Introduction
Traumatic brain injury (TBI) is the most common cause of death and disability in young people.1 Each year, TBI accounts for an estimated 1.1 million emergency department (ED) visits, 235,000 hospitalizations and 50,000 deaths in the United States (US).2 TBI is generally classified as mild (Glasgow coma scale [GCS] 13-15), moderate (GCS 9-12), and severe (GCS 3-8).3 Approximately 95% of patients with TBI are categorized as mild.4
Patients with moderate and severe TBI have a high morbidity and mortality and almost uniformly require intensive care unit (ICU) admission for neurologic monitoring and concentrated therapy.5 ICU care allows for early detection of secondary brain injury from cerebral edema, increased intracranial pressure, and cerebral ischemia.6 These secondary insults are the leading cause of inpatient death following TBI.7 However in patients with traumatic intracranial hemorrhage (tICH) and normal mental status, hemorrhage progression and neurosurgical intervention is infrequent.8, 9 Thus, the need for ICU admission in patients with mild TBI is less certain and routine ICU admission may lead to overutilization of critical care resources.
ICU admission is indicated if there is a decreased risk of morbidity and mortality associated with intensive care treatment.10 While guidelines suggest the need for in-patient observation for repeated neurological evaluations in patients with tICH, there are no clear recommendations when ICU admission is warranted.4 Admission criteria to ICUs vary widely and there is significant variability in the management of patients with mild TBI.11, 12 Previous attempts to determine evidence based criteria for ICU admission have failed due to the lack of scientific evidence.10
Given that intensive care in the USA is a costly and limited resource, appropriate utilization of ICU resources is important in providing efficient health care. Critical care beds in the US account for approximately 8% of hospital beds, but 28% of acute hospital care charges.13 Moreover, ICUs are often overcrowded, which lead to increased refusal for admissions to intensive care,14 prolonged ED boarding times, and adverse patient outcomes.15
The purpose of this study is to evaluate the need for ICU admission in adult ED patients with TBI and tICH. We hypothesize that a subset of ED patients with TBI and tICH can be considered low risk for requiring future inpatient critical care resources.
Materials and Methods
This is a retrospective cohort study of all adult trauma patients who sustained a tICH and were evaluated at a Level 1 trauma center from May 2006 through April 2008. The institutional review board at the study site approved the study.
Patients were identified from the hospital trauma registry using the International Statistical Classification of Diseases and Related Health Problems (ninth edition) (ICD-9) codes specific for tICH (codes 851-854). Following the initial query, the electronic medical record (EMR) was reviewed to determine eligibility for study inclusion. Patients were eligible if they were at least 18 years old and sustained a tICH on computerized tomography (CT) scan. The presence or absence of tICH was based on the dictated attending radiologist report of the cranial CT. tICH was defined as subarachnoid hemorrhage, epidural hematoma, subdural hematoma, intraventricular hemorrhage, intraparenchymal hemorrhage/contusion, and diffuse axonal injury.
Trauma patients at the study site are managed in the ED by both trauma surgeons and emergency medicine physicians. Patients that do not require immediate surgical intervention receive diagnostic and therapeutic management in the ED and are then triaged to home, an in-patient ICU bed, or an in-patient non-ICU bed. At the study site, patients with tICH detected on cranial CT are generally admitted to the ICU for neurologic monitoring for a minimum of 24 hours following injury.
We defined the need for ICU admission as the presence of a critical care intervention. The list of definitions of critical care interventions was derived from the Task Force of American College of Critical Care Medicine Guidelines for ICU admission 16 and represented specific interventions or patient conditions that would warrant intensive care monitoring or management. Definitions of critical care interventions are included in Table 1.
Table 1.
Definitions of critical care interventions
| Critical care intervention | Definition |
|---|---|
| Vasopressor or inotrope use | Use of dopamine, norepinephrine, epinephrine, dobutamine, phenylephrine, or vasopressin for vasopressor or inotropic support |
| Invasive monitoring | Use of central venous catheter to measure central venous pressure (not for venous access alone), or the use an arterial line to measure blood pressure, or the use of a pulmonary artery catheter to measure pulmonary artery wedge pressure |
| Mechanical ventilation | Mechanical ventilation for acute respiratory failure |
| Cardiac arrest | Cardiac arrest requiring cardiopulmonary resuscitation |
| Arrhythmia | Non-sinus arrhythmia less than 40 or greater than 120 beats/minute with the need for urgent intervention |
| RBC transfusion | Transfusion of packed red blood cells |
| FFP transfusion | Transfusion of fresh frozen plasma |
| Recombinant activated factor VII | Use of recombinant activated factor VII |
| Interventional angiography | Use of interventional angiography for therapeutic purposes |
| Neurosurgical intervention | Placement of an intracranial pressure monitor, burr hole, craniotomy, subdural drain, intraventricular catheter, or treatment with mannitol or hypertonic saline |
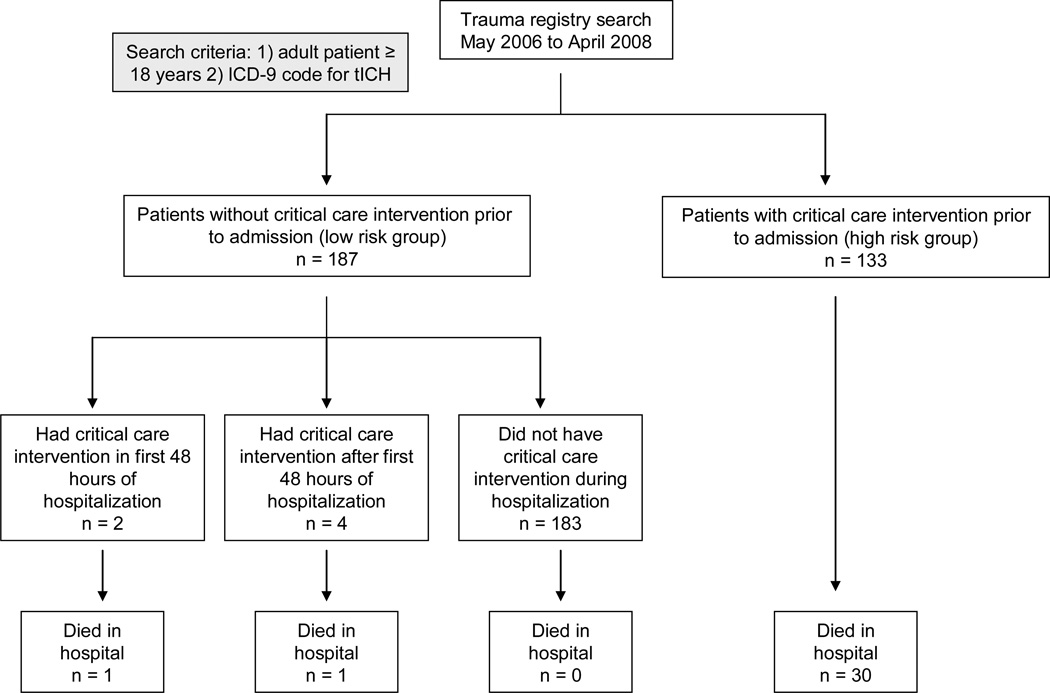
Patients were categorized into two cohorts based on the presence or absence of a critical care intervention prior to admission to the ICU. Time to admission to the ICU was defined as the time an admission bed request was placed while the patient was in the ED. Admission bed requests are placed with computerized order entry. We chose this cutoff to minimize potential bias from patients “boarding” in the ED while waiting for a bed that is geographically in the ICU. Once an ICU bed request is placed, the trauma service manages the patient in the ED. We considered the cohort of patients that received a critical care intervention prior to admission to the ICU as high risk to require ICU resources. Patients that did not receive a critical care intervention prior to admission were considered low risk. This low risk group was then further evaluated for the presence of a critical care intervention during their hospitalization. Any critical care intervention that occurred after admission in the low risk group was defined as a delayed critical care intervention. If a critical care intervention occurred while the patient was physically in the ED but after admission (bed request), it was coded as a delayed critical care intervention. Delayed critical care intervention was then further coded into either within 48 hours of admission (time from ICU bed request placement) or at any time during hospitalization (Figure 1).
Figure.
Flowchart of patient enrollment
ICD: international classification of diseases; tICH: traumatic intracranial hemorrhage
Data collection followed previously published guidelines on retrospective chart review.17 We abstracted data from the physician and nursing notes, laboratory and imaging studies, ED and inpatient orders, and procedures from the EMR. Data was collected on a standardized data collection form with pre-defined variables by a single data abstractor trained to the methodology of data collection (DKN). The data abstractor was not blinded to study hypothesis. Variables collected included age, gender, mechanism of injury, past medical history, initial ED GCS, loss of consciousness, initial ED blood pressure, initial ED respiratory rate, alcohol intoxication, treatment with packed red blood cells (PRBC), fresh frozen plasma (FFP), recombinant activated factor VII (rFVIIa), initial cranial CT results, initial platelet count and international normalized ratio (INR) level, and initial two hematocrit measurements. Blood urea nitrogen (BUN), creatinine (Cr), bicarbonate (HCO3-) and anion gap (AG) were also recorded. ICU and hospital length of stay were also recorded. Alcohol intoxication was considered positive if laboratory ethanol level ≥ 10 mg/dL. A unit of PRBC equals approximately 300 ml and a unit of FFP is approximately 400 ml. Cranial CT results were coded into anatomical categorical variables based on attending radiologist text report. ED data were abstracted prior to knowledge of patient outcomes. Data missing from the medical record was coded as missing in the dataset.
An Abbreviated Injury Score (AIS) for head and neck, face, chest, abdomen, extremities, and external body regions and the overall Injury Severity Score (ISS) were previously available from the trauma registry.18 These calculations were previously imputed into the trauma registry by data abstractors who were trained in these calculations. A Marshall Score was calculated for this study following previously described methodology.19 The AIS and ISS are scoring systems developed to measure injury severity based on anatomical injuries divided by body regions.18 The Marshall Score is a previously defined classification system that predicts patient outcome based on cranial CT findings (see Table 2 for classification of Marshall Score).19
Table 2.
Patient characteristics
| Characteristic | Patients | Median or Mean With IQR or 95%CI |
|---|---|---|
| Demographics | ||
| Age (yr) | 320/320 | 47.6 (5.4–89.8) |
| Male | 221/320 | 69.1% (63.1–74.1) |
| Mechanism of injury | ||
| Fall | 69/320 | 21.6% (17.2–26.5) |
| Fall from height | 23/320 | 7.2% (4.6–10.6) |
| Motor vehicle accident | 136/320 | 43.5% (37.0–48.1) |
| Pedestrian struck | 21/320 | 6.6% (4.1–10.0) |
| Bike | 13/320 | 4.1% (2.2–6.8) |
| Gunshot wound | 6/320 | 1.9% (0.7–4.0) |
| Direct blow | 43/320 | 13.4% (9.9–17.7) |
| Unknown mechanism | 9/320 | 2.8% (1.3–5.3) |
| Medication | ||
| Aspirin | 13/320 | 4.1% (21.8–68.5) |
| Clopidogrel | 4/320 | 1.3% (0.3–3.2) |
| Warfarin | 8/320 | 2.5% (10.9–48.7) |
| Any anticoagulant | 25/320 | 7.8% (5.1–11.3) |
| Injury severity | ||
| GCS | 317/320 | 14 (10–15) |
| Mild TBI: GCS 13–15 | 218/317 | 68.8% (63.6–73.9) |
| Moderate TBI: GCS 9–12 | 26/317 | 8.2% (5.2–11.2) |
| Severe TBI: GCS < 8 | 72/317 | 22.7% (18.1–27.4) |
| Isolated head injury | 231/320 | 72.2% (67.3–77.1) |
| Loss of consciousness reported | 104/320 | 32.5% (27.3–37.7) |
| Injury severity score | 320/320 | 17 (13–25) |
| Marshall Score† | 320/320 | 2 (2–2) |
| Cranial CT scan injuries | ||
| Depressed skull fracture | 2/320 | 0.6% (0–2.2) |
| Nondepressed skull fracture | 20/320 | 6.3% (3.6–8.9) |
| Intraparenchymal hemorrhage | 182/320 | 56.9% (51.4–62.3) |
| Subdural hemorrhage | 106/320 | 33.1% (28.0–38.3) |
| Epidural hemorrhage | 14/320 | 4.4% (2.1–6.6) |
| Herniation | 18/320 | 5.6% (3.1–8.2) |
| Diffuse axonal injury | 9/320 | 2.8% (1.0–4.6) |
| Subarachnoid hemorrhage | 146/320 | 45.6% (40.1–51.1) |
| Interventricular hemorrhage | 31/320 | 9.7% (6.4–12.9) |
| Presence of cerebral shift | 31/320 | 9.7% (6.4–12.9) |
| Presence of cerebral mass effect | 37/320 | 11.6% (8.0–15.1) |
CT Class 1, no lesions present; CT Class 2, lesions and/or midline shift ≤ 5 mm, cisterns present; no high- or mixed-density lesions > 25 mm; CT Class 3 (swelling), midline shift ≤ 5mm, cisterns compressed or absent, no high- or mixed-density lesions > 25 mm; CT Class 4 (shift), midline shift > 5 mm, no high- or mixed-density lesions >25 mm; CT Class 5, any surgically evacuated mass; CT Class 6, high- or mixed-density lesions >25 ml.
GCS: Glascow Coma Score; TBI: traumatic brain injury; CT: computed tomography; IQR: Interquartile range; CI: confidence interval
The primary outcome measure of this study is the presence of a delayed critical care intervention in the first 48 hours after admission in the group of patients that did not receive a critical care intervention prior to ICU admission (low risk group). A 48 hour cutoff was selected as this is a reasonable time frame for observation for neurological decline from a tICH. Secondary outcome measures included delayed critical care intervention at any point during hospitalization, 48 hour and in-hospital mortality, emergency surgery (defined as surgery requiring general anesthesia within 24 hours of admission), and discharge from the ICU or hospital within 24 and 48 hours.
Data was entered into a spreadsheet and analyzed using STATA 10.0 statistical software (STATA Corp, College Station, TX). Interval data were reported as the mean ± standard deviation (SDs) or median and interquartile range (IQRs). Proportions were presented with 95% confidence intervals. Categorical data was analyzed with chi-square test or Fisher’s exact test in cases of small cell size. Continuous data was analyzed with Student’s t-test for normally distributed data or wilcoxon rank-sum test for non-parametric data or ordinal data.
Results
A total of 320 medical records were identified from the trauma registry (Figure 1). Three hundred and seven (95.9%) patients were admitted to the ICU, 11 (3.4%) patients were admitted to a non-ICU setting, one patient died in the ED, and one patient was discharged home from the ED after a period of observation. Baseline characteristics are presented in Table 2. Mean age was 47.6 years old (± 21.1 years) and 221 patients (69.1%) were male. Twenty-five (7.8%) patients were taking an anticoagulant or antiplatelet medication (aspirin, clopidogrel, or warfarin). A total of 231 (72.2%) patients sustained an isolated head injury which was defined as AIS < 3 in non-head body region. The median GCS was 14 with the majority of patients having a GCS 13-15 (68.8%). The most common cranial injury was intraparenchymal hemorrhage (56.9%) and subdural hemorrhage (33.1%). See Table 2 for complete patient characteristics.
One hundred and thirty-three of 320 (41.6%) patients had a critical care intervention prior to admission (high risk group). Mechanical ventilation (35.6%) and RBC transfusion (15.6%) were the most common forms of critical care intervention (Table 3). When comparing the high and low risk groups, there were no significant differences in patient demographics, past medical history, medication use, and laboratory studies. Intraventricular hemorrhage, presence of cerebral shift, presence of cerebral mass effect, and presence of cerebral herniation were more prevalent in the high risk group. Patients in the high risk group were also more likely to have multi-system trauma, have an ISS > 15, and have a Marshall score > 2. Table 4 compares injury severity between groups.
Table 3.
Critical care intervention prior to admission
| Critical care intervention* | Patients | Mean With 95% CI |
|---|---|---|
| Critical care intervention in ED | 133/320 | 41.6% (36.1–47.0) |
| - Vasopressor or inotrope use | 3/320 | 0.9% (0.2–2.7) |
| - Invasive monitoring | 3/320 | 0.9% (0.2–2.7) |
| - Mechanical ventilation | 114/320 | 35.6% (30.4–41.1) |
| - Cardiac arrest | 0/320 | 0% (0–1.1%) |
| - Arrhythmias requiring treatment | 3/320 | 0.9% (0.2–2.7) |
| - RBC transfusion | 50/320 | 15.6% (11.8–20.1) |
| - FFP transfusion | 29/320 | 9.1% (6.2–12.8) |
| - rFVIIa | 4/320 | 1.3% (0.3–3.2) |
| - Interventional angiography | 1/320 | 0.3% (0–1.7) |
| - Neurosurgical intervention | 18/320 | 5.6% (3.4–8.7) |
see table 1 for definitions of critical care intervention
RBC: red blood cell; FFP: fresh frozen plasma; rFVIIa: recombinant activated factor VII; CI: confidence interval
Table 4.
Comparison of severity of injury between groups
| Characteristic | Group 1 (Low risk): Did not have a critical care intervention prior to admission (n=187) |
Group 2 (High risk): Had a critical care intervention prior to admission (n=133) |
p value | ||
|---|---|---|---|---|---|
| Patients | Median or Mean With IQR or 95% CI |
Patients | Median or Mean With IQR or 95% CI |
||
| GCS* | 184/187 | 15 (14–15) | 133/133 | 7 (3–15) | . |
| Mild TBI: GCS 13–15 | 179/184 | 97.3% (94.9–99.6) | 39/133 | 29.3% (21.6–37.1) | <0.001 |
| Moderate TBI: GCS 9–12 | 5/184 | 2.7% (0.4–5.1) | 21/133 | 15.8% (9.6–22.0) | <0.001 |
| Severe TBI: GCS < 8 | 0/184 | 0% (0–2.0) | 72/133 | 54.1% (45.7–62.6) | <0.001 |
| Isolated head | 155/187 | 83.8% (78.5–89.1) | 76/133 | 56.3% (47.9–64.7) | <0.001 |
| Loss of consciousness | 60/187 | 32.4% (25.7–39.2) | 44/133 | 32.6% (24.7–40.5) | 0.73 |
| Injury severity score | 187/187 | 16 (10–20) | 187/187 | 25 (17–33) | . |
| Injury severity score > 15 | 73/187 | 39.0% (32.0–46.4) | 118/133 | 88.7% (82.1–93.5) | <0.001 |
| Marshall Score | 187/187 | 2 (2–2) | 133/133 | 2 (2–3) | . |
| Marshall Score > 2 | 3/187 | 1.6% (0.3–4.6) | 44/133 | 33.1% (25.2–41.8) | <0.001 |
GCS: Glascow Coma Score; TBI: traumatic brain injury; LOC: computed tomography; ED: emergency department; IQR: interquartile range; CI: confidence interval
3 patients did not have GCS recorded
In the low risk group of 187 patients that did not receive a critical care intervention prior to admission, two patients (1.1%; 95% CI 0.1, 3.8%) met the primary outcome measure of delayed critical care intervention within the first 48 hours of hospitalization. Both patients received RBC transfusion as their critical care intervention with one patient eventually dying during hospitalization from pneumonia on hospital day number 21. None of the 146 patients (0%; 95%CI 0, 2.5) less than 70 years of age had a delayed critical care intervention within the first 48 hours of admission.
Four patients (2.1%, 95% CI 0.6, 5.4%) met the secondary outcome measure of delayed critical care intervention after 48 hours of hospitalization. These interventions occurred between 3 and 10 days post-injury. Of the 6 patients who sustained a delayed critical care intervention at any point during hospitalization, no patient suffered a neurological decline or the need for neurosurgical intervention due to worsening of the patient’s tICH (see Table 5 for patient details).
Table 5.
Patients that did not receive a critical care intervention prior to admission but required a critical care intervention during hospitalization
| Patient | GCS, ISS |
Had a critical care intervention prior to admission? |
Had a critical care intervention within the first 48 hours of admission? |
Had a critical care intervention after the first 48 hours of admission? |
Survived to discharge | LOS (ICU, hospital) |
|---|---|---|---|---|---|---|
| 62 year old male with fall from standing |
14, 32 | No | No | Yes, intubated for respiratory failure due to multiple rib fractures on HD #3 |
Yes | 18, 19 |
| 62 year old male in MVA | 15, 16 | No | No | Yes, intubated for delirium tremens on HD #3 | Yes | 13, 27 |
| 81 year old female with fall from standing |
15, 17 | No | Yes, transfused 1 unit of PRBC 16 hours after admission |
No | Yes | 4, 7 |
| 80 year old female with fall from standing |
13, 24 | No | No | Yes, transfused 1 unit PRBC on HD #4 | No, died due to respiratory failure from multiple rib fractures on HD #11 (made DNR during hospitalization) |
11, 11 |
| 49 year old male in MVA | 14, 21 | No | No | Yes, intubated for procedural sedation due to claustrophobia for MRI on HD #3 |
Yes | 6, 12 |
| 89 year old female in MVA |
9, 38 | No | Yes, PRBC transfusion 21 hours after admission |
Yes, intubated for respiratory failure due to pneumonia on HD #10 |
No, died from pneumonia on HD #21 | 5, 21 |
LOS: length of stay; ICU: intensive care unit; MVA: motor vehicle accident; GCS: Glascow Coma Score; ISS: injury severity score; HD: hospital day; PRBC: packed red blood cells; DNR: do not resuscitate; MRI = magnetic resonance imaging
No patients (0%, 95% CI 0, 2.0%) in the low risk group had an emergent surgery. One patient died in this group (0.5%; 95% CI 0, 2.9%) and no patients died within 48 hours of admission (0%, 95% CI 0, 2.0%). The mean hospital LOS was 4.7 days (95% CI 3.8, 5.6 days) and the mean ICU LOS was 2.0 days (95% CI 1.4, 3.0 days) in the low risk group. One hundred and twelve (59.4%) patients in the low risk group were discharged from either the ICU or hospital within 24 hours and 142 (75.9%) patients were discharged within 48 hours (Table 6).
Table 6.
Comparison of secondary outcomes between groups
| Characteristic | Group 1 (Low risk): Did not have a critical care intervention prior to admission (n=187) |
Group 2 (High risk): Had a critical care intervention prior to admission (n=133) |
p value | ||
|---|---|---|---|---|---|
| Patients | Median or Mean With IQR or 95% CI |
Patients | Median or Mean With IQR or 95% CI |
||
| Emergent surgery | 0/187 | 0% (0–2.0) | 28/133 | 21.1% (14.5–29.0) | <0.001 |
| Mortality in hospital | 2/187 | 1.1% (0.1–3.8) | 31/133 | 23.3% (14.5–31.4) | <0.001 |
| Mortality within 48 hours | 0/187 | 0 (0–2.0) | 15/133 | 11.3% (6.5–17.9) | <0.001 |
| Mean hospital LOS (days) | . | 4.7 (3.8–5.6) | . | 21.9 (14.2–29.7) | <0.001 |
| Mean ICU LOS (days) | . | 2.0 (1.4–3.0) | . | 8.1 (6.1–10.1) | <0.001 |
| Patients discharged in 24 hours* | 112/187 | 59.9% (52.5–67.0) | 17/133 | 12.8% (7.6–19.7) | <0.001 |
| Patients discharged in 48 hours* | 142/187 | 75.9% (69.2–81.9) | 24/133 | 18.0% (11.9–25.6) | <0.001 |
LOS: length of stay; ICU: intensive care unit; ED: emergency department; CI: confidence interval
discharge from either ICU or hospital
The majority (218/317, 68.8%, 95% CI 63.6, 73.9%) of patients had mild TBI (GCS 13-15). Patients with mild TBI were more likely to survive, have shorter hospital LOS, and not have a critical care intervention prior to admission compared to moderate and severe TBI.
Discussion
The initial goal of our study was to develop a model to predict ED patients with tICH who are at high risk of delayed critical care intervention and therefore would warrant ICU admission. However our primary outcome measure of delayed critical care intervention was identified in only 1.1% of the study cohort. This rate was much lower than expected and we were therefore unable to statistically develop a prediction model. These results indicate, however, that patients with mild TBI not receiving a critical care intervention prior to hospital admission have a low rate of requiring a critical care intervention in the next 48 hours.
Furthermore, these low risk patients are at low risk of requiring a critical care intervention during their entire hospitalization. The absence of a critical care intervention prior to admission may serve as a triage tool by itself to screen for a subset of patients with tICH that are low risk for requiring ICU resources and may be safely managed in a non-ICU setting. Triaging these low risk patients to a non-ICU setting may serve to decrease acute hospital costs as well as decompress overcrowded ICUs and decrease ED boarding times. Given that the majority of patients sustaining a tICH fall into this low risk category, the impact of this triage is likely substantial.
Using previously published data from US hospitals in 2004, the average cost per day of an ICU bed is $2,575 while a ward bed costs $1,488.20 If all patients who met the low risk criteria (no critical care intervention prior to admission) were admitted to the ward rather than the ICU for the mean duration of ICU stay (2.0 days) then the estimated savings during the study period would have been $203,268/year. This does not account for the potential additional cost from the patients mistriaged to the ward or the potential decreased length of hospital stay if patients are admitted directly to the ward. Future studies evaluating ICU resource utilization should include a cost-effectiveness analysis to adequately assess the cost versus benefit of implementation of an ICU triage model. A potential cost-effectiveness analysis might evaluate the cost of one misclassified patient (patient considered low risk but then requires a critical care intervention) or in-hospital death.
Prior studies have evaluated baseline characteristics to predict outcome in traumatic brain injury.21–23 However there has been only one study that attempted to predict critical care intervention in traumatic brain injury using baseline characteristics.22 This study however, had a relatively high threshold for requiring ICU admission (raised intracranial pressure and need for neurosurgical intervention) and those authors were unable to develop a sufficient prediction model.
Some clinicians may wish to identify all patients requiring delayed critical care interventions. To further increase the sensitivity of predicting delayed critical care interventions, an age cutoff may be implemented. Post hoc analysis demonstrated that if patients over 70 years of age were excluded from the low risk group, no patient undergoing delayed critical care intervention within 48 hours of admission would be missed in our cohort. In addition, many clinicians would wish to closely monitor anti-coagulated patients and patients with epidural hematomas in an ICU setting as these patients are believed at high risk for further bleeding and neurosurgical interventions.24
In this study, hospital and ICU length of stay in the low risk group was comparable to prior studies involving mild TBI patients.8 We found a high rate of low risk patients discharged from the ICU or hospital within 24 or 48 hours. This suggests a majority of low risk patients are being admitted and observed in the ICU for short time periods and are then transitioned to a ward bed or even home. However due to the low rate of admissions to non-ICU settings, we were unable to identify differences in hospital length of stay in low risk patients admitted to the ICU compared to non-ICU settings.
The study has several limitations. This study is retrospective and is subject to the limitations of medical record review. This study was also limited to a single center where local admission criteria and management of TBI might not be generalizable to other settings. Due to the lack of literature and guidelines for in-hospital triage of patients with TBI, the standard management of these patients is unclear. Prior studies demonstrate wide variability in the management of patients with TBI11, 12 and other studies suggest little benefit from hospital admission for minor head injury.25, 26
As the vast majority of patients were admitted to the ICU, we were unable to describe patients with tICH that were managed in a non-ICU setting. There may be unexpected advantages with ICU care in these patients that may have prevented a critical care intervention from occurring. For example, TBI patients admitted to the ICU at our center have neurological checks ordered to be performed by nursing staff every one hour compared to every two hours or greater in non-ICU settings.
The list of critical care interventions is based on prior guidelines which were based on expert opinion.16 Ideally, this list would be based on evidence demonstrating improved outcomes in an ICU setting versus a non-ICU setting. We defined this list of critical care interventions a priori based on interventions best managed in an ICU setting. Analysis of the six patients receiving a delayed critical care intervention during their hospitalization demonstrated that none of the critical care interventions were likely a result of the head injury sustained.
In conclusion, patients with tICH and the absence of a critical care intervention prior to ICU admission are at very low risk for a delayed critical care intervention during hospitalization. Future studies to validate whether the absence of a critical care intervention prior to admission in patients with tICH, can serve as a safe, cost-effective triage tool for ICU admission.
Acknowledgments
Study site: UC Davis Medical Center
Footnotes
Prior presentations: None
Contributor Information
Matthew J. Sena, Email: matthewsena2004@yahoo.com.
James F. Holmes, Email: jfholmes@ucdavis.edu.
References
- 1.Ghajar J. Traumatic brain injury. Lancet. 2000;356(9233):923–929. doi: 10.1016/S0140-6736(00)02689-1. [DOI] [PubMed] [Google Scholar]
- 2.Langlois JAR-BW, Thomas KE. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta, GA: US Department of Health and Human Services, CDC; 2006. [Google Scholar]
- 3.Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005;294(12):1511–1518. doi: 10.1001/jama.294.12.1511. [DOI] [PubMed] [Google Scholar]
- 4.Vos PE, Battistin L, Birbamer G, et al. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9(3):207–219. doi: 10.1046/j.1468-1331.2002.00407.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang MC, Linnau KF, Tirschwell DL, Hollingworth W. Utility of repeat head computed tomography after blunt head trauma: a systematic review. J Trauma. 2006;61(1):226–233. doi: 10.1097/01.ta.0000197385.18452.89. [DOI] [PubMed] [Google Scholar]
- 6.Graham DI, Ford I, Adams JH, et al. Ischaemic brain damage is still common in fatal non-missile head injury. J Neurol Neurosurg Psychiatry. 1989;52(3):346–350. doi: 10.1136/jnnp.52.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall LFGT, Klauber MR. The outcome of severe closed head injury. J Neurosurg. 1991;75:S28–S36. [Google Scholar]
- 8.Huynh T, Jacobs DG, Dix S, et al. Utility of neurosurgical consultation for mild traumatic brain injury. Am Surg. 2006;72(12):1162–1165. discussion 1166–7. [PubMed] [Google Scholar]
- 9.Sifri ZC, Livingston DH, Lavery RF, et al. Value of repeat cranial computed axial tomography scanning in patients with minimal head injury. Am J Surg. 2004;187(3):338–342. doi: 10.1016/j.amjsurg.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Bone RC, McElwee NE, Eubanks DH, Gluck EH. Analysis of indications for intensive care unit admission. Clinical efficacy assessment project: American College of Physicians. Chest. 1993;104(6):1806–1811. doi: 10.1378/chest.104.6.1806. [DOI] [PubMed] [Google Scholar]
- 11.Hesdorffer DC, Ghajar J. Marked improvement in adherence to traumatic brain injury guidelines in United States trauma centers. J Trauma. 2007;63(4):841–847. doi: 10.1097/TA.0b013e318123fc21. discussion 847–8. [DOI] [PubMed] [Google Scholar]
- 12.Hesdorffer DC, Ghajar J, Iacono L. Predictors of compliance with the evidence-based guidelines for traumatic brain injury care: a survey of United States trauma centers. J Trauma. 2002;52(6):1202–1209. doi: 10.1097/00005373-200206000-00031. [DOI] [PubMed] [Google Scholar]
- 13.Groeger JS, Guntupalli KK, Strosberg M, et al. Descriptive analysis of critical care units in the United States: patient characteristics and intensive care unit utilization. Crit Care Med. 1993;21(2):279–291. doi: 10.1097/00003246-199302000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Escher M, Perneger TV, Chevrolet JC. National questionnaire survey on what influences doctors' decisions about admission to intensive care. BMJ. 2004;329(7463):425. doi: 10.1136/bmj.329.7463.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalfin DB, Trzeciak S, Likourezos A, et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007;35(6):1477–1483. doi: 10.1097/01.CCM.0000266585.74905.5A. [DOI] [PubMed] [Google Scholar]
- 16.Guidelines for intensive care unit admission, discharge, and triage. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 1999;27(3):633–638. [PubMed] [Google Scholar]
- 17.Worster A, Bledsoe RD, Cleve P, et al. Reassessing the methods of medical record review studies in emergency medicine research. Ann Emerg Med. 2005;45(4):448–451. doi: 10.1016/j.annemergmed.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Copes WS, Champion HR, Sacco WJ, et al. The Injury Severity Score revisited. J Trauma. 1988;28(1):69–77. doi: 10.1097/00005373-198801000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57(6):1173–1182. doi: 10.1227/01.neu.0000186013.63046.6b. discussion 1173–82. [DOI] [PubMed] [Google Scholar]
- 20.Milbrandt EB, Kersten A, Rahim MT, et al. Growth of intensive care unit resource use and its estimated cost in Medicare. Crit Care Med. 2008;36(9):2504–2510. doi: 10.1097/CCM.0b013e318183ef84. [DOI] [PubMed] [Google Scholar]
- 21.Andrews PJ, Sleeman DH, Statham PF, et al. Predicting recovery in patients suffering from traumatic brain injury by using admission variables and physiological data: a comparison between decision tree analysis and logistic regression. J Neurosurg. 2002;97(2):326–336. doi: 10.3171/jns.2002.97.2.0326. [DOI] [PubMed] [Google Scholar]
- 22.Hukkelhoven CW, Steyerberg EW, Habbema JD, Maas AI. Admission of patients with severe and moderate traumatic brain injury to specialized ICU facilities: a search for triage criteria. Intensive Care Med. 2005;31(6):799–806. doi: 10.1007/s00134-005-2628-y. [DOI] [PubMed] [Google Scholar]
- 23.Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):329–337. doi: 10.1089/neu.2006.0035. [DOI] [PubMed] [Google Scholar]
- 24.Cohen DB, Rinker C, Wilberger JE. Traumatic brain injury in anticoagulated patients. J Trauma. 2006;60(3):553–557. doi: 10.1097/01.ta.0000196542.54344.05. [DOI] [PubMed] [Google Scholar]
- 25.af Geijerstam JL, Oredsson S, Britton M. Medical outcome after immediate computed tomography or admission for observation in patients with mild head injury: randomised controlled trial. BMJ. 2006;333(7566):465. doi: 10.1136/bmj.38918.669317.4F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norlund A, Marke LA, af Geijerstam JL, et al. Immediate computed tomography or admission for observation after mild head injury: cost comparison in randomised controlled trial. BMJ. 2006;333(7566):469. doi: 10.1136/bmj.38918.659120.4F. [DOI] [PMC free article] [PubMed] [Google Scholar]