Abstract
The ecological context in which mosquitoes and malaria parasites interact has received little attention, compared to the genetic and molecular aspects of malaria transmission. Plant nectar and fruits are important for the nutritional ecology of malaria vectors, but how the natural diversity of plant-derived sugar sources affects mosquito competence for malaria parasites is unclear. To test this, we infected Anopheles coluzzi, an important African malaria vector, with sympatric field isolates of Plasmodium falciparum, using direct membrane feeding assays. Through a series of experiments, we then examined the effects of sugar meals from Thevetia neriifolia and Barleria lupilina cuttings that included flowers, and fruit from Lannea microcarpa and Mangifera indica on parasite and mosquito traits that are key for determining the intensity of malaria transmission. We found that the source of plant sugar meal differentially affected infection prevalence and intensity, the development duration of the parasites, as well as the survival and fecundity of the vector. These effects are likely the result of complex interactions between toxic secondary metabolites and the nutritional quality of the plant sugar source, as well as of host resource availability and parasite growth. Using an epidemiological model, we show that plant sugar source can be a significant driver of malaria transmission dynamics, with some plant species exhibiting either transmission-reducing or -enhancing activities.
Author Summary
The Anopheles coluzzii mosquito is an effective vector of human Plasmodium falciparum malaria. Besides feeding on blood, females readily feed on natural sources of plant sugars; but how plant diversity affects their ability to transmit malaria parasites is currently unknown. Here we show that mosquito feeding on peridomestic plant- and fruit-derived sugar sources can affect its interaction with P. falciparum, and hence malaria transmission. The planting of anti-Plasmodium plant sugar sources may represent a promising alternative strategy to contribute to the control of malaria.
Introduction
The ability of anopheline mosquitoes to transmit Plasmodium falciparum malaria is a complex phenotypic trait, determined by mosquito and parasite genetic factors, environmental factors, as well as the interaction between these factors [1–6]. A key environmental variable for host-parasite relationships is the availability and quality of food resources, which have been shown to influence host susceptibility, parasite infectivity and virulence, and ultimately disease dynamics [7]. The influence of diet on infectious diseases is particularly apparent in tritrophic interactions involving herbivorous insects, their parasites and larval food plants [8]. Such plant-mediated effects have often been attributed to either the direct toxic effect of plant secondary metabolites on parasite development [9], or differences in nutritional value that, in turn, affect host immunocompetence [10].
Mosquitoes, like herbivorous insects, are part of a multitrophic system that includes plants and parasites. Although female mosquitoes are well known blood-feeders, sugar sources such as nectar from floral and extra-floral nectaries, fruits and phloem sap compose an important part of their diet and have significant biological implications [11,12]. Recent studies indicate that females of An. gambiae s.l., the main vector of P. falciparum in large areas of Africa, can locate and display preference for natural sources of plant sugar [13–16], and that environmental sugars may play a crucial role in malaria vectorial capacity (a standard measure of malaria transmission potential), through effects on mosquito survival [17–24] or blood-feeding rate [17,21–23]. However, feeding on plant tissues could also influence vectorial capacity by enhancing or mitigating infection in malaria mosquitoes.
Compared to the important efforts devoted to documenting plant-mediated interactions among herbivorous insects and their parasites [8], studies on the nutritional effects in mosquito-Plasmodium interactions have lagged behind despite obvious epidemiological importance. The few existing studies indicate that adult diet can indeed influence mosquito immunocompetence and susceptibility to malaria parasites [25–30]. However, these studies have focused on quantitative, as opposed to qualitative, changes in diet, and have used non-natural food sources (e.g. different concentrations of glucose solutions). Whether natural plant diversity affects mosquito susceptibility to malaria parasites therefore remains to be discovered.
The current study addresses questions of the impact of natural plant diversity on mosquito susceptibility to malaria parasites, using the natural tritrophic interaction between the parasite P. falciparum, responsible for causing the most severe form of human malaria, the mosquito An. coluzzii (formerly the M molecular form of An. gambiae s.s.), a major vector of P. falciparum in Africa, and a range of peridomestic plant- and fruit-derived sugar sources. We challenged An. coluzzi females with sympatric field isolates of P. falciparum using direct membrane feeding assays and, through a series of experiments, examined the effects of plant sugar sources on parasite and mosquito traits that are instrumental in determining the intensity of malaria transmission: (i) the early parasite development within mosquito guts, (ii) the proportion of mosquitoes harboring sporozoite transmissible stages (i.e. the sporozoite index), (iii) the extrinsic incubation period of the parasite (EIP), (iv) the survival and fecundity of infected mosquitoes, and (v) the costs and benefits to feed on these plant sugar sources for both infected and uninfected mosquitoes. Finally, these results were combined into an epidemiological model to predict the relative contribution of different plant species to overall malaria transmission. We provide evidence for malaria transmission-reducing and -enhancing activities of some natural plant sugar sources.
Methods
Mosquitoes
Laboratory-reared An. coluzzii were obtained from an outbred colony established in 2008 and repeatedly replenished with F1 from wild-caught mosquito females collected in Kou Valley (11°23'14"N, 4°24'42"W), 30 km from Bobo Dioulasso, south-western Burkina Faso (West Africa), and identified by routine PCR-RFLP. Mosquitoes were held in 30 cm × 30 cm × 30 cm mesh-covered cages at the IRSS insectary under standard conditions (27 ± 2°C, 70 ± 5% relative humidity, 12:12 LD). Females were maintained on rabbit blood by direct feeding, and adult males and females fed with 5% glucose. Larvae were reared at a density of about 300 first instar larvae in 700 ml of water in plastic trays and were fed with Tetramin Baby Fish Food (Tetrawerke, Melle, Germany).
Plant materials and mosquito feeding on plant sugar source
We selected two perennial flowering ornamental plants: Thevetia neriifolia and Barleria lupilina collected in the gardens and parkland of Bobo Dioulasso (S1A and S1B Fig); and two fruits: mango (Mangifera indica, variety “demoiselle”) and Lannea microcarpa, both locally produced and purchased from the market in Bobo Dioulasso (S1C and S1D Fig). The plants and fruits were selected based on (i) their wide distribution around human dwellings in villages and cities of western Burkina Faso, (ii) An. coluzzii females readily rest, probe and feed on them (inferred from both field and lab observations, S1 appendix and [31]), and (iii) they provide relatively high mosquito survival to allow for the development of Plasmodium falciparum.
Plant sugar sources were offered to mosquitoes in 30 cm × 30 cm × 30 cm mesh-covered cages. For T. neriifolia and B. lupilina, between five and ten fresh bundles of flowering cuttings were added within each cage. The base of the bunch was wrapped in moistened paper towels and an aluminum sheet (S2A and S2B Fig). For the mango treatment, ripe fruits were cut in half, held on two 20 cm long wooden stakes and individually placed in the mosquito cages (S2C Fig). One bunch of about 40 ripe L. microcarpa fruits was placed in a petri dish within the mosquito cages (S2D Fig). Finally, 5% glucose was provided on cotton pads wrapped around two 20 cm long wooden stick (S2E Fig). Cuttings, fruits, and glucose were replaced every day. To confirm sugar ingestion, the gut content of a subset of mosquitoes was determined by the cold anthrone test for fructose [32] (S1 Appendix). Mosquitoes can sugar feed from a wide range of plant parts (floral nectaries, extra-floral nectaries at the base of the flowers or on stems or leaves, tissues juices, phloem sap, and honeydew) [11]. Here, the exact sources of the sugar taken up by mosquito females on plant cuttings (T. neriifolia and B. lupilina) were not determined.
Parasite isolate and experimental infections
Plasmodium falciparum gametocyte carriers were recruited among 5–12-year-old school children in the villages of Soumousso and Dande, located respectively 30 km north east and 60 km North West of Bobo Dioulasso in southwestern Burkina Faso. Parasitological surveys were carried out in collaboration with the medical team in charge of malaria treatment at the local health center in these two villages. Thick blood smears were taken from each volunteer, air-dried, Giemsa-stained, and examined by microscopy for the presence of P. falciparum at the IRSS lab in Bobo Dioulasso. Asexual trophozoite parasite stages were counted against 200 leucocytes, while infectious gametocytes stages were counted against 1000 leukocytes. Children with asexual parasitemia of > 1,000 parasites per microliter (estimated based on an average of 8000 leucocytes/ml) were treated in accordance with national guidelines. Asymptomatic P. falciparum gametocyte-positive children were recruited for the study. Mosquito infections were performed by direct feeding membrane assays using whole donor blood (i.e. no serum replacement) [33,34]. Briefly, gametocyte carrier blood was collected by venipuncture into heparinized tubes. Three to four day old female mosquitoes, distributed in 500 ml paper cups at a density of 80 mosquitoes per cup, were allowed to feed on this blood for one hour. Mosquitoes were starved of glucose solution for 24 h prior to the infection, with only water available, in wet cotton wool. Non-fed or partially fed females were removed and discarded, while the remaining fully-engorged mosquitoes were maintained in a biosafety room under the same standard conditions of 27 ± 2°C, 70 ± 5% relative humidity and, 12:12 LD) on their assigned plant sugar source.
Experiment 1: To determine the effect of plant sugars on the early development of P. falciparum and on mosquito survival and fecundity
Upon emergence, batches of female mosquitoes from the colony were randomly assigned to one of four sugar sources: a 5% glucose solution, T. neriifolia, B. lupilina or M. indica, for three to four days. Thus, mosquitoes were given ample time to feed on their sugar source, and by 2–3 days, all mosquitoes would have acquired sugar meal at least once. Male and female mosquitoes were kept together to ensure insemination. Three to four day old females were then transferred to paper cups for infection (see above). After infection, fully-engorged females were placed in 30 cm × 30 cm × 30 cm mesh-covered cages with their assigned sugar source (S2 Fig). Females thus received the sugar treatment both before and after the infection. Seven days post blood-meal, mosquitoes were dissected to assess microscopically (×400) the presence of oocysts in the midgut stained with 2% mercurochrome. We also examined whether ovaries contained matured eggs (egg incidence). Mosquito survival was monitored from 1 to 7 days post-treatment (dpi). Twice a day, dead mosquitoes were removed and counted from each cage at 08:00 and 17:00. We performed four replicates using a total of seven gametocyte carriers. To avoid potential cage effect on survival, fecundity and susceptibility to infection, mosquitoes belonging to the same sugar treatment and fed on blood from the same gametocyte carrier were distributed in at least two different cages. On average, 30 (range 10–50) mosquitoes per sugar treatment and gametocyte carrier were dissected (except for the mango treatment, gametocyte carriers A and B for which only 1 and 4 mosquitoes were dissected, respectively, due to a low survival rate, see details in S1 Table).
Experiment 2: To determine the effect of plant sugars on sporozoite index and EIP
The same general procedure as that described in Experiment 1 was used in Experiment 2. Because P. falciparum takes an average of 14 days to disseminate sporozoite, and mosquito survival rate on M. indica was relatively low, we chose to replace this fruit with L. microcarpa. As in the previous experiment, mosquitoes were offered their assigned sugar sources both before and after the infection. Mosquitoes were sampled from day 9 to 13 dpi by dissecting the head and thorax of 20 mosquitoes from each treatment and time point to estimate the parasite’s extrinsic incubation period. On day 14 the remaining mosquitoes in each cage were similarly dissected. The head and thorax were individually stored at -20°C. Sporozoite dissemination in head and thorax was assessed using PCR [35]. Mosquito survival was monitored from 1 to 14 dpi. Twice a day, dead mosquitoes were removed and counted from each cage at 08:00 and 17:00. We performed 2 replicates using a total of 4 gametocyte carriers between April and June 2013. On average, 26 (range 8–50) mosquitoes per sugar treatment and gametocyte carrier were dissected (see details in S2 Table).
Experiment 3: To determine how parasite infection and host plant species interact to influence mosquito longevity
The general procedure was identical to that of the two previous experiments except that a group of uninfected control mosquitoes was added, and that survival was monitored until all the mosquitoes had died. We assigned female mosquitoes to a 5% glucose solution, M. indica, T. neriifolia or B. lupilina. Uninfected control mosquitoes received heat-treated gametocytic blood. Briefly, half of the venous blood drawn from the gametocyte carrier was heated at 45°C for 20 minutes to kill the parasite gametocytes. Such heat treatment does not affect the survival and fecundity of mosquito females [36]. Because the nutritive quality of blood substantially vary among people and especially between infected and uninfected individuals, this procedure allows experiments to avoid the potential confounding effects of different blood origins on the performance of infected and control mosquitoes [33,37].
Statistical analysis
All statistical analyses were performed in R (version 2.15.3). Logistic regression by Generalized Linear Mixed Models (GLMM, binomial errors, logit link; lme4 package) was used to investigate the effect of sugar treatment on oocyst infection rate (Experiment 1) and sporozoite index (Experiment 2). GLMM with negative binomial errors (glmmADMB package) was used to test the effect of sugar treatment on oocyst intensity (Experiment 1). For these GLMMs, full models included sugar treatment and gametocytemia and their interaction as fixed effects. Binomial GLMM was also used to test the effect of sugar treatment, infection and gametocytemia on mosquito egg incidence (presence/absence of mature eggs in ovaries, Experiment 1). In all GLMMs, gametocyte carrier identity was included as a random effect. The effect of sugar treatment on mosquito survivorship was analyzed using Cox’s proportional hazard regression models ("coxph" function in the "survival" package) with (experiments 1 and 2) or without (experiment 3) censoring. Sugar treatment, gametocytemia, infection (for experiment 3 only) and their interaction were considered as explanatory variables. Model simplification used stepwise removal of terms, followed by likelihood ratio tests (LRT). Term removals that significantly reduced explanatory power (p < 0.05) were retained in the minimal adequate model.
Mathematical modeling
To quantify the consequences of plant sugar source on malaria transmission, we designed a mathematical model, grounded within the SIR framework [38,39], that accounts for the essential transmission processes: vector competence (i.e. infection level) and infectious potential (the period during which the mosquito can transmit the pathogen). We assumed that the mosquito population studied could be categorized into Susceptible individuals (Sm, i.e. that could be infected), which then moved to the Exposed category upon infection (Em, i.e. infected, but not yet infectious) and became Infectious (Im, i.e. mosquitoes that can transmit the pathogen). Infectious potential was characterized by two phases: a first phase of low infection level (when the oocysts crack up and sporozoites begin to invade mosquito salivary glands i.e. day 9 to days 10–11) followed by a second phase of higher infection levels (from day 10–11 until mosquito death). The duration and vector competence for each phase and each plant are detailed in the S2 Appendix. We also assumed similar categories for the human population (S2 Appendix). We simulated the expected outbreak size in a human population (number of individuals that has been infected at the end of the season when one infectious human was introduced into a population of 100 individuals) for each plant sugar. We explored the parameter space through a Latin hypercube sampling with 10,000 replicates for which all parameters were randomly chosen within their confidence interval based on the data measurements obtained experimentally here. Data are deposited in the Dryad repository: (DOI: doi:10.5061/dryad.9s690) [40]
Ethics statement
Ethical approval was obtained from the Centre Muraz Institutional Ethics Committee (A003-2012/CE-CM) and National Ethics Committee of Burkina Faso (2014–0040). Before enrollment, legal guardians of each child participant provided written consent on behalf of the minors. The protocol conforms to the declaration of Helsinki on ethical principles for medical research involving human subjects (version 2002).
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by both the Office of Laboratory Animal Welfare of US Public Health Service (Assurance Number: A5928-01) and national committee of Burkina Faso (IRB registration #00004738 and FWA 00007038). Animals were cared for by trained personnel and veterinarians.
Results
Plant sugar source influences the early development of P. falciparum and the vector’s survival and fecundity
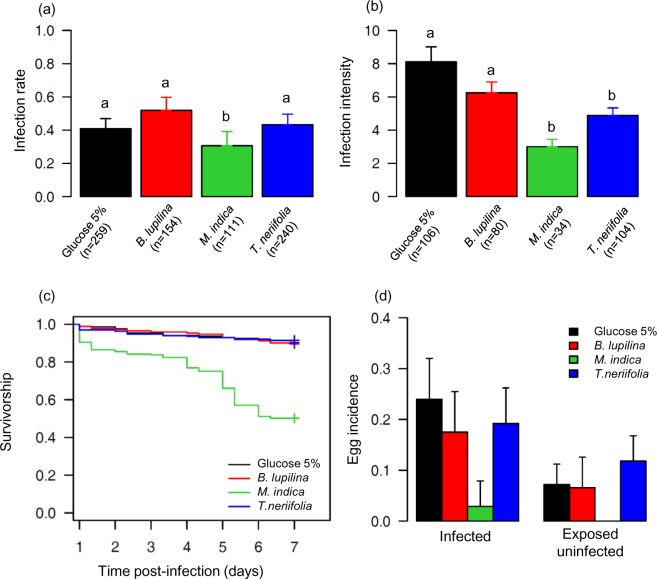
A total of 324 out of 764 (42.4%) An. coluzzii became successfully infected upon parasite exposure. The infection rate significantly varied among mosquitoes maintained on different sugar treatments (LRT X 2 3 = 13.2, P = 0.004; Fig 1A). Infection dose (i.e. gametocytemia) significantly affected parasite prevalence, with higher gametocyte density in blood leading to an increased likelihood of infection (X 2 1 inline = 5.2, P = 0.04). Finally, there was a significant gametocytemia by sugar treatment interaction (X 2 3 inline = 10.2, P = 0.017).
Fig 1. Effect of sugar treatment on the early development of P. falciparum, and on the survival and fecundity of malaria-exposed Anopheles coluzzii.
(a) Infection rate (± 95% CI), expressed as the proportion of mosquitoes exposed to an infectious blood meal and harboring at least one oocyst in their midgut, over 4 replicates and using a total of 7 gametocyte carriers. Numbers in brackets indicate the total number of mosquitoes dissected 7 days post infection (dpi) for each sugar treatment. Different letters above the bars denote statistically significant differences based on multiple pair-wise post-hoc tests. (b) Infection intensity (± se), expressed as the mean number of developing oocysts in the guts of infected females, over 4 replicates and using a total of 7 gametocyte carriers. Numbers in brackets indicate the total number of infected mosquitoes for each sugar treatment. Different letters above the bars denote statistically significant differences based on multiple pair-wise post-hoc tests. (c) Survivorship of malaria-exposed mosquitoes for each sugar treatment over 4 replicates and using a total of 7 gametocyte carriers. Survival was recorded twice a day from 1 to 7 dpi. (d) Egg incidence (± 95% CI) of malaria-exposed mosquitoes, expressed as the proportion of mosquito females carrying fully matured eggs inside their ovaries on 7 dpi for each sugar treatment and infection status.
The mean number of developing oocysts in infected females (i.e. intensity) significantly varied among sugar treatments (LRT X 2 3 = 19.8, P = 0.0002, Fig 1B). Gametocytemia had a positive effect on intensity (LRT X 2 1 = 4.6, P = 0.03), and there was a significant gametocytemia by sugar treatment interaction (LRT X 2 3 = 12.5, P = 0.006).
Sugar source was also linked to the probability of a mosquito surviving until 7 dpi (LRT X 2 3 = 80, P < 0.001; Fig 1C and S3 Table), with survivorship ranging from 92%, 90% and 89.5% on 5% glucose, T. neriifolia and B. lupilina, respectively, to 50% on M. indica. Egg incidence was reduced in mosquitoes fed on M. indica (LRT X 2 3 = 27, P < 0.001; Fig 1D and S3 Table). Infected females were more likely to carry eggs in their ovaries compared to females exposed to an infectious blood-meal but which remained uninfected (hereafter referred to as “exposed-uninfected”) (LRT X 2 1 = 8, P = 0.005; Fig 1D), irrespective of sugar treatment (i.e. no infection by sugar treatment interaction: LRT X 2 3 = 3.5, P = 0.32, Fig 1D). Among infected females, egg incidence was also positively associated with infection intensity (X2 1 = 5.8, P = 0.016, S3 Fig). Finally, there was no effect of gametocytemia on mosquito survival (LRT X 2 1 = 0.12, P = 0.72) and egg incidence (LRT X 2 1 = 1.3, P = 0.26).
Plant sugar source influences the sporozoite index and the parasite’s Extrinsic Incubation Period (EIP)
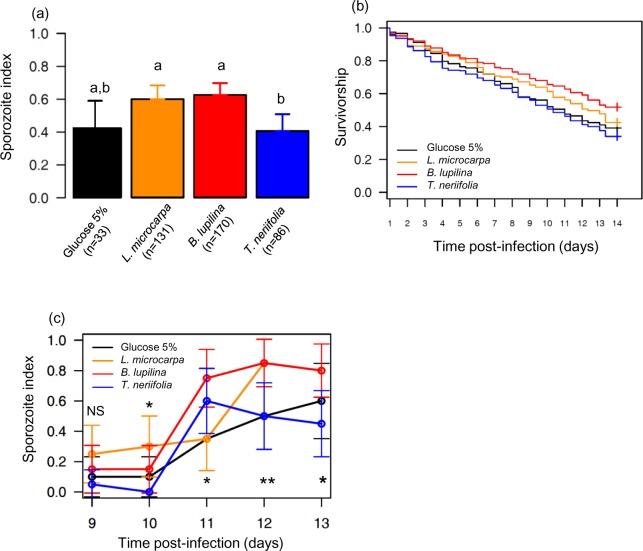
The aim of this experiment was to determine the extent to which natural plant sugar sources can affect the time of sporozoite release as well as the proportion of mosquitoes harboring sporozoites. A high mortality rate was observed in mosquitoes fed on M. indica (Fig 1C and preliminary data indicating a very low number of mosquitoes surviving until 14 dpi, the average incubation period of P. falciparum), hence, we replaced M. indica with Lannea microcarpa (S1 Fig), a less common fruit-derived sugar but one which provides relatively high mosquito survival rate.
Overall, a total of 235 out of 420 (56%) An. coluzzii harbored sporozoites 14 dpi. Sugar treatment had a significant effect on sporozoite index (LRT X 2 3 = 15; P = 0.002; Fig 2A), with mosquitoes fed on T. neriifolia being less likely to harbor disseminated sporozoites (Fig 2A). Gametocytemia positively influenced sporozoite index (LRT X 2 1 = 4, P = 0.046). We also found an effect of sugar treatment on mosquito survival (LRT X 2 3 = 26; P < 0.001, Fig 2B and S3 Table) with mosquitoes living longer when maintained on B. lupilina followed by L. microcarpa, 5% glucose and T. neriifolia.
Fig 2. Effect of sugar treatment on the sporozoite index and EIP.
(a) Sporozoite index (± 95% CI), expressed as the proportion of mosquitoes exposed to an infectious blood meal and having disseminated sporozoites in their head/thoraces, over 2 replicates and using a total of 4 gametocyte carriers. Numbers in brackets indicate, for each sugar treatment, the total number of mosquitoes analyzed with PCR on 14 days post infection (dpi). Different letters above the bars denote statistically significant differences based on multiple pair-wise post-hoc tests. (b) Survivorship of malaria-exposed mosquitoes for each sugar treatment over 2 replicates, and using a total of 4 gametocyte carriers. Survival was recorded twice a day from 1 to 14 dpi. (c) Sporozoite index (± 95% CI) over time and using a total of 2 gametocyte carriers. *p<0.05; **p < 0.01, NS: non-significant difference between sugar treatment
Sporozoites were observed as soon as 9 dpi (Fig 2C). Significant differences in sporozoite index between sugar treatments were observed from 10 to 14 dpi, indicating that the temporal dynamics of sporozoite dissemination varied (Fig 2C) depending on sugar treatment. As expected, there was a significant time effect with sporozoite index increasing over time (LRT X 2 1 = 94, P < 0.0001).
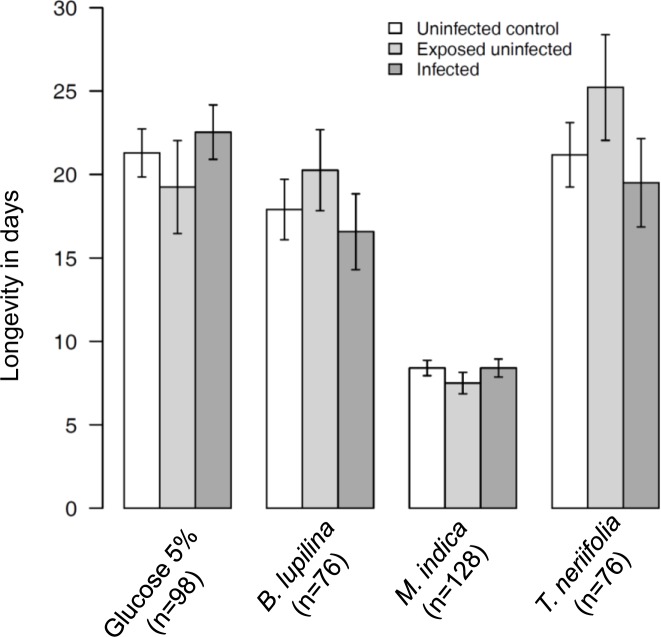
Plant sugar source similarly influences the longevity of infected and uninfected control mosquitoes
The previous experiment showed a reduced sporozoite index in T. neriifolia-fed mosquitoes (Fig 2A) compared to those fed on other sugar sources. Although, this can be an effect of the plant on parasite development and sporozoite dissemination, this observation is also consistent with an increased mortality of infected individuals compared to exposed-uninfected counterparts. In other words, there might be an interaction between infection and plant treatment, such that infected mosquitoes suffer increased mortality when reared on this species, and hence display reduced sporozoite index at 14 dpi. Therefore, we measured mosquito longevity using a factorial design, crossing infection status (infected with P. falciparum versus exposed-uninfected versus uninfected control) with sugar treatment. Uninfected control mosquitoes received a heat-treated gametocytic blood meal. Because M. indica showed an effect on both oocyst infection rate and intensity (Fig 1A and 1C), while L. microcarpa showed no effect on sporozoite dissemination (Fig 2A) compared to a 5% glucose solution, we used M. indica instead of L. microcarpa for this experiment.
Infection status had no effect on mosquito lifespan (LRT X 2 2 = 1; P = 0.6, Fig 3). Importantly, although mosquito longevity significantly varied among sugar treatment (LRT X 2 3 = 160; P < 0.001, Fig 3), there was no sugar treatment by infection status interactions (LRT X2 6 = 4.8; P = 0.56, Fig 3), demonstrating that the source of the sugar meals affected mosquito longevity irrespective of their infection status. We further analyzed the effect of infection status on the longevity of T. neriifolia-fed mosquitoes only and found no significant difference (LRT X2 2 = 1.6; P = 0.4). This suggests that the observed reduction in the sporozoite index of mosquitoes fed on T. neriifolia does not result from the influence of increased parasite virulence on mosquito lifespan.
Fig 3. Effects of sugar treatment and infection status on mosquito longevity.
Bars show the mean adult longevity ± 1 SE of uninfected control, exposed-uninfected and infected mosquitoes held on one of four sources of sugar. Numbers in brackets indicate, for each sugar treatment, the total number of mosquitoes monitored.
Plant sugar source affects the transmission intensity of malaria
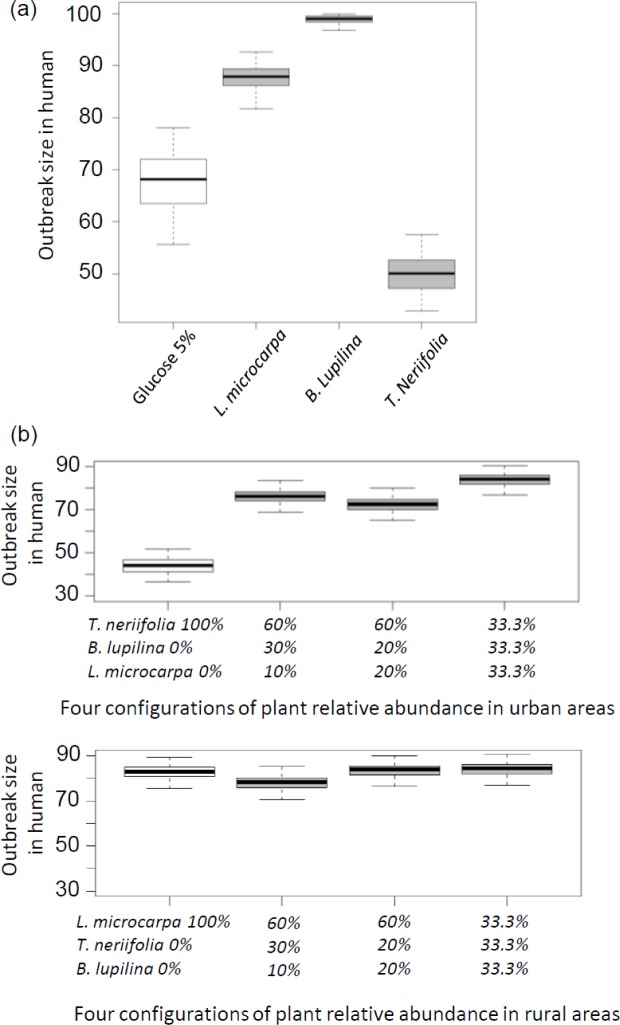
The epidemiological outcome predicted by the model for each plant species considered in isolation is consistent with intuitive expectations (Fig 4A). Compared to the baseline scenario with a 5% glucose solution, both L. microcarpa and B. lupilina caused an increased transmission potential, mainly because of increased infection rates among mosquitoes exposed to an infectious blood-meal (i.e. higher vector competence) (S4 Fig). In contrast, T. neriifolia induced a lower transmission potential, mainly because of a lower mosquito competence. as well as a longer infectious period (longevity).
Fig 4. Theoretical difference in the distribution of outbreak sizes among plant sugars.
(a) Epidemiological outcomes predicted by the model for each plant species considered in isolation, and (b) in a community perspective. In urban areas (upper panel), Thevetia neriifolia is relatively more abundant than Barleria lupilina and Lannea microcarpa. Four configurations with various relative abundances were considered: (i) a scenario of no diversity whereby T. neriifolia—the dominant plant—represents 100% of the feeding opportunities, (ii) a scenario of 60% T. neriifolia, 30% B. lupilina and 10% L. microcarpa, (iii) a scenario of 60–20–20%, and (iv) a scenario of equal feeding opportunities on each plant species. In rural areas (lower panel), L. microcarpa is relatively more abundant than T. neriifolia and B. lupilina. The same configurations as for urban areas were considered using this ranking. Outbreak size is the proportion of humans that has been infected at the end of the transmission season after one infectious human was introduced into the population. The box plots indicate the median (large horizontal bars), the 25th and 75th percentiles (squares), the minimum and maximum values (whiskers) and outliers (circles).
When considering these individual effects within a community perspective, including the various proportions of each plant species, contrasting results appeared (Fig 4B). We assumed that mosquitoes do not display preference for plant species, hence that feeding choice is solely dependent on plant relative abundance. First, when considering plant communities generally occurring in urban areas, malaria transmission potential was largely impacted by plant relative abundance (Fig 4B). In this setting, increasing plant diversity leads to a decrease in T. neriifolia relative abundance and hence results in higher levels of transmission. In contrast, the different configurations of plant relative abundance showed similar patterns of transmission in rural areas where T. neriifolia is less abundant.
Discussion
Feeding on different sources of natural plant-derived sugars can influence key traits that affect the capacity of mosquitoes to transmit malaria, including mosquito longevity and mosquito susceptibility to P. falciparum. When combined into an epidemiological model these effects have important consequences for malaria transmission. Compared to control mosquitoes fed on a 5% glucose solution, individuals fed on T. neriifolia showed a 30% decrease in malaria transmission. In contrast, mosquitoes fed on L. microcarpa and B. lupilina showed a 30% and 40% increase in transmission potential, respectively. Previous research has revealed the role of sugar in providing energy for flight, increasing mosquito survival and fecundity and decreasing biting rate on vertebrate hosts [12,23]. Our findings add a more direct effect of epidemiological importance by showing that plant-derived sugars can modulate mosquito-Plasmodium interactions.
The plant-mediated effect on infection could not be attributed to variation in blood meal size (S3 Appendix). Uptake of larger infectious blood meals may result in more gametocytes entering the mosquito midgut [41] and hence possibly increased infection. We tested whether plant sugar source could influence mosquito blood meal size. Mosquitoes fed with L. microcarpa prior to a blood meal took on average smaller blood meals than mosquitoes fed on glucose 5% or B. lupilina (S1 Appendix). However, infection in L. microcarpa-fed mosquitoes was not higher than that of glucose- or B. lupilina-fed individuals (Fig 2A). Furthermore, the results could not be attributed to mosquito inability to survive on particular plant sources such as M. indica owing to less feeding and eventual starvation (S4 Appendix).
At least three non-mutually exclusive mechanisms could explain the effects of plant sugar sources on mosquito competence for P. falciparum. First, there can be direct negative effects of plant secondary metabolites (e.g. alkaloids, terpenes, glycosides) on pathogen development. Secondary compounds generally play a role in plant defense against herbivores and thereby tend to be toxic and reduce host survivorship and reproduction [42,43]. These chemicals are found in all plant tissues, including floral nectars and fleshy fruits [44–47]. In bumblebees for example, the ingestion of alkaloids, terpenoids and iridoid glycosides contained in floral nectars can reduce infection by the protozoan parasite, Crithidia bombi [48,49]. Second, plants can enhance or reduce infection by modulating the host body condition and energetic status [50]. Feeding on poor quality resources may yield less energy to parasite growth and hence limit parasite infection [51]. Third, plants may indirectly influence infection through effects on host immune response or gut microbiota. Because immunological defenses are energetically expensive, hosts in poor condition may display reduced resistance to parasites [52]. In our experiments, mosquitoes fed with M. indica fruits or T. neriifolia flowers displayed reduced infection levels and reduced fitness (survivorship or fecundity), thus corroborating the first two mechanisms. T. neriifolia contain toxic cardiac glycosides, and mango fruits contain phenolic compounds, including mangiferin, two secondary metabolite groups suspected to have strong anti-protozoan properties [9,53–55]. In addition, previous studies have demonstrated that malaria parasites require sugars from the vector for an optimal development [56,57]. The relationships between nutrition, host general condition, toxic chemicals and infection are complex [58,59], and further studies are required to determine whether T. neriifolia and mango fruits have direct anti-plasmodium activities or have low nutritional value that limit both parasite growth and host fitness. The plant sugar source treatment was continuously provided in our study, thus it will be important to determine whether these effects result from the ingestion of plant-derived sugars prior (prophylactic effects) or post (therapeutic effects) infection. Mosquitoes from a colony continually replenished with F1 from wild-caught females were used here and it will also be important to study plant-mediated effects using field mosquitoes since rearing insects in the laboratory for many generations is unlikely to represent the genetic diversity observed in nature.
Under natural conditions, the frequency of contacts between a mosquito and a source of natural sugar will depend on the innate plant preference of the insect and environmental factors such as plant availability and accessibility. Our design did not allow the mosquitoes to choose between the different plants. We expect different epidemiological consequences when mosquitoes consume different proportions of each plant. Using our model, we simulated different scenarios of plant relative abundance in either urban (where T. neriifolia is relatively dominant compared to B. lupilina and L. microcarpa) or rural (where L. microcarpa dominates) areas. Our findings indicate that sharp epidemiological differences are indeed expected in urban areas where relatively small decreases in T. neriifolia abundance could lead to important increases in malaria transmission. This can be interpreted as an example of a dilution effect whereby poor-competent host species decreases the average efficiency of pathogen transmission [60,61]. In contrast, in rural areas, a decrease in L. microcarpa abundance in favor of T. neriifolia would only have a small negative impact on disease transmission (i.e. a weak dilution effect [60]).
Infected mosquitoes were more likely to carry eggs in their ovary compared to exposed-uninfected counterparts. This finding may illustrate a cost of resistance whereby the expression of an efficient immune response against Plasmodium establishment comes at a cost to egg production. Alternatively, this finding may illustrate the terminal investment hypothesis (also known as fecundity compensation), which postulates that when the future reproductive opportunities of an individual decline because of high mortality risk such as high infection levels, organisms increase their current reproductive investment [48]. We examined the first gonotrophic cycle only and further studies are needed to determine the mechanisms responsible for the increased probability of egg development in infected individuals.
Although we did not find any significant reductions in mosquito longevity or fecundity following Plasmodium infection, previous studies have demonstrated that this parasite can be costly to the mosquito host, especially in specific conditions reflecting what occurs in nature, such as nutritional, predation, insecticide or hydric stress [37,39,62–65]. Since Plasmodium infection decreases mosquito fitness, natural selection should favor the evolution of defenses against it. Besides immunological defenses, insect hosts can develop behavioral defenses including self-medication to better resist or tolerate their parasites [66,67]. Alternatively, malaria parasites might manipulate mosquito plant preference in ways that favor their own development and transmission [3,68]. A recent study found that P. falciparum infection increases both An. gambiae attraction to nectar sources and their total sugar uptake [69]. It will be of fundamental importance to better investigate the fitness costs and benefits of feeding on different natural sugar sources and to study plant preference by infected and uninfected mosquitoes. This will allow us to determine whether mosquito vectors or P. falciparum can exploit plant sugar sources to respectively self-medicate or manipulate the insect plant choice.
The interaction between malaria parasites and mosquito vector has drawn much attention for possible interventions to prevent transmission of this pathogen. Our findings have several implications for disease control. A number of different genetic manipulation strategies are now available to reduce mosquito competence for Plasmodium in the laboratory (refractory transgenic mosquitoes) [70–72]. In addition, the use of transmission-blocking vaccines against P. falciparum mosquito-stages has proven to be a promising strategy [73]. However, before translating these findings into the natural settings, it will be crucial to determine how the transgenes expression or vaccine efficacy can be affected by mosquito plant-feeding behavior. Finally, some novel perspective for malaria control including the cultivation of anti-Plasmodium plants may be truly sustainable. Concretely, fostering the planting of species such as T. neriifolia, which is commonly used by mosquitoes and which negatively affects vectorial capacity may locally reduce malaria transmission.
Supporting Information
(a) Thevetia neriifolia (syn: Thevetia peruviana. Casacabela thevetia; common name: yellow oleander. Fam: Apocynaceae) is an evergreen tropical shrub or small tree (up to 6 m) native of central South America. Yellow oleanders are common and widespread in villages and cities of West Africa where it is mostly used as a courtyard hedge. (b) Barleria lupilina (syn: Barleria macrostachys; common name: Hop-headed barleria; Fam: Acanthaceae) is a common ornamental shrub (about 1.5 m) in cities and villages of West Africa. (c) Mangifera indica (common name: mango; Fam: Anacardiaceae) is a large fruit-tree (up to 30 m) native of India, and widely distributed in West Africa for fruit consumption. In Burkina Faso, mango trees are commonly found in villages and cities within courtyards where they provide shade and fruit. (d) Lannea microcarpa (syn: L. microcarpa acida. L. microcarpa djalonica; common name: African grape. Fam: Anacardiaceae) is a tree (up to 15 m) indigenous of West Africa. Unlike mango, L. microcarpa species are not cultivated; they propagate naturally in the savanna vegetation and can occur in West African villages and cities.
(DOCX)
(a) Bundle of flowers of Thevetia neriifolia. (b) Bundle of flowers of Barleria lupilina. (c) Fruit of Mangifera indica. (d) Fruits of Lannea microcarpa microcarpa. (e) 5% glucose solution on cotton pads.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Raymond Hien, Jean Bazié, Fulgence Da and Baudoin Dabiré for technical help; all children and their parents for participating in this study and the local authorities for their support.
Data Availability
Data have been deposited in dryad: Plant-Mediated Effects on Mosquito Capacity to Transmit Human Malaria, doi:10.5061/dryad.9s690.
Funding Statement
The work was funded by the ANR grant 11-PDOC-006-01 to TL (http://www.agence-nationale-recherche.fr). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Li J, Wang X, Zhang G, Githure JI, Yan G, James AA. Genome-block expression-assisted association studies discover malaria resistance genes in Anopheles gambiae . Proc Natl Acad Sci U S A. 2013;110: 20675–80. 10.1073/pnas.1321024110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Molina-Cruz A, DeJong RJ, Ortega C, Haile A, Abban E, Rodrigues J, et al. Some strains of Plasmodium falciparum, a human malaria parasite, evade the complement-like system of Anopheles gambiae mosquitoes. Proc Natl Acad Sci U S A. 2012;109: E1957–62. 10.1073/pnas.1121183109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lefèvre T, Vantaux A, Dabiré KR, Mouline K, Cohuet A. Non-genetic determinants of mosquito competence for malaria parasites. PLoS Pathog. 2013;9: e1003365 10.1371/journal.ppat.1003365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lambrechts L, Halbert J, Durand P, Gouagna LC, Koella JC. Host genotype by parasite genotype interactions underlying the resistance of anopheline mosquitoes to Plasmodium falciparum . Malar J. 2005;4: 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Redmond SN, Eiglmeier K, Mitri C, Markianos K, Guelbeogo WM, Gneme A, et al. Association mapping by pooled sequencing identifies TOLL 11 as a protective factor against Plasmodium falciparum in Anopheles gambiae . BMC Genomics. 2015;16: 779 10.1186/s12864-015-2009-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gendrin M, Rodgers FH, Yerbanga RS, Ouédraogo JB, Basáñez M- G, Cohuet A, et al. Antibiotics in ingested human blood affect the mosquito microbiota and capacity to transmit malaria. Nat Commun. 2015;6: 5921 10.1038/ncomms6921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wolinska J, King KC. Environment can alter selection in host-parasite interactions. Trends Parasitol. 2009;25: 236–244. 10.1016/j.pt.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 8. Cory JS, Hoover K. Plant-mediated effects in insect-pathogen interactions. Trends Ecol Evol. 2006;21: 278–286. [DOI] [PubMed] [Google Scholar]
- 9. Lefèvre T, Oliver L, Hunter MD, De Roode JC, Letters E. Evidence for trans-generational medication in nature. Ecol Lett. 2010;13: 1485–1493. 10.1111/j.1461-0248.2010.01537.x [DOI] [PubMed] [Google Scholar]
- 10. Lee KP, Simpson SJ, Wilson K. Dietary protein-quality influences melanization and immune function in an insect. Funct Ecol. 2008;22: 1052–1061. [Google Scholar]
- 11. Foster WA. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol. 1995;40: 443–474. [DOI] [PubMed] [Google Scholar]
- 12. Stone CM, Foster WA. Plant-sugar feeding and vectorial capacity In: Takken W, Koenraadt CJM, editors. Ecology of parasite-vector interactions, Volume 3 Wageningen Academic Publishers; 2013. pp. 35–79. [Google Scholar]
- 13. Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, et al. Field experiments of Anopheles gambiae attraction to local fruits / seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malar J. 2010;9: 262 10.1186/1475-2875-9-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nyasembe VO, Teal PEA, Mukabana WR, Tumlinson JH, Torto B. Behavioural response of the malaria vector Anopheles gambiae to host plant volatiles and synthetic blends. Parasit Vectors. 2012;5: 234 10.1186/1756-3305-5-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nikbakhtzadeh MR, Ii JWT, Otienoburu PE, Foster WA. Olfactory basis of floral preference of the malaria vector Anopheles gambiae (Diptera : Culicidae) among common African plants. J Vector Ecol. 2014;39: 372–383. 10.1111/jvec.12113 [DOI] [PubMed] [Google Scholar]
- 16. Manda H, Gouagna LC, Nyandat E, Kabiru EW, Jackson RR, Foster W a, et al. Discriminative feeding behaviour of Anopheles gambiae s.s. on endemic plants in western Kenya. Med Vet Entomol. 2007;21: 103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gary RE, Foster WA. Effects of available sugar on the reproductive fitness and vectorial capacity of the malaria vector Anopheles gambiae (Diptera : Culicidae). J Med Entomol. 2001;38: 22–28. [DOI] [PubMed] [Google Scholar]
- 18. Impoinvil DE, Kongere JO, Foster WA, Njiru BN, Killeen GF, Githure JI, et al. Feeding and survival of the malaria vector Anopheles gambiae on plants growing in Kenya. Med Vet Entomol. 2004;18: 108–115. [DOI] [PubMed] [Google Scholar]
- 19. Gary RE, Foster WA. Anopheles gambiae feeding and survival on honeydew and extra-floral nectar of peridomestic plants. Med Vet Entomol. 2004;18: 102–107. [DOI] [PubMed] [Google Scholar]
- 20. Manda H, Gouagna LC, Foster WA, Jackson RR, Beier JC, Githure JI, et al. Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae . Malar J. 2007;6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stone CM, Jackson BT, Foster WA. Effects of plant-community composition on the vectorial capacity and fitness of the malaria mosquito Anopheles gambiae . Am J Trop Med Hyg. 2012;87: 727–736. 10.4269/ajtmh.2012.12-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gu WD, Muller G, Schlein Y, Novak RJ, Beier JC. Natural plant sugar sources of Anopheles mosquitoes strongly impact malaria transmission potential. PLoS One. 2011;6: e15996 10.1371/journal.pone.0015996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu L, Qualls W a, Marshall JM, Arheart KL, DeAngelis DL, McManus JW, et al. A spatial individual-based model predicting a great impact of copious sugar sources and resting sites on survival of Anopheles gambiae and malaria parasite transmission. Malar J. 2015;14: 59 10.1186/s12936-015-0555-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nyasembe VO, Cheseto X, Kaplan F, Foster WA, Teal PEA, Tumlinson JH, et al. The invasive American weed Parthenium hysterophorus can negatively impact malaria control in Africa. PLoS One. 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suwanchaichinda C, Paskewitz SM. Effects of larval nutrition, adult body size, and adult temperature on the ability of Anopheles gambiae (Diptera: Culicidae) to melanize sephadex beads. J Med Entomol. 1998;35: 157–161. [DOI] [PubMed] [Google Scholar]
- 26. Lambrechts L, Chavatte JJ- M, Snounou G, Koella JC. Environmental influence on the genetic basis of mosquito resistance to malaria parasites. Proc R Soc B Biol Sci. 2006;273: 1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferguson HM, Read AF. Genetic and environmental determinants of malaria parasite virulence in mosquitoes. Proc R Soc B Biol Sci. 2002;269: 1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Okech BA, Gouagna LC, Kabiru EW, Beier JC, Yan G, Githure JI. Influence of age and previous diet of Anopheles gambiae on the infectivity of natural Plasmodium falciparum gametocytes from human volunteers. J Insect Sci. 2004;4: 33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koella JC, Sorensen FL. Effect of adult nutrition on the melanization immune response of the malaria vector Anopheles stephensi . Med Vet Entomol. 2002;16: 316–320. [DOI] [PubMed] [Google Scholar]
- 30. Kelly R, Edman JD. Infection and transmission of Plasmodium gallinaceum (Eucoccida: Plasmodiidae) in Aedes aegypti (Diptera: Culicidae): effect of preinfection sugar meals and postinfection blood meals. J Vector Ecol. 1997;22: 36–42. [PubMed] [Google Scholar]
- 31. Gouagna L, Poueme RS, Dabiré KR, Ouédraogo J, Fontenille D, Simard F. Patterns of sugar feeding and host plant preferences in adult males of An. gambiae (Diptera : Culicidae). J Vector Ecol. 2010;35: 267–276. 10.1111/j.1948-7134.2010.00082.x [DOI] [PubMed] [Google Scholar]
- 32. Van Handel E. The detection of nectar in mosquitoes. Mosq News. 1972;32: 458. [Google Scholar]
- 33. Vantaux A, Dabiré KR, Cohuet A, Lefèvre T. A heavy legacy: offspring of malaria-infected mosquitoes show reduced disease resistance. Malar J. 2014;13: 442 10.1186/1475-2875-13-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vantaux A, de Sales Hien DF, Yameogo B, Dabiré KR, Thomas F, Cohuet A, et al. Host-seeking behaviors of mosquitoes experimentally infected with sympatric field isolates of the human malaria parasite Plasmodium falciparum: no evidence for host manipulation. Front Ecol Evol. 2015;3: 1–12. [Google Scholar]
- 35. Morassin B, Fabre R, Berry A, Magnaval JF. One year’s experience with the polymerase chain reaction as a routine method for the diagnosis of imported malaria. Am J Trop Med Hyg. 2002;66: 503–508. [DOI] [PubMed] [Google Scholar]
- 36. Sangare I, Michalakis Y, Yameogo B, Dabire R, Morlais I, Cohuet A. Studying fitness cost of Plasmodium falciparum infection in malaria vectors: validation of an appropriate negative control. Malar J. 2013;12: 2 10.1186/1475-2875-12-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alout H, Yameogo B, Djogbénou LS, Chandre F, Dabiré RK, Corbel V, et al. Interplay between Plasmodium infection and resistance to insecticides in vector mosquitoes. J Infect Dis. 2014;210: 1464–1470. 10.1093/infdis/jiu276 [DOI] [PubMed] [Google Scholar]
- 38. Keeling MJ, Rohani P. Modeling infectious diseases in humans and animals Princeton University Press; 2008. [Google Scholar]
- 39. Roux O, Vantaux A, Roche B, Yameogo B, Dabiré KR, Diabaté A, et al. Evidence for carry-over effects of predator exposure on pathogen transmission potential. Proc R Soc B Biol Sci. 2015;282: 20152430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hien DF, Dabiré K, Roche B, Diabaté A, Yerbanga R, Cohuet A, et al. Data from: Plant-mediated effects on mosquito capacity to transmit human malaria. Dryad Digital Repository. 10.5061/dryad.9s690. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pichon G, Awono-Ambene HP, Robert V. High heterogeneity in the number of Plasmodium falciparum gametocytes in the bloodmeal of mosquitoes fed on the same host. Parasitology. 2000;121: 115–120. [DOI] [PubMed] [Google Scholar]
- 42. Stam JM, Kroes A, Li Y, Gols R, van Loon JJ a, Poelman EH, et al. Plant interactions with multiple insect herbivores: from community to genes. Annu Rev Plant Biol. 2014;65: 689–713. 10.1146/annurev-arplant-050213-035937 [DOI] [PubMed] [Google Scholar]
- 43. Ode PJ. Plant chemistry and natural enemy fitness: effects on herbivore and natural enemy interactions. 2006;51: 163–185. [DOI] [PubMed] [Google Scholar]
- 44. Adler LS. The ecological significance of toxic nectar. Oikos. 2001;3: 409–420. [Google Scholar]
- 45. Cipollini ML, Levey DJ. Secondary metabolites of fleshy vertebrate-dispersed fruits: adaptive hypotheses and implications for seed dispersal. Am Nat. 1997;150: 346–372. 10.1086/286069 [DOI] [PubMed] [Google Scholar]
- 46. Baker HG. Non-sugar chemical constituents of nectar. Apidologie. 1977;8: 349–356. [Google Scholar]
- 47. Whitehead SR, Bowers MD. Evidence for the adaptive significance of secondary compounds in vertebrate-dispersed fruits. Am Nat. 2013;182: 563–577. 10.1086/673258 [DOI] [PubMed] [Google Scholar]
- 48. Manson JS, Otterstatter MC, Thomson JD. Consumption of a nectar alkaloid reduces pathogen load in bumble bees. Oecologia. 2010;162: 81–89. 10.1007/s00442-009-1431-9 [DOI] [PubMed] [Google Scholar]
- 49. Richardson LL, Adler LS, Leonard AS, Andicoechea J, Regan KH, Anthony WE, et al. Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proc R Soc B Biol Sci. 2015;282: 20142471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cressler CE, Nelson WA, Day T, Mccauley E. Disentangling the interaction among host resources, the immune system and pathogens. Ecol Lett. 2014;17: 284–293. 10.1111/ele.12229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hall SR, Knight CJ, Becker CR, Duffy MA, Tessier AJ, Caceres CE. Quality matters: resource quality for hosts and the timing of epidemics. Ecol Lett. 2009;12: 118–128. 10.1111/j.1461-0248.2008.01264.x [DOI] [PubMed] [Google Scholar]
- 52. Sheldon BC, Verhulst S. Ecological immunity: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol. 1996;11: 317–321. [DOI] [PubMed] [Google Scholar]
- 53. Gowler CD, Leon KE, Hunter MD, De Roode JC. Secondary defense chemicals in milkweed reduce parasite infection in monarch butterflies, Danaus plexippus . J Chem Ecol. 2015;41: 520–523. 10.1007/s10886-015-0586-6 [DOI] [PubMed] [Google Scholar]
- 54. Shah K, Patel M, Patel R, Parmar P. Mangifera Indica (Mango). Pharmacogn Rev. 2010;4: 42 10.4103/0973-7847.65325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ibrahim N, Mathew L. Comparative analysis of cardiac glycosides and quantification of peruvoside from Thevetia neriifolia, Juss fruit rind extracts through HPLTC fingerprinting. Int J Pharm Pharm Sci. 2014;6: 412–415. [Google Scholar]
- 56. Liu K, Dong Y, Huang Y, Rasgon JL, Agre P. Impact of trehalose transporter knockdown on Anopheles gambiae stress adaptation and susceptibility to Plasmodium falciparum infection. Proc Natl Acad Sci U S A. 2013;110: 17504–9. 10.1073/pnas.1316709110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mack SR, Samuels S, Vanderberg JP. Hemolymph of Anopheles stephensi from noninfected and Plasmodium berghei-infected mosquitoes. 3. Carbohydrates. J Parasitol. 1979;65: 217–221. [PubMed] [Google Scholar]
- 58. Ponton F, Wilson K, Cotter SC, Raubenheimer D, Simpson SJ. Nutritional immunology: a multi-dimensional approach. PLoS Pathog. 2011;7: e1002223 10.1371/journal.ppat.1002223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mason PA, Singer MS. Defensive mixology: combining acquired chemicals towards defence. Funct Ecol. 2015;29: 441–450. [Google Scholar]
- 60. Ostfeld RS, Keesing F. Effects of host diversity on infectious disease. Annu Rev Ecol Evol Syst. 2011;43: 157–182. [Google Scholar]
- 61. Roche B, Rohani P, Dobson AP, Guégan J- F. The impact of community organization on vector-borne pathogens. Am Nat. 2013;181: 1–11. 10.1086/668591 [DOI] [PubMed] [Google Scholar]
- 62. Sangare I, Dabire R, Yameogo B, Da DF, Michalakis Y, Cohuet A. Stress dependent infection cost of the human malaria agent Plasmodium falciparum on its natural vector Anopheles coluzzii . Infect Genet Evol. 2014;25: 57–65. 10.1016/j.meegid.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 63. Lalubin F, Delédevant A, Glaizot O, Christe P. Natural malaria infection reduces starvation resistance of nutritionally stressed mosquitoes. J Anim Ecol. 2014;83: 850–857. 10.1111/1365-2656.12190 [DOI] [PubMed] [Google Scholar]
- 64. Aboagye-Antwi F, Guindo A, Traore AS, Hurd H, Coulibaly M, Traore S, et al. Hydric stress-dependent effects of Plasmodium falciparum infection on the survival of wild-caught Anopheles gambiae female mosquitoes. Malar J. 2010;9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ahmed AM, Hurd H. Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiae via follicular apoptosis. Microbes Infect. 2006;8: 308–315. [DOI] [PubMed] [Google Scholar]
- 66. De Roode JC, Lefèvre T. Behavioral Immunity in Insects. Insects. 2012;3: 789–820. 10.3390/insects3030789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. de Roode JC, Lefèvre T, Hunter MD. Ecology. Self-medication in animals. Science (80-). 2013;340: 150–1. [DOI] [PubMed] [Google Scholar]
- 68. Lefèvre T, Thomas F. Behind the scene, something else is pulling the strings: Emphasizing parasitic manipulation in vector-borne diseases. Infect Genet Evol. 2008;8: 504–519. [DOI] [PubMed] [Google Scholar]
- 69. Nyasembe VO, Teal PEA, Sawa P, Tumlinson JH, Borgemeister C, Torto B. Plasmodium falciparum infection increases Anopheles gambiae attraction to nectar sources and sugar uptake. Curr Biol. 2014;24: 217–221. 10.1016/j.cub.2013.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Isaacs AT, Li F, Jasinskiene N, Chen X, Nirmala X, Vinetz JM, et al. Engineered resistance to Plasmodium falciparum development in transgenic Anopheles stephensi . PLoS Pathog. 2011;7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dong Y, Das S, Cirimotich CM, Souza-Neto JA, McLean KJ, Dimopoulos G. Engineered Anopheles immunity to Plasmodium infection. PLoS Pathog. 2011;7: e1002458 10.1371/journal.ppat.1002458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang S, Jacobs-Lorena M. Genetic approaches to interfere with malaria transmission by vector mosquitoes. Trends Biotechnol. 2013;31: 185–193. 10.1016/j.tibtech.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wu Y, Sinden RE, Churcher TS, Tsuboi T, Yusibov V. Development of malaria transmission-blocking vaccines: from concept to product. Adv Parasitol. 2015;89: 109–52. 10.1016/bs.apar.2015.04.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Thevetia neriifolia (syn: Thevetia peruviana. Casacabela thevetia; common name: yellow oleander. Fam: Apocynaceae) is an evergreen tropical shrub or small tree (up to 6 m) native of central South America. Yellow oleanders are common and widespread in villages and cities of West Africa where it is mostly used as a courtyard hedge. (b) Barleria lupilina (syn: Barleria macrostachys; common name: Hop-headed barleria; Fam: Acanthaceae) is a common ornamental shrub (about 1.5 m) in cities and villages of West Africa. (c) Mangifera indica (common name: mango; Fam: Anacardiaceae) is a large fruit-tree (up to 30 m) native of India, and widely distributed in West Africa for fruit consumption. In Burkina Faso, mango trees are commonly found in villages and cities within courtyards where they provide shade and fruit. (d) Lannea microcarpa (syn: L. microcarpa acida. L. microcarpa djalonica; common name: African grape. Fam: Anacardiaceae) is a tree (up to 15 m) indigenous of West Africa. Unlike mango, L. microcarpa species are not cultivated; they propagate naturally in the savanna vegetation and can occur in West African villages and cities.
(DOCX)
(a) Bundle of flowers of Thevetia neriifolia. (b) Bundle of flowers of Barleria lupilina. (c) Fruit of Mangifera indica. (d) Fruits of Lannea microcarpa microcarpa. (e) 5% glucose solution on cotton pads.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Data have been deposited in dryad: Plant-Mediated Effects on Mosquito Capacity to Transmit Human Malaria, doi:10.5061/dryad.9s690.