Abstract
Background
In Canada and elsewhere, pazopanib and sunitinib—tyrosine kinase inhibitors targeting the vascular endothelial growth factor receptors—are recommended as first-line treatment for patients with metastatic renal cell carcinoma (mrcc). A large randomized noninferiority trial of pazopanib versus sunitinib (comparz) demonstrated that the two drugs have similar efficacy; however, patients randomized to pazopanib experienced better health-related quality of life (hrqol) and nominally lower rates of non-study medical resource utilization.
Methods
The cost-effectiveness of pazopanib compared with sunitinib for first-line treatment of mrcc from a Canadian health care system perspective was evaluated using a partitioned-survival model that incorporated data from comparz and other secondary sources. The time horizon of 5 years was based on the maximum duration of follow-up in the final analysis of overall survival from the comparz trial. Analyses were conducted first using list prices for pazopanib and sunitinib and then by assuming that the prices of sunitinib and pazopanib would be equivalent.
Results
Based on list prices, expected costs were CA$10,293 less with pazopanib than with sunitinib. Pazopanib was estimated to yield 0.059 more quality-adjusted life-years (qalys). Pazopanib was therefore dominant (more qalys and lower costs) compared with sunitinib in the base case. In probabilistic sensitivity analyses, pazopanib was dominant in 79% of simulations and was cost-effective in 90%–100% of simulations at a threshold cost-effectiveness ratio of CA$100,000. Assuming equivalent pricing, pazopanib yielded CA$917 in savings in the base case, was dominant in 36% of probabilistic sensitivity analysis simulations, and was cost-effective in 89% of simulations at a threshold cost-effectiveness ratio of CA$100,000.
Conclusions
Compared with sunitinib, pazopanib is likely to be a cost-effective option for first-line treatment of mrcc from a Canadian health care perspective.
Keywords: Cost-effectiveness analyses, economic evaluations, partitioned-survival analyses, pazopanib, post-progression survival analyses, quality-adjusted life-years, sensitivity analyses, sunitinib
INTRODUCTION
Renal cell carcinomas (rccs) arise in the renal epithelium and account for approximately 85% of all kidney cancers1. The Canadian Cancer Society estimated that, in 2015, approximately 6200 Canadians were diagnosed with kidney cancer and approximately 1800 individuals succumbed to the disease2.
Locally advanced or metastatic rcc (mrcc) is not susceptible to chemotherapy3. Systemic immunotherapy involving the use of interferon alfa provided only modest survival benefits to selected patients with advanced rcc, highlighting the need for more effective systemic therapy4. The availability of targeted agents for mrcc, including the tyrosine kinase inhibitors pazopanib and sunitinib, and the mtor (mammalian target of rapamycin) inhibitor temsirolimus, has significantly affected treatment of the disease through improvements in response rates, progression-free survival (pfs), and overall survival (os), with manageable side effects5. Targeted therapy using pazopanib, sunitinib, and temsirolimus is therefore recommended for mrcc in first-line settings in Canada6.
The comparz trial (NCT00720941 at http://www.ClinicalTrials.gov/) was a phase iii randomized non-inferiority trial in which the efficacy and safety of pazopanib were compared with those of sunitinib in patients with clear-cell mrcc7. In comparz, 1100 patients were randomized to receive a continuous dose of pazopanib 800 mg once daily (n = 557) or sunitinib 50 mg once daily for 4 weeks, followed by 2 weeks without treatment (n = 553). The study was powered to show the noninferiority of pazopanib compared with sunitinib with respect to the primary endpoint of pfs as assessed by a blinded independent review committee (irc), with noninferiority predefined as the upper bound of the 95% confidence interval (ci) for the hazard ratio (hr) of pazopanib versus sunitinib being less than 1.25. Secondary endpoints included os, investigator-assessed pfs, safety, and health-related quality of life (hrqol). Based on the initial data cut-off in May 2012 after 659 disease-progression events, pazopanib was found to be noninferior to sunitinib with respect to pfs (hr for pazopanib vs. sunitinib: 1.05; 95% ci: 0.90 to 1.22)7. The os was similar in the two arms (hr: 0.91; 95% ci: 0.76 to 1.08). The incidences of fatigue and hand–foot syndrome were higher in patients receiving sunitinib, and changes in hair color, alopecia, and weight loss were observed more frequently in patients receiving pazopanib. The mean change from baseline in 11 of 14 hrqol domains—in particular, those related to fatigue or soreness in the mouth, throat, hands, or feet—during the first 6 months of treatment favoured pazopanib (p < 0.05 for all 11 comparisons). Based on those results, the comparz investigators concluded that pazopanib and sunitinib have similar efficacy, but that their adverse event (ae) and hrqol profiles favour pazopanib7–9. In the final analysis of os, conducted in September 2013 when more than 650 patients had died and 2 years after the last patient had been enrolled, and with a maximum reported follow-up for os of approximately 5 years, os was similar in the two groups (hr for pazopanib vs. sunitinib: 0.92; 95% ci: 0.79 to 1.06; p = 0.24). The results of comparz are supported by a smaller phase iii crossover trial of 168 patients, which demonstrated that, compared with 22% of patients who preferred sunitinib, 70% preferred pazopanib; 8% had no preference10.
Although the comparz study of pazopanib versus sunitinib as first-line treatment for mrcc demonstrated not only noninferiority with respect to efficacy but also favourable toxicity and hrqol profiles, the relative cost-effectiveness of the two treatments was not assessed. The objective of the present study was therefore to evaluate the cost-effectiveness of pazopanib compared with sunitinib as first-line treatment for patients with mrcc from the perspective of the Canadian publicly funded health care system, based on results of the comparz trial and other sources.
METHODS
Overview of the Model
A partitioned-survival model was used to assess the cost-effectiveness of pazopanib compared with sunitinib for the first-line treatment of mrcc. The model incorporated three health states: alive with no progression (“pre-progression”), alive with progression [“post-progression survival” (pps)], and dead. The population of interest was treatment-naïve patients with mrcc, which is consistent with the study population in the comparz trial7 and with the terms of the marketing authorizations for pazopanib and sunitinib in Canada11. The analysis was conducted from the perspective of the Canadian publicly funded health care system, consistent with the requirements for economic evaluations submitted to the pan-Canadian Oncology Drug Review. The pan-Canadian Oncology Drug Review makes recommendations to Canadian provinces and territories (with the exception of Quebec) about oncology drug funding decisions12,13. Accordingly, only health care costs related to the treatment of mrcc that would be materially affected by treatment with pazopanib and sunitinib were considered. Effectiveness was measured in terms of quality-adjusted life-years (qalys). A time horizon of 5 years, representing the approximate maximum duration of follow-up at the time of the final os analysis in the comparz trial, was used in the base case. A 5-year time horizon is appropriate if material differences in outcomes and costs are unlikely after 5 years, which is a reasonable assumption given the similarity of pfs and os for pazopanib and sunitinib in the comparz trial. A time horizon of 10 years, which approximates a lifetime projection (>90% of patients are projected to be dead after 10 years) was used in sensitivity analyses14.
The proportion of patients in each health state over time was calculated based on estimated survival distributions for pfs and os. The pps was calculated as the difference between os and pfs. Costs and hrqol were both assumed to be conditioned on treatment and expected time in the progression-free and post-progression states. To accommodate the 4-week cycle for pazopanib and the 6-week cycle for sunitinib, the cycle duration of the model was 1 week, eliminating the need for a half-cycle correction.
The model generated estimates of expected lifetime costs (costs of medication, dispensing, and administration; routine follow-up, monitoring, and supportive care; other costs associated with pazopanib and sunitinib treatment; and total costs), progression-free life-years (pflys), post-progression life-years, overall life-years, and qalys. Costs and qalys were discounted at 5% annually as recommended by the Canadian Agency for Drugs and Technologies in Health14. Effectiveness measures were reported on a discounted and undiscounted basis. The incremental cost-effectiveness ratio (icer) for pazopanib versus sunitinib was defined as the ratio of the difference in total costs (pazopanib – sunitinib) to the difference in qalys (incremental cost per qaly gained). The net monetary benefit (nmb) of pazopanib compared with sunitinib was also calculated at threshold cost-effectiveness values of CA$100,000, CA$150,000, and CA$200,000 per qaly gained.
Model Estimation
Model inputs are summarized in the subsections that follow and in Table i.
TABLE I.
Model inputs
| Variable | Pazopanib | Sunitinib |
|---|---|---|
| Progression-free survival (PFS) | ||
| Lambda | 0.0425 | 0.0530 |
| Gamma | 1.1781 | 1.0920 |
| Overall survival (OS) | ||
| Lambda | 0.0138 | 0.0176 |
| Gamma | 1.1467 | 1.0929 |
| Utility values [mean (SE)] | ||
| PFS | 0.7089 (0.0193) | 0.6832 (0.0236) |
| Post-progression vs. pre-progression survival | −0.1580 (0.0395) | −0.1323 (0.0331) |
| List price of drug (CA$) | 34.42 per 200-mg tablet | 256.16 per 50-mg tablet |
| Cost per 6 weeks of treatment (CA$) | 6,216.00 | 7,073.08 |
| Price of drug assuming equivalent pricing (CA$) | 34.42 per 200-mg tablet | 206.51 per 50-mg tablet |
| Other treatment-related cost, per month (CA$) [mean (SE)] | ||
| Hospital days | 75.78 (14.68) | 106.66 (20.42) |
| Medical office visits | 27.33 (3.55) | 28.79 (3.63) |
| Medical or surgical specialty visits | 34.58 (6.67) | 39.01 (7.43) |
| Telephone consultations | 7.99 (1.17) | 7.43 (0.87) |
| Urgent care | 3.98 (0.42) | 6.33 (0.60) |
| Home health care | 0.70 (0.25) | 2.84 (1.77) |
| Laboratory visits | 1.04 (0.13) | 1.34 (0.16) |
| Laboratory tests | 18.45 (2.41) | 23.57 (4.32) |
| Radiologic visits | 34.80 (2.41) | 45.24 (3.38) |
| TOTAL | 204.65 (12.98) | 261.21 (16.18) |
| Costs of routine care, disease progression, and terminal care (CA$) [mean (SE)] | ||
| PFS (per month) | 842 (201) | 805 (201) |
| Post-progression survival (per month) | 935 (234) | 935 (234) |
| Disease progression (one-time) | 8,043 (2,011) | 8,043 (2,011) |
| Cancer death (one-time) | 22,270 (5,567) | 22,270 (5,567) |
| Cost of PTACT per patient (mean CA$) | ||
| Axitinib | 1,333 | 1,930 |
| Bevacizumab | 1,952 | 1,343 |
| Everolimus | 7,289 | 7,039 |
| Pazopanib | 765 | 1,849 |
| Sirolimus | 17 | 18 |
| Sorafenib | 2,465 | 4,056 |
| Sunitinib | 6,223 | 3,440 |
| Temsirolimus | 1,444 | 2,060 |
| Cytokinea | 375 | 292 |
| Otherb | 690 | 561 |
| Unapprovedc | 0 | 0 |
| TOTAL | 22,553 | 22,587 |
Assumed to be interferon alfa.
Assigned same cost as interferon alfa.
Assumed zero cost.
PFS = progression-free survival; OS = overall survival; SE = standard error; PTACT = post-treatment anticancer therapy.
PFS and OS
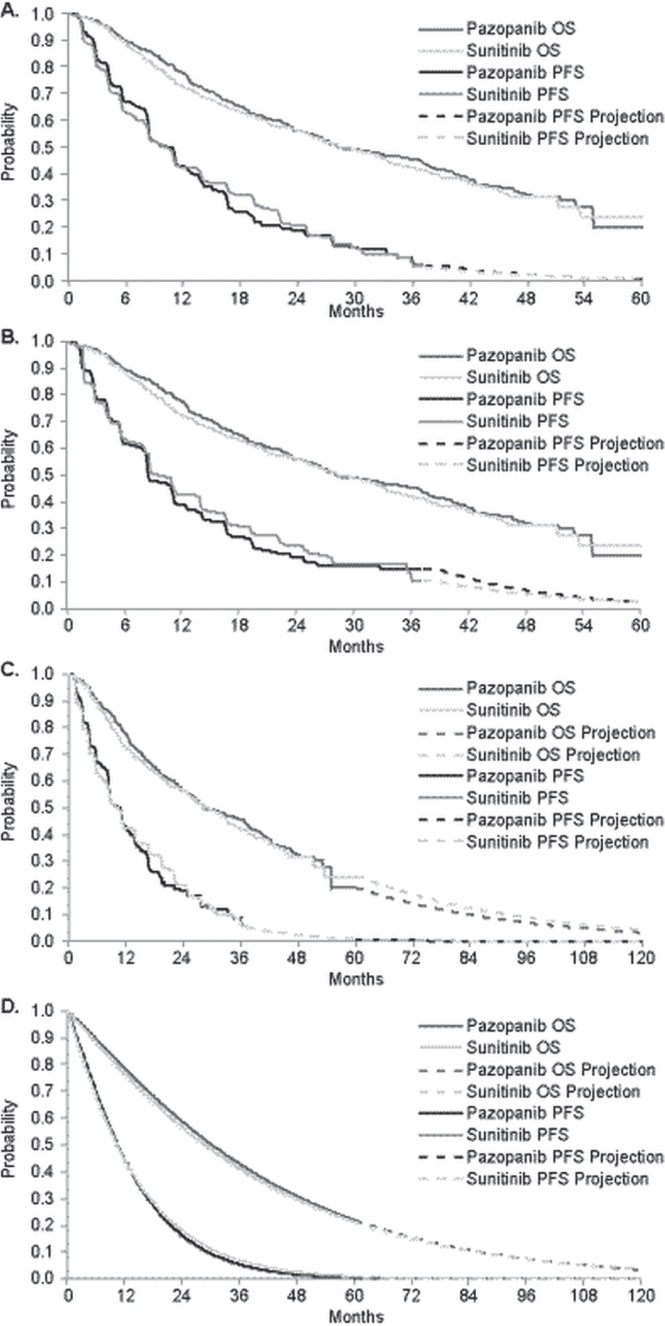
In the base case, pfs and os were estimated using Kaplan–Meier survival distributions from the comparz trial (Figure 1). For analyses requiring projections beyond the end of follow-up in comparz, pfs and os were projected based on parametric survival function fits to patient-level survival time data from the comparz trial, using accelerated failure-time regression. Exponential, Weibull, log-logistic, lognormal, and gamma distributions were considered. Goodness of fit was assessed by visual inspection, Akaike information criteria, and comparisons of restricted mean survival time for the parametric compared with the empirical distributions. For both pfs and os, the 1-parameter exponential model provided the worst fit; the 3-parameter gamma distribution provided the best fit. The 2-parameter models (Weibull, log-logistic, lognormal) all produced a similar fit to the pfs and os curves. The gamma distribution was not used because its long tails might overstate survival. For pfs, the Weibull was used for both arms because it closely matched the Kaplan–Meier distributions in terms of restricted mean survival time at the end of follow-up. The Weibull distribution was also used for os because it provided the most conservative estimate of the gain in expected os with pazopanib over the 10-year projection. Because pfs was not updated in the final os analysis from comparz, the pfs had to be projected from 36 to 60 months in the base case. In sensitivity analyses using a 10-year time horizon, pfs and os were estimated using two different approaches:
■ Kaplan-Meier distributions for the first 5 years and Weibull distributions for the remainder of the modelling horizon, and
■ Weibull distributions for the entirety of the modelling horizon.
FIGURE 1.
Survival distributions for progression-free (PFS) and overall survival (OS). (A) 5-Year time horizon, investigator-assessed PFS. (B) 5-Year time horizon, independent review committee–assessed PFS. (C) 10-Year time horizon, investigator-assessed PFS, with PFS and OS based on Kaplan–Meier distribution to the maximum follow-up period in the COMPARZ trial and with Weibull extrapolation thereafter. (D) 10-Year time horizon, investigator-assessed PFS, with PFS and OS based on Weibull distribution for the entire period.
Investigator-assessed pfs rather than irc-assessed pfs was used in the base case. The former is more likely to resemble patient assessments in routine clinical practice. Also, irc-assessed pfs might be biased by informative censoring of unconfirmed locally assessed progressions15. Analyses of hrs for pfs from controlled trials have found no evidence of bias with investigator-assessed compared with irc-assessed pfs16. In comparz, no systematic differences in the timing of the assessments by the investigators compared with the assessments by the irc that would have biased the comparison of pazopanib over sunitinib were discernable. Although irc-assessed pfs was the primary endpoint in comparz, the study reported here was a post hoc evaluation that did not involve hypothesis testing, and so the primacy of irc-assessed pfs in comparz is irrelevant. Nevertheless, to address the possibility that the use of investigator-assessed pfs biased the analysis, irc-assessed pfs was used in sensitivity analyses.
HRQOL Utility Values
Patients in the comparz trial were asked to complete the Functional Assessment of Chronic Illness Therapy–Fatigue with its Additional Concerns Module, the Functional Assessment of Cancer Therapy–Kidney Symptom Index 19, the Seville Quality of Life Questionnaire, and the Cancer Therapy Satisfaction Questionnaire7. An assessment of preference-based measures of hrqol such as the EQ-5D (EuroQol Group, Rotterdam, Netherlands) or the SF-6D (Health Economics and Decision Science, University of Sheffield, Sheffield, U.K.) was not included.
To the best of our knowledge, no currently published algorithms map the Functional Assessment of Cancer Therapy–Kidney Symptom Index 19 or the Cancer Therapy Satisfaction Questionnaire to utility values. Mean utility values for pazopanib and sunitinib during pfs were therefore estimated by combining data about the incidence and duration of aes from comparz with a regression equation relating the presence of aes to utility values (Table ii). The regression equation was estimated using data from the VEG105192 trial (NCT00334282), a phase iii randomized controlled trial of pazopanib compared with placebo in patients with mrcc17. Generalized linear model regression was used, with patients defined as clusters. The regression equation used EQ-5D utility values as the dependent variable and baseline patient characteristics [age (<65 or ≥65 years), sex, performance status, prior treatment (yes or no)], treatment group, and the presence of aes as independent variables. The aes were characterized by grade (grades 1–2 vs. grade 3 and greater) and whether the ae was observed more frequently in the sunitinib arm of comparz. Tests of the interaction between treatment group and aes were nonsignificant, and so data for both pazopanib and placebo patients in VEG105192 were used17. The results were similar whether using the absolute utility values or the change in utility values from baseline, and so, for simplicity, absolute values were used.
TABLE II.
Generalized linear regression model relating adverse events to EQ-5Da utility values in the VEG105192 trial
| Variable | Estimate | SE | 95% CI | p Value | |
|---|---|---|---|---|---|
|
| |||||
| Lower | Upper | ||||
| Intercept | 0.7794 | 0.0354 | 0.71 | 0.8487 | <0.0001 |
| Treatment | |||||
| Pazopanib (vs. placebo) | 0.0106 | 0.0259 | −0.0402 | 0.0615 | 0.6824 |
| First-line (vs. second-line) | −0.0365 | 0.0214 | −0.0785 | 0.0055 | 0.0885 |
| AEs (vs. no AEs) | |||||
| Grades 3–4 | |||||
| Observed more frequently with sunitinibb | −0.2044 | 0.0682 | −0.338 | −0.0708 | 0.0027 |
| Others | −0.1101 | 0.0448 | −0.1979 | −0.0222 | 0.014 |
| Grades 1–2 | |||||
| Observed more frequently with sunitinibb | −0.0202 | 0.0262 | −0.0715 | 0.0311 | 0.4395 |
| Others | −0.0075 | 0.0225 | −0.0516 | 0.0367 | 0.7399 |
| Age | |||||
| <65 years (vs. ≥65 years) | 0.0176 | 0.0232 | −0.0279 | 0.063 | 0.4488 |
| Sex | |||||
| Men (vs. women) | 0.0463 | 0.0237 | −0.0002 | 0.0929 | 0.051 |
| ECOG performance status (vs. 0) | |||||
| 1 | −0.1463 | 0.0217 | −0.1889 | −0.1038 | <0.0001 |
| Missing | −0.0768 | 0.075 | −0.2238 | 0.0701 | 0.3056 |
EuroQol Group, Rotterdam, Netherlands.
Includes all AEs observed in 10% or more of subjects in either arm of the COMPARZ trial and observed more frequently with sunitinib than with pazopanib in COMPARZ.
SE = standard error; CI = confidence interval; AEs = adverse events; ECOG = Eastern Cooperative Oncology Group.
Using the regression equation, utility values were then estimated for every day of the pre-progression follow-up period for all patients in comparz. Patient-level data from comparz were used for baseline patient characteristics and for the incidence and duration of aes. Only aes beginning during treatment were included. Any aes coded as unresolved or resolving were assigned an end date equal to the day of progression or death, and aes with missing start date information were excluded. Mean utility values for pfs were then estimated for each treatment group using Kaplan–Meier sample average methods18.
The standard error (se) for each utility value was obtained by bootstrapping. Mean utility values were estimated separately using investigator-assessed and irc-assessed pfs, with the latter being used in sensitivity analyses. Because the VEG105192 trial provided few post-progression assessments with EQ-5D utility values, post-progression utility values for both treatments were estimated based on the reported utility value for best supportive care after termination of second-line therapy in a cost-effectiveness analysis of sunitinib, which was based on data from the 014 phase iii trial of sunitinib19.
We also conducted sensitivity analyses in which utility values were derived from published studies. The utility value for pfs without aes was assumed to be 0.795 based on the value for stable disease without aes from a study by Swinburn et al.20 that used a vignettes approach and time tradeoff values to estimate U.K. community-based preferences for health states associated with mrcc. Disutilities for aes were also obtained from the Swinburn study, if available. For aes not included in the Swinburn study, we used disutility values identified from a systematic review of utility values for chemotherapy-related aes reported by Shabaruddin et al.21, which we supplemented with targeted (non-systematic) searches of PubMed, Google Scholar, references from retrieved studies, and the Internet. Shabaruddin et al. identified eighteen studies reporting utility values for chemotherapy- related aes. Where the studies from Shabaruddin did not report specific aes, did not include utilities for a referent state from which the incremental effect of the ae on utilities could be calculated, included only Asian patients, or focused only on chemotherapy-induced nausea and vomiting, and where estimates from the Swinburn mrcc study20 were already available, the Shabaruddin studies were not included. A total of fifteen studies, including the study by Swinburn, were identified20,22–34.
The aes reported in the identified studies were recoded to a uniform set of descriptors (for example, fatigue and asthenia were recoded as “fatigue/asthenia”). All aes that were classified as “severe” were considered to be grades 3–4, and aes that were not otherwise classified were considered either grades 1–2 or grades 3–4 based on a review of the vignettes or the relative magnitudes of the utility decrements compared with decrements reported in other studies. For aes with multiple estimates available, the mean was used. Any aes for which disutility values were not available were assigned values based on the mean disutility value for all aes of that grade (−0.0947 for grades 1–2 aes and −0.2001 for grades 3–4 aes). Using the utility values thus derived from the published studies, utility values were then estimated for every day of pre- progression follow-up for all patients in comparz. For patients with more than 1 ae on a given day, the maximum disutility was used (that is, effects of multiple aes were not additive). Mean (se) utilities for pfs for pazopanib and sunitinib were then estimated using Kaplan–Meier sample average methods as already described.
Costs
Costs considered in the evaluation—including those for pazopanib and sunitinib, dispensing and administration, routine follow-up care, disease progression and terminal care, and other direct medical costs—were based on published sources35–38. Planned doses of pazopanib and sunitinib were assumed to be the same as the per-protocol doses in the comparz trial7. Unit costs of pazopanib (CA$34.42 per 200-mg tablet) and sunitinib (CA$206.51 per 50-mg tablet) were based on the population-weighted average of province-specific wholesale prices (IMS Brogan, Ottawa, ON)39. Administration costs were based on the Ontario fee schedule for physician services (G388—Management of special oral chemotherapy for malignant disease40). Dispensing costs were estimated based on the dispensing fee payable to most pharmacies under the Ontario Drug Benefit Program41.
In the model, the cost of a full pazopanib or sunitinib prescription was assumed to be incurred on the first day of each treatment cycle for all patients remaining alive and progression-free. Expected medication costs were then adjusted for dose modifications, treatment interruptions, and discontinuation before or after progression as follows: the full cost was multiplied by the treatment group–specific dose intensity factors reflecting the ratio of the actual to the planned doses of pazopanib and sunitinib received in comparz (222,424 mg / 328,604 mg = 67.7% for pazopanib, and 9,435 mg / 13,980 mg = 67.5% for sunitinib). Actual and planned doses in comparz were estimated using the Kaplan–Meier sample average method18. Administration and dispensing costs were also assumed to be incurred at the beginning of each cycle and were similarly adjusted by dose intensity factors reflecting the ratio of the mean actual to the planned number of treatment cycles (11.37 cycles / 14.67 cycles = 77.5% for pazopanib, and 7.97 cycles / 9.99 cycles = 79.8% for sunitinib).
Monthly costs of routine care during pfs and pps, as well as “one-off” costs associated with disease progression and death, were assumed to be the same whether patients received pazopanib or sunitinib and were derived from a published economic evaluation of sunitinib for treatment-naïve mrcc patients in Canada19. Costs of routine care were based on clinical expert opinion and fee schedules. “Routine care” included physician visits; blood work; thyroid-stimulating hormone, triiodothyronine, amylase, and lipase tests; and computed tomography and bone scans. Costs of disease progression and death were derived from a previously published study of the cost-effectiveness of breast cancer treatment42, assuming that such costs would be independent of cancer type.
To account for the differences in non-study medical resource utilization (mru) between pazopanib and sunitinib that were observed in comparz, we included an additional cost category denoted “Other treatment–related costs.” Those costs were estimated by combining monthly rates of non-study mru from comparz with unit cost estimates from published or publicly available sources. The mru data collected in comparz included hospital days, medical office visits, emergency department visits, home health visits, laboratory visits and tests, medical or surgical procedures, and radiology visits and tests. Protocol-specified resource use was not included. Unit cost estimates for each of the foregoing categories were obtained from published or publicly available sources35–38. No attempt was made to attribute costs to specific aes, because differences in non-study mru observed in comparz were assumed to be a consequence of differences in the efficacy or safety (or both) of pazopanib and sunitinib.
In comparz, patients randomized to pazopanib were significantly more likely to receive post-treatment anti-cancer therapy (ptact) with sunitinib (29.4% vs. 16.3%, chi-square p < 0.001), and patients randomized to sunitinib were significantly more likely to receive ptact with pazopanib (10.9% vs. 4.5%, p = 0.001) and sorafenib (17.7% vs. 10.8, p = 0.012). To account for those and other differences in the use of ptact observed in comparz, ptact was included as a one-time cost at disease progression. Use of ptact was taken from comparz. Unit costs of ptact were taken from IMS Brogan (Table i). The mean duration of ptact was assumed to be 6 months, based on the approximate restricted mean survival time for pfs among patients receiving everolimus in record-1, a randomized double-blind placebo-controlled trial of everolimus in patients with mrcc whose disease had progressed despite prior treatment with sunitinib, sorafenib, or both43. Treatment regimens for ptact were based on published studies.
All cost estimates from prior years were updated to 2014 Canadian dollars using the Consumer Price Index for health care44.
Analyses
To account for the possibility that the actual price of sunitinib could differ from the quoted list price, two sets of analyses were conducted: in one, the list prices of pazopanib and sunitinib were used, and in the other, the cost of 6 weeks of sunitinib was assumed to equal the cost of 6 weeks of pazopanib at its list price. For each of the analyses, results were generated for a variety of scenarios in which key model parameters and assumptions were varied from their base case values. For each scenario, we generated costs, qalys, incremental costs and qalys, and the icer and nmb. For each scenario (including the base case), we conducted probabilistic sensitivity analyses (psas) by simultaneously sampling from the estimated probability distributions of the model parameters to obtain 1000 sets of model input estimates45,46. For each simulation, all model results were generated. Those results were then used to calculate 95% credible intervals (cris)47,48 for model results, as well as the proportion of simulations in each quadrant of the cost-effectiveness plane (that is, with qalys on the x axis and costs on the y axis: northeast, cost>0 / qalys>0; southeast, cost<0 / qalys>0; southwest, costs<0 / qalys<0; and northwest, cost>0 / qalys<0) and the proportion of simulations for which pazopanib was preferred to sunitinib at various threshold values of cost-effectiveness (that is, the acceptability curve for pazopanib).
In the psas, pfs and os were sampled from bootstrapped survival distributions. Utility values for pfs were assumed to be distributed as beta random variables; the decrements in utility for pps compared with pfs were assumed to be distributed as normal random variables. Unit costs of pazopanib and sunitinib, and the costs associated with dispensing and administering those drugs, were not sampled. Other costs were sampled as lognormal variables. Parameters for which se estimates were unavailable were assumed to have a se equal to 25% of the point estimate.
Funding Source
Funding for this research was provided to Policy Analysis Inc. (pai) by GlaxoSmithKline (gsk) and Novartis. Authors at gsk had a role in the conception and design of the study; the collection, analysis, and interpretation of data; final manuscript approval; and decisions regarding submission of the manuscript for publication.
RESULTS
Analysis Based on List Prices of Pazopanib and Sunitinib
Compared with sunitinib, pazopanib yielded (discounted, Table iii) 0.013 fewer pflys, 0.070 more post-progression life-years, and 0.057 more life-years (0.68 months). The qalys gained with pazopanib were estimated to be 0.059 (95% cri: −0.076 to 0.213 qalys). Based on list prices, medication costs were CA$10,902 less with pazopanib than with sunitinib, primarily because of the lower daily price and the expected pflys. Administration and dispensing costs were CA$128 higher with pazopanib because of its assumed shorter cycle duration (4 weeks vs. 6 weeks). Other costs during pfs were CA$949 lower with pazopanib, largely because of lower other treatment-related costs (based on mru data from the comparz trial). Other costs during pps were CA$1,430 higher with pazopanib, reflecting a longer expected pps. Expected total costs were CA$10,293 lower with pazopanib than with sunitinib (95% cri: −CA$16,994 to −CA$3,083). Because pazopanib was estimated to provide more qalys at a lower cost, the pazopanib icer was dominant in the base case. At threshold cost-effectiveness values of CA$100,000, CA$150,000, and CA$200,000 per qaly gained, the nmb of pazopanib compared with sunitinib was CA$16,179 (95% cri: CA$4,288 to CA$28,883), CA$19,122 (95% cri: CA$885 to CA$39,268), and CA$22,065 (95% cri: −CA$3,422 to CA$49,494) respectively. At a threshold value of CA$100,000 per qaly gained, 64% of the nmb was a consequence of reduced costs (savings of CA$10,293), and 36% was a consequence of increased qalys (0.059 qalys gained “monetized” at a value of CA$100,000 per qaly equals approximately CA$5,900).
TABLE III.
Base case results for analysis using list prices for pazopanib and sunitinib
| Result | Pazopanib | Sunitinib | Differencea |
|---|---|---|---|
| Effectiveness, not discounted (n) | |||
| Life-years | 2.704 | 2.645 | 0.059 |
| Progression-free life-years | 1.177 | 1.192 | −0.014 |
| Post-progression life-years | 1.527 | 1.453 | 0.074 |
| Quality-adjusted life-years (QALYs) | 1.676 | 1.615 | 0.061 |
| Effectiveness, discounted | |||
| Life-years | 2.529 | 2.473 | 0.057 |
| Progression-free life-years | 1.144 | 1.157 | −0.013 |
| Post-progression life-years | 1.385 | 1.316 | 0.070 |
| QALYs | 1.574 | 1.515 | 0.059 |
| Costs, discounted (CA$) | |||
| Study medication | 40,151 | 51,053 | −10,902 |
| Administration and dispensing | 434 | 306 | 128 |
| Other costs | 14,366 | 15,315 | −949 |
| Post-progression | 61,475 | 60,045 | 1,430 |
| TOTAL | 116,427 | 126,719 | −10,293 |
| Cost per QALY gained | Dominant | ||
| Net monetary benefit, by threshold for ICER (CA$) | |||
| CA$100,000 per QALY gained | 16,179 | ||
| CA$150,000 per QALY gained | 19,122 | ||
| CA$200,000 per QALY gained | 22,065 | ||
| Probability that pazopanib is cost-effective compared with sunitinib by threshold for ICER (%) | |||
| CA$100,000 per QALY gained | 100 | ||
| CA$150,000 per QALY gained | 98 | ||
| CA$200,000 per QALY gained | 96 |
Difference was calculated before rounding, and so values could differ by ±1.
ICER = incremental cost-effectiveness ratio.
In psas using base case assumptions, pazopanib was projected to yield more qalys in 79% of the simulations and lower costs in 100% of the simulations. Pazopanib was therefore projected to be dominant (that is, yielding more qalys and lower costs) compared with sunitinib in 79% of the simulations. Sunitinib was not projected to be dominant in any simulations. The probability that pazopanib was cost-effective compared with sunitinib at threshold values of cost-effectiveness of CA$100,000, CA$150,000, and CA$200,000 per qaly gained was 100%, 98%, and 96% respectively.
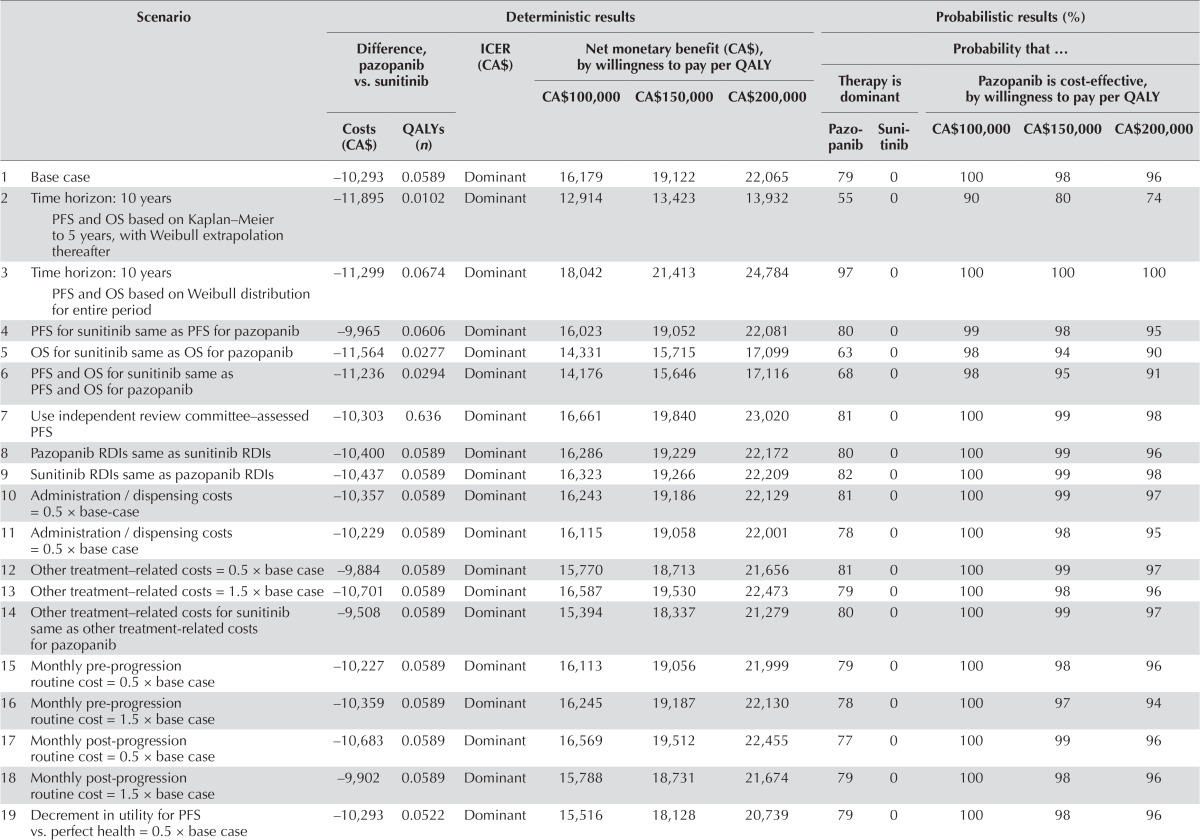
The results were relatively insensitive to changes in model parameters and assumptions. Given a threshold value for cost-effectiveness of CA$100,000 per qaly gained, the nmb was positive in all scenarios examined, ranging from CA$11,236 (assuming that pfs, os, and utility during pfs for sunitinib were equal to those for pazopanib) to CA$18,419 (assuming the decrement in utility for pps vs. pfs was 0.5 × base case value, Table iv). The nmb was most sensitive to assumptions regarding the utility values. The nmb was less favourable when the pfs and os for sunitinib were assumed to equal those for pazopanib. In no instance was the nmb less than CA$0. At an icer threshold of CA$100,000 per qaly gained, the estimated probability that pazopanib was cost-effective compared with sunitinib ranged from 90% to 100% across all scenarios examined.
TABLE IV.
Scenario analyses for analysis using list prices for pazopanib and sunitinib
| Scenario | Deterministic results | Probabilistic results (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Difference, pazopanib vs. sunitinib | ICER (CA$) | Net monetary benefit (CA$), by willingness to pay per QALY | Probability that … | ||||||||
|
| |||||||||||
|
|
Therapy is dominant | Pazopanib is cost-effective, by willingness to pay per QALY | |||||||||
| CA$100,000 | CA$150,000 | CA$200,000 | |||||||||
|
|
|
|
|||||||||
| Costs (CA$) | QALYs (n) | Pazopanib | Sunitinib | CA$100,000 | CA$150,000 | CA$200,000 | |||||
| 1 Base case | −10,293 | 0.0589 | Dominant | 16,179 | 19,122 | 22,065 | 79 | 0 | 100 | 98 | 96 |
| 2 Time horizon: 10 years PFS and OS based on Kaplan–Meier to 5 years, with Weibull extrapolation thereafter |
−11,895 | 0.0102 | Dominant | 12,914 | 13,423 | 13,932 | 55 | 0 | 90 | 80 | 74 |
| 3 Time horizon: 10 years PFS and OS based on Weibull distribution for entire period |
−11,299 | 0.0674 | Dominant | 18,042 | 21,413 | 24,784 | 97 | 0 | 100 | 100 | 100 |
| 4 PFS for sunitinib same as PFS for pazopanib | −9,965 | 0.0606 | Dominant | 16,023 | 19,052 | 22,081 | 80 | 0 | 99 | 98 | 95 |
| 5 OS for sunitinib same as OS for pazopanib | −11,564 | 0.0277 | Dominant | 14,331 | 15,715 | 17,099 | 63 | 0 | 98 | 94 | 90 |
| 6 PFS and OS for sunitinib same as PFS and OS for pazopanib | −11,236 | 0.0294 | Dominant | 14,176 | 15,646 | 17,116 | 68 | 0 | 98 | 95 | 91 |
| 7 Use independent review committee–assessed PFS | −10,303 | 0.636 | Dominant | 16,661 | 19,840 | 23,020 | 81 | 0 | 100 | 99 | 98 |
| 8 Pazopanib RDIs same as sunitinib RDIs | −10,400 | 0.0589 | Dominant | 16,286 | 19,229 | 22,172 | 80 | 0 | 100 | 99 | 96 |
| 9 Sunitinib RDIs same as pazopanib RDIs | −10,437 | 0.0589 | Dominant | 16,323 | 19,266 | 22,209 | 82 | 0 | 100 | 99 | 98 |
| 10 Administration / dispensing costs = 0.5 × base-case | −10,357 | 0.0589 | Dominant | 16,243 | 19,186 | 22,129 | 81 | 0 | 100 | 99 | 97 |
| 11 Administration / dispensing costs = 0.5 × base case | −10,229 | 0.0589 | Dominant | 16,115 | 19,058 | 22,001 | 78 | 0 | 100 | 98 | 95 |
| 12 Other treatment–related costs = 0.5 × base case | −9,884 | 0.0589 | Dominant | 15,770 | 18,713 | 21,656 | 81 | 0 | 100 | 99 | 97 |
| 13 Other treatment–related costs = 1.5 × base case | −10,701 | 0.0589 | Dominant | 16,587 | 19,530 | 22,473 | 79 | 0 | 100 | 98 | 96 |
| 14 Other treatment–related costs for sunitinib same as other treatment-related costs for pazopanib | −9,508 | 0.0589 | Dominant | 15,394 | 18,337 | 21,279 | 80 | 0 | 100 | 99 | 97 |
| 15 Monthly pre-progression routine cost = 0.5 × base case | −10,227 | 0.0589 | Dominant | 16,113 | 19,056 | 21,999 | 79 | 0 | 100 | 98 | 96 |
| 16 Monthly pre-progression routine cost = 1.5 × base case | −10,359 | 0.0589 | Dominant | 16,245 | 19,187 | 22,130 | 78 | 0 | 100 | 97 | 94 |
| 17 Monthly post-progression routine cost = 0.5 × base case | −10,683 | 0.0589 | Dominant | 16,569 | 19,512 | 22,455 | 77 | 0 | 100 | 99 | 96 |
| 18 Monthly post-progression routine cost = 1.5 × base case | −9,902 | 0.0589 | Dominant | 15,788 | 18,731 | 21,674 | 79 | 0 | 100 | 98 | 96 |
| 19 Decrement in utility for PFS vs. perfect health = 0.5 × base case | −10,293 | 0.0522 | Dominant | 15,516 | 18,128 | 20,739 | 79 | 0 | 100 | 98 | 96 |
| 20 Decrement in utility for PFS vs. perfect health = 1.5 × base case | −10,293 | 0.0655 | Dominant | 16,841 | 20,116 | 23,390 | 78 | 0 | 99 | 99 | 96 |
| 21 Decrement in utility for PPS vs. PFS = 0.5 × base case | −10,293 | 0.0813 | Dominant | 18,419 | 22,483 | 26,546 | 81 | 0 | 100 | 98 | 95 |
| 22 Decrement in utility for PPS vs. PFS = 1.5 × base case | −10,293 | 0.0365 | Dominant | 13,938 | 15,761 | 17,583 | 79 | 0 | 100 | 99 | 96 |
| 23 Utility values based on published studies | −10,293 | 0.0639 | Dominant | 16,738 | 20,006 | 23,273 | 87 | 0 | 100 | 100 | 99 |
| 24 Utility during PFS for sunitinib same as utility during PFS for pazopanib | −10,293 | 0.0291 | Dominant | 13,206 | 14,662 | 16,118 | 67 | 0 | 99 | 95 | 90 |
| 25 PFS, OS, and utility during PFS for sunitinib same as that for pazopanib | −11,236 | 0.0000 | Dominant | 11,236 | 11,236 | 11,236 | 49 | 0 | 96 | 87 | 80 |
| 26 Time horizon: 10 years PFS and OS based on Weibull distribution for entire period, utility during PFS for sunitinib same as utility during PFS for pazopanib |
−11,299 | 0.0377 | Dominant | 15,065 | 16,947 | 18,830 | 87 | 0 | 100 | 100 | 100 |
| 27 Discount rate: 0% | −10,436 | 0.0610 | Dominant | 16,534 | 19,583 | 22,632 | 76 | 0 | 100 | 98 | 95 |
| 28 Discount rate: 3% | −10,351 | 0.0597 | Dominant | 16,318 | 19,302 | 22,286 | 76 | 0 | 100 | 98 | 96 |
QALY(s) = quality-adjusted life-year(s); ICER = incremental cost-effectiveness ratio; PFS = progression-free survival; OS = overall survival; RDIs = relative dose intensities; PPS = post-progression survival.
Based on published studies, mean (se) utility values for pazopanib and sunitinib in pfs were estimated to be 0.7386 (0.0049) and 0.7082 (0.0060) respectively [difference: 0.0303 (0.0077)]. When those values were used in the model, the gain in qalys with pazopanib was estimated to be 0.064. The nmb at a threshold value of CA$100,000 was estimated to be CA$16,738.
Analyses Assuming Equal Pricing for Pazopanib and Sunitinib
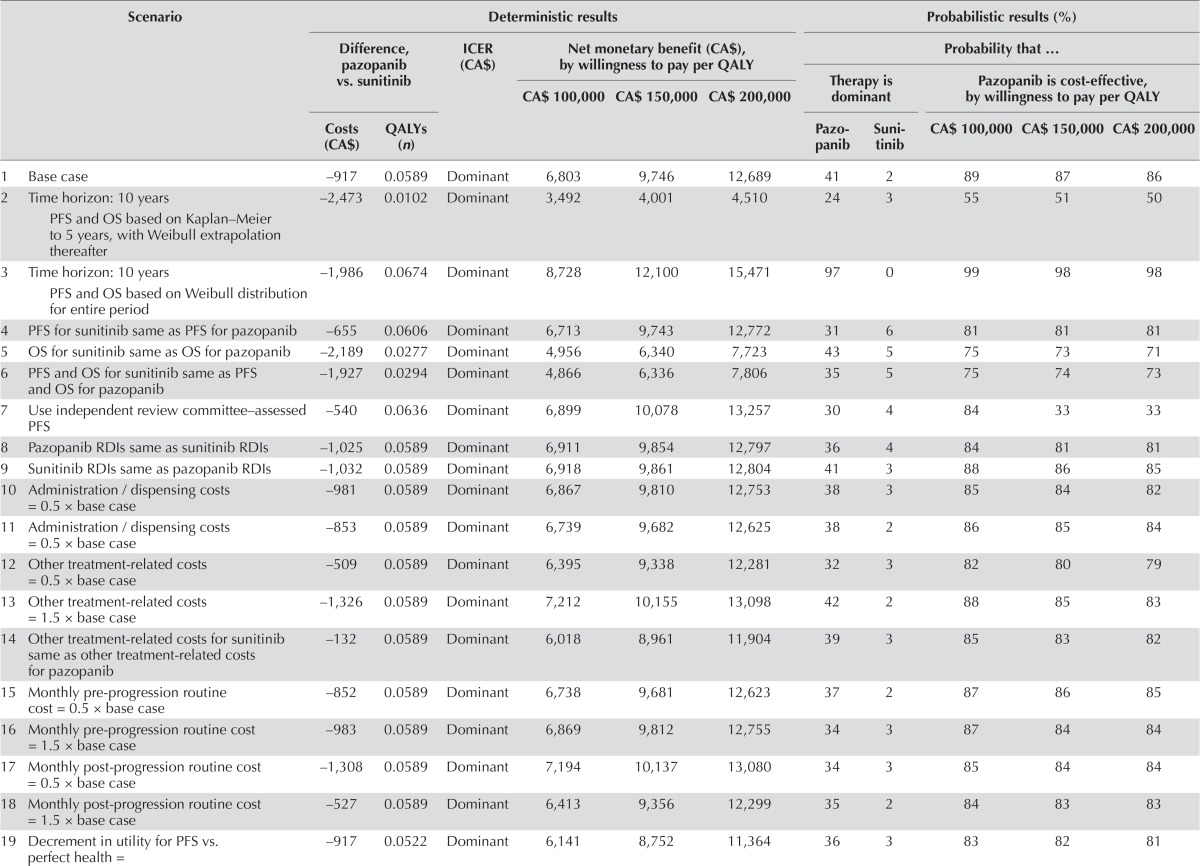
In the analysis assuming that the cost of sunitinib over a 6-week period was equal to that of pazopanib, expected total costs were estimated to be CA$917 lower with pazopanib in the base case (Table v; 95% cri: −CA$6,849 to CA$5,755). As with the analysis using list prices, pazopanib was dominant in the base case. At threshold values of cost-effectiveness of CA$100,000, CA$150,000, and CA$200,000 per qaly gained, the nmb of pazopanib compared with sunitinib was CA$6,803 (95% cri: − CA$4,615 to CA$19,130), CA$9,746 (95% cri: −CA$7,977 to CA$28,543), and CA$12,689 (95% cri: −CA$11,306 to CA$38,346) respectively. Pazopanib was expected to yield more qalys than sunitinib in 80% of the simulations and was associated with costs lower than those for sunitinib in 54%. Pazopanib was expected to be dominant in 36% of the simulations. The probability that pazopanib was cost-effective compared with sunitinib was 89%, 87%, and 86% at threshold values of cost-effectiveness of CA$100,000, CA$150,000, and CA$200,000 per qaly gained respectively.
TABLE V.
Scenario analyses for analysis using equivalent prices for pazopanib and sunitinib
| Scenario | Deterministic results | Probabilistic results (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Difference, pazopanib vs. sunitinib | ICER (CA$) | Net monetary benefit (CA$), by willingness to pay per QALY | Probability that … | ||||||||
|
| |||||||||||
|
|
Therapy is dominant | Pazopanib is cost-effective, by willingness to pay per QALY | |||||||||
| CA$ 100,000 | CA$ 150,000 | CA$ 200,000 | |||||||||
|
|
|
|
|||||||||
| Costs (CA$) | QALYs (n) | Pazopanib | Sunitinib | CA$ 100,000 | CA$ 150,000 | CA$ 200,000 | |||||
| 1 Base case | −917 | 0.0589 | Dominant | 6,803 | 9,746 | 12,689 | 41 | 2 | 89 | 87 | 86 |
| 2 Time horizon: 10 years PFS and OS based on Kaplan–Meier to 5 years, with Weibull extrapolation thereafter |
−2,473 | 0.0102 | Dominant | 3,492 | 4,001 | 4,510 | 24 | 3 | 55 | 51 | 50 |
| 3 Time horizon: 10 years PFS and OS based on Weibull distribution for entire period |
−1,986 | 0.0674 | Dominant | 8,728 | 12,100 | 15,471 | 97 | 0 | 99 | 98 | 98 |
| 4 PFS for sunitinib same as PFS for pazopanib | −655 | 0.0606 | Dominant | 6,713 | 9,743 | 12,772 | 31 | 6 | 81 | 81 | 81 |
| 5 OS for sunitinib same as OS for pazopanib | −2,189 | 0.0277 | Dominant | 4,956 | 6,340 | 7,723 | 43 | 5 | 75 | 73 | 71 |
| 6 PFS and OS for sunitinib same as PFS and OS for pazopanib | −1,927 | 0.0294 | Dominant | 4,866 | 6,336 | 7,806 | 35 | 5 | 75 | 74 | 73 |
| 7 Use independent review committee–assessed PFS | −540 | 0.0636 | Dominant | 6,899 | 10,078 | 13,257 | 30 | 4 | 84 | 33 | 33 |
| 8 Pazopanib RDIs same as sunitinib RDIs | −1,025 | 0.0589 | Dominant | 6,911 | 9,854 | 12,797 | 36 | 4 | 84 | 81 | 81 |
| 9 Sunitinib RDIs same as pazopanib RDIs | −1,032 | 0.0589 | Dominant | 6,918 | 9,861 | 12,804 | 41 | 3 | 88 | 86 | 85 |
| 10 Administration / dispensing costs = 0.5 × base case | −981 | 0.0589 | Dominant | 6,867 | 9,810 | 12,753 | 38 | 3 | 85 | 84 | 82 |
| 11 Administration / dispensing costs = 0.5 × base case | −853 | 0.0589 | Dominant | 6,739 | 9,682 | 12,625 | 38 | 2 | 86 | 85 | 84 |
| 12 Other treatment-related costs = 0.5 × base case | −509 | 0.0589 | Dominant | 6,395 | 9,338 | 12,281 | 32 | 3 | 82 | 80 | 79 |
| 13 Other treatment-related costs = 1.5 × base case | −1,326 | 0.0589 | Dominant | 7,212 | 10,155 | 13,098 | 42 | 2 | 88 | 85 | 83 |
| 14 Other treatment-related costs for sunitinib same as other treatment-related costs for pazopanib | −132 | 0.0589 | Dominant | 6,018 | 8,961 | 11,904 | 39 | 3 | 85 | 83 | 82 |
| 15 Monthly pre-progression routine cost = 0.5 × base case | −852 | 0.0589 | Dominant | 6,738 | 9,681 | 12,623 | 37 | 2 | 87 | 86 | 85 |
| 16 Monthly pre-progression routine cost = 1.5 × base case | −983 | 0.0589 | Dominant | 6,869 | 9,812 | 12,755 | 34 | 3 | 87 | 84 | 84 |
| 17 Monthly post-progression routine cost = 0.5 × base case | −1,308 | 0.0589 | Dominant | 7,194 | 10,137 | 13,080 | 34 | 3 | 85 | 84 | 84 |
| 18 Monthly post-progression routine cost = 1.5 × base case | −527 | 0.0589 | Dominant | 6,413 | 9,356 | 12,299 | 35 | 2 | 84 | 83 | 83 |
| 19 Decrement in utility for PFS vs. perfect health = | −917 | 0.0522 | Dominant | 6,141 | 8,752 | 11,364 | 36 | 3 | 83 | 82 | 81 |
| 20 Decrement in utility for PFS vs. perfect health = 1.5 × base case | −917 | 0.0655 | Dominant | 7,466 | 10,740 | 14,014 | 38 | 3 | 86 | 84 | 83 |
| 21 Decrement in utility for PPS vs. PFS = 0.5 × base case | −917 | 0.0813 | Dominant | 9,044 | 13,107 | 17,171 | 38 | 3 | 85 | 84 | 84 |
| 22 Decrement in utility for PPS vs. PFS = 1.5 × base case | −917 | 0.0365 | Dominant | 4,563 | 6,385 | 8,208 | 37 | 3 | 85 | 84 | 83 |
| 23 Utility values based on published studies | −917 | 0.0639 | Dominant | 6,859 | 10,128 | 13,398 | 40 | 1 | 91 | 89 | 88 |
| 24 Utility during PFS for sunitinib same as utility during PFS for pazopanib | −917 | 0.0291 | Dominant | 3,830 | 5,287 | 6,743 | 29 | 6 | 75 | 73 | 72 |
| 25 PFS, OS, and utility during PFS for sunitinib same as that for pazopanib | −1,927 | 0.0000 | Dominant | 1,927 | 1,927 | 1,927 | 23 | 10 | 57 | 54 | 53 |
| 26 Time horizon: 10 years PFS and OS based on Weibull distribution for entire period, utility during PFS for sunitinib same as utility during PFS for pazopanib |
−1,986 | 0.0377 | Dominant | 5,751 | 7,634 | 9,516 | 87 | 0 | 94 | 91 | 90 |
| 27 Discount rate = 0% | −789 | 0.0610 | Dominant | 6,887 | 9,936 | 12,985 | 36 | 3 | 84 | 83 | 82 |
| 28 Discount rate = 3% | −871 | 0.0597 | Dominant | 6,839 | 9,882 | 12,806 | 38 | 3 | 86 | 84 | 83 |
QALY(s) = quality-adjusted life-year(s); ICER = incremental cost-effectiveness ration; PFS = progression-free survival; OS = overall survival; RDIs = relative dose intensities; PPS = post-progression survival.
Assuming equivalent pricing for pazopanib and sunitinib, pazopanib was dominant in all of the scenarios examined. The nmb for pazopanib compared with sunitinib calculated at a threshold value of CA$100,000 per qaly gained ranged from CA$1,927 (assuming equivalent pfs, os, and utility during pfs for sunitinib and for pazopanib) to CA$9,044 (assuming a decrement in utility for pps vs. pfs equal to 0.5 × the base case). At an icer threshold of CA$100,000 per qaly gained, the estimated probability that pazopanib was cost-effective compared with sunitinib ranged from 55% to 99%. When utility values based on published studies were used, the nmb at a threshold value of CA$100,000 was estimated to be CA$6,859.
DISCUSSION
We evaluated the cost-effectiveness of pazopanib compared with sunitinib as first-line treatment for mrcc from the perspective of the Canadian public health care system. In one set of analyses, the list price of sunitinib was used. In a second set, the cost of 6 weeks of sunitinib treatment was assumed to be the same as that of pazopanib treatment. In both analyses, pazopanib was projected, in the base case, to yield more qalys at a lower cost than sunitinib would. In the first set of analyses, the estimated cost savings with pazopanib (CA$10,293) were largely attributable to its lower list price. In the second set of analyses, the savings with pazopanib treatment (CA$917) were largely attributable to a shorter expected pfs and lower costs for other treatment-related care, which were partly offset by the higher costs of ptact.
Because of the similarity of pazopanib and sunitinib with respect to efficacy, it is important that the model results be evaluated in the context of the uncertainty associated with the base case estimates. In both sets of analyses, the psas suggested a relatively high probability that pazopanib represents a cost-effective treatment compared with sunitinib. Results of deterministic sensitivity analyses suggest that those findings are robust to changes in specific parameter estimates.
The model used in the present study is similar to one used in a recent evaluation of the cost-effectiveness of pazopanib compared with sunitinib from a U.S. health care system perspective49; however, the two studies have several important differences. First, the two studies took different perspectives (one U.S. and the other Canadian), and the cost estimates differ accordingly. Second, the U.S. study used a 3-year time horizon in the base case, consistent with the maximum follow-up as of the initial data cut-off for comparz7, whereas the study reported here used a 5-year time horizon in the base case, consistent with the maximum follow-up in the final analysis of os for comparz9. Estimates of os in the present study therefore have greater precision than those used in the U.S. evaluation. Also, the U.S. study took utility values for pazopanib and sunitinib from the pisces trial10, a randomized controlled double-blind crossover trial assessing treatment preferences for pazopanib or sunitinib in patients with mrcc. To address potential limitations in those estimates, the analysis presented here used utility values estimated by combining data on the incidence and duration of aes in comparz, with a regression model that related aes to utility values. The regression model was estimated using EQ-5D utility data and aes from the phase iii pivotal trial of pazopanib17. In a sensitivity analysis, disutility values for aes were derived from published studies.
Although the difference in mean utility values for pazopanib compared with sunitinib in the present study (0.0257 based on data from the VEG105192 trial17 and 0.0303 based on the vignettes studies) is less than that used in the earlier one (0.0569 based on pisces)10, the analyses reported here nevertheless support the hypothesis that, compared with sunitinib treatment, pazopanib treatment is associated with improved hrqol (although the magnitude of the difference might be less than that reported previously).
When equivalent pricing for pazopanib and sunitinib was assumed, the cost savings with pazopanib could be attributed other treatment-related costs. Those costs were estimated based on a post hoc analysis of data on non-study mru during the comparz trial50. Differences in mru between groups were not statistically significant. The uncertainty in the differences is reflected in the psas. Also, comparz was a multinational clinical trial, and patterns of resource use in that trial might not be representative of use in typical clinical practice in Canada. Although the estimated savings should be interpreted cautiously, they are not inconsistent with expectations given the observed statistically significant benefits with respect to tolerability for patients receiving pazopanib in comparz.
In comparz, no protocol-specified crossover from pazopanib to sunitinib or vice versa occurred. However, many patients received additional ptact, including pazopanib, sunitinib, other anti–vascular endothelial growth factor therapies, or mtor inhibitors. Patients randomized to pazopanib were significantly more likely to receive ptact with sunitinib, and patients randomized to sunitinib were more likely to receive ptact with pazopanib, sorafenib, or both. The likelihood of a patient receiving any anti–vascular endothelial growth factor therapy (39% for pazopanib and 37% for sunitinib) or any mtor inhibitor (31% vs. 30%) was similar. We addressed potential differences in the use of ptact between pazopanib and sunitinib by including the estimated costs of those medications in the analysis. Our estimates of effectiveness and cost are therefore internally consistent. The total estimated costs of ptact were virtually identical in the two groups.
CONCLUSIONS
Our analyses suggest that, compared with sunitinib, pazopanib is likely to be a cost-effective option in first-line treatment for mrcc from a Canadian health care system perspective.
ACKNOWLEDGMENTS
The authors thank Michelle D. Hackshaw bsc pharm mshs phd, formerly of gsk, for contributions to the study design and initial manuscript drafts. They also thank Nancy Price phd and Prasad Kulkarni phd cmpp of aoi Communications, LP, for editorial assistance (assembling tables and figures, collating author comments, copyediting, fact-checking, referencing, and graphic services).
Funding for this study was provided to pai by gsk and Novartis. Pazopanib is an asset of Novartis ag as of 2 March 2015. Editorial support and graphic services were provided by aoi Communications, LP, and were funded by gsk.
Two sister publications on the cost-effectiveness of pazopanib compared with sunitinib from the Italian and U.K. health care system perspectives are also submitted or are in progress. Those manuscripts are based on the same clinical studies and use the same cost-effectiveness model. Some of the methods in those manuscripts are similar to the methods described in the present work.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: TED and JA are employees of Policy Analysis Inc. (pai), which has received research funding and consulting fees from gsk and Novartis, and support for travel to meetings. TED’s institution also received consulting fees and research funding from gsk and Novartis for activities unrelated to the present study. JP is an employee of Novartis. JD and HRN were employees of gsk at the time of the analysis. JD holds stock in gsk.
REFERENCES
- 1.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–90. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2015. Toronto, ON: Canadian Cancer Society; 2015. [Available online at: http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/canadian-cancer-statistics-2015-EN.pdf; cited 31 March 2016] [Google Scholar]
- 3.Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol. 2000;163:408–17. doi: 10.1016/S0022-5347(05)67889-5. [DOI] [PubMed] [Google Scholar]
- 4.Coppin C, Porzsolt F, Awa A, Kumpf J, Coldman A, Wilt T. Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev. 2005:CD001425. doi: 10.1002/14651858.CD001425.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Coppin C, Kollmannsberger C, Le L, Porzsolt F, Wilt TJ. Targeted therapy for advanced renal cell cancer (rcc): a Cochrane systematic review of published randomised trials. BJU Int. 2011;108:1556–63. doi: 10.1111/j.1464-410X.2011.10629.x. [DOI] [PubMed] [Google Scholar]
- 6.North S, Basappa N, Bjarnason G, et al. Management of advanced kidney cancer: Canadian Kidney Cancer Forum 2013 consensus update: Canadian Kidney Cancer Forum 2013. Can Urol Assoc J. 2013;7:238–43. doi: 10.5489/cuaj.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–31. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 8.Cella D, Hackshaw MD, Diaz J, et al. Quality of life (qol) among patients with renal cell carcinoma (rcc) treated with pazopanib versus sunitinib in the comparz study [abstract 346] J Clin Oncol. 2013;31 [Available online at: http://meetinglibrary.asco.org/content/107460-134; cited 10 May 2015] [Google Scholar]
- 9.Motzer RJ, Hutson TE, McCann L, Deen K, Choueiri TK. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib. N Engl J Med. 2014;370:1769–70. doi: 10.1056/NEJMc1400731. [DOI] [PubMed] [Google Scholar]
- 10.Escudier B, Porta C, Bono P, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: pisces study. J Clin Oncol. 2014;32:1412–18. doi: 10.1200/JCO.2013.50.8267. [DOI] [PubMed] [Google Scholar]
- 11.Health Canada, Health Products and Food Branch . Summary Basis of Decision: Votrient. Ottawa, ON: Health Canada; 2010. [Available online at: http://www.hc-sc.gc.ca/dhp-mps/alt_formats/pdf/prodpharma/sbd-smd/phase1-decision/drug-med/sbd_smd_2010_votrient_128332-eng.pdf; cited 31 March 2016] [Google Scholar]
- 12.Vogel L. Pan-Canadian review of cancer drugs will not be binding on provinces. CMAJ. 2010;182:887–8. doi: 10.1503/cmaj.109-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canadian Agency for Drugs and Technologies in Health (cadth), pan-Canadian Oncology Drug Review (pcodr) Pan-Canadian Oncology Drug Review Submission Guidelines. Toronto, ON: CADTH-pCODR; 2016. [Available online at: https://www.cadth.ca/sites/default/files/pcodr/pCODR’s%20Drug%20Review%20Process/pcodr-submission-guidelines.pdf; cited 31 March 2016] [Google Scholar]
- 14.Mittmann N, Evans WK, Rocchi A, et al. Addendum to CADTH’s Guidelines for the Economic Evaluation of Health Technologies: Specific Guidance for Oncology Products. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2009. [Available online at: https://www.cadth.ca/media/pdf/H0405_Guidance_for_Oncology_Prodcuts_gr_e.pdf; cited 31 March 2016] [Google Scholar]
- 15.Dodd LE, Korn EL, Freidlin B, et al. Blinded independent central review of progression-free survival in phase iii clinical trials: important design element or unnecessary expense? J Clin Oncol. 2008;26:3791–6. doi: 10.1200/JCO.2008.16.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United States, Department of Health and Human Services, Food and Drug Administration (fda), Oncologic Drug Advisory Committee . Evaluation of Radiologic Review of Progression-Free Survival in Non-hematologic Malignancies [meeting briefing document] Silver Spring MD: FDA; 2012. [Available online at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM311141.pdf; cited 31 March 2016] [Google Scholar]
- 17.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase iii trial. J Clin Oncol. 2010;28:1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 18.Lin DY, Feuer EJ, Etzioni R, Wax Y. Estimating medical costs from incomplete follow-up data. Biometrics. 1997;53:419–34. doi: 10.2307/2533947. [DOI] [PubMed] [Google Scholar]
- 19.Chabot I, Rocchi A. How do cost-effectiveness analyses inform reimbursement decisions for oncology medicines in Canada? The example of sunitinib for first-line treatment of metastatic renal cell carcinoma. Value Health. 2010;13:837–45. doi: 10.1111/j.1524-4733.2010.00738.x. [DOI] [PubMed] [Google Scholar]
- 20.Swinburn P, Lloyd A, Nathan P, Choueiri TK, Cella D, Neary MP. Elicitation of health state utilities in metastatic renal cell carcinoma. Curr Med Res Opin. 2010;26:1091–6. doi: 10.1185/03007991003712258. [DOI] [PubMed] [Google Scholar]
- 21.Shabaruddin FH, Chen LC, Elliott RA, Payne K. A systematic review of utility values for chemotherapy-related adverse events. Pharmacoeconomics. 2013;31:277–88. doi: 10.1007/s40273-013-0033-x. [DOI] [PubMed] [Google Scholar]
- 22.Ossa DF, Briggs A, McIntosh E, Cowell W, Littlewood T, Sculpher M. Recombinant erythropoietin for chemotherapy-related anaemia: economic value and health-related quality-of-life assessment using direct utility elicitation and discrete choice experiment methods. Pharmacoeconomics. 2007;25:223–37. doi: 10.2165/00019053-200725030-00005. [DOI] [PubMed] [Google Scholar]
- 23.Hutton J, Brown R, Borowitz M, Abrams K, Rothman M, Shakespeare A. A new decision model for cost-utility comparisons of chemotherapy in recurrent metastatic breast cancer. Pharmacoeconomics. 1996;9(suppl 2):8–22. doi: 10.2165/00019053-199600092-00004. [DOI] [PubMed] [Google Scholar]
- 24.Brown RE, Hutton J, Burrell A. Cost effectiveness of treatment options in advanced breast cancer in the U.K. Pharmacoeconomics. 2001;19:1091–102. doi: 10.2165/00019053-200119110-00003. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd A, van Hanswijck de Jonge P, Doyle S, Cornes P. Health state utility scores for cancer-related anemia through societal and patient valuations. Value Health. 2008;11:1178–85. doi: 10.1111/j.1524-4733.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 26.Havrilesky LJ, Broadwater G, Davis DM, et al. Determination of quality of life–related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113:216–20. doi: 10.1016/j.ygyno.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preference values associated with stage iii colon cancer and adjuvant chemotherapy. Qual Life Res. 2010;19:391–400. doi: 10.1007/s11136-010-9589-5. [DOI] [PubMed] [Google Scholar]
- 28.Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95:683–90. doi: 10.1038/sj.bjc.6603326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84. doi: 10.1186/1477-7525-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doyle S, Lloyd A, Walker M. Health state utility scores in advanced non-small cell lung cancer. Lung Cancer. 2008;62:374–80. doi: 10.1016/j.lungcan.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Beusterien KM, Szabo SM, Kotapati S, et al. Societal preference values for advanced melanoma health states in the United Kingdom and Australia. Br J Cancer. 2009;101:387–9. doi: 10.1038/sj.bjc.6605187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beusterien KM, Davies J, Leach M, et al. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual Life Outcomes. 2010;8:50. doi: 10.1186/1477-7525-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swinburn P, Wang J, Chandiwana D, Mansoor W, Lloyd A. Elicitation of health state utilities in neuroendocrine tumours. J Med Econ. 2012;15:681–7. doi: 10.3111/13696998.2012.670175. [DOI] [PubMed] [Google Scholar]
- 34.Shingler SL, Swinburn P, Lloyd A, et al. Elicitation of health state utilities in soft tissue sarcoma. Qual Life Res. 2013;22:1697–706. doi: 10.1007/s11136-012-0301-9. [DOI] [PubMed] [Google Scholar]
- 35.Ontario Ministry of Health and Long-Term Care (mohltc) Schedule of Benefits: Physician Services Under the Health Insurance Act. Toronto, ON: MOHLTC; 2015. [Available online at: http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/sob_master20160229.pdf; cited 31 March 2016] [Google Scholar]
- 36.Canadian Institute for Health Information (cihi) Hospital Cost Drivers Technical Report: What Factors Have Determined Hospital Expenditure Trends in Canada? Ottawa, ON: CIHI; 2012. [Available online at: https://www.cihi.ca/en/hospital_costdriver_tech_en.pdf; cited 31 March 2016] [Google Scholar]
- 37.Home Care Ontario. Home Care Services. Facts and Figures – Publicly Funded Home Care [Web page]. Hamilton, ON: Home Care Ontario; n.d. [Available at: http://www.homecareontario.ca/public/about/home-care/system/facts-and-figures.cfm; cited 31 March 2016] [Google Scholar]
- 38.Ontario Ministry of Health and Long-Term Care (mohltc) Ontario Case Costing Initiative (OCCI) [Web resource] Toronto, ON: MOHLTC; 2000. [Available at: http://ophid.scholarsportal.info/details/view.html?q=re&uri=/phirn/occi_PHIRN_e.xml; cited 31 March 2016] [Google Scholar]
- 39.Statistics Canada Table 051–0001 . Ottawa, ON: Statistics Canada; 2015. Estimates of population, by age group and sex for July 1, Canada, provinces and territories: annual (persons unless otherwise noted) [Web resource] [Available at http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=510001; cited 31 March 2016] [Google Scholar]
- 40.Ontario Ministry of Health and Long-Term Care (mohltc) Bulletin 4540–2008 Physician Services Agreement – Changes Effective September 1, 2011. Toronto, ON: MOHLTC; 2011. Chart 4: New Fee Codes. [Available online at: http://health.gov.on.ca/en/pro/programs/ohip/bulletins/4000/bul4540_4.pdf; cited 31 March 2016] [Google Scholar]
- 41.Ontario Ministry of Health and Long-Term Care (mohltc) Ontario Drug Benefit Program: Dispensing fees [Web page] Toronto, ON: MOHLTC; 2013. [Current version available online at: http://www.health.gov.on.ca/en/public/programs/drugs/programs/odb/opdp_dispensing_fees.aspx; cited 31 March 2016] [Google Scholar]
- 42.Rocchi A, Verma S. Anastrozole is cost-effective vs tamoxifen as initial adjuvant therapy in early breast cancer: Canadian perspectives on the atac completed-treatment analysis. Support Care Cancer. 2006;14:917–27. doi: 10.1007/s00520-006-0035-8. [DOI] [PubMed] [Google Scholar]
- 43.Motzer RJ, Escudier B, Oudard S, et al. on behalf of the record-1 Study Group Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116:4256–65. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 44.Statistics Canada. Table 326-0021. Consumer Price Index (CPI), annual [Web resource] Ottawa, ON: Statistics Canada; 2016. [Available at: http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=3260021; cited 31 March 2016] [Google Scholar]
- 45.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17:479–500. doi: 10.2165/00019053-200017050-00006. [DOI] [PubMed] [Google Scholar]
- 46.Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5:157–77. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 47.Löthgren M, Zethraeus N. Definition, interpretation and calculation of cost-effectiveness acceptability curves. Health Econ. 2000;9:623–30. doi: 10.1002/1099-1050(200010)9:7<623::AID-HEC539>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 48.Spiegelhalter D, Abrams K, Myles J. Bayesian Approaches to Clinical Trials and Health-Care Evaluation. Chichester, U.K: John Wiley and Sons; 2004. [Google Scholar]
- 49.Delea TE, Amdahl J, Diaz J, Nakhaipour HR, Hackshaw MD. Cost-effectiveness of pazopanib versus sunitinib for renal cancer in the United States. J Manag Care Spec Pharm. 2015;21:46–54. 54a–b. doi: 10.18553/jmcp.2015.21.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansen RN, Hackshaw MD, Nagar SP, et al. Health care costs among renal cancer patients using pazopanib and sunitinib. J Manag Care Spec Pharm. 2015;21:37–44. 44a–d. doi: 10.18553/jmcp.2015.21.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]