subset in mice, represent no more than 1% of liver lymphocytes in humans (Kenna et al., 2003). Conversely, MAIT cells comprise up to 45% of liver T cells in humans but only a small percentage in the common C57BL/6J strain of laboratory mouse (though they are 20-fold more abundant in wild-derived CAST/EiJ mice than in C57BL/6J mice) (Cui et al., 2015). Species differences in tissue-resident lymphocytes are perhaps even more striking for γδ T cells; γδ T cells are less abundant in humans and mice than in cattle and swine, and the diversity or oligoclonality of γδ T cells differs significantly in different mammals (Hein and Dudler, 1997; Hein and Mackay, 1991; Mackay and Hein, 1989; Van Rhijn et al., 2007). Furthermore, Vγ3Vδ1+ DETCs are absent in humans, while Vγ9Vδ2+ T cells, which are the most abundant population of γδ T cells in human peripheral blood, are absent in mice. Going forward, a challenge will be to elucidate the relative contributions and non-redundant roles of individual tissue lymphocyte subsets, especially ILCs, for which specific genetic depletion strategies that preserve adaptive lymphocytes are only beginning to emerge. The first studies using such tools have suggested both redundant and non-redundant roles for ILCs in various experimental settings (Oliphant et al., 2014; Rankin et al., 2016; Song et al., 2015).
Superposition of Tissue-Resident Memory T Cells with Innate and Innate-like Lymphocyte Subsets
The innate and innate-like lymphocytes cohabit non-lymphoid organs with long-lived CD4+ or CD8+ tissue-resident memory T (TRM) cells expressing conventional, diverse, MHC-restricted αβTCRs. TRM cells seed tissues early during the immune response to infection and represent a subset distinct from recirculating memory T cells in blood and secondary lymphoid organs (Clark, 2015; Schenkel and Masopust, 2014; Stary et al., 2015). Like innate tissue-resident lymphocytes, TRM cells are numerically prominent in the mucosal tissues where pathogen encounter first occurs, with the highest seeding of TRM found at the site of infection; upon repeated infections, some degree of cross-protection of adjacent epithelium and distant epithelial tissues can also occur (Jiang et al., 2012). Tissue microenvironmental factors, mostly as yet undefined, likely have a role in shaping the phenotype of embedded TRM cells. For example, epidermal TRM cells adopt a dendritic morphology reminiscent of Langerhans cells and DETCs, which share the same niche, and distinct from TRM cells of the same TCR specificity that have seeded the dermis (Zaid et al., 2014). In the absence of reinfection, these epidermal TRM cells have been shown to scan swathes of epidermis in a matter of hours through slow, non-directed Brownian motion (Zaid et al., 2014). Upon encounter with cognate antigen, TRM cells elaborate soluble factors, which rapidly engender an antimicrobial state in parenchymal cells, and recruit and activate other immune cells (Ariotti et al., 2014; Schenkel et al., 2013, 2014). Although the initial trigger for TRM cell reactivation is cognate antigen, the generation of a nonspecific, tissue-wide state of alarm can offer protection against non-cognate pathogens. Thus, the differentiation of antigen-specific lymphocytes into TRM cells enables them to mount an innate-like response with the rapidity and frontline surveillance features of innate lymphocytes. At the same time, TRM cells retain the diversity, specificity, and amplifying function characteristic of adaptive T cells. It must be noted that tissue-resident innate and innate-like lymphocytes and TRM cells may be differentially important at different stages of development. For example, it has been seen that after infection, TRM cells displace DETCs from the skin (Zaid et al., 2014). Likewise, intestinal induced IELs gain numerical prominence over natural IELs with age (Cheroutre et al., 2011). Thus, lymphocyte subsets that seed tissues in fetal life and have limited regeneration potential may be functionally and even physically superseded by adaptive cells later in life after exposure to serial infections or injuries.
Concluding Remarks
It seems reasonable to assume that, taken in isolation, TRM cells function primarily in antimicrobial immunity and eschew the tissue-supportive roles of their innate and innate-like cousins. However, we favor the broader view of tissues as diverse “ecosystems” composed of multiple cell types, where TRM and other tissue-resident lymphocytes, in coordination with macrophages and additional cell types, provide a robust and multifaceted protection of tissue function and integrity upon diverse inflammatory insults and injuries. A previously proposed crosstalk between tissue-resident innate and adaptive lymphocytes can be viewed as a part of the still poorly understood web of communications between parenchymal, immune, and other accessory cells in an organ (Carnaud et al., 1999; Gasteiger and Rudensky, 2014; Okabe and Medzhitov, 2014). Beyond the common hallmarks shared by diverse classes of tissue-resident lymphocytes, specific tissues may imprint resident lymphocytes with unique features tailored to those tissues. This imprinting has been well appreciated for specialized embryonic-derived macrophages (e.g., microglia versus Langerhans cells), but only a few examples have been observed for resident lymphocytes (Amit et al., 2015; Okabe and Medzhitov, 2015; Perdiguero and Geissmann, 2015). As one example, Treg cells in visceral adipose tissue express the transcription factor PPAR-γ, which contributes to their distinct transcriptional profile and enables their accumulation in adipose tissue (Bapat et al., 2015; Cipolletta et al., 2012, 2015). However, the environmental factors that drive this imprinting in the adipose tissue remain largely unknown, although IL-33 and TCR signaling may help maintain some aspects of this transcriptional program (Kolodin et al., 2015; Vasanthakumar et al., 2015). The specific mediators that imprint resident cells have been best worked out for the intestine. In the intestine and related organs, retinoic acid from dietary sources has a major role in imprinting naive T cells, ILCs, IELs, peritoneal macrophages, and tolerogenic dendritic cells, the last of which additionally support extrathymic Treg cell differentiation, IgA class switching, and expression of gut-homing molecules (Coombes et al., 2007; Iwata et al., 2004; Mora et al., 2006; Mucida et al., 2009; Okabe and Medzhitov, 2014; Reis et al., 2014; Spencer et al., 2014; Sun et al., 2007; Suzuki et al., 2010; van de Pavert et al., 2014). However, despite these advances in our understanding of tissue-associated factors, a comprehensive framework for understanding the tissue microenvironments that shape identity of resident lymphocyte subsets is still lacking. The elucidation of the relationships between parenchymal cells, tissue-resident innate and adaptive lymphocytes, and other cells in this complex ecosystem, their hierarchy, and their means of communication would inform an integrated view of tissue function in health and disease and may offer novel means of therapeutic intervention in diverse inflammatory disorders and cancer.
Acknowledgments
We apologize to the many scientists whose work could not be cited in this Review due to space constraints. We thank Ruslan Medzhitov for inspiring ideas and Ashutosh Chaudhry for helpful discussions. This work was supported by the National Institutes of Health (R37 AI034206 to A.Y.R.; F30 AI122721 to X.F.; MSTP grant T32 GM07739 to the Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Program), the Ludwig Cancer Center at Memorial Sloan-Kettering Cancer Center (A.Y.R.), Hilton Foundation (A.Y.R.), and the Howard Hughes Medical Institute (AY.R.).
References
- Adachi S, Yoshida H, Kataoka H, Nishikawa S. Three distinctive steps in Peyer’s patch formation of murine embryo. Int Immunol. 1997;9:507–514. doi: 10.1093/intimm/9.4.507. [DOI] [PubMed] [Google Scholar]
- Allen JE, Sutherland TE. Host protective roles of type 2 immunity: parasite killing and tissue repair, flip sides of the same coin. Semin Immunol. 2014;26:329–340. doi: 10.1016/j.smim.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo ES, Gottschalk RA, Das J, Egawa T, Hobbs RM, Pandolfi PP, Pereira P, Nichols KE, Koretzky GA, Jordan MS, Sant’Angelo DB. Development of promyelocytic zinc finger and ThPOK-expressing innate gamma delta T cells is controlled by strength of TCR signaling and Id3. J Immunol. 2010;184:1268–1279. doi: 10.4049/jimmunol.0903218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol. 2015;17:18–25. doi: 10.1038/ni.3325. [DOI] [PubMed] [Google Scholar]
- Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, Jacobs H, Haanen JB, Schumacher TN. T cell memory. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–105. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- Asarnow DM, Kuziel WA, Bonyhadi M, Tigelaar RE, Tucker PW, Allison JP. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55:837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaña S, Roach K, Turner S, Paul J, Kovats S. IRF4 promotes cutaneous dendritic cell migration to lymph nodes during homeostasis and inflammation. J Immunol. 2012;189:3368–3377. doi: 10.4049/jimmunol.1102613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira A, Mota-Santos T, Itohara S, Degermann S, Heusser C, Tonegawa S, Coutinho A. Localization of γ/δ T cells to the intestinal epithelium is independent of normal microbial colonization. J Exp Med. 1990;172:239–244. doi: 10.1084/jem.172.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bando JK, Liang HE, Locksley RM. Identification and distribution of developing innate lymphoid cells in the fetal mouse intestine. Nat Immunol. 2015;16:153–160. doi: 10.1038/ni.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapat SP, Myoung Suh J, Fang S, Liu S, Zhang Y, Cheng A, Zhou C, Liang Y, LeBlanc M, Liddle C, et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature. 2015;528:137–141. doi: 10.1038/nature16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beagley KW, Fujihashi K, Lagoo AS, Lagoo-Deenadaylan S, Black CA, Murray AM, Sharmanov AT, Yamamoto M, McGhee JR, Elson CO, et al. Differences in intraepithelial lymphocyte T cell subsets isolated from murine small versus large intestine. J Immunol. 1995;154:5611–5619. [PubMed] [Google Scholar]
- Bendelac A, Killeen N, Littman DR, Schwartz RH. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263:1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Tatituri RVV, Brigl M, Kim EY, Tuli A, Sanderson JP, Gadola SD, Hsu FF, Besra GS, Brenner MB. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011;12:1202–1211. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Tatituri RVV, Heiss C, Watts GFM, Hsu FF, Veerapen N, Cox LR, Azadi P, Besra GS, Brenner MB. Activation of iNKT cells by a distinct constituent of the endogenous glucosylceramide fraction. Proc Natl Acad Sci USA. 2014;111:13433–13438. doi: 10.1073/pnas.1415357111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, Artis D. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- Brigl M, Tatituri RVV, Watts GFM, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, Brenner MB. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011;208:1163–1177. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, Mathis D. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JKM, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci USA. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba A, Dascher CC, Besra GS, Brenner MB. Rapid NKT cell responses are self-terminating during the course of microbial infection. J Immunol. 2008;181:2292–2302. doi: 10.4049/jimmunol.181.4.2292. [DOI] [PubMed] [Google Scholar]
- Chien YH, Iwashima M, Wettstein DA, Kaplan KB, Elliott JF, Born W, Davis MM. T-cell receptor delta gene rearrangements in early thymocytes. Nature. 1987;330:722–727. doi: 10.1038/330722a0. [DOI] [PubMed] [Google Scholar]
- Chien YH, Meyer C, Bonneville M. γδ T cells: first line of defense and beyond. Annu Rev Immunol. 2014;32:121–155. doi: 10.1146/annurev-immunol-032713-120216. [DOI] [PubMed] [Google Scholar]
- Chua WJ, Truscott SM, Eickhoff CS, Blazevic A, Hoft DF, Hansen TH. Polyclonal mucosa-associated invariant T cells have unique innate functions in bacterial infection. Infect Immun. 2012;80:3256–3267. doi: 10.1128/IAI.00279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolletta D, Cohen P, Spiegelman BM, Benoist C, Mathis D. Appearance and disappearance of the mRNA signature characteristic of Treg cells in visceral adipose tissue: age, diet, and PPARγ effects. Proc Natl Acad Sci USA. 2015;112:482–487. doi: 10.1073/pnas.1423486112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA. Resident memory T cells in human health and disease. Sci Transl Med. 2015;7:269rv1. doi: 10.1126/scitranslmed.3010641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- Cohen NR, Tatituri RVV, Rivera A, Watts GFM, Kim EY, Chiba A, Fuchs BB, Mylonakis E, Besra GS, Levitz SM, et al. Innate recognition of cell wall β-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe. 2011;10:437–450. doi: 10.1016/j.chom.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides MG, Bendelac A. Transcriptional regulation of the NKT cell lineage. Curr Opin Immunol. 2013;25:161–167. doi: 10.1016/j.coi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides MG, Gudjonson H, McDonald BD, Ishizuka IE, Verhoef PA, Dinner AR, Bendelac A. PLZF expression maps the early stages of ILC1 lineage development. Proc Natl Acad Sci USA. 2015;112:5123–5128. doi: 10.1073/pnas.1423244112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1-NKT cell population. Proc Natl Acad Sci USA. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett AJ, Eckle SBG, Birkinshaw RW, Liu L, Patel O, Mahony J, Chen Z, Reantragoon R, Meehan B, Cao H, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509:361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- Cortez VS, Fuchs A, Cella M, Gilfillan S, Colonna M. Cutting edge: Salivary gland NK cells develop independently of Nfil3 in steady-state. J Immunol. 2014;192:4487–4491. doi: 10.4049/jimmunol.1303469. [DOI] [PubMed] [Google Scholar]
- Cui Y, Franciszkiewicz K, Mburu YK, Mondot S, Le Bourhis L, Premel V, Martin E, Kachaner A, Duban L, Ingersoll MA, et al. Mucosal-associated invariant T cell-rich congenic mouse strain allows functional evaluation. J Clin Invest. 2015;125:4171–4185. doi: 10.1172/JCI82424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Granger SW, Mucida D, Graddy R, Leclercq G, Zhang W, Honey K, Rasmussen JP, Cheroutre H, Rudensky AY, Kronenberg M. Mouse TCRalphabeta+CD8alphaalpha intraepithelial lymphocytes express genes that down-regulate their antigen reactivity and suppress immune responses. J Immunol. 2007;178:4230–4239. doi: 10.4049/jimmunol.178.7.4230. [DOI] [PubMed] [Google Scholar]
- Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, West ML, Smith OM, Holland AM, Tsai JJ, et al. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 2012;336:91–95. doi: 10.1126/science.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, Lantz O. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- Eberl G, Colonna M, Di Santo JP, McKenzie ANJ. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348:aaa6566. doi: 10.1126/science.aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciotti F, Ramanjaneyulu GS, Lepore M, Sansano S, Cavallari M, Kistowska M, Forss-Petter S, Ni G, Colone A, Singhal A, et al. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat Immunol. 2012;13:474–480. doi: 10.1038/ni.2245. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie ANJ. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- Gangadharan D, Lambolez F, Attinger A, Wang-Zhu Y, Sullivan BA, Cheroutre H. Identification of pre- and postselection TCRalphabeta+ intraepithelial lymphocyte precursors in the thymus. Immunity. 2006;25:631–641. doi: 10.1016/j.immuni.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Gasteiger G, Rudensky AY. Interactions between innate and adaptive lymphocytes. Nat Rev Immunol. 2014;14:631–639. doi: 10.1038/nri3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science. 2015;350:981–985. doi: 10.1126/science.aac9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013;13:607–614. doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgel P, Radosavljevic M, Macquin C, Bahram S. The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol Immunol. 2011;48:769–775. doi: 10.1016/j.molimm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gober HJ, Kistowska M, Angman L, Jenö P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16:1114–1123. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186:6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanash AM, Dudakov JA, Hua G, O’Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339–350. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- Havran WL, Allison JP. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature. 1990;344:68–70. doi: 10.1038/344068a0. [DOI] [PubMed] [Google Scholar]
- Havran WL, Grell S, Duwe G, Kimura J, Wilson A, Kruisbeek AM, O’Brien RL, Born W, Tigelaar RE, Allison JP. Limited diversity of T-cell receptor gamma-chain expression of murine Thy-1+ dendritic epidermal cells revealed by V gamma 3-specific monoclonal antibody. Proc Natl Acad Sci USA. 1989;86:4185–4189. doi: 10.1073/pnas.86.11.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran WL, Chien YH, Allison JP. Recognition of self antigens by skin-derived T cells with invariant gamma delta antigen receptors. Science. 1991;252:1430–1432. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- Hein WR, Dudler L. TCR gamma delta+ cells are prominent in normal bovine skin and express a diverse repertoire of antigen receptors. Immunology. 1997;91:58–64. doi: 10.1046/j.1365-2567.1997.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein WR, Mackay CR. Prominence of gamma delta T cells in the ruminant immune system. Immunol Today. 1991;12:30–34. doi: 10.1016/0167-5699(91)90109-7. [DOI] [PubMed] [Google Scholar]
- Helgeland L, Vaage JT, Rolstad B, Midtvedt T, Brandtzaeg P. Microbial colonization influences composition and T-cell receptor V beta repertoire of intraepithelial lymphocytes in rat intestine. Immunology. 1996;89:494–501. doi: 10.1046/j.1365-2567.1996.d01-783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, Beck E, Wiesner J, Eberl M, Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett. 2001;509:317–322. doi: 10.1016/s0014-5793(01)03191-x. [DOI] [PubMed] [Google Scholar]
- Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016 doi: 10.1126/science.aaf1648. Published online February 4, 2016. http://dx.doi.org/10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed]
- Hung LY, Lewkowich IP, Dawson LA, Downey J, Yang Y, Smith DE, Herbert DR. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc Natl Acad Sci USA. 2013;110:282–287. doi: 10.1073/pnas.1206587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AS, Severson KM, Vaishnava S, Behrendt CL, Yu X, Benjamin JL, Ruhn KA, Hou B, DeFranco AL, Yarovinsky F, Hooper LV. Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci USA. 2011;108:8743–8748. doi: 10.1073/pnas.1019574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Bonneville M, Takagaki Y, Nakanishi N, Kanagawa O, Krecko EG, Tonegawa S. Different gamma delta T-cell receptors are expressed on thymocytes at different stages of development. Proc Natl Acad Sci USA. 1989;86:631–635. doi: 10.1073/pnas.86.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara S, Nakanishi N, Kanagawa O, Kubo R, Tonegawa S. Monoclonal antibodies specific to native murine T-cell receptor gamma delta: analysis of gamma delta T cells during thymic ontogeny and in peripheral lymphoid organs. Proc Natl Acad Sci USA. 1989;86:5094–5098. doi: 10.1073/pnas.86.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara S, Farr AG, Lafaille JJ, Bonneville M, Takagaki Y, Haas W, Tonegawa S. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990;343:754–757. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- Jenkins MK, Chu HH, McLachlan JB, Moon JJ. On the composition of the preimmune repertoire of T cells specific for Peptide-major histocompatibility complex ligands. Annu Rev Immunol. 2010;28:275–294. doi: 10.1146/annurev-immunol-030409-101253. [DOI] [PubMed] [Google Scholar]
- Jensen KDC, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon γ. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Sun S, Xu A, Bhargava P, Yang L, Lam KSL, Gao B, Lee CH, Kersten S, Qi L. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J Biol Chem. 2012;287:13561–13571. doi: 10.1074/jbc.M112.350066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain L, Costanzo A, Webb B, Holt M, Bendelac A, Savage PB, Teyton L. Endogenous ligands of natural killer T cells are alpha-linked glycosylceramides. Mol Immunol. 2015;68(2 Pt A):94–97. doi: 10.1016/j.molimm.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KA, Scollay R. Seeding of neonatal lymph nodes by T cells and identification of a novel population of CD3-CD4+ cells. Eur J Immunol. 1992;22:329–334. doi: 10.1002/eji.1830220207. [DOI] [PubMed] [Google Scholar]
- Kenna T, Golden-Mason L, Porcelli SA, Koezuka Y, Hegarty JE, O’Farrelly C, Doherty DG. NKT cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT cells. J Immunol. 2003;171:1775–1779. doi: 10.4049/jimmunol.171.4.1775. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MREI, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Illarionov P, Vela JL, Pei B, Girardi E, Li X, Li Y, Imamura M, Kaneko Y, Okawara A, et al. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol. 2011;12:966–974. doi: 10.1038/ni.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- Klose CSN, Blatz K, d’Hargues Y, Hernandez PP, Kofoed-Nielsen M, Ripka JF, Ebert K, Arnold SJ, Diefenbach A, Palmer E, Tanriver Y. The transcription factor T-bet is induced by IL-15 and thymic agonist selection and controls CD8αα(+) intraepithelial lymphocyte development. Immunity. 2014a;41:230–243. doi: 10.1016/j.immuni.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Klose CSN, Flach M, Möhle L, Rogell L, Hoyler T, Ebert K, Fabiunke C, Pfeifer D, Sexl V, Fonseca-Pereira D, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014b;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, Germain RN, Benoist C, Mathis D. Antigen-and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015;21:543–557. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori HK, Witherden DA, Kelly R, Sendaydiego K, Jameson JM, Teyton L, Havran WL. Cutting edge: dendritic epidermal γδ T cell ligands are rapidly and locally expressed by keratinocytes following cutaneous wounding. J Immunol. 2012;188:2972–2976. doi: 10.4049/jimmunol.1100887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y, Cao W, Xi X, Ma C, Cui L, He W. The NKG2D ligand ULBP4 binds to TCRgamma9/delta2 and induces cytotoxicity to tumor cells through both TCRgammadelta and NKG2D. Blood. 2009;114:310–317. doi: 10.1182/blood-2008-12-196287. [DOI] [PubMed] [Google Scholar]
- Kotas ME, Lee HY, Gillum MP, Annicelli C, Guigni BA, Shulman GI, Medzhitov R. Impact of CD1d deficiency on metabolism. PLoS ONE. 2011;6:e25478. doi: 10.1371/journal.pone.0025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalovsky D, Alonzo ES, Uche OU, Eidson M, Nichols KE, Sant’Angelo DB. PLZF induces the spontaneous acquisition of memory/effector functions in T cells independently of NKT cell-related signals. J Immunol. 2010;184:6746–6755. doi: 10.4049/jimmunol.1000776. [DOI] [PubMed] [Google Scholar]
- Kumar H, Belperron A, Barthold SW, Bockenstedt LK. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J Immunol. 2000;165:4797–4801. doi: 10.4049/jimmunol.165.9.4797. [DOI] [PubMed] [Google Scholar]
- Le Bourhis L, Martin E, Péguillet I, Guihot A, Froux N, Coré M, Lévy E, Dusseaux M, Meyssonnier V, Premel V, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol. 2011;32:212–218. doi: 10.1016/j.it.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Lee WY, Moriarty TJ, Wong CHY, Zhou H, Strieter RM, van Rooijen N, Chaconas G, Kubes P. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol. 2010;11:295–302. doi: 10.1038/ni.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015a;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Wang H, Starrett GJ, Phuong V, Jameson SC, Hogquist KA, et al. Tissue-Specific Distribution of iNKT Cells Impacts Their Cytokine Response. Immunity. 2015b;43:566–578. doi: 10.1016/j.immuni.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leishman AJ, Naidenko OV, Attinger A, Koning F, Lena CJ, Xiong Y, Chang HC, Reinherz E, Kronenberg M, Cheroutre H. T cell responses modulated through interaction between CD8alphaalpha and the nonclassical MHC class I molecule, TL. Science. 2001;294:1936–1939. doi: 10.1126/science.1063564. [DOI] [PubMed] [Google Scholar]
- Leishman AJ, Gapin L, Capone M, Palmer E, MacDonald HR, Kronenberg M, Cheroutre H. Precursors of functional MHC class I- or class II-restricted CD8alphaalpha(+) T cells are positively selected in the thymus by agonist self-peptides. Immunity. 2002;16:355–364. doi: 10.1016/s1074-7613(02)00284-4. [DOI] [PubMed] [Google Scholar]
- Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Cao X, Zhang X, Kovalovsky D. PLZF Controls the Development of Fetal-Derived IL-17+Vγ6+ γδ T Cells. J Immunol. 2015;195:4273–4281. doi: 10.4049/jimmunol.1500939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch L, O’Shea D, Winter DC, Geoghegan J, Doherty DG, O’Farrelly C. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol. 2009;39:1893–1901. doi: 10.1002/eji.200939349. [DOI] [PubMed] [Google Scholar]
- Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O’Shea D, O’Farrelly C, Exley MA. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–587. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay CR, Hein WR. A large proportion of bovine T cells express the gamma delta T cell receptor and show a distinct tissue distribution and surface phenotype. Int Immunol. 1989;1:540–545. doi: 10.1093/intimm/1.5.540. [DOI] [PubMed] [Google Scholar]
- Mackley EC, Houston S, Marriott CL, Halford EE, Lucas B, Cerovic V, Filbey KJ, Maizels RM, Hepworth MR, Sonnenberg GF, et al. CCR7-dependent trafficking of RORγ+ ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat Commun. 2015;6:5862. doi: 10.1038/ncomms6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanski JL, Jongeneel CV, Bucher P, Casanova JL, Walker PR. Single-cell PCR analysis of TCR repertoires selected by antigen in vivo: a high magnitude CD8 response is comprised of very few clones. Immunity. 1996;4:47–55. doi: 10.1016/s1074-7613(00)80297-6. [DOI] [PubMed] [Google Scholar]
- Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- Mayans S, Stepniak D, Palida SF, Larange A, Dreux J, Arlian BM, Shinnakasu R, Kronenberg M, Cheroutre H, Lambolez F. αβT cell receptors expressed by CD4(−)CD8αβ(−) intraepithelial T cells drive their fate into a unique lineage with unusual MHC reactivities. Immunity. 2014;41:207–218. doi: 10.1016/j.immuni.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BD, Bunker JJ, Ishizuka IE, Jabri B, Bendelac A. Elevated T cell receptor signaling identifies a thymic precursor to the TCRαβ(+) CD4(−)CD8β(−) intraepithelial lymphocyte lineage. Immunity. 2014;41:219–229. doi: 10.1016/j.immuni.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BD, Bunker JJ, Erickson SA, Oh-Hora M, Bendelac A. Crossreactive αβ T Cell Receptors Are the Predominant Targets of Thymocyte Negative Selection. Immunity. 2015;43:859–869. doi: 10.1016/j.immuni.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3− LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- Meierovics A, Yankelevich WJC, Cowley SC. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc Natl Acad Sci USA. 2013;110:E3119–E3128. doi: 10.1073/pnas.1302799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes JS, Mucida DS, Cara DC, Alvarez-Leite JI, Russo M, Vaz NM, de Faria AM. Stimulation by food proteins plays a critical role in the maturation of the immune system. Int Immunol. 2003;15:447–455. doi: 10.1093/intimm/dxg043. [DOI] [PubMed] [Google Scholar]
- Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DMW, Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci USA. 2015;112:10762–10767. doi: 10.1073/pnas.1509070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Mucida D, Pino-Lagos K, Kim G, Nowak E, Benson MJ, Kronenberg M, Noelle RJ, Cheroutre H. Retinoic acid can directly promote TGF-β-mediated Foxp3(+) Treg cell conversion of naive T cells. Immunity. 2009;30:471–472. doi: 10.1016/j.immuni.2009.03.008. author reply 472–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Husain MM, Muroi S, van Wijk F, Shinnakasu R, Naoe Y, Reis BS, Huang Y, Lambolez F, Docherty M, et al. Transcriptional reprogramming of mature CD4+ helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat Immunol. 2013;14:281–289. doi: 10.1038/ni.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Förster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol. 2015;17:9–17. doi: 10.1038/ni.3320. [DOI] [PubMed] [Google Scholar]
- Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, Englezakis A, Barlow JL, Hams E, Scanlon ST, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson C, Thornton EE, McKenzie B, Schaupp AL, Huskens N, Griseri T, West N, Tung S, Seddon BP, Uhlig HH, Powrie F. ILC3 GM-CSF production and mobilisation orchestrate acute intestinal inflammation. eLife. 2016;5:1–21. doi: 10.7554/eLife.10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier D, Hafler DA. Fingolimod for multiple sclerosis. N Engl J Med. 2012;366:339–347. doi: 10.1056/NEJMct1101691. [DOI] [PubMed] [Google Scholar]
- Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X, Sun R, Yokoyama WM, Tian Z. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol. 2015;17:2–8. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobezinsky LA, Angelov GS, Tai X, Jeurling S, Van Laethem F, Feigenbaum L, Park JH, Singer A. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nat Immunol. 2012;13:569–578. doi: 10.1038/ni.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poussier P, Edouard P, Lee C, Binnie M, Julius M. Thymus-independent development and negative selection of T cells expressing T cell receptor alpha/beta in the intestinal epithelium: evidence for distinct circulation patterns of gut- and thymus-derived T lymphocytes. J Exp Med. 1992;176:187–199. doi: 10.1084/jem.176.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raberger J, Schebesta A, Sakaguchi S, Boucheron N, Blomberg KEM, Berglöf A, Kolbe T, Smith CIE, Rülicke T, Ellmeier W. The transcriptional regulator PLZF induces the development of CD44 high memory phenotype T cells. Proc Natl Acad Sci USA. 2008;105:17919–17924. doi: 10.1073/pnas.0805733105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimpour A, Koay HF, Enders A, Clanchy R, Eckle SBG, Meehan B, Chen Z, Whittle B, Liu L, Fairlie DP, et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J Exp Med. 2015;212:1095–1108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin LC, Girard-Madoux MJH, Seillet C, Mielke LA, Kerdiles Y, Fenis A, Wieduwild E, Putoczki T, Mondot S, Lantz O, et al. Complementarity and redundancy of IL-22-producing innate lymphoid cells. Nat Immunol. 2016;17:179–186. doi: 10.1038/ni.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. Natalizumab for multiple sclerosis. N Engl J Med. 2007;356:2622–2629. doi: 10.1056/NEJMct071462. [DOI] [PubMed] [Google Scholar]
- Reis BS, Hoytema van Konijnenburg DP, Grivennikov SI, Mucida D. Transcription factor T-bet regulates intraepithelial lymphocyte functional maturation. Immunity. 2014;41:244–256. doi: 10.1016/j.immuni.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B, von Boehmer H, Guy-Grand D. Selection of intraepithelial lymphocytes with CD8 alpha/alpha co-receptors by self-antigen in the murine gut. Proc Natl Acad Sci USA. 1992;89:5336–5340. doi: 10.1073/pnas.89.12.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio M, Silk JD, Jones EY, Cerundolo V. Biology of CD1- and MR1-restricted T cells. Annu Rev Immunol. 2014;32:323–366. doi: 10.1146/annurev-immunol-032713-120243. [DOI] [PubMed] [Google Scholar]
- Sandstrom A, Peigné CM, Léger A, Crooks JE, Konczak F, Gesnel MC, Breathnach R, Bonneville M, Scotet E, Adams EJ. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity. 2014;40:490–500. doi: 10.1016/j.immuni.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage AK, Constantinides MG, Bendelac A. Promyelocytic leukemia zinc finger turns on the effector T cell program without requirement for agonist TCR signaling. J Immunol. 2011;186:5801–5806. doi: 10.4049/jimmunol.1100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8+ T cells. Nat Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper HS, Rakhshandehroo M, van de Graaf SFJ, Venken K, Koppen A, Stienstra R, Prop S, Meerding J, Hamers N, Besra G, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122:3343–3354. doi: 10.1172/JCI62739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selin LK, Santolucito PA, Pinto AK, Szomolanyi-Tsuda E, Welsh RM. Innate immunity to viruses: control of vaccinia virus infection by gamma delta T cells. J Immunol. 2001;166:6784–6794. doi: 10.4049/jimmunol.166.11.6784. [DOI] [PubMed] [Google Scholar]
- Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6:73–79. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- Shires J, Theodoridis E, Hayday AC. Biological insights into TCRgammadelta+ and TCRalphabeta+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE) Immunity. 2001;15:419–434. doi: 10.1016/s1074-7613(01)00192-3. [DOI] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, Zhong C, Chase JM, Rothman PB, Yu J, et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife. 2014;3:e01659. doi: 10.7554/eLife.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Lee JS, Gilfillan S, Robinette ML, Newberry RD, Stappenbeck TS, Mack M, Cella M, Colonna M. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J Exp Med. 2015;212:1869–1882. doi: 10.1084/jem.20151403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF, Jr, Wang J, Ramalingam TR, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, Perro M, Vrbanac VD, Tager AM, Shi J, et al. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 2015;348:aaa8205. doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyártó BZ, Southern PJ, Masopust D. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell. 2015;161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara S, Shimizu T, Yoshida Y, Aiba T, Yamagiwa S, Asakura H, Abo T. Extrathymic derivation of gut lymphocytes in parabiotic mice. Immunology. 1999;96:57–65. doi: 10.1046/j.1365-2567.1999.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, Shklovskaya E, Fazekas de St Groth B, Triccas JA, Weninger W. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med. 2011;208:505–518. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Sugahara S, Shimizu T, Tada T, Minagawa M, Maruyama S, Watanabe H, Saito H, Ishikawa H, Hatakeyama K, Abo T. Low level of mixing of partner cells seen in extrathymic T cells in the liver and intestine of parabiotic mice: its biological implication. Eur J Immunol. 1998;28:3719–3729. doi: 10.1002/(SICI)1521-4141(199811)28:11<3719::AID-IMMU3719>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, Agace WW, Fagarasan S. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 2010;33:71–83. doi: 10.1016/j.immuni.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA, Meng F, Luster AD, Bendelac A. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J Exp Med. 2011;208:1179–1188. doi: 10.1084/jem.20102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura M, Hata A, Matsuoka S, Shand FHW, Nakanishi Y, Ikebuchi R, Ueha S, Tsutsui H, Inaba K, Matsushima K, et al. Tracking and quantification of dendritic cell migration and antigen trafficking between the skin and lymph nodes. Sci Rep. 2014;4:6030. doi: 10.1038/srep06030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- Tupin E, Benhnia MREI, Kinjo Y, Patsey R, Lena CJ, Haller MC, Caimano MJ, Imamura M, Wong CH, Crotty S, et al. NKT cells prevent chronic joint inflammation after infection with Borrelia burgdorferi. Proc Natl Acad Sci USA. 2008;105:19863–19868. doi: 10.1073/pnas.0810519105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J Immunol. 2008;181:4452–4456. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesaki Y, Setoyama H, Matsumoto S, Okada Y. Expansion of alpha beta T-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ-free mice and its independence from thymus. Immunology. 1993;79:32–37. [PMC free article] [PubMed] [Google Scholar]
- van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rhijn I, Spiering R, Smits M, van Blokland MTM, de Weger R, van Eden W, Rutten VPMG, Koets AP. Highly diverse TCR δ chain repertoire in bovine tissues due to the use of up to four D segments per δ chain. Mol Immunol. 2007;44:3155–3161. doi: 10.1016/j.molimm.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, Mielke LA, Afshar-sterle S, Masters SL, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 2015;16:276–285. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian UH, Engelhardt B. Alpha4 integrins as therapeutic targets in autoimmune disease. N Engl J Med. 2003;348:68–72. doi: 10.1056/NEJMe020157. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, Yoshida H, Kubo M, Kawamoto H, Koseki H, Taniguchi M. Development and function of invariant natural killer T cells producing T(h)2-and T(h)17-cytokines. PLoS Biol. 2012;10:e1001255. doi: 10.1371/journal.pbio.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, et al. Transcription factor RORα is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- Wu J, Groh V, Spies T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J Immunol. 2002;169:1236–1240. doi: 10.4049/jimmunol.169.3.1236. [DOI] [PubMed] [Google Scholar]
- Yamagata T, Mathis D, Benoist C. Self-reactivity in thymic double-positive cells commits cells to a CD8 alpha alpha lineage with characteristics of innate immune cells. Nat Immunol. 2004;5:597–605. doi: 10.1038/ni1070. [DOI] [PubMed] [Google Scholar]
- Zaid A, Mackay LK, Rahimpour A, Braun A, Veldhoen M, Carbone FR, Manton JH, Heath WR, Mueller SN. Persistence of skin-resident memory T cells within an epidermal niche. Proc Natl Acad Sci USA. 2014;111:5307–5312. doi: 10.1073/pnas.1322292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]


