ABSTRACT
The proper development of the vertebrate retina relies heavily on producing the correct number and type of differentiated retinal cell types. To achieve this, proliferating retinal progenitor cells (RPCs) must exit the cell cycle at an appropriate time and correctly express a subset of differentiation markers that help specify retinal cell fate. Homeobox genes, which encode a family of transcription factors, have been accredited to both these processes, implicated in the transcriptional regulation of important cell cycle components, such as cyclins and cyclin-dependent kinases, and proneural genes. This dual regulation of homeobox genes allows these factors to help co-ordinate the transition from the proliferating RPC to postmitotic, differentiated cell. However, understanding the exact molecular targets of these factors remains a challenging task. This commentary highlights the current knowledge we have about how these factors regulate cell cycle progression and differentiation, with particular emphasis on a recent discovery from our lab demonstrating an antagonistic relationship between Vsx2 and Dmbx1 to control RPC proliferation. Future studies should aim to further understand the direct transcriptional targets of these genes, additional co-factors/interacting proteins and the possible recruitment of epigenetic machinery by these homeobox genes.
KEYWORDS: cell cycle, development, dmbx1, epigenetic, gene expression, mouse, neurogenesis, progenitor cell, retina, vsx2, zebrafish
Introduction
The developing vertebrate neural retina emerges through a complex process of morphogenesis of the anterior neuroepithelium and initially consists of proliferating retinal progenitor cells (RPCs), which are multipotent cells capable of limited self-renewal. Differentiation begins in the center of the neural retina and expands toward the periphery as RPCs differentiate into one of the five main classes of retinal neurons (ganglion cells, amacrine cells, bipolar cells, horizontal cells, and photoreceptor cells), most of which are highly specialized into multiple subtypes, or Müller glial. In the mature retina, these cells are organized into 3 cellular layers [ganglion cell layer (GCL), inner nuclear layer (INL) and outer nuclear layer (ONL)], which must be properly formed so that the cells are capable of relaying visual sensory information to the brain via the optic nerve.8 Differentiation also occurs in a specific temporal order that is well conserved across vertebrate species, with ganglion cells appearing first while Müller glia are the last to appear.57,76,83 Due to this birth order, the proportion of cells that exit the cell cycle and differentiate must be appropriately timed to ensure the correct number and type of cells are produced.
Therefore, proper neurogenesis of the retina relies on appropriately regulating the transition from a proliferating RPC to a post-mitotic differentiated retinal cell. At the heart of this process, two activities must be carefully coordinated: (1) cell cycle exit and (2) activation of an appropriate differentiation program. Failure to do so can result in proliferation defects resulting in microphthalmia or hyperplasia, differentiation defects and disruption to the lamination of the retina, and apoptosis (refs. 2,7,22,67,93). It is commonly believed that cell cycle exit and differentiation are intimately coupled, and that halting cell cycle progression is a prerequisite for differentiation. This is for the most part true, considering that they are temporally coupled: when cell cycle or proliferation-related genes are down regulated (i.e. vsx2, cyclinD1), differentiation specific genes are concurrently up-regulated.14 Cell cycle kinetics is also observed to change during differentiation with a lengthening of the cell cycle as the retina matures.4 Genetic manipulations that lengthen or reduce the G1 phase are observed to result in premature terminal differentiation or increased progenitor proliferation, respectively.52,78 In addition, genetic perturbations that prevent cell cycle exit in the retina often lead to a delay in retinal differentiation.93 Although still controversial, evidence suggests that the two processes can be uncoupled and that cell cycle exit is not required for, or a consequence of, differentiation.46,52,66 Supporting this idea, RPCs start to express differentiation markers before the onset of cell cycle exit and cell cycle proteins are still expressed well after terminal mitosis.33,74,75 In addition, major perturbations on important cell cycle factors (i.e., Rb, CKIs) can cause ectopic division of differentiated neurons28,67). Therefore, the proliferation to differentiation transition is not as rigid as the classic perspective posits, although further research is required to fully explore this notion.
A flexible relationship likely represents the independence of the underlying molecular mechanisms governing cell cycle control and differentiated programs. As such, elucidating the molecular players is important to understand how each process is separately governed. Although these are separate processes, there may be unique opportunities for cross talk between these pathways. Advantageously, this can help ensure that cell cycle exit is properly coordinated with differentiation. For example, the proneural gene ATOH7/ATH5, which is required for the production of RGCs, also regulates cell cycle length via Notch signaling.24 Many other transcription factors (TFs), including a broad class of homeobox genes, have also been associated with both cell cycle regulation and differentiation. Homeobox TFs are therefore properly positioned to activate/repress cell cycle genes while concurrently repressing/activating differentiation programs. This review will focus on understanding the molecular mechanisms controlling cell cycle exit and/or differentiation during retinal neurogenesis, and in particular the role of homeobox TFs including dmbx1 and vsx2, which may contribute to understanding the etiology of retinal developmental disorders.
Cell cycle control in the developing retina
Increasing evidence indicates that transcription factors that regulate retinal neurogenesis impinge on major cell cycle regulators. Terminal differentiation is characterized by stalling in the G1 phase as cells arrest and exit the cell cycle into an irreversible G0 phase.19 Due to this, the G1/S phase progression is an important regulatory point that determines whether a cell will transition from a proliferative to post-mitotic state. The key molecular event dictating whether a cell progresses from G1 to S phase or exits the cell cycle culminates on the phosphorylation status of retinoblastoma (Rb) protein.37 The main function of Rb is to inhibit division, promote cell cycle exit and suppress cell cycle re-entry of differentiated cells.17 In agreement, loss of retinoblastoma 1 (rb1) function in zebrafish leads to a delay of cell cycle exit and delay of differentiation of early-born RGCs, which negatively affects proper retinotectal connectivity and phototactic behaviors.51 In mice this phenotype is more severe, with Rb1 knockouts exhibiting retinal hypoplasia, ectopic division, and considerable apoptosis of retinal cells.67
The phosphorylation status of Rb during G1 depends on the activity of cyclin-dependent kinases (CDK), which are active when bound to a corresponding cyclin protein.37 When active, the cyclin-CDK complex phosphorylates Rb, and dissociates Rb from the transcription factor, E2F, allowing E2F to transcribe genes important for the S and G2 phases, leading to cell cycle progression (Fig. 1).88 The major cyclin important in retinal development is CyclinD1 (Ccnd1), which bind to and activates Cdk4/6. Ccnd1 is highly expressed in RPCs but its expression is downregulated in differentiated retinal cells.6,37,84 Consistent with a function in maintaining proliferation, Ccnd1 loss in mice causes severe microphthalmia due to reduced RPC proliferation.29,41 In addition, the cell cycle is prolonged, RPCs prematurely exit the cell cycle and retinas display differentiation defects showing a greater proportion of RGCs and photoreceptors at the expense of horizontal and amacrine cells.28 In zebrafish, knockdown of ccnd1 also results in microphthalmia although differentiation is not severely affected since all major cell types are produced, suggesting a role in cell cycle regulation independent of differentiation.35 Conversely, ectopic ccnd1 prevents normal cell cycle exit, causing excessive cell proliferation and apoptosis.85 It is interesting to highlight that recently Ccnd1 has also more broadly been associated with transcriptional regulation in mouse retinal development and therefore may have non-cell cycle related functions.12 The role of other cyclins, including other D-type cyclins (D2 and D3) and E-type cyclins is not as pronounced in the retina, although cyclin E, but not D2/D3, is capable of rescuing RPC defects in Ccnd1 knockouts.20,29,30,47 Interestingly, CyclinD3 levels are up regulated in Ccnd1 knockouts although retinal proliferation is not rescued, indicating a compensating mechanism that cannot functionally substitute for Ccnd1.48 As a result, the major cell cycle activator in the G1 phase of the retina appears to be Ccnd1 and high expression in RPCs normally promotes cell cycle progression.
Figure 1.
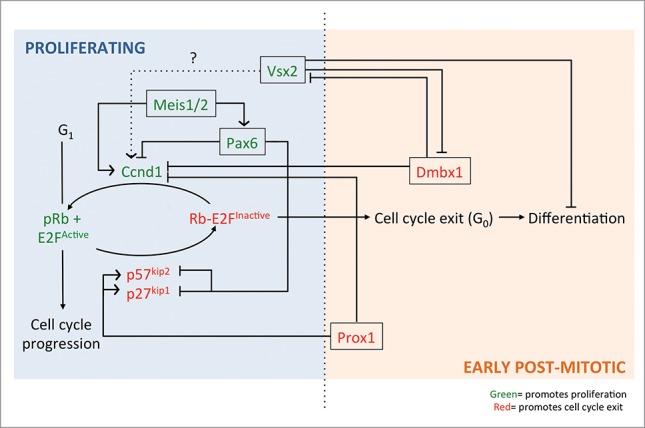
Schematic representing how the homeobox genes Pax6, Meis1/2, Prox1, Dmbx1 and Vsx2 control the cell cycle progression or exit of an RPC into an early post-mitotic neuron. Some of these genes (Pax6, vsx2 and Meis1/2) are expressed early in a proliferating RPC (seen on the ‘blue’ proliferating side) whereas others (Dmbx1 and Prox1) are expressed as a RPC exits the cell cycle (seen on the ‘orange’ early post-mitotic side). Each homeobox gene highlighted has been implicated in activating/inhibiting certain cell cycle factors/other homeobox genes via direct or indirect transcriptional regulation (see text for more details). Pointed arrows indicate an activating role whereas straight edge arrows indicate an inhibiting role.
However, a mechanism must be in place to counter cyclin-CDK activity to promote cell cycle exit. One of the most prominent mechanisms involves the activity of cyclin kinase inhibitors (CKIs) (Fig. 1). Two families of CKIs have been identified including the Ink4 family and Cip/Kip family,89 but only three CKIs have been implicated in retinal development including p27kip1 and p57kip2, and p19ink4d. Consistent with their role in cell cycle exit, p27kip1, p57kip2 and p19ink4d are upregulated and highly expressed in a subset of RPCs undergoing cell cycle exit and in newly post-mitotic cells, although each CKI shows distinct spatial and temporal expression.28,36,37 A loss of any of these CKIs results in an increase in proliferation of RPCs, whereas overexpression leads to premature cell cycle exit.27,36,61 The main mechanism behind how CKIs exert their cell cycle inhibition appears to be by directly binding and inactivating cyclin-CDKs89 For example, p27kip1 can directly interact with Ccnd1-Cdk4/6 or cyclin E-Cdk2 and prevent their activity.37 However, if there are sufficiently high levels of Ccnd1, excessive Ccnd1 can bind and sequester p27kip1 and free other cyclin-cdk complexes (i.e. cyclin E-cdk2) to promote cell cycle progression. In agreement, simultaneous knockout of p27kip1 in Ccnd1 knockout mice can rescue retinal cellularity48,87 Analysis indicates this is because cyclinE-cdk2 is freed from inhibition by p27kip1, allowing partial restoration of pRb and promotion of the cell cycle.87 It appears, therefore, that the balance between the levels of cell cycle activators (i.e., cyclin-CDK) and repressors (i.e. CKIs) is crucial in controlling the cell cycle of the RPCs. Moreover, the timing of expression of these positive and negative regulators is key to understanding this balance.
Homeobox genes control of cell cycle exit via regulation of cell cycle components
Homeobox genes encode a family of transcription factor proteins characterized by the presence of a DNA binding motif called the homeodomain and over the years, have been seen to have critical roles in retinal neurogenesis.32,94 Traditionally, homeobox genes were predominantly linked to the transcription of genes responsible for maintaining a proliferative RPC identity, specifying cell fate of RPCs and/or dictating differentiation. However, given that the cell cycle needs to be dynamically regulated as a cell transitions from a RPC to a post-mitotic cell, the question of whether homeobox genes could also directly regulate the expression of cell cycle components gained interest. Now, multiple homeobox TFs have been observed to have direct or indirect roles in regulating cyclins and CKIs. This indicates that TFs are multifaceted factors capable of intervening and coordinating different, distinct molecular pathways. The importance of these roles is highlighted by the fact that homeobox genes are increasingly being implicated in human retinal disorders including microphthalmia and therefore understanding their multi-faceted function could help understand causes and possible treatments of these disorders.94 The focus here will be on highlighting examples of homeobox genes controlling the cell cycle and differentiation in the retina with only minor consideration given to evidence for similar roles found in the central nervous system. However, it is important to note that a number of homeobox genes have also been implicated in other tissues and notably, carcinogenesis, given their intimate relationship with cell cycle components (reviewed in ref. 1).
One of the best recent examples of a retinal homeobox gene that regulates the cell cycle is the paired box (Pax) gene, Pax6. Pax6 is highly expressed in the developing eye, and is predominately viewed as an early retinal determination gene with homozygous mutations resulting in a complete absence of eye structures (congenital aniridia) in humans.15,54 However, Pax6 overexpression also causes microphthalmia and retinal dysplasia, consistent with a cell cycle effect.98 Pax6 expression is found throughout the RPC population and subsequently in differentiated RGCs, amacrine and horizontal cells.56 In controlling differentiation, Pax6 is required to maintain RPC multipotency with Pax6-deficient RPCs showing limited differentiation, producing exclusively amacrine cells.68 Failure to create other neuron subtypes stems from the fact that Pax6 directly regulates the transcription of proneural genes, including RGC specification factor Atoh7, and neurogenesis factors neurogenin-2 and Ascl1.61,73,77,80 Pax6 expression in RPCs is also required to promote cell cycle progression. Pax6 loss in the retina results in a significant reduction in the amount of cells in the S phase and an increase in the number of cells exiting the cell cycle.42,77 Interestingly, Pax6-deficient mouse retinas show an increase in factors that both promote (cyclinD1-3) and inhibit (p27kip1 and p57kip2) the cell cycle, with Pax6-deficient cells typically co-expressing these opposing factors42 This implies that Pax6s regulation of the cell cycle is complex, possessing the ability to either promote or inhibit the cell cycle. Evidence of its role in the retina versus the brain seems to support this idea. Although Pax6 appears to normally promote cell cycle progression/proliferation in RPCs, Pax6 in cortical progenitors actually inhibits proliferation.9,40,42,68 Therefore, this may represent a context-specific difference that results from different extrinsic/intrinsic cues, or a presence of other TFs/proteins that interact with and affect Pax6s specific function. It is not clear from studies in the retina whether Pax6 can directly regulate the transcription of these cell cycle factors since other TFs, such as Vsx2, which are known to regulate cell cycle factors, are reduced in expression in Pax6-deficient RPCs.42 Although this implies it is indirectly regulated, investigation in cortical progenitor cells indicate that Pax6 is capable of directly repressing the transcription of cell cycle factors, such as Cdk6, to attenuate proliferation.69
Other highly studied TFs are Meis1/2, which belong to the TALE-class of homeobox TFs and are the only Meis family members expressed in the eye;16,53 Bumsted-O'Brien, 2007). Meis proteins are the vertebrate homolog of the homothorax (Hth) gene in Drosophila which is expressed in multipotent cells of the eye and is required to maintain proliferation and prevent premature differentiation.10 A similar role in controlling RPC proliferation for Meis1/2 in the retina of chick, mouse and zebrafish has also been seen, suggesting a highly conserved role in retinal development.6,53,55 In these 3 species, Meis1/2 is also expressed in proliferating RPCs, but down regulated when cells begin to differentiate.6,53 Knockout or knockdown of Meis1/2 in mice, chick and zebrafish all result in microphthalmia due to impairment in the proliferation of RPCs.6,53 Moreover, meis1 and meis2 work synergistically in zebrafish since the amount of mitotic cells are even more reduced when both genes are knocked down.53 Evidence suggests that the mechanism by which Meis1 maintains proliferation is by promoting the G1 to S transition of the cell cycle. This is because knockdown of meis1 in zebrafish results in a significantly higher proportion of cells stalled in the G1 phase.11 Consistent with this cell cycle effect, ccnd1 expression is reduced in meis1/2 knockdowns. The proliferation defect in the retina can also be rescued by expressing ccnd1, implying that meis1/2 regulates ccnd1 expression.6,53 If this regulation is direct or indirect is not known. This regulatory relationship is also observed in the retina where Meis1 knockdown results in reduced Pax6 transcript levels.11,54 However, expression of Pax6 in Meis1 knockdown does not rescue the loss of ccnd1 expression, suggesting that loss of ccnd1 is independent of the loss of Pax6.11 In contrast to Meis1's regulation of Pax6, biochemical and functional analysis of a Meis1 eye enhancer has identified Pax6 binding sites, suggesting that Pax6 may also regulate Meis1 expression.82 However, the importance of this regulation in the retina is uncertain, considering that pax6 morphants do not exhibit alterations in meis1 expression.82 However, in light of this evidence and the fact that Meis and Pax6 are expressed in similar domains within the eye, it suggests that homeobox TFs are capable of regulating each other's expression.6
Unlike the previous examples above, Prox1 homeobox TF negatively regulates RPC proliferation and is required to promote cell cycle exit. Prox1 is the vertebrate homolog of the prospero gene in Drosophila, which promotes cell cycle exit via repression of the transcription of cell cycle progression genes, cdc25, cyclinE and cyclinA during neurogenesis.63 In the vertebrate retina, a similar role in promoting cell cycle exit also occurs. Prox1 expression is found in proliferating RPCs preceding the expression of p27kip1 and p57kip2.39 Loss of Prox1 results in expansion of Ccnd1 expression, more proliferating RPCs, and fewer differentiated cells with a bias toward the production of later-born cell types.39 Conversely, ectopic expression of Prox1 results in premature cell cycle exit and bias toward early born cell types, and horizontal cells. This indicates that Prox1 may promote CKI expression to cause cell cycle exit, although whether Prox1 has a direct role in their transcription remains to be investigated. A similar role for Prox1-mediated cell cycle exit is also reported in the mouse lens, where p27kip1 and p57kip2 expression is decreased in Prox1-deficient lens, and in spinal interneurons.70,91 Prox1 has also been observed to have a critical role in suppressing malignant neuroblastoma transformation by suppressing Ccnd1, CyclinA, CyclinB1, Cdc25A and inducing p27kip1.45 These all indicate that Prox1 has important effects on the cell cycle factors in development and disease. Interestingly, Prox1 expression in RPCs is extinguished in cells exiting the cell cycle at the G1/G0 phase, and reinitiated in horizontal and amacrine cells, being necessary and sufficient for horizontal cell genesis.39 This segregated expression patterns seems to suggest that regulation of the cell cycle and differentiation program by Prox1 are completely independent processes. Indeed, expression of Prox1 has also been reported in bipolar cells, rod precursors and Muller glia, although the requirement of Prox1 in these cells is not fully elucidated.26,39 The role of Pax6, Meis1 and Prox1 in the cell cycle is summarized in the schematic in Figure 1.
Vsx2 and dmbx1: Coordinating cell cycle exit through antagonistic gene expression
The examples of Pax6, Meis1/2 and Prox1 highlight how homeobox TFs can have a profound effect on retinal development through the regulation of cell cycle machinery. In our lab, we have added to this understanding by investigating the role of visual system homeobox 2 (vsx2) and diencephalon/mesencephalon homeobox 1 (dmbx1) in zebrafish retinal development. We have shown how they function antagonistically to control the transition from a RPC to post-mitotic cell by affecting each other's expression, and cell cycle factors to ultimately affect proliferation and cell cycle exit92 (summarized in Fig. 1).
Vsx2 (also previously referred to as Chx10 in mice or alx in zebrafish) is a member of the paired-like homeobox TF family and contains both a homeodomain and an adjacent CVC domain. Both domains are required for high specificity DNA binding, with Vsx2 generally mediating transcriptional repression of its target genes.34,65,97 Vsx2 is expressed in RPCs in the developing retina, but is downregulated as RPCs exit the cell cycle. In the mature retina, remaining RPCs found in the ciliary or circumferential marginal zone (CMZ) and differentiated bipolar interneurons, and Muller glia maintain Vsx2 expression.50,64,81 Null mutations in VSX2, are associated with severe microphthalmia and congenital blindness in humans and have also been previously identified as the cause of microphthalmia in the ocular retardation (orJ) mouse model.18,43 Knockdown in zebrafish also results in microphthalmia highlighting a conserved role of vsx2 in retinal development.5,90,92 More specific analysis of Vsx2 loss of function experiments in the retina shows there are two major defects: (1) a reduction in the number of proliferating RPCs; and (2) an absence of bipolar cells due to defects in bipolar differentiation.18 The early effect of Vsx2 on proliferation and later effect on bipolar development appear to be separable processes since Vsx2 is dispensable for cell proliferation but required to control bipolar cell genesis (by inhibition of rod differentiation) in the postnatal mouse retina.65 However, transient ectopic vsx2 expression in zebrafish is sufficient to prolong RPC proliferation in the central retina.92 To maintain an RPC identity over a postmitotic cell state, Vsx2 partly acts to repress genes (i.e., Vsx1 and Ath5) that would otherwise specify the fate of the cell.26,90 Vsx2 maintains RPC proliferation via repression of the inhibitory cell cycle component, p27kip1. Supporting this, Vsx2-null retinas express a higher portion of p27kip1-positive cells, but when p27kip1 is concurrently knocked out with Vsx2, there is a rescue in the proliferation defect, hypocellular retinal phenotype, and lamination defects.50 It is proposed that Vsx2 regulates p27kip1 at the post-transcriptional level through Ccnd1- mediated repression of p27kip1.50 This implies that Vsx2 may directly regulate Ccnd1 levels, but this would require Vsx2 to act as a transcriptional activator, which has not yet been reported. Given these functions, Vsx2 must be appropriately down regulated to facilitate the transition from a RPC to postmitotic cell, although the factors responsible for this regulation remain unclear.
A promising candidate was Dmbx1 since contrary to Vsx2, Dmbx1 is required for cell cycle exit during retinal development. Dmbx1 also belongs to the paired-like homeobox gene family and is thought to act as a transcriptional repressor.60 However, its transcriptional targets have not been well defined. Dmbx1 has been implicated in regulating neurogenesis in both the retina and midbrain.71,93 Sequence analysis has indicated that this gene is conserved in mouse, humans and zebrafish although there is functional divergence in dmbx1 between species. In particular, mouse Dmbx1 is predominantly expressed and important in midbrain and hindbrain development, but its expression and function in the retina has not been thoroughly investigated.49,71,72 In contrast, the zebrafish genome harbours two dmbx1 paralogs, dmbx1a and dmbx1b, and both are expressed in the midbrain (pretectum/tectum), hindbrain and retina, with knockdown resulting in microphthalmia and defects in the retinotectal pathway.21,59,93 Interestingly, a recently identified mutation in human DMBX1 was associated with hyperopia indicating that this gene is relevant to human retinal disorders as well.3 Zebrafish dmbx1a expression onset in the neural retina coincides with when RPCs start exiting the cell cycle and is found to coincide predominately in newly post-mitotic cells in the INL.21,93 Knockdown of dmbx1a results in significant reduction in retinal growth, and disruption to retinal differentiation and lamination,93 Underlying this phenotype, more retinal cells remain proliferative, possess a prolonged cell cycle, and fail to differentiate.93 These results suggest that dmbx1 promotes cell cycle exit, although the exact molecular mechanism responsible, remains to be fully elucidated.
Wong et al. (2015)92 provide insight into this mechanism by establishing evidence for a signaling axis involving antagonistic functions of vsx2 and dmbx1. Since vsx2 is expressed in RPCs, but downregulated at the onset of dmbx1 expression, this suggested that these TFs might mutually repress each other. To investigate this, Wong et al. (2015)92 analyzed changes in transcript levels in dmbx1/vsx2 overexpression and knockdown embryos. Consistent with the hypothesis, vsx2 expression is upregulated when dmbx1 is knocked down, with vsx2 expression expanding beyond RPCs of the CMZ and into the central retina. Conversely, knockdown of vsx2 correspondingly results in an upregulation of dmbx1, while overexpression of vsx2 significantly decreases dmbx1 expression. This antagonistic regulation of expression supports the hypothesized mutual repression, with manipulation of gene expression either fostering or removing this repression.
Importantly, the changes in vsx2 or dmbx1 expression levels result in obvious changes to proliferation or timing of cell cycle exit, indicating that the proper temporal expression of these genes have important implications on the state of RPCs. In particular, vsx2 overexpression is sufficient to prolong proliferating RPC identity in the central retina, since central retinal cells continue to incorporate BrdU for longer when vsx2 is transiently induced. In contrast, overexpression of dmbx1 results in premature cell cycle exit. A decrease in the number of mitotic cells (pHH3 and BrdU-positive cells) in an early retina (24hpf), normally dominated by mitotic cells, supports this conclusion. In addition, premature cell cycle exit results in an abundance of earlier born retinal cells type, RGCs, and decrease in later born cell types, including Müller glia and cone photoreceptors. This indicates that controlling the balance of vsx2 and dmbx1 expression down regulation and onset, respectively, is crucial in establishing proper retinal development and the mutual antagonism between these genes helps ensure that this balance is met.
The question now remained, what other factors fit into this molecular pathway, upstream and downstream of vsx2/dmbx1? Upstream of vsx2, we show that FGF signaling is necessary to maintain vsx2 expression. Inhibition of FGF signaling causes vsx2 levels to decrease, suggesting that FGF signaling is a positive regulator of vsx2 in zebrafish, a relationship which is conserved with mammals.27 Interestingly, we also found that this positive regulation by FGF must be sustained in order for vsx2 levels to be up regulated in the absence of dmbx1, since the simultaneous inhibition of FGF signaling and dmbx1a knockdown did not result in an up regulation of vsx2.92 This suggests that vsx2 level is not directly regulated by dmbx1 but requires continued FGF signaling to be upregulated. It is possible that dmbx1 regulates the repression of FGF signaling components that lead to reduced vsx2, but future studies are required to address if there is direct binding of dmbx1 to the vsx2 promoter, or other promoters, in order to fully understand this regulation.
In addition, understanding the downstream factors affected, provide insight into why the cell cycle and proliferation ability of the cells are affected in dmbx1 knockdown. We found that ccnd1 was prominently affected in dmbx1 morphants,92 showing a substantial increase in expression throughout the central retina. Conversely, overexpression of dmbx1 decreases the expression of ccnd1. Furthermore, combined knockdown of dmbx1 and ccnd1 is able to partially rescue the differentiation defect observed in dmbx1 morphants. This suggests that dmbx1 negatively regulates ccnd1 to promote cell cycle exit, but does not seem to be necessary for controlling retinal differentiation per se. Therefore, dmbx1 represents a homeobox TF whose role is uniquely accredited to controlling cell cycle exit of proliferating RPCs by negatively regulating ccnd1.
It is important to note that due to the antagonistic nature of the vsx2-dmbx1 relationship, vsx2 expression needs to be down regulated before dmbx1 expression predominates and furthermore, dmbx1 cannot reduce vsx2 until it is more highly expressed. What is responsible for initially tipping the balance toward dmbx1 expression is unknown. Extrinsic factors implicated in retinal development (i.e. Wnt, FGF, Shh) may be responsible for prompting RPCs to start to exit the cell cycle, ultimately leading to changes in the transcription of factors including vsx2 and dmbx1. We know from our results that vsx2 is influenced by FGF signaling, but it is unknown what other factors are capable of regulating dmbx1.
Further understanding cell cycle control: Transcriptional targets, protein interactions and epigenetic regulation
Most of the effects of homeobox genes, including vsx2 and dmbx1 on retinal development, are associated with the genes they transcriptionally regulate. As such, discovering the transcriptional targets is crucial to understanding their function. We conclude from our findings that vsx2 likely transcriptionally represses dmbx1, and dmbx1 likely transcriptionally represses vsx2 and ccnd1. However direct evidence, using a biochemical technique such as chromatin immunoprecipitation (ChIP), is needed to show that these TFs are capable of binding to the promoters of these genes. Moreover, this influence is not necessarily an independent process and may require the activity of additional co-factors. In particular, TFs commonly hetero-dimerize with other TFs to affect their DNA binding specificity and targets. For example, Meis1 and Pbx, another homeobox gene implicated in controlling retinal RGC axon outgrowth, have been seen to interact and cooperatively bind to target genes.13,45 In addition, Dmbx1 has been shown to form a heterodimer with Otx2.60 Homeobox proteins can also physically interact with other types of proteins, most notably, including cell cycle factors. Prox1 has been shown to interact with proliferating cell nuclear antigen (PCNA) and this was seen to inhibit Prox1s ability to transcriptionally activate the betaB1-crystallin promoter.23 RPC proliferation promoting factor, Six3/6 has been shown physically interact with geminin, a DNA replication-inhibitor that promotes cell cycle arrest, to inhibit its activity and ultimately control the balance between proliferation and differentiation.31,86 Whether other homeobox genes, including vsx2 and dmbx1, control RPC proliferation by physically interacting with cell cycle proteins remains an exciting question to address in the future. Discovering these new protein-protein interactions can add a complexity that aids in better understanding the function of these homeobox TFs, especially in different developmental contexts.
TFs can also recruit epigenetic machinery to affect retinal development. Given that the transition from a RPC to postmitotic, differentiated cell is accompanied by dramatic changes in gene expression, epigenetic regulation can promote long-term repression of cell cycle/progenitor related genes and represents an evolving field of study in retinal development. Highlighting the importance of epigenetics, studies support the idea that chromatin modifiers, including histone methyltransferases (HMT) have a substantial influence on the RPC proliferation and differentiation. For example, loss of Ezh2 activity, a subunit in polycomb repressive complex 2 (PRC2) responsible for di- and trimethylation of lysine 27 of histone H3, reduces RPC proliferation, disrupts retinal lamination and delays differentiation in the mouse retina.95 A specific type of HMT, G9a, is also implicated in brain and retinal development, with g9a loss in zebrafish causing notable reductions in eye and brain size (Rai et al., 2010). In the mouse retina, G9a mediated dimethylation of lysine 9 of histone H3 in RPCs on progenitor cell-related genes, including cyclins and progenitor TFs (such as Vsx2) is essential to mediate repression and promote normal terminal differentiation and survival of retinal cells.57 Since transcription factors are capable of recruiting/interacting with chromatin modifiers, it is possible that homeobox TFs interact with HMTs/other modifiers. In support of this motion, the TF PRDM4, recruits the HMT, PRMT5 to maintain neural stem cells in a proliferative state.25 Whether dmbx1 interacts with epigenetic machinery, to stabilize repression of ccnd1, vsx2 and other targets, and promote cell cycle exit is an exciting question to explore.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was funded by the Canadian Institutes of Health Research (VT) and the Vision Science Research Program Scholarship (AM).
References
- [1].Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer 2002; 2(10):777-85; PMID:12360280; http://dx.doi.org/ 10.1038/nrc907 [DOI] [PubMed] [Google Scholar]
- [2].Agathocleous M, Harris WA. From progenitors to differentiated cells in the vertebrate retina. Annu Rev Cell Dev Biol 2009; 25:45-69; PMID:19575661; http://dx.doi.org/ 10.1146/annurev.cellbio.042308.113259 [DOI] [PubMed] [Google Scholar]
- [3].Alazami AM, Patel N, Shamseldin HE, Anazi S, Al-Dosari MS, Alzahrani F, Hijazi H, Alshammari M, Aldahmesh MA, Salih MA, et al.. Accelerating novel candidate gene discovery in neurogenetic disorders via whole-exome sequencing of prescreened multiplex consanguineous families. Cell Rep 2015; 10(2):148-61; PMID:25558065; http://dx.doi.org/ 10.1016/j.celrep.2014.12.015 [DOI] [PubMed] [Google Scholar]
- [4].Alexiades MR, Cepko C. Quantitative analysis of proliferation and cell cycle length during development of the rat retina. Dev Dyn 1996; 205(3):293-307; PMID:8850565; http://dx.doi.org/ 10.1002/(SICI)1097-0177(199603)205:3%3c293::AID-AJA9%3e3.0.CO;2-D [DOI] [PubMed] [Google Scholar]
- [5].Barabino SM, Spada F, Cotelli F, Boncinelli E. Inactivation of the zebrafish homologue of Chx10 by antisense oligonucleotides causes eye malformations similar to the ocular retardation phenotype. Mech Dev 1997; 63(2):133-43; PMID:9203137; http://dx.doi.org/ 10.1016/S0925-4773(97)00036-1 [DOI] [PubMed] [Google Scholar]
- [6].Barton K, Levine E. Expression patterns and cell cycle profiles of PCNA, MCM6, cyclin D1, cyclin A2, cyclin B1, and phosphorylated histone H3 in the developing mouse retina. Dev Dyn 2008; 237:672-82; PMID:18265020; http://dx.doi.org/ 10.1002/dvdy.21449 [DOI] [PubMed] [Google Scholar]
- [7].Baye LM, Link BA. The disarrayed mutations results in cell cycle and neurogenesis defects during retinal development in zebrafish. BMC Dev Biol 2007; 7:28; PMID:17411431; http://dx.doi.org/ 10.1186/1471-213X-7-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bejarano-Escobar R, Blasco M, Martin-Partido G, Francisco-Morcillo J. Molecular characterization of cell types in the developing, mature and regenerating fish retina. Rev Fish Biol Fisheries 2014; 24:127-58; http://dx.doi.org/ 10.1007/s11160-013-9320-z [DOI] [Google Scholar]
- [9].Berger J, Berger S, Tuoc TC, D'amelio M, Cecconi F, Gorski JA, Jones KR, Gruss P, Stoykova A. Conditional activation of Pax6 in the developing cortex of trans- genic mice causes progenitor apoptosis. Development 2007; 134:1311-22; PMID:17329367; http://dx.doi.org/ 10.1242/dev.02809 [DOI] [PubMed] [Google Scholar]
- [10].Bessa J, Gebelein B, Pichaud F, Casares F, Mann RS. Combinatorial control of Drosophila eye development by eyeless, homothorax and teashirt. Genes Dev 2002; 16:2415-27; PMID:12231630; http://dx.doi.org/ 10.1101/gad.1009002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bessa J, Tavares MJ, Santos J, Kikuta H, Laplante M, Becker TS, Gomez- Skarmeta JL, Casares F. Meis1 regulates cyclin D1 and c-myc expression, and controls the proliferation of multipotent cells in the early developing zebrafish eye. Development 2008; 135(5):799-803; PMID:18216175; http://dx.doi.org/ 10.1242/dev.011932 [DOI] [PubMed] [Google Scholar]
- [12].Bienvenu F, Jirawatnotai S, Elias JE, Meyer CA, Mizeracka K, Marson A, Frampton GM, Cole MF, Odom DT, Odajima J, et al.. Transcriptional role of cyclin D1 in development revealed by a genetic-proteomic screen. Nature 2010; 463(7279):374-8; PMID:20090754; http://dx.doi.org/ 10.1038/nature08684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bischof LJ, Kagawa N, Moskow JJ, Takahashi Y, Iwamatsu A, Buchberg AM, Waterman MR. Members of the meis1 and pbx homeodomain protein families cooperatively bind a cAMP-responsive sequence (CRS1) from bovine CYP17. J Biol Chem 1998; 273(14):7941-8; PMID:9525891; http://dx.doi.org/ 10.1074/jbc.273.14.7941 [DOI] [PubMed] [Google Scholar]
- [14].Blackshaw S, Harpavat S, Trimarchi J, Cai L, Huang H, Kuo WP, Weber G, Lee K, Fraioli RE, Cho SH, et al.. Genomic analysis of mouse retinal development. PLoS Biol 2004; 2(9):E247; PMID:15226823; http://dx.doi.org/ 10.1371/journal.pbio.0020247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brown A, McKie M, van Heyningen V, Prosser J. The human PAX6 mutation database. Nucleic Acids Res 1998; 26:259-64; PMID:9399848; http://dx.doi.org/ 10.1093/nar/26.1.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bumsted-O'Brien KM, Hendrickson A, Haverkamp S, Ashery-Padan R, Schulte D. Expression of the homeodomain transcription factor Meis2 in the embryonic and postnatal retina. J Comp Neurol 2007; 505(1):58-72; http://dx.doi.org/ 10.1002/cne.21458 [DOI] [PubMed] [Google Scholar]
- [17].Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer 2008; 8(9):671-82; PMID:18650841; http://dx.doi.org/ 10.1038/nrc2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Burmeister M, Novak J, Liang MY, Basu S, Ploder L, Hawes NL, Vidgen D, Hoover F, Goldman D, Kalnins VI, et al.. Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat Genet 1996; 12(4):376-84; PMID:8630490; http://dx.doi.org/ 10.1038/ng0496-376 [DOI] [PubMed] [Google Scholar]
- [19].Buttitta LA, Edgar BA. Mechanisms controlling cell cycle exit upon terminal differentiation. Curr Opin Cell Biol 2007; 19(6):697-704; PMID:18035529; http://dx.doi.org/ 10.1016/j.ceb.2007.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Carthon BC, Neumann CA, Das M, Pawlyk B, Li T, Geng Y, Sicinski P. Genetic replacement of cyclin D1 function in mouse development by cyclin D2. Mol Cell Biol. 2005; 25(3):1081-8; PMID:15657434; http://dx.doi.org/ 10.1128/MCB.25.3.1081-1088.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chang L, Khoo B, Wong L, Tropepe V. Genomic sequence and spatiotemporal expression comparison of zebrafish mbx1 and its paralog, mbx2. Dev Genes Evol 2006; 216:647-54; PMID:16733737; http://dx.doi.org/ 10.1007/s00427-006-0082-7 [DOI] [PubMed] [Google Scholar]
- [22].Chen D, Opavsky R, Pacal M, Tanimoto N, Wenzel P, Seeliger MW, Leone G, Bremner R. Rb-mediated neuronal differentiation through cell-cycle-independent regulation of E2f3a. PLoS Biol 2007; 5(7):e179; PMID:17608565; http://dx.doi.org/ 10.1371/journal.pbio.0050179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen X, Patel TP, Simirskii VI, Duncan MK. PCNA interacts with Prox1 and represses its transcriptional activity. Mol Vis 2008; 14:2076-86; PMID:19023449. [PMC free article] [PubMed] [Google Scholar]
- [24].Chiodini F, Matter-Sadzinski L, Rodrigues T, Skowronska-Krawczyk D, Brodier L, Schaad O, Bauer C, Ballivet M, Matter JM. A positive feedback loop between ATOH7 and a notch effector regulates cell-cycle progression and neurogenesis in the retina. Cell Rep 2013; 3(3):796-807; PMID:23434507; http://dx.doi.org/ 10.1016/j.celrep.2013.01.035 [DOI] [PubMed] [Google Scholar]
- [25].Chittka A, Nitarska J, Grazini U, Richardson WD. Transcription factor postivie regulatory domain 4 (PRDM4) recruits protein arginine methyltransferase 5 (PRMT5) to mediate histone arginine methylation and control neural stem cell proliferation and differentiation. J Bio Chem 2012; 287(51):42995-3006; http://dx.doi.org/ 10.1074/jbc.M112.392746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cid E, Santos-Ledo A, Parrilla-Monge M, Lillo C, Arévalo R, Lara JM, Aijón J, Velasco A. Prox1 expression in rod precursors and Müller cells. Exp Eye Res 2010; 90(2):267-76. [DOI] [PubMed] [Google Scholar]
- [27].Clark AM, Yun S, Veien ES, Wu YY, Chow RL, Dorsky RI, Levine EM. Negative regulation of Vsx1 by its paralog Chx10/Vsx2 is conserved in the vertebrate retina. Brain Res 2008; 1192:99-113; PMID:17919464; http://dx.doi.org/ 10.1016/j.brainres.2007.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cunningham JJ, Levine EM, Zindy F, Goloubeva O, Roussel MF, Smeyne RJ. The cyclin-dependent kinase inhibitors p19(Ink4d) and p27(Kip1) are coexpressed in select retinal cells and act cooperatively to control cell cycle exit. Mol Cell Neurosci 2002; 19(3):359-74; PMID:11906209; http://dx.doi.org/ 10.1006/mcne.2001.1090 [DOI] [PubMed] [Google Scholar]
- [29].Das G, Choi Y, Sicinski P, Levine EM. Cyclin D1 fine-tunes the neurogenic output of embryonic retinal progenitor cells. Neural Dev 2009; 4:15; PMID:19416500; http://dx.doi.org/ 10.1186/1749-8104-4-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Das G, Clark AM, Levine EM. Cyclin D1 inactivation extends proliferation and alters histogenesis in the postnatal mouse retina. Dev Dyn 2012; 241(5):941-52; PMID:22434780; http://dx.doi.org/ 10.1002/dvdy.23782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Del Bene F, Tessmar-Raible K, Wittbrodt J. Direct interaction of geminin and Six3 in eye development. Nature 2004; 427:745-9; PMID:14973488; http://dx.doi.org/ 10.1038/nature02292 [DOI] [PubMed] [Google Scholar]
- [32].Del Bene F, Wittbrodt J. Cell cycle control by homeobox genes in development and disease. Semin Cell Dev Biol 2005; 16:449-60; PMID:15840452; http://dx.doi.org/ 10.1016/j.semcdb.2005.02.001 [DOI] [PubMed] [Google Scholar]
- [33].Dixit R, Tachibana N, Touahri Y, Zinyk D, Logan C, Schuurmans C. Gene expression is dynamically regulated in retinal progenitor cells prior to and during overt cellular differentiation. Gene Expr Patterns 2014; 14:42-54; PMID:24148613; http://dx.doi.org/ 10.1016/j.gep.2013.10.003 [DOI] [PubMed] [Google Scholar]
- [34].Dorval KM, Bobechko BP, Ahmad KF, Bremner R. Transcriptional activity of the paired-like homeodomain proteins CHX10 and VSX1. J Biol Chem 2005; 280(11):10100-8; PMID:15647262; http://dx.doi.org/ 10.1074/jbc.M412676200 [DOI] [PubMed] [Google Scholar]
- [35].Duffy KT, McAleer MF, Davidson WR, Kari L, Kari C, Lui CG, Farber SA, Cheng KC, Mest JR, Wickstrom E., et al.. Coordinate control of cell cycle regulatory genes in zebrafish development tested by cyclin D1 knockdown with morpholino phosphorodiamidates and hydroxyprolyl-phosphono peptide nucleic acids. Nucleic Acids Res 2005; 33(15):4914-21; PMID:16284195; http://dx.doi.org/ 10.1093/nar/gki799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dyer MA, Cepko CL. p57(Kip2) regulates progenitor cell proliferation and amacrine interneuron development in the mouse retina. Development 2000; 127(16):3593-605; PMID:10903183. [DOI] [PubMed] [Google Scholar]
- [37].Dyer MA, Cepko CL. Regulating proliferation during retinal development. Nat Rev Neurosci 2001a; 2(5):333-42; http://dx.doi.org/ 10.1038/35072555 [DOI] [PubMed] [Google Scholar]
- [38].Dyer MA, Cepko CL. p27Kip1 and p57Kip2 regulate proliferation in distinct retinal progenitor cell populations. J Neurosci 2001b; 21(12):4259-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dyer MA, Livesey FJ, Cepko CL, Oliver G. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet 2003; 34:53-8; PMID:12692551; http://dx.doi.org/ 10.1038/ng1144 [DOI] [PubMed] [Google Scholar]
- [40].Estivill-Torrus G, Pearson H, Van Heyningen V, Price DJ, Rashbass P. Pax6 is required to regulate the cell cycle and the rate of progression from symmetrical to asymmetrical division in mammalian cortical progenitors. Development 2002; 129:455-66; PMID:11807037. [DOI] [PubMed] [Google Scholar]
- [41].Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Gene Dev 1995; 9(19):2364-72; PMID:7557388; http://dx.doi.org/ 10.1101/gad.9.19.2364 [DOI] [PubMed] [Google Scholar]
- [42].Farhy C, Elgart M, Shapira Z, Oron-Karni V, Yaron O, Menuchin Y, Rechavi G, Ashery-Padan R. Pax6 is required for normal cell-cycle exit and the differentiation kinetics of retinal progenitor cells. PLoS One 2013; 8(9):e76489; PMID:24073291; http://dx.doi.org/ 10.1371/journal.pone.0076489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ferda Percin E, Ploder LA, Yu JJ, Arici K, Horsford DJ, Rutherford A, Bapat B, Cox DW, Duncan AM, Kalnins VI, et al.. Human microphthalmia associated with mutations in the retinal homeobox gene CHX10. Nat Genet 2000; 25(4):397-401; PMID:10932181; http://dx.doi.org/ 10.1038/78071 [DOI] [PubMed] [Google Scholar]
- [44].Foskolou IP, Stella D, Rozani I, Lavigne MD, Politis PK. Prox1 suppresses the proliferation of neuroblastoma cells via a dual action in p27-kip1 and cdc25A. Oncogene 2013; 32:947-60; PMID:22508481; http://dx.doi.org/ 10.1038/onc.2012.129 [DOI] [PubMed] [Google Scholar]
- [45].French CR, Erickson T, Callander D, Berry KM, Koss R, Hagey DW, Stout J, Wuennenberg-Stapleton K, Ngai J, Moens CB, et al.. Pbx homeodomain proteins pattern both the zebrafish retina and tectum. BMC Dev Biol 2007; 7:85; PMID:17634100; http://dx.doi.org/ 10.1186/1471-213X-7-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Garcia-Dominguez M, Poquet C, Garel S, Charnay P. Ebf gene function is required for coupling neuronal differentiation and cell cycle exit. Development 2003; 130(24):6013-25; PMID:14573522; http://dx.doi.org/ 10.1242/dev.00840 [DOI] [PubMed] [Google Scholar]
- [47].Geng Y, Whoriskey W, Park MY, Bronson RT, Medema RH, Li T, Weinberg RA, Sicinski P. Rescue of cyclin D1 deficiency of knockin cyclin E. Cell 1999; 97(6):767-77; PMID:10380928; http://dx.doi.org/ 10.1016/S0092-8674(00)80788-6 [DOI] [PubMed] [Google Scholar]
- [48].Geng Y, Yu Q, Sicinska E, Das M, Bronson RT, Sicinski P. Deletion of p27kip1 gene restores normal development in cyclin D1-deficient mice. Proc Natl Acad Sci USA 2001; 98(1):194-9; PMID:11134518; http://dx.doi.org/ 10.1073/pnas.98.1.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gogoi RN, Schubert FR, Martinez-Barbera JP, Acampora D, Simeone A, Lumsden A. The paired-type homeobox gene Dmbx1 marks the midbrain and pretectum. Mech Dev 2002; 114(1-2):213-7; PMID:12175514; http://dx.doi.org/ 10.1016/S0925-4773(02)00067-9 [DOI] [PubMed] [Google Scholar]
- [50].Green ES, Stubbs JL, Levine EM. Genetic rescue of cell number in a mouse model of microphthalmia: interactions between Chx10 and G1-phase cell cycle regulators. Development 2003; 130(3):539-52; PMID:12490560; http://dx.doi.org/ 10.1242/dev.00275 [DOI] [PubMed] [Google Scholar]
- [51].Gyda M, Wolman M, Lorent K, Granato M. The tumor suppressor gene retinoblastoma-1 is required for retinotectal development and visual function in zebrafish. PLoS Genet 2012; 8(11):e1003106; PMID:23209449; http://dx.doi.org/ 10.1371/journal.pgen.1003106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hardwick LJA, Philpott A. Nervous decision-making: to divide or differentiate. Cell Press 2014; 30(6):254-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Heine P, Dohle E, Bumsted,-O'Brien K, Engelkamp D, Schulte D. Evidence for an evolutionary conserved role of homothorax/Meis1/2 during vertebrate retina development. Development 2008; 135(5):805-11; PMID:18216174; http://dx.doi.org/ 10.1242/dev.012088 [DOI] [PubMed] [Google Scholar]
- [54].Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature 1991; 354:522-5; PMID:1684639; http://dx.doi.org/ 10.1038/354522a0 [DOI] [PubMed] [Google Scholar]
- [55].Hisa T, Spence SE, Rachel RA, Fujita M, Nakamura T, Ward JM, Devor-Henneman DE, Saiki Y, Kutsuna H, Tessarollo L, et al.. Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. EMBO J 2004; 23:450-9; PMID:14713950; http://dx.doi.org/ 10.1038/sj.emboj.7600038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hsieh YW, Yang XJ. Dynamic Pax6 expression during the neurogenic cell cycle influences proliferation and cell fate choices of retinal progenitors. Neural Dev 2009; 4:32; PMID:19686589; http://dx.doi.org/ 10.1186/1749-8104-4-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hu M, Easter SS. Retinal neurogenesis: the formation of the initial central patch of postmitotic cells. Dev Biol 1999; 207:309-21; PMID:10068465; http://dx.doi.org/ 10.1006/dbio.1998.9031 [DOI] [PubMed] [Google Scholar]
- [58].Katoh K, Yamazaki R, Onishi A, Sanuki R, Furukawa T. G9a histone methyltransferase activity in retinal progenitors is essential for proper differentiation and survival of mouse retinal cells. J Neurosci 2012; 32(49):17658-70; PMID:23223288; http://dx.doi.org/ 10.1523/JNEUROSCI.1869-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kawahara A, Chien CB, Dawid IB. The homeobox gene mbx is involved in eye and tectum development. Dev Biol 2002; 248:107-17; PMID:12142024; http://dx.doi.org/ 10.1006/dbio.2002.0709 [DOI] [PubMed] [Google Scholar]
- [60].Kimura K, Miki T, Shibasaki T, Zhang Y, Ogawa M, Saisho H, Okuno M, Iwanaga T, Seino S. Functional analysis of transcriptional repressor Otx3/Dmbx1. FEBS Lett 2005; 579(13):2926-32; PMID:15890343; http://dx.doi.org/ 10.1016/j.febslet.2005.04.042 [DOI] [PubMed] [Google Scholar]
- [61].Le TT, Wroblewski E, Patel S, Riesenberg AN, Brown NL. Math5 is required for both early retinal neuron differentiation and cell cycle progression. Dev Biol 2006; 295:764-78; PMID:16690048; http://dx.doi.org/ 10.1016/j.ydbio.2006.03.055 [DOI] [PubMed] [Google Scholar]
- [62].Levine EM, Close J, Fero M, Ostrovsky A, Reh TA. p27(Kip1) regulates cell cycle withdrawal of late multipotent progenitor cells in the mammalian retina. Dev Biol 2000; 219(2):299-314; PMID:10694424; http://dx.doi.org/ 10.1006/dbio.2000.9622 [DOI] [PubMed] [Google Scholar]
- [63].Li L, Vaessin H. Pan-neural Prospero terminates cell proliferation during Drosphila neurogenesis. Genes Dev 1999; 14:147-151. [PMC free article] [PubMed] [Google Scholar]
- [64].Liu IS, Chen JD, Ploder L, Vidgen D, van der Kooy D, Kalnins VI, McInnes RR. Developmental expression of a novel murine homeobox gene (Chx10): evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron 1994; 13(2):377-93; PMID:7914735; http://dx.doi.org/ 10.1016/0896-6273(94)90354-9 [DOI] [PubMed] [Google Scholar]
- [65].Livne-Bar I, Pacal M, Cheung MC, Hankin M, Trogadis J, Chen D, Dorval KM, Bremner R. Chx10 is required to block photoreceptor differentiation but is dispensable for progenitor proliferation in the postnatal retina. Proc Natl Acad Sci USA 2006; 103(13):4988-93; PMID:16547132; http://dx.doi.org/ 10.1073/pnas.0600083103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lobjois V, Bel-Vialar S, Trousse F, Pituello F. Forcing neural progenitors cells to cycle is insufficient to alter cell-fate decision and timing of neuronal differentiation in the spinal cord. Neural Dev 2008; 3:4; PMID:18271960; http://dx.doi.org/ 10.1186/1749-8104-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].MacPherson D, Sage J, Kin T, Ho D, McLaughin ME, Jacks T. Cell type-specific effects of Rb deletion in the murine retina. Gene Dev 2004; 18(14):1681-94; PMID:15231717; http://dx.doi.org/ 10.1101/gad.1203304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillenmot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell 2001; 105:43-55; PMID:11301001; http://dx.doi.org/ 10.1016/S0092-8674(01)00295-1 [DOI] [PubMed] [Google Scholar]
- [69].Mi D, Carr CB, Georgala PA, Huang YT, Manuel MN, Jeanes E, Niisato E, Sansom SN, Livesey FJ, Theil T, et al.. Pax6 exerts regional control of cortical progenitor proliferation via direct repression of Cdk6 and hypophosphorylation of pRb. Neuron 2013; 78(2):269-84; PMID:23622063; http://dx.doi.org/ 10.1016/j.neuron.2013.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mishra K, Gui H, Matise MP. Prox1 regulates a transitory state for interneuron neurogenesis in the spinal cord. Dev Dyn 2007; 237:393-402; http://dx.doi.org/ 10.1002/dvdy.21422 [DOI] [PubMed] [Google Scholar]
- [71].Ohtoshi A, Behringer RR. Neonatal lethality, dwarfism, and abnormal brain development in Dmbx1 mutant mice. Mol Cell Biol 2004; 24:7548-58; PMID:15314164; http://dx.doi.org/ 10.1128/MCB.24.17.7548-7558.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ohtoshi A, Bradley A, Behringer RR, Nishijima I. Generation and maintenance of Dmbx1 gene-targeted mutant alleles. Mamm Genome 2006; 17(7):744-50; PMID:16845469; http://dx.doi.org/ 10.1007/s00335-006-0021-y [DOI] [PubMed] [Google Scholar]
- [73].Oron-Karni V, Farhy C, Elgart M, Marquardt T, Remizova L, Yaron O, Xie Q, Cvekl A, Ashery-Padan R. Dual requirement for Pax6 in retinal progenitor cells. Development 2008; 135:4037-3047; PMID:19004853; http://dx.doi.org/ 10.1242/dev.028308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Pacal M, Bremner R. Induction of the ganglion cell differentiation program in human retinal progenitors before cell cycle exit. Dev Dyn 2014; 243(5):712-29; PMID:24339342; http://dx.doi.org/ 10.1002/dvdy.24103 [DOI] [PubMed] [Google Scholar]
- [75].Pacal M, Bremner R. Mapping differentiation kinetics in the mouse retina reveals an extensive period of cell cycle protein expression in post-mitotic newborn neurons. Dev Dyn 2012; 241(10):1525-44; PMID:22837015; http://dx.doi.org/ 10.1002/dvdy.23840 [DOI] [PubMed] [Google Scholar]
- [76].Peterson RE, Fadool JM, McClintock J, Linser PJ. Muller cell differentiation in the zebrafish neural retina: evidence of distinct early and late stages in cell maturation. J Comp Neurol 2001; 429:530-40; PMID:11135233; http://dx.doi.org / [DOI] [PubMed] [Google Scholar]
- [77].Phillips GT, Stair CN, Young Lee, Wroblewski E, Berberoglu MA, Brown NL, Mastick GS. Precocious retinal neurons: Pax6 controls timing of differentiation and determination of cell type. Dev Biol 2005; 279:308-21; PMID:15733660; http://dx.doi.org/ 10.1016/j.ydbio.2004.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pilaz LJ, Patti D, Marcy G, Ollier E, Pfister S, Douglas RJ, Betizeau M, Gautier E, Cortay V, Doerflinger N, et al.. Forced G1-phase reduction alters mode of division, neuron number and laminar phenotype in the cerebral cortex. Proc Natl Acad Sci USA 2009; 106:21924-9; PMID:19959663; http://dx.doi.org/ 10.1073/pnas.0909894106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rai K, Jafri IF, Chidester S, James SR, Karpf AR, Cairns BR, Jones DA. Dnmt3 and G9a cooperate for tissue-specific development in zebrafish. J Biol Chem 2009; 285:4110-21; PMID:19946145; http://dx.doi.org/ 10.1074/jbc.M109.073676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Riesenberg AN, Le TT, Willardsen MI, Blackburn DC, Vetter ML, Brown NL. Pax6 regulation of Math5 during mouse retinal neurogenesis. Genesis 2009; 47:175-87; PMID:19208436; http://dx.doi.org/ 10.1002/dvg.20479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Rowan S, Cepko CL. Genetic analysis of the homeodomain transcription factor Chx10 in the retina using a novel multifunctional BAC transgenic mouse reporter. Dev Biol 2004; 271(2):388-402; PMID:15223342; http://dx.doi.org/ 10.1016/j.ydbio.2004.03.039 [DOI] [PubMed] [Google Scholar]
- [82].Royo JL, Bessa J, Hidalgo C, Fernandez-Minan A, Tena JJ, Roncero Y, Gomez-Skarmeta JL, Casares F. Identification and analysis of conserved cis-regulatory regions of the meis1 gene. PLoS One 2012; 7(3):e33617; PMID:22448256; http://dx.doi.org/ 10.1371/journal.pone.0033617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Schmitt EA, Dowling JE. Early reinal development in the zebrafish. Danio rerio: light and electron microscopic analyses. J Comp Neurol 1999; 404:515-36; PMID:9987995; http://dx.doi.org/ 10.1002/(SICI)1096-9861(19990222)404:4%3c515::AID-CNE8%3e3.0.CO;2-A [DOI] [PubMed] [Google Scholar]
- [84].Sicinski P, Donaher JL, Parker SB, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 1995; 82(4):621-30; PMID:7664341; http://dx.doi.org/ 10.1016/0092-8674(95)90034-9 [DOI] [PubMed] [Google Scholar]
- [85].Skapek SX, Lin SC, Jablonski MM, McKeller RN, Tan M, Hu H, Lee EY. Persistent expression of cyclin D1 disrupts normal photoreceptor differentiation and retina development. Oncogene 2001; 20(46):6742-51; PMID:11709709; http://dx.doi.org/ 10.1038/sj.onc.1204876 [DOI] [PubMed] [Google Scholar]
- [86].Tessmar K, Loosli F, Wittbrodt J. A screen for co-factors of Six3. Mech Dev 2002; 117:103-13; PMID:12204251; http://dx.doi.org/ 10.1016/S0925-4773(02)00185-5 [DOI] [PubMed] [Google Scholar]
- [87].Tong W, Pollard JW. Genetic evidence for the interactions of cyclin D1 and p27(Kip1) in mice. Mol Cell Biol 2001; 21(4):1319-28; PMID:11158317; http://dx.doi.org/ 10.1128/MCB.21.4.1319-1328.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Van den Heuvel S., Dyson NJ. Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol 2008; 9(9):713-24; PMID:18719710; http://dx.doi.org/ 10.1038/nrm2469 [DOI] [PubMed] [Google Scholar]
- [89].Vidal A, Koff A. Cell-cycle inhibitors: three families united by a common cause. Gene 2000; 247(1–2):1-15; PMID:10773440; http://dx.doi.org/ 10.1016/S0378-1119(00)00092-5 [DOI] [PubMed] [Google Scholar]
- [90].Vitorino M, Jusuf PR, Maurus D, Kimura Y, Higashijima S, Harris WA. Vsx2 in the zebrafish retina: restricted lineages through derepression. Neural Dev 2009; 4:14; PMID:19344499; http://dx.doi.org/ 10.1186/1749-8104-4-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nature Genet 1999; 21:318-22; PMID:10080188; http://dx.doi.org/ 10.1038/6844 [DOI] [PubMed] [Google Scholar]
- [92].Wong L, Power N, Miles A, Tropepe V. Mutual antagonism of the paired-type homeobox genes, vsx2 and dmbx1, regulates retinal progenitor cell cycle exit upstream of ccnd1 expression. Dev Biol 2015; 402(2):216-28; PMID:25872183; http://dx.doi.org/ 10.1016/j.ydbio.2015.03.020 [DOI] [PubMed] [Google Scholar]
- [93].Wong L, Weadick C, Kuo C, Chang BSW, Tropepe V. Duplicate dmbx1 genes regulate progenitor cell cycle and differentiation during zebrafish midbrain and retinal development. BMC Dev Biol 2010; 10:100; PMID:20860823; http://dx.doi.org/ 10.1186/1471-213X-10-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zagozewski JL, Zhang Q, Pinto VI, Wigle JT, Eisenstat DD. The role of homeobox genes in retinal development and disease. Dev Biol 2014; 393:195-208; PMID:25035933; http://dx.doi.org/ 10.1016/j.ydbio.2014.07.004 [DOI] [PubMed] [Google Scholar]
- [95].Zhang J, Taylor RJ, Torre AL, Wilken MS, Cox KE, Reh TA, Vetter ML. Ezh2 maintains retinal progenitor proliferation, transcriptional integrity and the timing of late differentiation. Dev Biol 2015; 403(2):128-38; PMID:25989023; http://dx.doi.org/ 10.1016/j.ydbio.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zhang X, Friedman A, Heaney S, Purcell P, Mass RL. Meis homeoproteins directly regulate Pax6 during vertebrate lens morphogenesis. Genes Dev 2002; 16(16):2097-107; PMID:12183364; http://dx.doi.org/ 10.1101/gad.1007602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zou C, Levine EM. Vsx2 controls eye organogenesis and retinal progenitor identity via homeodomain and non-homeodomain residues required for high affinity DNA binding. PLoS Genet 2012; 8(9):e1002924; PMID:23028343; http://dx.doi.org/ 10.1371/journal.pgen.1002924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Manuel M, Pratt T, Liu M, Jeffery G, Price DJ Overexpression of Pax6 results in microphthalmia, retinal dysplasia and defective retinal ganglion cell axon guidance. BMC Dev Biol. 2008; 8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]


