Abstract
Background
Cryptococcal meningitis (CM) is a leading cause of HIV-associated mortality. In clinical trials evaluating treatments for CM, biomarkers of early fungicidal activity (EFA) in cerebrospinal fluid (CSF) have been proposed as candidate surrogate endpoints for all- cause mortality (ACM). However, there has been no systematic evaluation of the group-level or trial-level evidence for EFA as a candidate surrogate endpoint for ACM.
Methods
We conducted a systematic review of randomized trials in treatment of CM to evaluate available evidence for EFA measured as culture negativity at 2 weeks/10 weeks and slope of EFA as candidate surrogate endpoints for ACM. We performed sensitivity analysis on superiority trials and high quality trials as determined by Cochrane measures of trial bias.
Results
Twenty-seven trials including 2854 patients met inclusion criteria. Mean ACM was 15.8% at 2 weeks and 27.0% at 10 weeks with no overall significant difference between test and control groups. There was a statistically significant group-level correlation between average EFA and ACM at 10 weeks but not at 2 weeks. There was also no statistically significant group-level correlation between CFU culture negativity at 2weeks/10weeks or average EFA slope at 10 weeks. A statistically significant trial-level correlation was identified between EFA slope and ACM at 2 weeks, but is likely misleading, as there was no treatment effect on ACM.
Conclusions
Mortality remains high in short time periods in CM clinical trials. Using published data and Institute of Medicine criteria, evidence for use of EFA as a surrogate endpoint for ACM is insufficient and could provide misleading results from clinical trials. ACM should be used as a primary endpoint evaluating treatments for cryptococcal meningitis.
Introduction
Cryptococcal meningitis (CM) is a neglected disease, although it is a leading cause of HIV/AIDS-associated mortality in sub-Saharan Africa with an estimated half million deaths yearly. [1] Combined antiretroviral therapy (cART) has decreased HIV/AIDS–associated mortality in the developed world such as the United States [2]; however, access to cART is still limited in many regions globally and is often a first presentation of HIV infection. In addition, even after ‘standard of care’ therapy, mortality remains high; thus, effective antifungal therapies are still needed to decrease mortality and disability caused by CM.[3] To facilitate drug development, authors have hypothesized that microbiological biomarkers may serve as candidate trial-level surrogate endpoints replacing all-cause mortality (ACM) in assessing treatment effects of antifungal drugs in CM to decrease the number of enrolled participants in trials.[4] Differences in proportions of cerebrospinal fluid (CSF) cultures below the level of detection (CFU<LOD; referred to here as CSF culture negativity) at 2 and 10 weeks and the difference in mean slope of quantitative cultures based on the number of colony forming units (CFUs) in the CSF over time (EFA) have been the most commonly used measurements of microbiological clearance in patients with CM.[5–12] Indeed, this biomarker was utilized as part of a composite endpoint to recommend trial suspension in a recent trial of adjunctive corticosteroids in treating CM [13]. However, prior to implementation, rigorous analysis of such endpoints will help delineate the advantages and limitations of biomarkers in clinical studies and patient care. Indeed, confusion in the use of a microbial biomarker in the treatment of AIDS-associated mycobacterial disease with clarithromycin may have contributed to excess trial mortality in one recent study [14].
The Institute of Medicine (IOM) framework for evaluation of biomarkers as candidate surrogate endpoints recommends both analytical validation and qualification assessments for biomarker evaluation. Evaluation of analytical validity of biomarkers includes assessments of reliability, reproducibility, standardization and quality controls. Qualification requires two further criteria to consider a biomarker as a valid candidate surrogate endpoint. The biomarker should serve as a group-level correlate with the true direct clinical endpoint (ACM in this disease) regardless of therapy. This requires evaluation of a correlation between quantitative changes in the biomarker with changes in the direct measure of patient benefit independent of treatment (higher or lower concentrations of CFUs in the CSF or higher/lower slope of EFA with ACM regardless of which, or any, treatment received without a test or control group) across multiple studies. The next criteria is that the biomarker should serve as a trial-level surrogate in that a net treatment effect (difference in outcome between the test and control group related to treatment) of interventions on the biomarker should capture the net treatment effect of the intervention on the clinical endpoint [15]. In other words, there should be treatment related changes between the test and control group on both the biomarker and the direct outcome of interest. This is based on an evaluation of differences between test and control group on the biomarker (EFA) compared to differences in the direct patient outcome (ACM). Implicit in this construction is that the treatment has an effect on both the biomarker and the direct patient endpoint.[16] For general applicability, the biomarker should be valid across a number of study conditions with different drugs and different patient populations. The IOM recommends an initial evaluation of a biomarker as a surrogate endpoint should consider all studies in a disease area in a trial-level meta-analysis.[17] [18] Such analyses have been carried out for various surrogate endpoints like progression free survival in oncology and HIV viral load in AIDS.[18] [19,20]
Although EFA has been shown to correlate with ACM independent of treatment in several individual clinical trials (group-level surrogacy) [21], group-level correlations have not been assessed across all trials. Furthermore, while these correlations represent a first step in evaluation of a biomarker they do not assess the relationship between treatment related changes in EFA and treatment effects on ACM (trial-level surrogacy). Further evaluation at the trial level of treatment effects across all trial evidence is needed to recommend the use of EFA as a reliable surrogate endpoint to assess treatment effects on ACM. To date there has been no systematic trial-level analysis of EFA as a candidate surrogate endpoint in CM. Therefore, we evaluated the available evidence for the biomarkers of cryptococcal EFA slope and culture negativity on both criteria for candidate surrogate endpoints: 1) group-level correlations of measures of EFA with ACM independent of treatment across high quality, randomized control trials, and 2) trial-level treatment effects (differences between test and control groups) on the surrogate of EFA analyzed by two different methods (CSF culture negativity at fixed time points and slope of change in CFUs in CSF [EFA slope]) compared to treatment effects on ACM at 2 and 10 weeks in a systematic review of the literature.
Materials and Methods
Search strategy
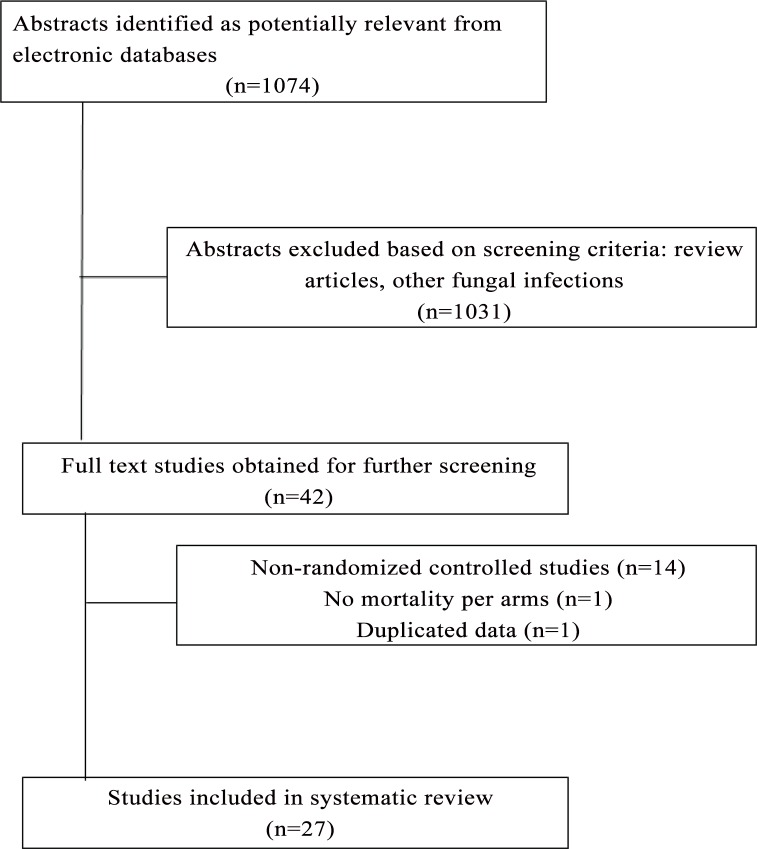
The search sources included Embase, WOS, Scopus, Pubmed, Cochrane Library and Google Scholar through April 22, 2013. The search terms included “cryptococcal meningitis” and “cryptococcosis”. Studies were included if they were randomized trials on treatment of CM. Non-randomized studies, duplicate data, or prevention or maintenance therapy trials for CM were excluded. Microbiological outcomes including average EFA slope and proportions of patients with CSF culture negativity at 2 or 10 weeks and all-cause mortality data at 2 or 10 weeks were extracted (Fig 1).
Fig 1. Flow diagram of search strategy and studies selected.
Data extraction
Extraction of data was conducted by one investigator and was reviewed by 2 independent investigators. The protocol for the present review was registered in PROSPERO, international prospective register of systematic review from the Centre for Review and Dissemination, University of York (PROSPERO2013:CRD42013003726).
Risk of bias assessment
To evaluate the quality of the studies we used the Cochrane Collaboration´s tool for assessing risk of bias [22]. Each study was evaluated for 7 types of potential biases. The category of “other bias” included studies with no stated hypotheses and studies with small numbers of patients randomized as studies with less than 20 patients per study arm were considered to have high risk for bias. “Unclear risk” was assigned when information was not available for the respective bias assessment.
Included studies were conducted in the US, Canada, India, Vietnam, Thailand, Indonesia, Laos, Vietnam, Uganda, South Africa, Malawi, Botswana, Zimbabwe and Netherlands.
Antifungal interventions evaluated in the studies were: Amphotericin B deoxycolate (A), liposomal Amphotericin B, lipid complex Amphotericin B, fluconazole (FLU), voriconazole, itraconazole, interferon gamma, rifampin and flucytosine (F) alone or in combination. Early versus delayed cART was evaluated in two studies. One study evaluated the effect of dexamethasone in patients receiving antifungal standard of care. Nineteen studies compared 2 treatment groups, 5 studies compared 3 treatment groups and 3 studies compared 4 treatment groups. (S1 Table)
We defined higher quality studies as those performed in HIV patients in which 3 or more elements were listed as “low risk for bias” and 2 or less elements were listed as “high risk” of bias. The number of “unclear risk” elements in the quality assessment was not considered in evaluating studies for the sensitivity analysis on higher quality studies.
We evaluated correlations of ACM with average EFA slope and proportion of patients with CSF culture negativity at 2 and 10 weeks, and analyses were repeated limited to higher quality studies according to the Cochrane Collaboration´s tool and studies with superiority hypotheses, since trials with non-inferiority hypotheses may minimize differences between test and control groups.
Statistical Analysis
Descriptive statistics were used to evaluate the results of each treatment group on EFA and ACM. A weighted least squares method with generalized estimating equations (GEE) was used to estimate the relationship between ACM and various measures of EFA. The data from each study arm was weighted by the sample size. Group-level correlation between the mortality rates from treatment groups within the same study was addressed by GEE using study as a cluster variable with a working independence model. The method of Fay and Graubard was used to provide accurate p-values for hypothesis testing.[23]
Analyses to assess trial-level treatment effects were performed in studies comparing control groups usually consisting of fluconazole (FLC, or Amphotericin B (A) regimens alone or in combination with flucytosine (AF) compared to test interventions including various drug combinations, different doses of drugs, lipid formulations of Amphotericin B, interferon, or dexamethasone. (S1 Table)
Between treatment-assignment group contrasts were formed for the test groups compared to the control groups. We tested for trial-level treatment effects on ACM and measures of EFA using weighted least squares as described above with weights proportional to the estimated variance of the contrasts. Study was used as a cluster variable.
We conducted and reported this study using the PRISMA guidelines for systematic reviews.[24]
Results
Study characteristics
One-thousand seventy four publications were retrieved from the search, of which 1047 were excluded based on a priori exclusion criteria (Fig 1). Twenty-seven studies (n = 2854 patients) were included in this review, encompassing 65 treatment-assignment groups (study arms) and 38 comparisons of test and control groups. Twenty-five studies were in HIV/AIDS patients and 2 studies in non-HIV/AIDS patients; 13 higher quality studies (low bias highlighted in green) were selected for further analysis based on the Cochrane Collaboration tool for assessing risk of bias (Table 1) [22].
Table 1. Cochrane´s assessment tool for assessing risk of bias.
| Author(s) | Year of publication | Random Sequence generation (Selection bias) | Allocation Concealment (Selection bias) | Blinding of participants and researchers (Performance bias) | Blinding of outcome assessment (Detection bias) | Incomplete outcome data (Attrition bias) | Selective reporting (Reporting bias) | Other bias |
|---|---|---|---|---|---|---|---|---|
| Studies in HIV patients | ||||||||
| Jarvis et al [5] | 2012 | Low | Unclear | High | Unclear | Low | Low | Low |
| Techapornroong et al [31] | 2007 | Low | Low | Low | Unclear | High | High | High |
| Brouwer et al [6] | 2004 | Low | Unclear | High | Unclear | Low | Low | High |
| Nussbaum et al [7] | 2009 | Low | Unclear | Unclear | Unclear | Low | Low | Low |
| Mayanja-Kizza et al [32] | 1998 | Low | Unclear | Unclear | Unclear | Low | High | High |
| Hamill et al [33] | 2010 | Low | Unclear | Low | Unclear | Low | Low | Low |
| Chotmongkol et al [34] | 1997 | High | Unclear | Unclear | Unclear | Low | Low | High |
| Tansuphaswadikul et al [35] | 2006 | Unclear | Unclear | Unclear | Unclear | High | High | High |
| Loyse et al [8] | 2012 | Low | Unclear | Unclear | Unclear | Low | Low | High |
| Bicanic et al [9] | 2008 | Low | Unclear | High | Unclear | Low | Low | Low |
| Chotmongkol et al [36] | 2005 | Unclear | Unclear | High | Unclear | Low | Low | High |
| Jadhav et al [37] | 2010 | Low | Low | Unclear | Unclear | High | High | High |
| Jackson et al [10] | 2012 | Low | Unclear | Unclear | Unclear | Low | Low | High |
| Pappas et al [38] | 2009 | Unclear | Unclear | High | Unclear | Low | Low | Low |
| Pappas et al [39] | 2004 | Unclear | Unclear | Unclear | Unclear | Low | Low | High |
| Bisson et al [11] | 2013 | Unclear | Unclear | High | Unclear | Low | Low | High |
| Larsen et al [40] | 1990 | Unclear | Unclear | Unclear | Unclear | Low | Low | High |
| de Gans et al [41] | 1992 | Unclear | Unclear | Unclear | Unclear | Low | Low | High |
| Leenders et al [42] | 1997 | Low | Low | Unclear | Unclear | High | High | High |
| van der Horst et al [43] | 1997 | Unclear | Unclear | Unclear | Unclear | Low | Low | Low |
| Day et al [44] | 2013 | Low | Unclear | High | Unclear | Low | Low | Low |
| Makadzange et al [45] | 2010 | Low | Low | High | Unclear | Low | Low | Low |
| Saag et al [46] | 1992 | Low | Unclear | Unclear | Unclear | Low | Low | Low |
| Sharkey et al [47] | 1996 | Unclear | Unclear | Unclear | Unclear | High | High | High |
| Beardsley et al [13] | 2016 | Low | Low | Unclear | Unclear | Low | Low | Low |
| Studies including non-HIV patients | ||||||||
| Bennett et al [48] | 1979 | Low | Unclear | Unclear | Unclear | Low | Low | Low |
| Dismukes et al [49] | 1987 | Unclear | Unclear | Unclear | Unclear | High | High | High |
The mean CD4 count was reported in 19 of 25 studies in HIV/AIDS and ranged from 9 to 100 cells mm3. Mean HIV viral load reported in 8 studies ranged from 98,752 to 398,107 copies/ml. Mean age among all studies ranged from 28 to 52 years old.
Event rates for microbiological and mortality outcomes
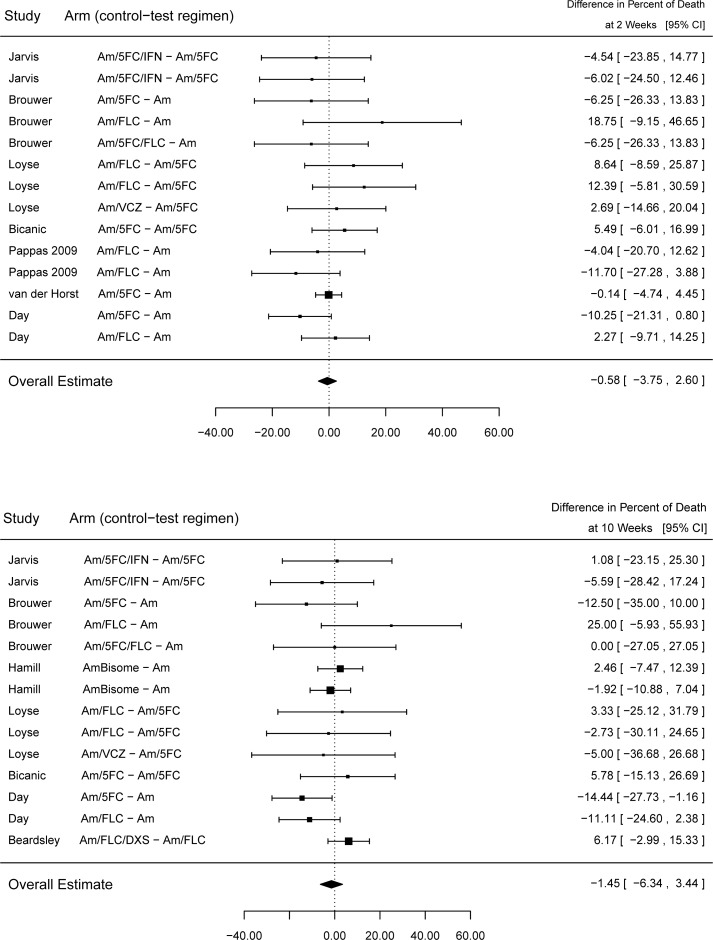
Not all data was available in every study. Mean ACM was 15.8% and 27.0% at 2 weeks (17 studies, 41 arms) and 10 weeks (17 studies, 40 arms). There was no statistically significant overall treatment effect among trials on ACM with a mortality difference of -0.58% (95% CI -3.75% to +2.60%) at 2 weeks and 1.45% (95% CI -6.34% to +3.44%) at 10 weeks. (Fig 2, top panel)
Fig 2. ACM differences between test and control groups at 2 weeks and 10 weeks.
All studies with non-missing data are displayed. There are 7 studies and 21 arms.
Mean rates of CSF culture negativity were 42.5% (SD ±0.24) and 63.3% (SD ±0.20) at 2 weeks (11 studies, 27 treatment groups) and 10 weeks (5 studies, 10 treatment groups), respectively. Mean EFA slope was -0.41 cfu/ml CSF/day (SD ±0.13) among studies that measured this microbiological outcome (9 studies, 24 treatment groups).
Among studies that reported CSF culture negativity at 2 weeks, 8 studies reported ACM at 2 weeks, and 5 studies at 10 weeks. Mean EFA slope was reported in 9 studies, of which 8 reported ACM at 2 weeks and 8 reported ACM at 10 weeks.
Group-level correlations of EFA biomarkers with all-cause mortality independent of treatment
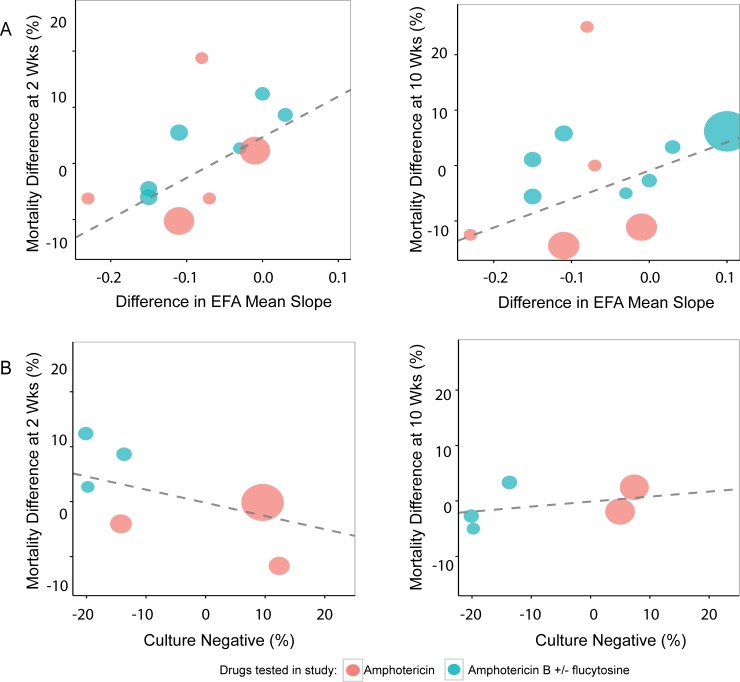
Considering each analysis separately, there was no statistically significant correlation between average EFA slope and ACM at 2 weeks (slope 29.15, 95%CI -24.99 to +83.18, P = 0.18) or between CSF culture negativity at 2 weeks and ACM at 2 weeks (slope -0.18, 95% CI -0.44 to +0.09, P = 0.10) and or 10 weeks (slope -0.02, 95% CI -0.48 to +0.43, P = 0.88). There was a statistically significant correlation between EFA and ACM at 10 weeks (slope 54.2, 95% CI 6.66 to 101.75, P = 0.04) (Fig 3).
Fig 3. Group-level correlation of EFA and ACM regardless of treatment, all studies.
All studies with non-missing data are displayed. N = 16 studies and 40 arms A) Mean EFA slope vs ACM at 2 and 10 weeks (9 studies and 24 arms). B) % CSF culture negative vs. ACM at 2 and 10 weeks (9 studies and 22 arms).
Based on the Cochrane assessment of risk for bias, 13 higher quality studies were selected to perform sensitivity analysis. (Table 1) There was no significant correlation between average EFA slope and ACM at 2 weeks (P = 0.14) or between CSF culture negativity at 2 weeks and ACM at 2 or 10 weeks (P = 0.18 and 0.71 respectively). (S1 Fig, S2 Table). A significant correlation between EFA and ACM was again noted at 10 wks (P = 0.04). This relationship was again found significant between EFA and ACM at 10 weeks, considering only trials with superiority hypotheses (P = 0.04). (S2 Fig, S3 Table).
Trial-level correlation of treatment effects on EFA biomarkers and all-cause mortality
In the absence of a consistent significant effect on ACM independent of treatment (Fig 3), the current data do not provide a strong rationale to support testing biomarkers as treatment surrogates. However, since the biomarker is being used as a treatment surrogate and trials have been stopped based in part on this surrogate endpoint [13], we conducted such an analysis. Restricting analyses to high quality studies, there were no statistically significant trial-level correlations between treatment effects on CSF culture negativity at 2 weeks and 2 week ACM (estimate -0.24, 95%CI -0.99 to +0.52, P = 0.23) or 10 week ACM (estimate 0.09, 95% CI -0.03 to +0.22, P = 0.07). There was also no statistically significant correlation between average EFA slope and ACM at 10 weeks (slope estimate 51.31, 95% CI -104.04 to +206.66, P = 0.18). However, there was a statistically significant correlation between EFA slope and ACM at 2 weeks (slope estimate 72.57, 95% CI +11.12 to +134.02, P = 0.03; Fig 4A)
Fig 4. Trial–level correlations of treatment effect on EFA compared to treatment effects on ACM: All studies with non-missing data are displayed.
N = 9 studies and 26 arms A) Average EFA slope vs ACM at 2 and 10 weeks (6 studies and 18 arms). B) % CSF culture negative vs ACM at 2 and 10 weeks (4 studies and 12 arms).
Discussion
Theoretically, EFA has an important characteristic as a surrogate endpoint in that it is on the causal pathway of disease. Correlation of EFA and mortality has been reported in individual randomized trials, which supports the concept of EFA as a potential candidate surrogate endpoint. According to the IOM, for a biomarker to be a candidate surrogate endpoint it should correlate at the group-level with the direct endpoint regardless of treatment (no comparison of outcomes between test and control groups). If such a correlation is shown, four further criteria are required on trial-level treatment effects: 1) treatment has effect on microbiological outcomes, 2) treatment has an effect on mortality 3) microbiological outcomes have an effect on mortality and 4) the treatment effect on mortality is captured by the treatment effect on microbiological outcomes.[15,17,25] Our analyses utilizing IOM guidelines and published data showed that EFA passes the first of these criteria; however, there was a lack of support for the second criterion (no effect on mortality) and no consistent support for the third criterion (microbiological effects and mortality), making examination of the fourth criterion (treatment effects on microbiological outcomes and mortality) moot. Negative CSF cultures at 2 weeks and 10 weeks also did not meet any of these criteria. Study quality assessed by Cochrane criteria did not affect the outcomes of the analyses as correlation of negative CSF cultures at 2 weeks with ACM at 2 or 10 weeks was not statistically significant in higher quality studies. Evaluation of studies with superiority hypotheses (since non-inferiority hypotheses may minimize differences between groups) also revealed no consistent statistically significant correlation between EFAs or negative CSF cultures with ACM. This lack of correlation shows that EFA does not consistently meet the first criteria and, according to IOM criteria, does not support its role as a candidate treatment surrogate for ACM based on current evidence.
While EFA might present a misleading picture of effects on ACM, because of the significant association at 10 wks and decisions on current trials made based on EFA, we compared trial-level treatment effects on EFA and negative CSF cultures at 2 weeks and 10 weeks, to treatment effects on ACM at 2 and 10 weeks. Our analyses show that treatment again demonstrates inconsistent effects on EFA and ACM, significant (without adjustment for multiple end point comparisons) at 2 weeks but not at 10 weeks in contrast to the group-level analysis. There was no treatment effect on negative CSF cultures at 2 weeks or 10 weeks. Since there is no treatment effect on ACM, the apparent effects on average EFA slope at 2 weeks could present a misleading picture of benefit on the patient-centered outcome of ACM. Thus, one cannot substitute a candidate surrogate endpoint for a direct measure of patient benefit without an effect on the direct endpoint of ACM, according to IOM guidelines. The precision around the estimates of ACM shows that lack of demonstrated differences in ACM is not related to insufficient sample size across all the studies combined.
It is important to note that the present study utilized only randomized controlled trials to facilitate testing for treatment surrogates. Inclusion of lower quality studies such as non-controlled observational studies utilizing clearly inferior therapies such as oral fluconazole that results in a lack of CSF fungal clearance and 100% mortality [26] may improve a group-level prognostic relationship between EFA and ACA but is not valid for evaluating trial-level treatment effects. In addition, a relationship valid principally for therapies largely abandoned (fluconazole monotherapy) is less likely to be useful for identifying therapies superior or equal to current standard-of-care therapies such as intravenous Amphotericin B, nor is applicable as a surrogate endpoint for current trials. Despite its potential correlation within selected patient groups [21], lack of an association of EFA as a group-level prognostic marker across all available studies may also be due to issues with analytical validity reflected in an inability to replicate the EFA assay among different investigators that could hamper its use in clinical practice. Since lack of assay standardization can be a cause of poor correlations, assay validity is the first step in the IOM recommended evaluation. Indeed, we could not find any published literature testing widely utilized reliability measures of EFA testing. We also did not find consistent measurement in trials of other direct patient centered outcomes other than ACM such as patient morbidity to compare to EFA. Mortality is a competing risk for measures of morbidity, as symptoms and patient function cannot be measured in patients who have died; thus, any analysis of morbidity would still need to include mortality. Regarding the analysis of EFA as a treatment surrogate, microbiological biomarkers of treatment efficacy may fail to correlate with direct patient outcomes because of off-target toxicities or benefits, issues with measurement of the biomarker (analytical validity), or alternative mechanisms of disease or treatment benefit not captured by the biomarker.[15,27] While theoretically compelling, microbiological biomarkers do not account for host response to infection, drug toxicities and interactions.[28] The utility of biomarkers of EFA and culture negativity in pre-clinical studies to demonstrate biological activity and prioritize candidates for further development is unclear, as we did not perform analyses to evaluate this as done in oncology.[20] However, the evidence does not support the use of EFA biomarkers as surrogate endpoints for ACM in trials used to confirm patient benefit.
Future studies could be performed to improve the use of EFA as a predictor of individual patient outcomes measured at the patient-level. Patient-level data was not available to perform analyses to evaluate EFA as a prognostic indicator for individual patients in clinical practice. However, trial-level data are necessary to evaluate the use of a biomarker as a treatment surrogate endpoint as we have evaluated here. We found potential biases in many of the randomized trials in CM. Trials in CM often consisted of multiple intervention groups and small numbers of patients enrolled, decreasing the precision of trial results. Poor funding has been implicated as limiting more extensive study of a neglected disease that, nevertheless, kills over a million individuals yearly [29]. More focused trials comparing a smaller number of regimens with an adequate number of patients followed for a sufficient duration of time using ACM as a primary endpoint, and microbiological outcomes as secondary endpoints may be an alternative approach to provide informative surrogate validation. Other direct measures of patient morbidity could be evaluated in addition to ACM, for instance in a ranked ordinal scale [30].
In summary, we utilized published evidence to evaluate EFA biomarkers as candidate surrogate endpoints for ACM in CM trials. Such trial-level meta-analyses have been widely performed to evaluate candidate surrogate endpoints in areas like oncology.[18,20] However, despite widespread use of microbiological biomarkers as surrogate endpoints in infectious diseases trials such as urinary tract infections, streptococcal pharyngitis, gonorrheal urethritis, tuberculosis, and hepatitis C (and others), this is the first trial-level meta-analysis of surrogate endpoints in infectious diseases published in the medical literature outside of HIV.[19] Application of such rigorous analyses of treatment surrogates thus may be important to facilitate the development of anti-infectives to combat the world-wide deficiency of effective antimicrobials.
Supporting Information
1A) EFA mean slope vs ACM at 2 and 10 wks: 8 studies, 22 arms 1B) CSF culture negativity vs. ACM at 2 and 10 wks: 5 studies, 14 arms.
(EPS)
1A) EFA mean slope vs ACM at 2 and 10 wks: 7 studies, 16 arms. 1B) CSF culture negativity vs. ACM at 2 and 10 wks: 7 studies, 15 arms.
(EPS)
(DOCX)
(DOCX)
(XLSX)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
JHP works for Leidos Biomedical Research under the FFRDC contract and receives compensation in the form of salary. Leidos Biomedical Research is a FFRDC (Federally Funded Research and Development Contractor) to NIH and is a fully separate subsidiary of corporate Leidos Inc. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health (Contract No. HHSN261200800001E). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This project was also supported by the Intramural Research Program of the NIH, National Institutes of Allergy and Infectious Diseases (NIH AI001123, AI001124-PRW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids 23: 525–530. 10.1097/QAD.0b013e328322ffac [DOI] [PubMed] [Google Scholar]
- 2.Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR (2013) Epidemiology of Cryptococcal Meningitis in the US: 1997–2009. PLoS One 8: e56269 10.1371/journal.pone.0056269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhein J BD (2012) Prognosis and management of cryptococcal meningitis in patients with human immunodeficiency virus infection. Neurobehavioral HIV Medicine 2012: 45–61. [Google Scholar]
- 4.Montezuma-Rusca JM (2014) Outcomes assessments in clinical trials of cryptococcal meningitis: consideration on use of early fungicidal activity as a potential surrogate endpoint for all-cause mortality. Current Treatment Options in Infectious Diseases 6: 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis JN, Meintjes G, Rebe K, Williams GN, Bicanic T, Williams A, et al. (2012) Adjunctive interferon-gamma immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS 26: 1105–1113. 10.1097/QAD.0b013e3283536a93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouwer AE, Rajanuwong A, Chierakul W, Griffin GE, Larsen RA, White NJ, et al. (2004) Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet 363: 1764–1767. [DOI] [PubMed] [Google Scholar]
- 7.Nussbaum JC, Jackson A, Namarika D, Phulusa J, Kenala J, Kanyemba C, et al. (2010) Combination flucytosine and high-dose fluconazole compared with fluconazole monotherapy for the treatment of cryptococcal meningitis: a randomized trial in Malawi. Clin Infect Dis 50: 338–344. 10.1086/649861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loyse A, Wilson D, Meintjes G, Jarvis JN, Bicanic T, Bishop L, et al. (2012) Comparison of the early fungicidal activity of high-dose fluconazole, voriconazole, and flucytosine as second-line drugs given in combination with amphotericin B for the treatment of HIV-associated cryptococcal meningitis. Clin Infect Dis 54: 121–128. 10.1093/cid/cir745 [DOI] [PubMed] [Google Scholar]
- 9.Bicanic T, Wood R, Meintjes G, Rebe K, Brouwer A, Loyse A, et al. (2008) High-dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV-infected patients: a randomized trial. Clin Infect Dis 47: 123–130. 10.1086/588792 [DOI] [PubMed] [Google Scholar]
- 10.Jackson AT, Nussbaum JC, Phulusa J, Namarika D, Chikasema M, Kanyemba C, et al. (2012) A phase II randomized controlled trial adding oral flucytosine to high-dose fluconazole, with short-course amphotericin B, for cryptococcal meningitis. AIDS 26: 1363–1370. 10.1097/QAD.0b013e328354b419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bisson GP, Molefi M, Bellamy S, Thakur R, Steenhoff A, Tamuhla N, et al. (2013) Early versus delayed antiretroviral therapy and cerebrospinal fluid fungal clearance in adults with HIV and cryptococcal meningitis. Clin Infect Dis 56: 1165–1173. 10.1093/cid/cit019 [DOI] [PubMed] [Google Scholar]
- 12.Day JN, Chau TT, Wolbers M, Mai PP, Dung NT, Mai NH, et al. (2013) Combination antifungal therapy for cryptococcal meningitis. N Engl J Med 368: 1291–1302. 10.1056/NEJMoa1110404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beardsley J, Wolbers M, Kibengo FM, Ggayi AB, Kamali A, Cuc NT, et al. (2016) Adjunctive Dexamethasone in HIV-Associated Cryptococcal Meningitis. N Engl J Med 374: 542–554. 10.1056/NEJMoa1509024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohn DL, Fisher EJ, Peng GT, Hodges JS, Chesnut J, Child CC, et al. (1999) A prospective randomized trial of four three-drug regimens in the treatment of disseminated Mycobacterium avium complex disease in AIDS patients: excess mortality associated with high-dose clarithromycin. Terry Beirn Community Programs for Clinical Research on AIDS. Clin Infect Dis 29: 125–133. [DOI] [PubMed] [Google Scholar]
- 15.Fleming TR, DeMets DL (1996) Surrogate end points in clinical trials: are we being misled? Ann Intern Med 125: 605–613. [DOI] [PubMed] [Google Scholar]
- 16.Lassere MN (2008) The Biomarker-Surrogacy Evaluation Schema: a review of the biomarker-surrogate literature and a proposal for a criterion-based, quantitative, multidimensional hierarchical levels of evidence schema for evaluating the status of biomarkers as surrogate endpoints. Stat Methods Med Res 17: 303–340. [DOI] [PubMed] [Google Scholar]
- 17.Institute of Medicine (2010) Evaluation of biomarkers and surrogate endpoints in chronic disease. [PubMed]
- 18.Prasad V, Kim C, Burotto M, Vandross A (2015) The Strength of Association Between Surrogate End Points and Survival in Oncology: A Systematic Review of Trial-Level Meta-analyses. JAMA Intern Med 175: 1389–1398. 10.1001/jamainternmed.2015.2829 [DOI] [PubMed] [Google Scholar]
- 19.Kim S, Hughes MD, Hammer SM, Jackson JB, DeGruttola V, Katzenstein DA (2000) Both serum HIV type 1 RNA levels and CD4+ lymphocyte counts predict clinical outcome in HIV type 1-infected subjects with 200 to 500 CD4+ cells per cubic millimeter. AIDS Clinical Trials Group Study 175 Virology Study Team. AIDS Res Hum Retroviruses 16: 645–653. [DOI] [PubMed] [Google Scholar]
- 20.Korn EL, Sachs MC, McShane LM (2016) Statistical controversies in clinical research: assessing pathologic complete response as a trial-level surrogate end point for early-stage breast cancer. Ann Oncol 27: 10–15. 10.1093/annonc/mdv507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bicanic T, Muzoora C, Brouwer AE, Meintjes G, Longley N, Taseera K, et al. (2009) Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis 49: 702–709. 10.1086/604716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343: d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fay MP, Graubard BI (2001) Small-sample adjustments for Wald-type tests using sandwich estimators. Biometrics 57: 1198–1206. [DOI] [PubMed] [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339: b2700 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buyse M, Burzykowski T, Michiels S, Carroll K (2008) Individual- and trial-level surrogacy in colorectal cancer. Stat Methods Med Res 17: 467–475. 10.1177/0962280207081864 [DOI] [PubMed] [Google Scholar]
- 26.Bicanic T, Meintjes G, Wood R, Hayes M, Rebe K, Bekker LG, et al. (2007) Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis 45: 76–80. [DOI] [PubMed] [Google Scholar]
- 27.Fleming TR, Powers JH (2012) Biomarkers and surrogate endpoints in clinical trials. Stat Med 31: 2973–2984. 10.1002/sim.5403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casadevall A, Pirofski LA (2014) Microbiology: Ditch the term pathogen. Nature 516: 165–166. 10.1038/516165a [DOI] [PubMed] [Google Scholar]
- 29.Adams P (2016) How to stop crypto, a deadly disease so neglected it's missed on the 'neglected' list. Newsweek. [Google Scholar]
- 30.Evans SR, Rubin D, Follmann D, Pennello G, Huskins WC, Powers JH, et al. (2015) Desirability of Outcome Ranking (DOOR) and Response Adjusted for Duration of Antibiotic Risk (RADAR). Clin Infect Dis 61: 800–806. 10.1093/cid/civ495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Techapornroong M, Suankratay C (2007) Alternate-day versus once-daily administration of amphotericin B in the treatment of cryptococcal meningitis: a randomized controlled trial. Scand J Infect Dis 39: 896–901. [DOI] [PubMed] [Google Scholar]
- 32.Mayanja-Kizza H, Oishi K, Mitarai S, Yamashita H, Nalongo K, Watanabe K, et al. (1998) Combination therapy with fluconazole and flucytosine for cryptococcal meningitis in Ugandan patients with AIDS. Clin Infect Dis 26: 1362–1366. [DOI] [PubMed] [Google Scholar]
- 33.Hamill RJ, Sobel JD, El-Sadr W, Johnson PC, Graybill JR, Javaly K, et al. (2010) Comparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS-associated acute cryptococcal meningitis: a randomized, double-blind clinical trial of efficacy and safety. Clin Infect Dis 51: 225–232. 10.1086/653606 [DOI] [PubMed] [Google Scholar]
- 34.Chotmongkol V, Sukeepaisarncharoen W, Thavornpitak Y (1997) Comparison of amphotericin B, flucytosine and itraconazole with amphotericin B and flucytosine in the treatment of cryptococcal meningitis in AIDS. J Med Assoc Thai 80: 416–425. [PubMed] [Google Scholar]
- 35.Tansuphaswadikul WN S., Phonrat B., Boonpok L., Getahun A., and Pitisuttithum P. (2006) Comparison of one week with two week regimens of amphotericin B both followed by fluconazole in the treatment of cryptococcal meningitis among AIDS patients. Journal of the Medical Association of Thailand 89: 1677–1685. [PubMed] [Google Scholar]
- 36.Chotmongkol V, Arayawichanont A, Sawanyawisuth K, Thavornpitak Y (2005) Initial treatment of cryptococcal meningitis in AIDS. Southeast Asian J Trop Med Public Health 36: 170–173. [PubMed] [Google Scholar]
- 37.Jadhav MP, Bamba A, Shinde VM, Gogtay N, Kshirsagar NA, Bichile LS, et al. (2010) Liposomal amphotericin B (Fungisome) for the treatment of cryptococcal meningitis in HIV/AIDS patients in India: a multicentric, randomized controlled trial. J Postgrad Med 56: 71–75. 10.4103/0022-3859.65276 [DOI] [PubMed] [Google Scholar]
- 38.Pappas PG, Chetchotisakd P, Larsen RA, Manosuthi W, Morris MI, Anekthananon T, et al. (2009) A phase II randomized trial of amphotericin B alone or combined with fluconazole in the treatment of HIV-associated cryptococcal meningitis. Clin Infect Dis 48: 1775–1783. 10.1086/599112 [DOI] [PubMed] [Google Scholar]
- 39.Pappas PG, Bustamante B, Ticona E, Hamill RJ, Johnson PC, Reboli A, et al. (2004) Recombinant interferon- gamma 1b as adjunctive therapy for AIDS-related acute cryptococcal meningitis. J Infect Dis 189: 2185–2191. [DOI] [PubMed] [Google Scholar]
- 40.Larsen RA, Leal MA, Chan LS (1990) Fluconazole compared with amphotericin B plus flucytosine for cryptococcal meningitis in AIDS. A randomized trial. Ann Intern Med 113: 183–187. [DOI] [PubMed] [Google Scholar]
- 41.de Gans J, Portegies P, Tiessens G, Eeftinck Schattenkerk JK, van Boxtel CJ, van Ketel RJ, et al. (1992) Itraconazole compared with amphotericin B plus flucytosine in AIDS patients with cryptococcal meningitis. Aids 6: 185–190. [DOI] [PubMed] [Google Scholar]
- 42.Leenders AC, Reiss P, Portegies P, Clezy K, Hop WC, Hoy J, et al. (1997) Liposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS-associated cryptococcal meningitis. Aids 11: 1463–1471. [DOI] [PubMed] [Google Scholar]
- 43.van der Horst CM, Saag MS, Cloud GA, Hamill RJ, Graybill JR, Sobel JD, et al. (1997) Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. N Engl J Med 337: 15–21. [DOI] [PubMed] [Google Scholar]
- 44.Day JN, Chau TT, Lalloo DG (2013) Combination antifungal therapy for cryptococcal meningitis. N Engl J Med 368: 2522–2523. [DOI] [PubMed] [Google Scholar]
- 45.Makadzange AT, Ndhlovu CE, Takarinda K, Reid M, Kurangwa M, Gona P, et al. (2010) Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub-saharan Africa. Clin Infect Dis 50: 1532–1538. 10.1086/652652 [DOI] [PubMed] [Google Scholar]
- 46.Saag MS, Powderly WG, Cloud GA, Robinson P, Grieco MH, Sharkey PK, et al. (1992) Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. N Engl J Med 326: 83–89. [DOI] [PubMed] [Google Scholar]
- 47.Sharkey PK, Graybill JR, Johnson ES, Hausrath SG, Pollard RB, Kolokathis A, et al. (1996) Amphotericin B lipid complex compared with amphotericin B in the treatment of cryptococcal meningitis in patients with AIDS. Clin Infect Dis 22: 315–321. 8838189 [Google Scholar]
- 48.Bennett JE, Dismukes WE, Duma RJ, Medoff G, Sande MA, Gallis H, et al. (1979) A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptoccal meningitis. N Engl J Med 301: 126–131. [DOI] [PubMed] [Google Scholar]
- 49.Dismukes WE, Cloud G, Gallis HA, Kerkering TM, Medoff G, Craven PC, et al. (1987) Treatment of cryptococcal meningitis with combination amphotericin B and flucytosine for four as compared with six weeks. N Engl J Med 317: 334–341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1A) EFA mean slope vs ACM at 2 and 10 wks: 8 studies, 22 arms 1B) CSF culture negativity vs. ACM at 2 and 10 wks: 5 studies, 14 arms.
(EPS)
1A) EFA mean slope vs ACM at 2 and 10 wks: 7 studies, 16 arms. 1B) CSF culture negativity vs. ACM at 2 and 10 wks: 7 studies, 15 arms.
(EPS)
(DOCX)
(DOCX)
(XLSX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.