Abstract
Background
Glucocorticoid therapy is used widely in patients with rheumatoid arthritis (RA) with good efficacy but concerns about safety including fractures. Estimates of fracture risk for any given patient are complicated by the dynamic pattern of glucocorticoid use, where patients vary in their dose, duration and timing of glucocorticoid use.
Objective
To investigate which methods are currently used to attribute fractures to glucocorticoid exposure and investigate whether such methods can consider individual treatment patterns.
Results
Thirty-eight studies used five common definitions of risk attribution to glucocorticoid exposure: “current use”, “ever use”, “daily dose”, “cumulative dose” and “time variant”. One study attempted to combine multiple definitions where “cumulative dose” was nested within “daily dose”, covering the effects of dose and duration but not timing. The majority of results demonstrated an equivocal or increased risk of fracture with increased exposure, although there was wide variation, with odds ratios, hazard ratios and relative risks ranging from 0.16 to 8.16. Within definitions there was also variability in the results with the smallest range for “time variant”, 1.07 to 2.8, and the largest for “cumulative dose”, ranging from risk estimates of 0.88 to 8.12.
Conclusion
Many studies have looked into the effect of glucocorticoids on fracture risk in patients with RA. Despite this, there is no clear consensus about the magnitude of risk. This is a consequence of the varied analysis models and their different assumptions. Moreover, no current analysis method allows consideration of dose, duration and timing of glucocorticoid therapy, preventing a clear understanding of fracture risk for patients and their individual treatment patterns.
Keywords: Glucocorticoids, Rheumatoid arthritis, Fracture, Rheumatology, Epidemiology
Highlights
-
•
A literature review on which methods are used to define risk attribution of glucocorticoid use to fractures was undertaken.
-
•
Five common methods of defining risk attribution of glucocorticoids were found.
-
•
No currently used method considers dose, duration and timing.
-
•
Dose, duration and timing are thought to affect the risk of fracture due to the dynamic patterns of glucocorticoid therapy.
1. Introduction
Rheumatoid arthritis (RA) is an autoimmune disease which affects between 0.5 and 1% of the population [1]. Glucocorticoids were identified as a treatment for RA over 60 years ago [2] and approximately 2/3 of patients have ever used glucocorticoids [3]. They have been found to reduce joint tenderness and pain [4], and to reduce the rate of disease progression when used in addition to standard therapies [5]. Whilst reducing disease progression, there are adverse effects associated with the use of glucocorticoids, including bone fracture, infection, cataracts, and diabetes [6]. Glucocorticoid use tends to be dynamic, with patients switching between periods of use and non-use, and with varying doses through time in response to their disease severity. Thus most patients have a personalised treatment plan.
Glucocorticoids primarily affect bone health and fracture risk by acting on functions critical in the regeneration and healing cycles [7]. Glucocorticoids have been shown to affect the function of both osteoclasts and osteoblasts, resulting in disruption to bone repair. This impact on bone remodelling weakens the bone making it more brittle and at higher risk of fracture [8]. The brittleness of the bones also causes a reduction in bone mineral density (BMD) for those on glucocorticoids and hence an increase in the risk of fracture has been found at levels of BMD where the patient does not have osteoporosis [9] suggesting they affect fracture risk above and beyond the usual effect of decreasing BMD. Despite the acceptance that glucocorticoid therapy increases the risk of fracture, estimates about the size of the effect vary widely. This may be because a wide range of definitions has been used to attribute fractures to glucocorticoid exposure.
It is likely that the impact of glucocorticoids on fracture risk relates to the dose administered, the duration of exposure, the latency between administration and effect on bone regeneration, and post-exposure recovery [10]. This review will therefore investigate the range of different definitions used to attribute fractures to glucocorticoid exposure in patients with RA, and investigate the impact of these different definitions on the results. The assumptions of each definition of glucocorticoid exposure will be assessed for suitability with regards to the dynamic patterns of glucocorticoid exposure experience by patients with RA by reviewing their consideration of dose, duration and timing.
2. Methods
A literature search was carried out in Ovid using the databases Medline and Embase. For both databases the years covered by the search were from the conception of the database until the end of October 2014.
The search criteria used for inclusion of papers included terms for glucocorticoids, fractures, and RA, (see Appendix) and were the same for both databases. Searches were initially limited to English language, humans, adults with further limitations made to the publication type. In Medline, publication types removed included: case reports, Phase I or II clinical trials, reviews, meta analyses, duplicate publications, retracted publications and any other non-research article publications. In Embase, publication types removed included books, book series, conference papers, editorials, notes, reviews and short surveys. Following exclusions, abstracts were screened to ensure the topic of the paper was suitable and if no abstract was available within Ovid, attempts were made to find the paper online.
3. Results
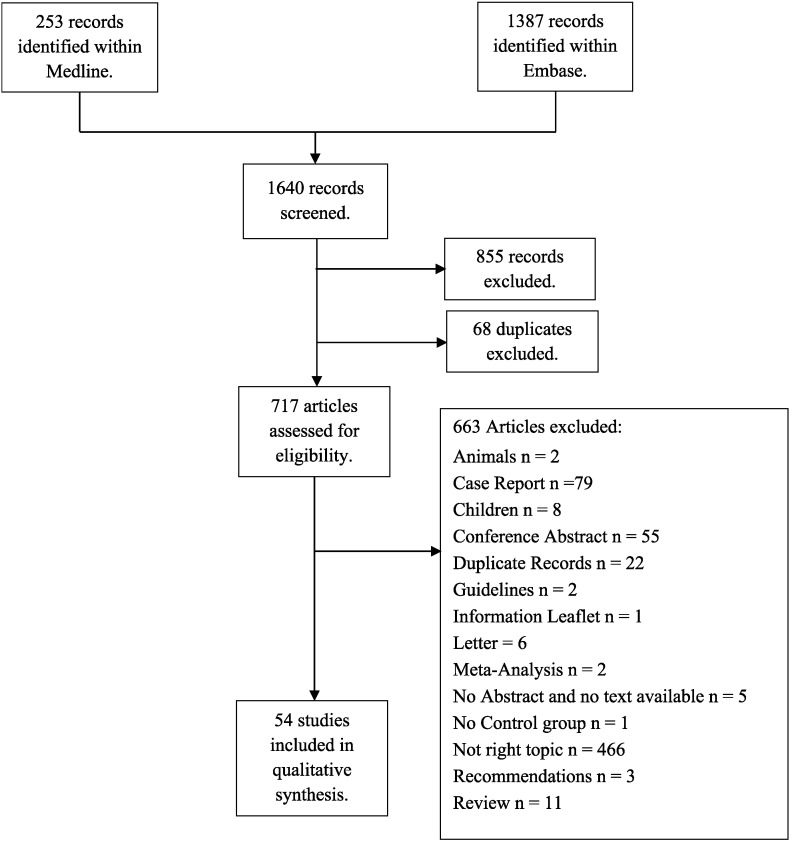
Fig. 1 describes the number of papers found at each stage of the literature search with the reasons papers were discarded.
Fig. 1.
Flow diagram.
During a qualitative synthesis stage, a further 16 papers were excluded either because there was no analysis of effect of glucocorticoids on fracture reported (5 papers), only 1 fracture occurring in either the glucocorticoid exposed or unexposed cohorts (2 papers), no association between glucocorticoids and fracture was reported (5 papers), RA was not considered in the paper (2 papers) or if the English full text version was not available (2 papers).
Thirty eight papers were selected for review (Table 1). The definitions for attributing fractures to glucocorticoid exposure were as follows: “current use” (n = 19), “ever use” (n = 15), “daily dose” (n = 13), “cumulative dose” (n = 8), “multi-variable” (n = 2), “time variant” (n = 8) and other definitions of glucocorticoid exposure (n = 3). Multiple definitions were reported in 17 papers. These models are described further in Fig. 2 using a hypothetical dynamic exposure pattern for an individual patient.
Table 1.
Results of literature search.
| Ref. | Design | Population type | Comparator population | Study methodology |
n | Fracture type | OR/RR/HR/P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CU | EU | DD | CD | ML | TV | OT | |||||||
| Van Everdingen [11] | Double blinded randomised control trial | RA patients given prednisone | RA patients given placebo | ✓ | 40 + 41 | Vertebral fracture | Not signif, OR not given | ||||||
| Furuya [12] | Prospective cohort | RA patients using GC | RA patients not using GC | ✓ | 1733 | Vertebral, main non vertebral and any non-vertebral | RR: Vt 1.90 (0.61, 5.94), any Non-Vt 1.69 (1.01,2.83) |
||||||
| Coulson [13] | Retrospective cohort | RA patients, GC use | RA patients, non GC use | ✓ | 8419 | Any, vertebral, hip and non-vertebral, non-hip fracture | RR: any fracture 1.325 signif, Vt 1.211 not signif no CI given | ||||||
| Hooyman [14] | Retrospective cohort | Females with RA with fracture of interest | Female with RA without fracture of interest | ✓ | 388 | Femur, humerus, pelvis, forearm, vertebral | RR: femur fracture 2.15 (0.79, 4.67) | ||||||
| Orstavik [15] | Retrospective cohort | Early RA patients long term GC use | Early RA patients none or short term GC use | ✓ | 249 | Vertebral fracture | OR: 4.15 (1.70,10.07) | ||||||
| Verstraeten [16] | Retrospective cohort | Postmenopausal women with RA | Postmenopausal controls | ✓ | 147 | Vertebral fracture | P value < 0.01 | ||||||
| Cooper [17] | Case control | RA patients admitted to orthopaedic unit | Community controls 1:2 matched | ✓ | 300 + 600 | Hip fracture | OR: 2.5 (1.1, 5.5) | ||||||
| Butler [18] | Case control from cohort | Rheumatology patients taking low dose GC therapy | Rheumatology patients not taking GC therapy | ✓ | 142 | Any fracture | P-value < 0.05 | ||||||
| Araia[19] | Cross sectional | RA patients | Healthy patients admitted for osteoporosis evaluation | ✓ | 117 | Vertebral fracture | OR: 3.82 (3.01, 4.85) | ||||||
| Orstavik [20] | Retrospective cohort | Established RA patients | General population | ✓ | ✓ | ✓ | 528 | Vertebral fracture | P-values CU & EU < 0.001 RR: TV 1.55 (1.03, 2.33) |
||||
| Kay [21] | Case control | RA patients with fracture | RA patients no fracture | ✓ | ✓ | ✓ | ✓ | 18 + 18 | Stress fracture | P-values: CU 0.32, EU 0.02, DD 0.0003, TV 0.06 | |||
| Furuya [22] | Prospective cohort | RA patients using GC | RA patients not using GC | ✓ | ✓ | 9720 | Hip fracture | HR: CU not signif. DD: men no change, women 1.03 (1.00, 1.06) |
|||||
| Furuya [23] | Prospective cohort | RA patients using GC | RA patients not using GC | ✓ | ✓ | 1020 | Non-vertebral and vertebral fracture | HR: CU not signif. DD: Vt 1.28 (1.14, 1.45) Non-Vt 1.01 (0.86, 1.18) |
|||||
| Ghazi [24] | Case control | RA patients | General population | ✓ | ✓ | 101 + 303 | Vertebral fracture | OR: CU 0.17 (0.04, 0.66) DD not reported |
|||||
| de Nijs [25] | Cross sectional | RA patients using oral GC on daily basis ≥ 1 month | RA patients not using GC matched 1:1 | ✓ | ✓ | ✓ | 410 | Vertebral fracture | OR: CD 1.00 no CI CU 4.31 (1.13, 16.47)⁎ DD: 1.16 (1.05, 1.28)⁎ |
||||
| De Vries [26] | Retrospective cohort | RA patients with current GC use | RA patients with past GC use | ✓ | ✓ | ✓ | ✓ | 191,752 | Osteoporotic, hip and vertebral fracture | RR: Osteo CU 1.68 (1.6, 1.76) DD < 7.5 mg 1.60 (1.50, 1.71) 7.5–15 mg 2.15 (1.97, 2.34) CD < 1gb 1.38 (0.59, 3.22) 1–5gb 8.12 (5.19, 12.74) |
|||
| Nampei [27] | Prospective cohort | RA patients taking GC | Different strength or duration of GC use | ✓ | ✓ | ✓ | 209 | Any, vertebral and “lower leg and pelvis” fracture | OR: all DD 1.174 (1.054, 1.306) TV 1.095 (1.015, 1.181) pelvis DD 1.195 (1.043, 1.370) TV 1.131 (1.033, 1.239) |
||||
| Maghraoui [28] | Cross sectional | RA patients using GC | RA patients, non-current use and different category of cumulative use | ✓ | ✓ | 172 | Vertebral fracture | P-value CD < 0.0001 | |||||
| Mazzantini [29] | Retrospective cohort | GC users ≥ 6 months | Never use of GC | ✓ | ✓ | 2359 | Osteoporotic fracture | P-value CU < 0.02 TV > 5 years < 0.001 < 2 and 2–5 years > 0.05 |
|||||
| Michel [30] | Retrospective cohort | RA patients with GC use | RA patients not using GC | ✓ | 395 | Any fracture | RR: 1.9, P-value 0.026 | ||||||
| Wright [31] | Prospective cohort | Arthritis patients using GC | Patients not using GC | ✓ | 147,657 | Any, hip and clinical spine fracture | P-value > 0.05 | ||||||
| Araia[19] | Prospective cohort | RA patients using GC | RA patients not using GC | ✓ | 112 | Vertebral fracture | P-value < 0.05 for age groups 50–54 and 60–64 | ||||||
| Saville [32] | Cross sectional | RA patients taking GC | RA patients not taking GC | ✓ | 164 | Vertebral fractures | P-value > 0.05 | ||||||
| Laan [33] | Cross sectional | RA patients taking GC | RA patients not taking GC | ✓ | 77 | Vertebral fractures | P-value 0.03 | ||||||
| Vis [34] | Prospective cohort | Established RA patients using GC | RA patients not on GC | ✓ | 102 | Vertebral and non-vertebral fracture | P-value: V 0.04, Non-V 0.30 |
||||||
| Lapi [35] | Retrospective cohort | Those taking GC | Those not taking GC | ✓ | 271,121 | Osteoporotic and hip fracture | OR: Osteo: male 1.39 (0.98, 1.97) female 1.69 (1.42, 2.01) | ||||||
| Peel [36] | Case control | Postmenopausal women with RA | Population based | ✓ | 76 + 347 | Vertebral fracture | Non signif | ||||||
| Ochi [37] | Prospective cohort | RA patients with fracture | RA patients no fracture | ✓ | ✓ | 9987 | Distal radius | HR: DD 1.07 (1.01, 1.13) EU: higher % GC users in fracture group |
|||||
| Saag [38] | Case control | Early RA, > 1 year CS use | Early RA, 1:1 matched, no GC use | ✓ | ✓ | 112 + 112 | Any fracture | P-value EU > 0.05 5–10 mg/d 4.5 (2.1, 9.6) |
|||||
| Lems [39] | Case control | RA patients treated with GC on daily basis ≥ 1 month | Rheumatology patients not using GC | ✓ | ✓ | 52 + 55 | Vertebral or peripheral fracture | P-values: Vt 0.03, peripheral > 0.05 | |||||
| Baskan [40] | Cross sectional | RA patients | Healthy patients | ✓ | ✓ | ✓ | 156 | Vertebral fracture | P-value > 0.05 | ||||
| Sugiyama [41] | Prospective cohort | Rheumatic disease patients on high dose GC | Rheumatic disease patients not on GC | ✓ | ✓ | ✓ | ✓ | 2631 | Vertebral fracture | HR: EU 8.16 (1.09, 60.86) DD 2.03 (1.43, 2.88) CD 0.88 (0.84, 0.93) OT various |
|||
| Sugiyama [42] | Prospective cohort | Early RA patients high dose GC | Early RA patients not on GC | ✓ | ✓ | ✓ | 700 | Vertebral fracture (symptomatic) | HR: DD 1.24 (1.16, 1.33) CD 0.92 (0.91, 0.94) OT various |
||||
| Sinigaglia [43] | Retrospective cohort | Established RA patients GC use | Established RA patients no GC use | ✓ | 925 | Vertebral fracture | OR: CD 1.03 (1.006, 1.07) | ||||||
| Lespessailles [44] | Cross sectional | RA patients using GC | Non GC users | ✓ | ✓ | 146 | Vertebral and any fracture | OR: any: CD 0–5 vs 15 + 4.04 (1.5, 11.2) TV 0–12 vs 60 + months 2.8 (1.07, 7.3) |
|||||
| Angeli [45] | Cross sectional | RA patients with high cumulative dose | RA patients with low cumulative dose | ✓ | ✓ | 551 | Vertebral fracture | P-values all > 0.05 | |||||
| Van Staa [46] | Retrospective cohort | Oral GC patients | No GC prescription in past 3 months | ✓ | 191,752 | Osteoporotic, hip and vertebral fracture | RR: Vt: various results: e.g. DD < 2.5, CD < 1 2.11 (0.87, 5.10) DD < 2.5, CD > 1 3.22 (2.09, 4.95) |
||||||
| Michel [47] | Case control | RA patients with fracture | RA patients without fracture | ✓ | 226 + 884 | Any fracture | OR: 1.07 (1.04, 1.11) | ||||||
| Van Staa [9] | Retrospective cohort | RA patients | Population based 1:3 matched | ✓ | 121,045 | Osteoporotic, hip and vertebral fracture | RR: N. prescriptions 0 prescript 1.5 (1.2, 1.9) 1–2 prescript 2.9 (1.9, 4.4) >2 prescript 5.5 (4.4, 6.8) |
||||||
Abbreviations: CU = current use, EU = ever use, DD = daily dose, CD = cumulative dose, ML = multi-level, TV = time variant, OT = other, OR = odds ratio, RR = relative risks, HR = hazard ratio, RA = rheumatoid arthritis, GC = glucocorticoid(s), Vt = vertebral fracture, Non-Vt = non-vertebral fracture, Osteo = osteoporotic fracture, signif = significant, N. = number.
The two results by Arai [19] were produced in the same paper in a study which originally started as a cross sectional study but extended in most patients to a Prospective Cohort study.
Crude odds ratios.
Fig. 2.
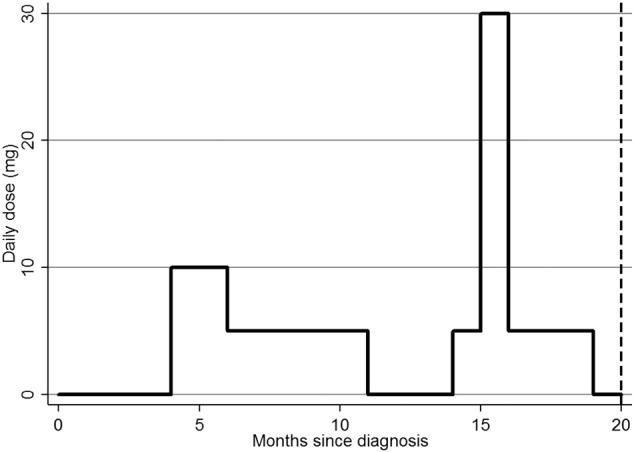
A hypothetical patient's exposure to glucocorticoids. Footnote: at 20 months: current use = No, ever use = Yes, daily dose = 0 mg, cumulative dose = 2.85 g, time variant = 12 months on glucocorticoids.
3.1. Descriptions with binary response
Two definitions of glucocorticoid exposure were binary variables. Firstly the “current use” definition, which meant the participant was on glucocorticoids at the time of fracture and secondly the “ever use” definition, which attributed an incident fracture to glucocorticoid use if a participant had ever taken glucocorticoids during the study period. In the exposure pattern for a hypothetical patient shown in Fig. 2, “current use” at the time of fracture would be 0 (i.e. the patient is off drug at the time of fracture and the fracture is thus not attributed to their glucocorticoid exposure) and “ever exposed” would be 1 (i.e. they HAVE ever been exposed to glucocorticoid therapy and the fracture would be attributed to their historical exposure.)
Of the 19 papers that reported results for “current use” of glucocorticoids, nine [15], [16], [17], [18], [19], [20], [25], [26], [28], [29] found a statistically significant increase in the risk of fracture, five [11], [14], [21], [22], [23] found no significant change in the risk of fracture, one [24] found a decreased risk of fracture, one found an increased risk in women but not men and the remaining three [12], [13], [27] found an increased risk at some fracture sites but not others. Those who found an increased risk of fracture had an odds ratio, relative risk or hazard ratio between 1.33 [13] and 4.15 [15] for current users compared to non-users whereas the paper that reported a decreased risk had an odds ratio of 0.17 for current users compared to the general population [24].
Within the 15 papers reporting “ever use” of glucocorticoids, six [20], [21], [30], [33], [37], [41] found an increased risk of fracture, five [31], [32], [36], [38], [40] found no statistically significant change in the risk of fracture, two [34], [39] found an increased risk at some fracture sites but not others, one [19] found an increased risk for certain age groups and one [35] reported an increased risk in women but not men. Within the six papers that reported an increased risk, only two reported odds ratios, relative risk or hazard ratios with values of 1.69 [35] and 8.16 [41].
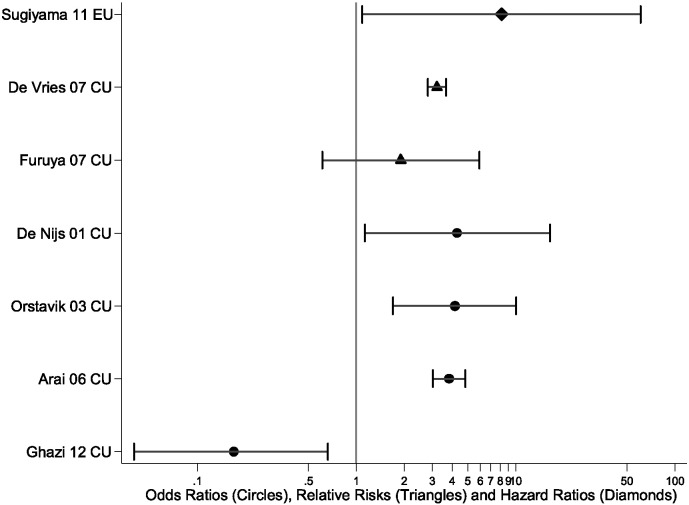
Fig. 3 illustrates the range of results obtained for both the “current use” and “ever use” methods of attributing fractures to glucocorticoid exposure, limited to vertebral fractures. A single fracture type was selected to reduce other causes of heterogeneity, enabling a comparison of the impact of analysis methodology on the results. Vertebral fractures were chosen since the majority of papers (29/39 studies (74%)) reported on either clinical or radiographic vertebral fractures.
Fig. 3.
The odds ratios (circles), relative risks (triangles) and hazard ratios (diamonds) with 95% confidence intervals showing the information given from the two binary methods, current use and ever use using results defining vertebral fractures.
Fig. 3 demonstrates that the increased risk of vertebral fracture was not consistent within the “current use” definition, with one “current use” paper having a relative risk around 2, with one “current use” paper having a relative risk around 3, and three “current use” papers having an odds ratio about 4. Fig. 3 demonstrates that the increased risk of vertebral fracture was not consistent within the “current use” definition, with one “current use” paper having a relative risk around 2, with one “current use” paper having a relative risk around 3 Three “current use” papers had an odds ratio about 4 whilst one had an odds ratio of around 0.2. A meta-analysis of these odds ratios for “current use” would generate approximately a fourfold increased risk of vertebral fracture. Furthermore, Fig. 3 illustrates through their absence that 12/15 publications considering “ever use” reported P-values only and no point estimate, and are hence missing.
3.2. Descriptions of dose
Two methods were used to attribute fractures to definitions of glucocorticoid exposure which demonstrated the effect of dose. Firstly the “daily dose” definition which described the dose the participant was on at the time of fracture and secondly the “cumulative dose” definition which described the total dose taken during the patients follow-up. In the hypothetical exposure pattern described in Fig. 2, the “daily dose” would be 0 mg and the “cumulative dose” 2.85 g. These two dose-specific definitions were also combined in two papers describing the effect of dose and duration in a multi-variable model.
Of the 13 papers using “daily dose” of glucocorticoid, nine [21], [23], [25], [26], [27], [37], [38], [41], [42] papers found an increased risk of fracture with increasing dose, one [40] found no statistically significant change in risk, one [22] found an increased risk for women but not men and two [24], [39] did not report statistics regarding “daily dose” although they mentioned recording “daily dose”. The odds ratios for those who found an increased risk ranged from 1.03 [22] to 2.03 [41] when “daily dose” was considered as a continuous variable (per mg per day) and 1.60 [26] (for < 7.5 mg/day compared to past use) to 4.5 [38] (for 5–10 mg/day compared to never treated patients) when “daily dose” was considered categorically.
Of the eight papers reporting “cumulative dose”, four [26], [28], [43], [44] reported an increased risk of fracture with increasing cumulative exposure, two [25], [45] reported no change to the risk of fracture and two [41], [42] reported a decreased risk of fracture. The odds ratios for those who found an increased risk ranged from 1.03 [43] to 4.31 [26] per gram increase as a continuous variable whilst those who found a decreased risk ranged from 0.88 [41] to 0.92 [42] per gram increase. When considered as a categorical variable the results ranged from 1.38 [29] if < 1 g had been consumed compared to past glucocorticoid use to 8.12 [29] when between 1 and 5 g had been consumed compared to past glucocorticoid use.
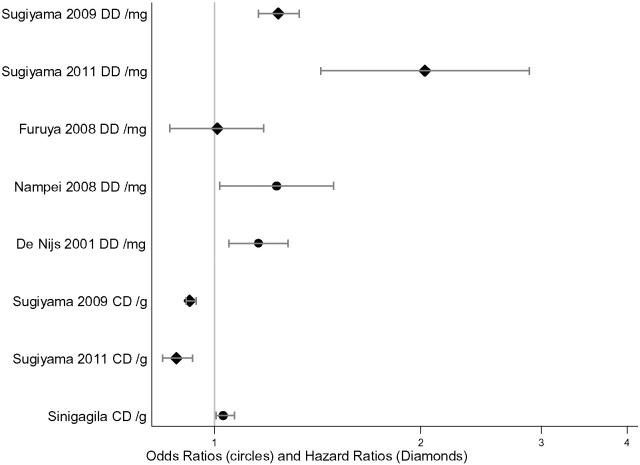
A plot of the differences within estimates of fracture risk from “daily dose” and “cumulative dose” are demonstrated in Fig. 4.
Fig. 4.
The odds ratios (circles), and hazard ratios (diamonds) with 95% confidence intervals showing the information given from the daily dose and cumulative dose methods of defining glucocorticoid exposure using results defining vertebral fractures.
Two [26], [46] papers nested “cumulative dose” within “daily dose” to provide a multi-variable model. This method showed a statistically significant increase risk of fracture for most combinations of “daily dose” and “cumulative dose” suggesting that there is a combination of effect from both “daily dose” and “cumulative dose”.
3.3. Descriptions of duration
One commonly used definition described duration of glucocorticoid therapy, the “time variant” definition which described the change in risk of fracture by the length of time spent on glucocorticoids. In the hypothetical exposure pattern described in Fig. 2, the duration spent on glucocorticoids was 12 month. Eight papers reported a time variant model of which four [20], [27], [44], [47] reported an increased risk of fracture with increasing exposure, three [21], [40], [45] reported no significant change and one [29] found an increased risk for those who had taken glucocorticoids for greater than 5 years compared to non- users. The odds ratios for those who found an increased risk ranged significantly from 1.07/year [47] to 2.8/month [44].
3.4. Other methods for attributing fractures to glucocorticoid exposure
Of the three papers who reported other methods of defining glucocorticoid exposure, one [9] investigated the effect of the number of prescriptions in the past six months and two [41], [42] investigated multiple methods of glucocorticoid exposure including pulse therapy, initial daily dose and number of dose increases.
No meta-analysis was attempted due to heterogeneity in the studies, such as the different fracture types, different comparator populations, study designs, different confounders, and small study numbers once stratified by risk attribution model.
4. Discussion
Five common methods were identified within this literature review that defined risk attribution of fracture risk to oral glucocorticoids in patients with RA: “current use”, “ever use”, “daily dose”, “cumulative dose” and “time-variant”. A multi-variable model was also identified where “cumulative dose” was nested within “daily dose”. Whilst the majority of papers showed an increased risk of fracture, regardless of the method defining risk attribution, the magnitude of this risk varied greatly. For example, estimates of increased risk ranged from 1.03 per gram increase in cumulative dose [43] to 8.16 [41] in patients ever exposed compared to never exposed. Conversely, the lowest estimate of risk was 0.17 [24] for patients currently using glucocorticoids compared to those not currently using glucocorticoids and suggested a protective effect. However, this contradicted most other findings. Even within analytic models, there was marked variation in the results.
The appropriateness of the analysis model is important to consider for drugs that are taken dynamically through time such as glucocorticoids (see Fig. 2).
The “current use” definition examines the association between the fracture and whether the patient was exposed to glucocorticoids on the day of the fracture, hence important assumptions for this model are that any prior glucocorticoid exposure does not affect the risk of fracture, and the dose of glucocorticoids on the day of fracture is not important. The wide range of results for “current use”, from 1.33 [13] to 4.15 [15], may reflect variability in patterns of prior use between studies. Indeed, the mean length of follow up ranged from 1 year to 12.6 years for papers reporting “current use”, allowing very different prior exposure patterns. The “ever use” definition, conversely, assumes that all historical therapy affects the risk of fracture, but this is regardless of how recently the therapy was taken. The range of results was harder to compare for this model since only P-values were reported in most cases.
The “daily dose” definition assumes that the strength of the dose on the day of fracture has the largest effect on fracture risk however it typically does not consider historical doses. This method has an advantage in that it provides clinicians and patients with information about the extent to which increasing dose is likely to affect the risk of fracture. For example, de Nijs et al. [25] showed that there was an increased fracture risk of about 16% per mg per day. Furthermore, this definition of glucocorticoid exposure assumes that the risk of fracture increases linearly with the increase of dose. This means that it is impossible to examine whether the change to the risk of fracture tapers at some value of daily dose.
The “cumulative dose” method assumes that all current and prior glucocorticoids have equal impact on fracture risk regardless of how recently they were taken. However, calculating cumulative dose can be difficult which may lead to misclassification and imprecision of estimates. The dynamic regeneration and repair of bone suggests cessation of glucocorticoids might be followed recovery in bone health as shown by Van Staa et al. [10]. The effect of historic doses probably, therefore, has less of an impact on fracture risk compared to more recent doses. Furthermore, the “cumulative dose” method is unable to distinguish between long term, low dose treatments and short-term, high dose treatments. This disadvantage can be overcome by using a multi-variable model and nesting cumulative dose within daily dose such as the models used by Van Staa et al. [46] and De Vries et al. [26]. For example, this allowed for differentiation between patients who had taken between 2.5 and 4.9 mg/day with a total cumulative dose of > 1 g and 15–29.9 mg/day with a cumulative dose of > 1 g. In this case the relative risk (95% CI) were 1.41 (1.23, 1.62) and 2.84 (2.45, 3.30) suggesting the higher dose for short periods of time has a greater impact on fracture risk.
The “time variant” method assumes that the duration spent on glucocorticoids affects fracture risk. Within this model, the majority of results found an increased fracture risk with increasing duration of use. However, the results are difficult to compare as they consider the unit of time as years [20], [21], [27], [29], [47], months [40], [44] and days [45].
Beyond the five models described above, and illustrated in Fig. 1, Sugiyama [41], [42] also included the effect of pulse therapy, initial daily dose and “number of glucocorticoid dose increases” as methods for defining glucocorticoid exposure. Van Staa et al. [9] considered the number of prescriptions received in the past 6 months as a measure of glucocorticoid dose with the participants split into 3 categories (0, 1–2, > 2 prescriptions). They found that patients with 2 or more prescriptions increased their risk by 160% whereas patients with no prescriptions increased their risk by only 30% compared to patients without RA.
None of the models described above considered dose, duration and timing simultaneously, despite it being probable that all are important. Furthermore, no study made a comparison of how recency of glucocorticoid exposure affects the risk of fractures. One novel method that does consider dose, duration, and timing is the weighted cumulative dose model [48]. This method has previously been used to investigate the risk of infection with glucocorticoid therapy [49]. This method weights the dose by how recent it is to the occurrence of the adverse event of interest. A cubic spline curve is fitted allowing the data to define the shape of the weighting curve and hence determine which time points provide the largest effect of the glucocorticoids, in combination with the dosage. Whilst many studies consider the dose-dependent risk irrespective of treatment duration, this method allows risk estimates for any given pattern of glucocorticoid use and can thus allow the comparison of the same dose but taken for different durations. For example, Dixon et al. [49] found that 5 mg prednisolone equivalent taken for 3, 6, 12 months or three years conferred an increased risk of serious infection of 11%, 30%, 55 and 100%, respectively, compared to non-use. This shows that historical doses, and the duration of such use, are important. It also allows consideration of the same dose and duration pattern, but taken at different times with respect to the event of interest, thereby allowing the exploration of recovery from risk (for example, six months at 5 mg/day for the last six months, versus six months at 5 mg/day started a year ago and discontinued six months ago).
Considering the limitations of this review, abstracts were screened and data extracted by a single reviewer. Only the two leading databases were included in the search for publications and papers not written in the English language were also excluded from the review. As the key focus of the publication was to identify the differences in methodology, this was felt to be reasonable. Within the publications, it was unclear whether the glucocorticoids were prescribed for RA or another illness. Despite a possible alternative indication within an RA population, any observed relationship would remain valid unless the effect of glucocorticoid therapy is modified significantly by the indication. Furthermore, this review spans the development of anti-TNF therapy for use in inflammatory conditions. Due to the potential direct impact of biologic therapy on fracture risk, a comparison between studies undertaken before and after 2000 would be useful. However, due to the heterogeneity of the study designs included within this review it is difficult to make a direct comparison within any given method between pre and post 2000 since there maybe unmeasured confounding.
5. Summary
There are five main methods by which fracture risk has been attributed to glucocorticoid exposure, none of which consider the dose, duration and timing of treatment. This means risk estimates will rarely consider the complex individual patterns of steroid treatment, and will thus not give an accurate fracture risk assessment for an individual patient. There are now opportunities with advanced analytical methods to incorporate all these factors into a single model, allowing the generation of risk estimates for any given pattern of steroid exposure.
Disclosures
DER and WGD report no conflicts of interest. EMD has received speaker fees from Eli Lilly. CC has received consultancy fees and honoraria from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda and UCB. TPvS reports grants on an observational research project from GSK and participation in expert meetings with GSK, Sanofi and Roche, all outside the submitted work.
Acknowledgements
We thank the Arthritis Research UK for their support: Arthritis Research UK grant number 20380. WGD was supported by an MRC Clinician Scientist Award (G0902272). This report includes independent research supported by the National Institute for Health Research Biomedical Research Unit Funding Scheme, Medical Research Council, National Institute of Health Research, Arthritis Research UK and the International Osteoporosis Foundation. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Appendix 1: Search terms used
The explode option (exp) was used for terms of particular interest where the papers of interest may fit into multiple subcategories.
The terms categorised under glucocorticoids included:
-
•
Triamcinolone
-
•
Dexamethasone
-
•
Prednisone
-
•
Prednisolone
-
•
Methylprednisolone
-
•
Budesonide
-
•
Bethamethasone
-
•
Cortisone
-
•
Glucocorticoids
-
•
exp Corticosteroids
-
•
Terms for the drug adjacent within 2 words (adj2) to methods of taking the drug, where terms for the drug included glucocorticoid*, Glucocorticosteroid*, Corticosteroid* and steroid* and methods of taking the drug included therap*, prescript*, use*, treat*, oral, and tablet.
-
•
The terms categorised under fracture included:
-
•
Fracture*
-
•
Terms categorised under rheumatoid arthritis included:
-
•
Rheumatoid arthritis
-
•
exp. Arthritis, Rheumatoid
-
•
Inflammatory polyarthritis
-
•
Inflammatory arthritis
References
- 1.Kvien T.K., Glennas A., Knudsrod O.G., Smedstad L.M., Mowinckel P., Forre O. The prevalence and severity of rheumatoid arthritis in Oslo. Results from a county register and a population survey. Scand. J. Rheumatol. 1997;26:412–418. doi: 10.3109/03009749709065712. [DOI] [PubMed] [Google Scholar]
- 2.Hench P.S., Kendall E.C., Slocumb C.H., Polley H.F. Effects of cortisone acetate and pituitary ACTH on rheumatoid arthritis, rheumatic fever and certain other conditions. Arch. Intern. Med. (Chic.) 1950;85:545–666. doi: 10.1001/archinte.1950.00230100002001. [DOI] [PubMed] [Google Scholar]
- 3.Caplan L., Wolfe F., Russell A.S., Michaud K. Corticosteroid use in rheumatoid arthritis: prevalence, predictors, correlates, and outcomes. J. Rheumatol. 2007;34:696–705. [PubMed] [Google Scholar]
- 4.Gotzsche P.C., Johansen H.K. Meta-analysis of short term low dose prednisolone versus placebo and non-steroidal anti-inflammatory drugs in rheumatoid arthritis. Br. Med. J. 1998;316:811–818. doi: 10.1136/bmj.316.7134.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirwan J.R., Bijlsma J.W., Boers M., Shea B.J. Effects of glucocorticoids on radiological progression in rheumatoid arthritis. Cochrane Database Syst. Rev. 2007 doi: 10.1002/14651858.CD006356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonough A.K., Curtis J.R., Saag K.G. The epidemiology of glucocorticoid-associated adverse events. Curr. Opin. Rheumatol. 2008;20:131–137. doi: 10.1097/BOR.0b013e3282f51031. [DOI] [PubMed] [Google Scholar]
- 7.Olney R.C. Mechanisms of impaired growth: effect of steroids on bone and cartilage. Horm. Res. Paediatr. 2009;72(Suppl. 1):30–35. doi: 10.1159/000229761. [DOI] [PubMed] [Google Scholar]
- 8.Teitelbaum S.L. Osteoclasts: what do they do and how do they do it? Am. J. Pathol. 2006;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Staa T.P., Geusens P., Bijlsma J.W.J., Leufkens H.G.M., Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3104–3112. doi: 10.1002/art.22117. [DOI] [PubMed] [Google Scholar]
- 10.Van Staa T.P., Leufkens H.G., Abenhaim L., Zhang B., Cooper C. Use of oral corticosteroids and risk of fractures. J. Bone Miner. Res. 2000;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 11.van Everdingen A.A., Siewertsz van Reesema D.R., Jacobs J.W.G., Bijlsma J.W.J. Low-dose glucocorticoids in early rheumatoid arthritis: discordant effects on bone mineral density and fractures? Clin. Exp. Rheumatol. 2003;21:155–160. [PubMed] [Google Scholar]
- 12.Furuya T., Kotake S., Inoue E., Nanke Y., Yago T., Kobashigawa T., Ichikawa N., Tanaka E., Momohara S., Nakajima A., Hara M., Tomatsu T., Yamanaka H., Kamatani N. Risk factors associated with incident clinical vertebral and nonvertebral fractures in Japanese women with rheumatoid arthritis: a prospective 54-month observational study. J. Rheumatol. 2007;34:303–310. [PubMed] [Google Scholar]
- 13.Coulson K.A., Reed G., Gilliam B.E., Kremer J.M., Pepmueller P.H. Factors influencing fracture risk, T score, and management of osteoporosis in patients with rheumatoid arthritis in the consortium of rheumatology researchers of north america (CORRONA) registry. J. Clin. Rheumatol. 2009;15:155–160. doi: 10.1097/RHU.0b013e3181a5679d. [DOI] [PubMed] [Google Scholar]
- 14.Hooyman J.R., Melton I.L.J., Nelson A.M. Fractures after rheumatoid arthritis. A population-based study. Arthritis Rheum. 1984;27:1353–1361. doi: 10.1002/art.1780271205. [DOI] [PubMed] [Google Scholar]
- 15.Orstavik R.E., Haugeberg G., Uhlig T., Falch J.A., Halse J.I., Hoiseth A., Lilleas F., Kvien T.K. Vertebral deformities in 229 female patients with rheumatoid arthritis: associations with clinical variables and bone mineral density. Arthritis Care Res. 2003;49:355–360. doi: 10.1002/art.11118. [DOI] [PubMed] [Google Scholar]
- 16.Verstraeten A., Dequeker J. Vertebral and peripheral bone mineral content and fracture incidence in postmenopausal patients with rheumatoid arthritis: effect of low dose corticosteroids. Ann. Rheum. Dis. 1986;45:852–857. doi: 10.1136/ard.45.10.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper C., Coupland C., Mitchell M. Rheumatoid arthritis, corticosteroid therapy and hip fracture. Ann. Rheum. Dis. 1995;54:49–52. doi: 10.1136/ard.54.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler R.C., Davie M.W., Worsfold M., Sharp C.A. Bone mineral content in patients with rheumatoid arthritis: relationship to low-dose steroid therapy. Br. J. Rheumatol. 1991;30:86–90. doi: 10.1093/rheumatology/30.2.86. [DOI] [PubMed] [Google Scholar]
- 19.Arai K., Hanyu T., Sugitani H., Murai T., Fujisawa J., Nakazono K., Kondo N., Endo N. Risk factors for vertebral fracture in menopausal or postmenopausal Japanese women with rheumatoid arthritis: a cross-sectional and longitudinal study. J. Bone Miner. Metab. 2006;24:118–124. doi: 10.1007/s00774-005-0657-9. [DOI] [PubMed] [Google Scholar]
- 20.Orstavik R.E., Haugeberg G., Mowinckel P., Hoiseth A., Uhlig T., Falch J.A., Halse J.I., McCloskey E., Kvien T.K. Vertebral deformities in rheumatoid arthritis: a comparison with population-based controls. Arch. Intern. Med. 2004;164:420–425. doi: 10.1001/archinte.164.4.420. [DOI] [PubMed] [Google Scholar]
- 21.Kay L.J., Holland T.M., Platt P.N. Stress fractures in rheumatoid arthritis: a case series and case-control study. Ann. Rheum. Dis. 2004;63:1690–1692. doi: 10.1136/ard.2003.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuya T., Inoue E., Hosoi T., Taniguchi A., Momohara S., Yamanaka H. Risk factors associated with the occurrence of hip fracture in Japanese patients with rheumatoid arthritis: a prospective observational cohort study. Osteoporos. Int. 2013;24:1257–1265. doi: 10.1007/s00198-012-2080-0. [DOI] [PubMed] [Google Scholar]
- 23.Furuya T., Kotake S., Inoue E., Nanke Y., Yago T., Hara M., Tomatsu T., Kamatani N., Yamanaka H. Risk factors associated with incident fractures in Japanese men with rheumatoid arthritis: a prospective observational cohort study. J. Bone Miner. Metab. 2008;26:499–505. doi: 10.1007/s00774-007-0836-y. [DOI] [PubMed] [Google Scholar]
- 24.Ghazi M., Kolta S., Briot K., Fechtenbaum J., Paternotte S., Roux C. Prevalence of vertebral fractures in patients with rheumatoid arthritis: revisiting the role of glucocorticoids. Osteoporos. Int. 2012;23:581–587. doi: 10.1007/s00198-011-1584-3. [DOI] [PubMed] [Google Scholar]
- 25.de Nijs R.N., Jacobs J.W., Bijlsma J.W., Lems W.F., Laan R.F., Houben H.H., ter Borg E.J., Huisman A.M., Bruyn G.A., van Oijen P.L., Westgeest A.A., Algra A., Hofman D.M., Osteoporosis Working Group DSfR Prevalence of vertebral deformities and symptomatic vertebral fractures in corticosteroid treated patients with rheumatoid arthritis. Rheumatology. 2001;40:1375–1383. doi: 10.1093/rheumatology/40.12.1375. [DOI] [PubMed] [Google Scholar]
- 26.De Vries F., Bracke M., Leufkens H.G.M., Lammers J.W.J., Cooper C., Van Staa T.P. Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum. 2007;56:208–214. doi: 10.1002/art.22294. [DOI] [PubMed] [Google Scholar]
- 27.Nampei A., Hashimoto J., Koyanagi J., Ono T., Hashimoto H., Tsumaki N., Tomita T., Sugamoto K., Nishimoto N., Ochi T., Yoshikawa H. Characteristics of fracture and related factors in patients with rheumatoid arthritis. Mod. Rheumatol. 2008;18:170–176. doi: 10.1007/s10165-008-0032-5. [DOI] [PubMed] [Google Scholar]
- 28.Maghraoui A.E., Rezqi A., Mounach A., Achemlal L., Bezza A., Ghozlani I. Prevalence and risk factors of vertebral fractures in women with rheumatoid arthritis using vertebral fracture assessment. Rheumatology. 2010;49:1303–1310. doi: 10.1093/rheumatology/keq084. [DOI] [PubMed] [Google Scholar]
- 29.Mazzantini M., Talarico R., Doveri M., Consensi A., Cazzato M., Bazzichi L., Bombardieri S. Incident comorbidity among patients with rheumatoid arthritis treated or not with low-dose glucocorticoids: a retrospective study. J. Rheumatol. 2010;37:2232–2236. doi: 10.3899/jrheum.100461. [DOI] [PubMed] [Google Scholar]
- 30.Michel B.A., Bloch D.A., Fries J.F. Predictors of fractures in early rheumatoid arthritis. J. Rheumatol. 1991;18:804–808. [PubMed] [Google Scholar]
- 31.Wright N.C., Lisse J.R., Walitt B.T., Eaton C.B., Chen Z., Nabel E., Rossouw J., Ludlam S., Pottern L., McGowan J., Ford L., Geller N., Prentice R., Anderson G., LaCroix A., Kooperberg C.L., Patterson R.E., McTiernan A., Shumaker S., Stein E., Cummings S., Wassertheil-Smoller S., Rajkovic A., Manson J., Assaf A.R., Phillips L., Beresford S., Hsia J., Chlebowski R., Whitlock E., Caan B., Kotchen J.M., Howard B.V., Van Horn L., Black H., Stefanick M.L., Lane D., Jackson R., Lewis C.E., Bassford T., Wactawski-Wende J., Robbins J., Hubbell F.A., Nathan L., Langer R.D., Gass M., Limacher M., Curb D., Wallace R., Ockene J., Lasser N., O’Sullivan M.J., Margolis K., Brunner R., Heiss G., Kuller L., Johnson K.C., Brzyski R., Sarto G.E., Vitolins M., Hendrix S. Arthritis increases the risk for fractures - results from the women's health initiative. J. Rheumatol. 2011;38:1680–1688. doi: 10.3899/jrheum.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saville P.D., Kharmosh O. Osteoporosis of rheumatoid arthritis: influence of age, sex and corticosteroids. Arthritis Rheum. 1967;10:423–430. doi: 10.1002/art.1780100504. [DOI] [PubMed] [Google Scholar]
- 33.Laan R.F.J.M., Van Riel P.L.C.M., Van Erning L.J.T.O., Lemmens J.A.M., Ruijs S.H.J., Van de Putte L.B.A. Vertebral osteoporosis in rheumatoid arthritis patients: effect of low dose prednisone therapy. Br. J. Rheumatol. 1992;31:91–96. doi: 10.1093/rheumatology/31.2.91. [DOI] [PubMed] [Google Scholar]
- 34.Vis M., Haavardsholm E.A., Boyesen P., Haugeberg G., Uhlig T., Hoff M., Woolf A., Dijkmans B., Lems W., Kvien T.K. High incidence of vertebral and non-vertebral fractures in the OSTRA cohort study: a 5-year follow-up study in postmenopausal women with rheumatoid arthritis. Osteoporos. Int. 2011;22:2413–2419. doi: 10.1007/s00198-010-1517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lapi F., Simonetti M., Michieli R., Pasqua A., Brandi M.L., Frediani B., Cricelli C., Mazzaglia G. Assessing 5-year incidence rates and determinants of osteoporotic fractures in primary care. Bone. 2012;50:85–90. doi: 10.1016/j.bone.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 36.Peel N.F.A., Moore D.J., Barrington N.A., Bax D.E., Eastell R. Risk of vertebral fracture and relationship to bone mineral density in steroid treated rheumatoid arthritis. Ann. Rheum. Dis. 1995;54:801–806. doi: 10.1136/ard.54.10.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ochi K., Go Y., Furuya T., Ikari K., Taniguchi A., Yamanaka H., Momohara S. Risk factors associated with the occurrence of distal radius fractures in Japanese patients with rheumatoid arthritis: a prospective observational cohort study. Clin. Rheumatol. 2014;33:477–483. doi: 10.1007/s10067-013-2415-z. [DOI] [PubMed] [Google Scholar]
- 38.Saag K.G., Koehnke R., Caldwell J.R., Brasington R., Burmeister L.F., Zimmerman B., Kohler J.A., Furst D.E. Low dose long-term corticosteroid therapy in rheumatoid arthritis: an analysis of serious adverse events. Am. J. Med. 1994;96:115–123. doi: 10.1016/0002-9343(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 39.Lems W.F., Jahangier Z.N., Jacobs J.W., Bijlsma J.W. Vertebral fractures in patients with rheumatoid arthritis treated with corticosteroids. Clin. Exp. Rheumatol. 1995;13:293–297. [PubMed] [Google Scholar]
- 40.Baskan B.M., Sivas F., Alemdaroglu E., Duran S., Ozoran K. Association of bone mineral density and vertebral deformity in patients with rheumatoid arthritis. Rheumatol. Int. 2007;27:579–584. doi: 10.1007/s00296-007-0323-8. [DOI] [PubMed] [Google Scholar]
- 41.Sugiyama T., Suzuki S., Yoshida T., Mayama T., Hashimoto N., Suyama K., Tanaka T., Sueishi M., Tatsuno I. Age, initial dose and dose increase are independent risk factors for symptomatic vertebral fractures in glucocorticoid-treated male patients. Intern. Med. 2011;50:817–824. doi: 10.2169/internalmedicine.50.4443. [DOI] [PubMed] [Google Scholar]
- 42.Sugiyama T., Tatsuno I., Suzuki S., Yoshida T., Tanaka T., Sueishi M., Saito Y. Incidence of symptomatic vertebral fracture with high-dose glucocorticoid treatment in the Chiba-Shimoshizu Rheumatic Cohort between 1986 and 2006. Endocr. J. 2009;56:591–599. doi: 10.1507/endocrj.k08e-318. [DOI] [PubMed] [Google Scholar]
- 43.Sinigaglia L., Nervetti A., Mela Q., Bianchi G., Del Puente A., Di Munno O., Frediani B., Cantatore F., Pellerito R., Bartolone S., La Montagna G., Adami S. A multicenter cross sectional study on bone mineral density in rheumatoid arthritis. J. Rheumatol. 2000;27:2582–2589. [PubMed] [Google Scholar]
- 44.Lespessailles E., Poupon S., Adriambelosoa N., Pothuaud L., Siroux V., Bouillon S., Benhamou C.L. Glucocorticoid-induced osteoporosis: is the bone density decrease the only explanation? Joint Bone Spine. 2000;67:119–126. [PubMed] [Google Scholar]
- 45.Angeli A., Guglielmi G., Dovio A., Capelli G., de Feo D., Giannini S., Giorgino R., Moro L., Giustina A. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39:253–259. doi: 10.1016/j.bone.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Van Staa T.P., Geusens P., Pols H.A.P., De Laet C., Leufkens H.G.M., Cooper C. A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. QJM. 2005;98:191–198. doi: 10.1093/qjmed/hci029. [DOI] [PubMed] [Google Scholar]
- 47.Michel B.A., Bloch D.A., Wolfe F., Fries J.F. Fractures in rheumatoid arthritis: an evaluation of associated risk factors. J. Rheumatol. 1993;20:1666–1669. [PubMed] [Google Scholar]
- 48.Sylvestre M.-P., Abrahamowicz M. Flexible modeling of the cumulative effects of time-dependent exposures on the hazard. Stat. Med. 2009;28:3437–3453. doi: 10.1002/sim.3701. [DOI] [PubMed] [Google Scholar]
- 49.Dixon W.G., Abrahamowicz M., Beauchamp M.-E., Ray D.W., Bernatsky S., Suissa S., Sylvestre M.-P. Immediate and delayed impact of oral glucocorticoid therapy on risk of serious infection in older patients with rheumatoid arthritis: a nested case–control analysis. Ann. Rheum. Dis. 2012;71:1128–1133. doi: 10.1136/annrheumdis-2011-200702. [DOI] [PMC free article] [PubMed] [Google Scholar]