Abstract
Background:
Although a number of monoimmunotherapies and targeted therapies are available to treat BRAF+ advanced melanoma, response rates remain relatively low in the range of 22–53% with progression-free survival (PFS) in the range of 4.8–8.8 months. Recently, combination targeted therapies have improved response rates to about 66–69%, PFS to 11.0–12.6 months and overall survival (OS) to 25.1–25.6 months. While combination immunotherapies have improved response rates of 67 compared with 19–29% with monotherapies and improved PFS of 11.7 compared with 4.4–5.8 months with monotherapies, the OS benefit is yet to be established in phase 3 trials. As healthcare costs continue to rise, US payers have a predominant interest in assessing the value of available treatments. Therefore, a cost-benefit model was developed to evaluate the value of treating BRAF+ advanced melanoma with two combination therapies: nivolumab + ipilimumab (N+I) and dabrafenib + trametinib (D+T).
Scope:
The model was used to estimate total costs, total costs by expenditure category, cost per month of PFS and cost per responder for the payer, and societal perspectives of treating advanced melanoma patients with the BRAF V600 mutation using combination targeted therapy (D+T) or combination immunotherapy (N+I). The model followed patients from initiation of treatment to the point of progression or death. Deterministic and probabilistic sensitivity analyses were conducted to evaluate the robustness of the results and to understand the dispersion of simulated results.
Findings:
Based on a hypothetical payer with one million covered lives, it was expected that fourteen metastatic melanoma patients with the BRAF V600 mutation would be treated each year. Cost-benefit with N+I and D+T was simulated from the payer perspective. The cost per month of PFS for N+I was $22,162, while that for D+T was $17,716 (−$4,446 cost difference); the cost per responder for N+I was $388,746 and that for D+T was $282,429 (−$106,316 cost difference). The cost per month of PFS and per responder from the societal perspective resembled the patterns observed from the payer’s perspective: the cost per month of PFS for N+I was $22,843, while that for D+T was $18,283 (−$4,560 cost difference). The cost per responder for N+I was $400,695 and that for D+T was $291,473 (−$109,222 cost difference). The totals of travel and treatment time for N+I and D+T were 58 hours and 3.9 hours per patient, respectively, of which total infusion time for N+I accounted for a majority – 59% – of the 58 hours. Sensitivity analyses indicated that results were most sensitive to model inputs for median PFS, body weight, and drug cost. Moreover, D+T is likely associated with a lower cost per month of PFS and cost per responder than N+I, except at low body weights (less than 57 kg).
Conclusion:
The model presented in this study was used to analyze the clinical and economic benefit of using combination therapies in advanced melanoma patients with the BRAF V600 mutation. This analysis suggests D+T therapy is associated with less patient time and lower costs relative to N+I to gain similar PFS and overall response rate (ORR) benefits.
Keywords: metastatic melanoma, BRAF V600E mutation-positive, cost-benefit model, targeted therapy, immunotherapy, dabrafenib, trametinib, combination therapy
Introduction
Approximately 0.02% of patients in the USA have melanoma, of which 15% have advanced melanoma and 40–55% have BRAF mutation [1,2]. The prevalence of melanoma continues to increase, and it is estimated that about 2.1% of men and women will be diagnosed with melanoma during their lifetime [3]. In recent years, substantial progress has been made in the treatment of BRAF V600-mutant unresectable or metastatic melanoma. Both targeted therapies and immunotherapies have demonstrated efficacy in this patient population and gained recommendations as first-line therapy options in the 2016 National Comprehensive Cancer Network (NCCN) Guidelines [1]. Targeted therapies that inhibit BRAF or MEK have shown to be effective as monotherapies in randomized clinical trials (RCTs) [1,4]. Among patients with the relevant genetic mutation, the combination of dabrafenib1 (a BRAF inhibitor) and trametinib1 (a MEK inhibitor) (henceforth D+T) has been shown to increase progression-free survival (PFS), overall survival (OS), and overall response rate (ORR) without increasing toxicity relative to BRAF-inhibitor monotherapy [5,6]. Table 1 describes the clinical efficacy available for mPFS, OS, and ORR for the above-mentioned first-line therapies in BRAF+ advanced melanoma patients.
Table 1.
Clinical efficacy from phase III RCTs of selected first-line therapies in BRAF+ advanced melanoma patients.
| Drug name | mPFS [source] | mOS [source] | Response rate in % [source] |
|---|---|---|---|
| Darafinib | 8.8 [6] | 18.7 [6] | 53 [6] |
| Trametinib | 4.8 [19] | 15.6 [39] | 22 [19] |
| Ipilimumab | 4.0 [10] | 10.0* [17] | 19* [10] |
| Nivolumab | 5.6 [10] | Not reported | 36.7 [15] |
| Nivolumab + ipilimumab | 11.7 [10] | Not reported | 67 [15] |
| Dabrafenib + trametinib | 11.0–12.6 [6,34] | 25.1–25.6 [6,34] | 66–69 [6,34] |
Including both BRAF+ and BRAF wild type.
Immunotherapy is an alternative treatment approach recommended by NCCN as systemic therapy in advanced melanoma patients [1]. Ipilimumaba (an anticytotoxic, T lymphocyte–associated antigen 4 [CTLA-4] antibody) is the first FDA-approved immune check-point inhibitor for the treatment of advanced melanoma patients. Patients see a response rate of approximately 15% with ipilimumab monotherapy, and many patients experience serious immune-mediated adverse events [7,8]. Nivolumab1 is a new and improved immunotherapy that inhibits programmed death 1 (PD-1) and has demonstrated greater PFS and OS benefits, as well as reduced rates of adverse events relative to ipilimumab monotherapy. Nivolumab and ipilimumab (N+I) combination therapy has recently been shown to produce further improvements in PFS and ORR relative to N or I monotherapy [9–11]. OS benefits have not yet been demonstrated in the phase 3 RCT setting for the N+I combination therapy. Table 1 describes the clinical efficacy available for mPFS, OS, and ORR. Less clear, however, are the benefits relative to adverse events, as well as the payer and societal costs of the targeted combination (D+T) and immunotherapy combination (N+I) therapies. Recently American Society of Clinical Oncology, Memorial Sloan Kettering Cancer Center, and NCCN have released several frameworks proposing value assessment of cancer drugs, which in part are driven by rising healthcare costs. Although there is no consensus in methodology, all frameworks use efficacy (PFS, OS, or both) as one of the key components in the value assessment. These value assessments all share the common goal of aiming to make better decisions about what treatments to use [12–14]. To provide evidence of value for US payer–decision makers in advanced melanoma, we systematically measured the costs and benefits of each combination therapy. Our framework generated estimates of total cost, cost per month of PFS, and cost per responder for combination targeted therapies (D+T) and immunotherapies (N+I) in BRAF+ advanced melanoma.
Methods
Model overview
As healthcare costs continue to rise, US payers have a predominant interest in assessing not only the budgetary impact but also the value of available treatments. A decision analytic model was developed to estimate the cost benefit to a US Payer for a combination of targeted therapies (D+T) and for a combination of immunotherapies (N+I) in first-line advanced melanoma. Patient burden was included in the model as a secondary perspective. Patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutation were included in the model. The model followed patients from initiation of treatment to the point of progression or death. The model excludes post-progression treatments and costs, as this phase of melanoma care is confounded by other therapies and cannot be attributed specifically to a first-line therapy option. The decision-analytic model was developed using Excel 2010 software (Microsoft Corp., Redmond, WA, USA).
Model inputs
All clinical inputs, which include ORR, mPFS, adverse events (AE), health resource utilization (HRU), and AE discontinuation data were acquired from published literature or based on assumptions when data was not available [5–6,9–10,15–19]. For the base case analysis, PFS and ORR inputs of D+T were derived from the COMBI-d trial [6]. The literature does not report treatment duration which can be used to estimate treatment cost appropriately, so mPFS was used as a proxy, consistent with therapy labels that recommend treatment until progression [16–19]. In the real world setting, patients may continue to receive treatment beyond trial-defined progression, causing the proxy to underestimate treatment duration, or treatment may end before progression due to toxicity, causing an overestimate. We examined the impact of the choice of mPFS as a proxy for treatment duration in sensitivity analyses.
Grade 3 and 4 AE rates were obtained from published literature [5,6,9,10]. HRU comprised of laboratory monitoring requirements, drug administration resources, and patient burden data [16–22]. Table 2 describes the clinical inputs used in the model.
Table 2.
Clinical inputs.
| N+I [source] | D+T [source] | |
|---|---|---|
| Efficacy inputs | ||
| ORR in % [source] | 67.0% [15] | 69% [6] |
| Median PFS in months [source] | 11.7 [10] | 11.0 [6] |
| Grade 3 and 4 adverse events | N+I pooled RCT AEs in % [sources 9–10] | D+T pooled RCT AEs in % [sources 5–6] |
| Rash | 4.91 | 0.72 |
| Diarrhea | 9.58 | 0.89 |
| Colitis | 9.83 | 0.00 |
| Nausea | 1.97 | 0.18 |
| Vomiting | 2.21 | 0.89 |
| Arthralgia | 0.25 | 0.72 |
| CuSCC | 0.00 | 1.97 |
| Pyrexia | 1.23 | 5.37 |
| Decreased ejection fraction | 0.00 | 2.86 |
| Increase in AST | 8.11 | 1.07 |
| Increase in ALT | 7.13 | 0.72 |
| Constipation | 0.25 | 0.00 |
| Fatigue | 4.42 | 0.72 |
| Pruritus | 1.72 | 0.00 |
| Decreased appetite | 0.98 | 0.00 |
| Hyperthyroidism | 0.25 | 0.00 |
| Headache | 0.74 | 0.00 |
| Hypophysitis | 0.49 | 0.00 |
| Pneumonitis | 0.49 | 0.00 |
| Maculopapular rash | 0.74 | 0.00 |
| Dyspnea | 1.23 | 0.00 |
| Odema peripheral | 0.00 | 0.36 |
| Bleeding events | 0.00 | 0.18 |
| Non-cutaneous malignancies | 0.00 | 0.18 |
| New primary melanoma | 0.00 | 0.18 |
| Hand–foot syndrome | 0.00 | 0.18 |
| Chills | 0.00 | 0.54 |
| Increased lipase | 1.97 | 0.00 |
Laboratory tests and monitoring frequencies were based on drug package inserts and assumptions, respectively [16–19]. Resource use associated with drug administration for each therapy was obtained from package inserts and published dosing regimens [16–22]. N+I was administered intravenously and was assumed to require a peripherally inserted central catheter (and associated activities for placement) for drug administration. The duration of each intravenous infusion was based on the published protocol [9]. Patient burden data was found in the published literature and included travel time and time required for receiving infusions (1.0 hours/infusion) or filling prescriptions at the pharmacy (0.35 hours/refill) [16,20,21,23]. Table 3 describes the health resource utilization inputs used in the model.
Table 3.
Health resurce utilization.
| Laboratory monitoring (monthly frequency of testing) | ||
|---|---|---|
| N+I [source] | D+T [source] | |
| Renal | 2.09† | 1 |
| Hepatic | 2.09† | 1† |
| Complete blood count | 0† | 1† |
| Thyroid | 2.09† | 0† |
| Electrolyte | 0† | 1† |
| Other HRUs | N+I [source] | D+T [source] |
| PICC lines per regimen | 1* | N/A |
| Infusions/month | 2.1 | N/A |
| MD visits/month | 2.1** | 0*** |
| Infusion time | 1.0 Hr; nivolumab [16] 1.5 Hr; ipilimumab [17] |
N/A |
| Wait time between infusions | 30 min† | N/A |
| Time lost for travel to infusion clinic | 1.0 h [20] | N/A |
| Time lost for travel to pharmacy and wait time for filling prescription | N/A | 0.35 h [21,23] |
Assumption.
Assumed one PICC line per patient for the full course of therapy.
Assumed one MD visit for each infusion.
Assumed one MD visit assumed for D+T for the initial prescription.
Economic inputs included in the model were AE costs, drug costs, drug administration costs, laboratory monitoring costs, and patient costs. The model uses a calculated weighted average total AE cost/patient. The weighted average AE cost/patient is based on the rate and cost of AEs. AE costs were obtained from published literature [24,25]. Event costs that were not available (non-cutaneous malignancies, new primary melanoma, and hand–foot syndrome) in the literature were estimated based on published HRU and treatment specific to the event [26–28]. Patient coinsurance was calculated at 2.5 and 3.1% for N+I and D+T, respectively and was based on the Affordable Care Act’s out of pocket maximum of $6,850 [29]. Laboratory monitoring and drug administration costs were obtained from the 2015 American Medical Association Clinical Diagnostic Laboratory Fee Schedule using the 50% National Limitation rate [30]. Drug costs were acquired from Micromedex Solutions, and patient burden costs were obtained from published literature and the Bureau of Labor Statistics [16,20,21,23,31–33]. All costs obtained from the literature were adjusted for inflation to reflect 2015 USD using the medical component of the Consumer Price Index [28]. Table 4 describes the economic inputs used in the model.
Table 4.
Economic inputs.
| Drug costs (WAC per vial or pill) | Value in USD [source] |
| Nivolumab(100 mg/10 mL) | 2,434 [31] |
| Ipilimumab (50 mg/10 mL) | 6,659 [31] |
| Dabrafenib (75 mg) | 73 [31] |
| Trametinib (2 mg) | 336 [31] |
|
| |
| Lab monitoring costs | |
| Renal panel (CPT 80069) | 5.91 [30] |
| Hepatic panel (CPT 80076) | 5.56 [30] |
| Complete blood count (85025) | 5.29 [30] |
| Thyroid (84443) | 11.44 [30] |
| Electrolytes (80051) | 4.78 [30] |
|
| |
| Drug administration resource costs | |
| Insertion of IV (PICC line, CPT36569) | 127.93 [30] |
| Imaging guided placement of PICC Line (CPT 76937) | 16.35 [30] |
| MD visit (level 2, established patient, CPT 99212) | 22.10 [30] |
| First hour of infusion (CPT 96413) | 68.28 [30] |
| Each additional hour of infusion (CPT 96415) | 14.20 [30] |
|
| |
| Patient/indirect costs | |
| Average hourly wage | 25.20 [32] |
| Cost of transportation to infusion clinic | 2.66 [20,33] |
| Cost of transportation to pharmacy | 0.24 [23,33] |
|
| |
| Adverse event costs (per event) | |
| Rash | 14,346 [24] |
| Diarrhea | 26,260 [24] |
| Colitis | 26,260 [24] |
| Nausea | 13,729 [24] |
| Vomiting | 6,338 [25] |
| Arthralgia | 5,078 [25] |
| CuSCC | 24,530 [24] |
| Pyrexia | 15,093 [24] |
| Decreased ejection fraction | 6,476 [25] |
| Increase in AST | 18,863 [24] |
| Increase in ALT | 18,863 [24] |
| Constipation | 6,338 [25] |
| Fatigue | 0† |
| Pruritus | 0† |
| Decreased appetite | 0† |
| Hyperthyroidism | 9,135 [25] |
| Headache | 0† |
| Hypophysitis | 0† |
| Pneumonitis | 27,697 [24] |
| Maculopapular rash | 14,346 [24] |
| Dyspnea | 13,284 [24] |
| Odema peripheral | 0† |
| Bleeding events | 8,450 [25] |
| Non-cutaneous malignancies | 39,123 [27–28] |
| New primary melanoma | 7,692 [27–28] |
| Hand-foot-syndrome | 10,718 [6,10,26,31] |
| Chills | 15,093 [24] |
| Increased lipase | 9,135 [25] |
Assumption.
Patient coinsurance was calculated to be 2.5% for N+I and 3.1% for D+T, based off of the Affordable Care Act out of pocket maximum of $6,850 for individuals [29].
Sensitivity analysis
Several sensitivity analyses were conducted to evaluate the robustness of the model and to understand the dispersion of simulated results. First, in the univariate deterministic sensitivity analysis (DSA), all model parameters were varied by ±25%, holding the other inputs fixed. 25% was selected to reflect plausible ranges observed in the literature review.
To test the robustness of the model, a probabilistic sensitivity analysis (PSA) was done using Monte Carlo simulation. The model was replicated 1,000 times where all parameters were as allowed to vary across a prespecified range, as determined by draws from a beta distribution. The range for costs and resource use were determined using a gamma distribution. In all cases except for mPFS and ORR, we used an assumed standard error of 10%. For estimates of mPFS and ORR, standard errors were obtained by bootstrapping, using 1,000 replications of a fitted Weibull, Lognormal, Gompertz, and Exponential functions of the Kaplan–Meier curves. Using the Akaike Information Criterion test, the Weibull function was found to be the best-fitting parametric survival function.
Using the fitted curves for both data sets, we constructed samples corresponding to the number of patients in each RCT that the Kaplan–Meier data was obtained from (N+I sample size=102; D+T sample size=211). Bootstrapping was done by sampling with replacement from each sample. The mPFS was calculated for each bootstrapped sample. Based on the bootstrapped sample, the standard error of the mPFS and 95% confidence interval were calculated. Similarly for the ORR estimate, bootstrapping was used to estimate the standard error of the average and 95% confidence interval.
Additional sensitivity analyses were also conducted to evaluate the robustness of assumptions and impact on results from specific scenarios, including time on treatment and body weight, using data from the COMBI-v trial [34].
Results
The model was used to estimate total costs, cost by cost category, cost per month of PFS and cost per responder for the payer, and societal perspectives of treating advanced melanoma patients with the V600 BRAF mutation. The incidence of melanoma in the USA is estimated at 0.02%, of which 15% of the patients have advanced melanoma and 40% have the BRAF-mutant type [1,5,35]. Cost estimates in this analysis are based on a hypothetical US payer with 1,000,000 members. Using these estimates, about 14 patients are expected to have advanced melanoma with the BRAF mutation.
Payer perspective
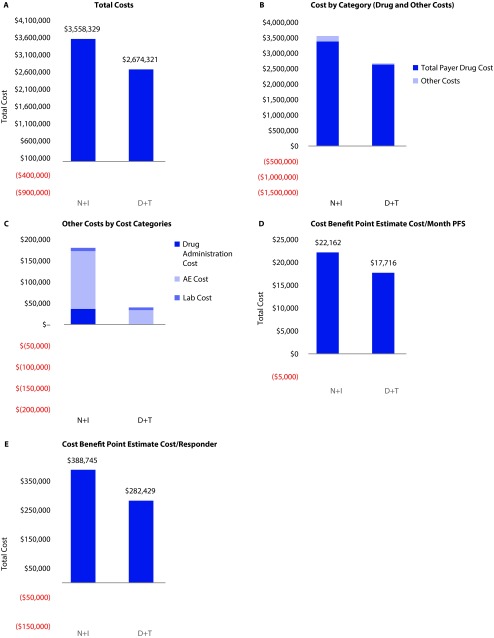
Budget impact results: D+T resulted in total costs of $2,674,321; N+I resulted in total costs of $3,558,329 to the payer across the hypothetical 1,000,000-member enrollment. On a per treated-patient basis, D+T per-patient cost was $194,876, and N+I per patient cost was $259,293. Lab costs were similar between N+I and D+T ($7,685 vs $6,137, respectively). Drug costs were $2,634,292 and $3,378,124 for D+T and N+I, respectively. Drug administration costs were $0 and $36,576. And AE costs were $33,892 and $135,944 for D+T and N+I, respectively.
Cost-benefit results: Incorporating duration of PFS, the total cost per month of PFS was $17,716/PFS month for D+T and $22,162/PFS month for N+I. Incorporating ORR, the total cost per responder was $282,429/responder for D+T and $388,745 for N+I. Figure 1.
Figure 1.
Payer costs.
*All model outputs are based on a sample size of 13.7 patients for each regimen.
Societal perspective
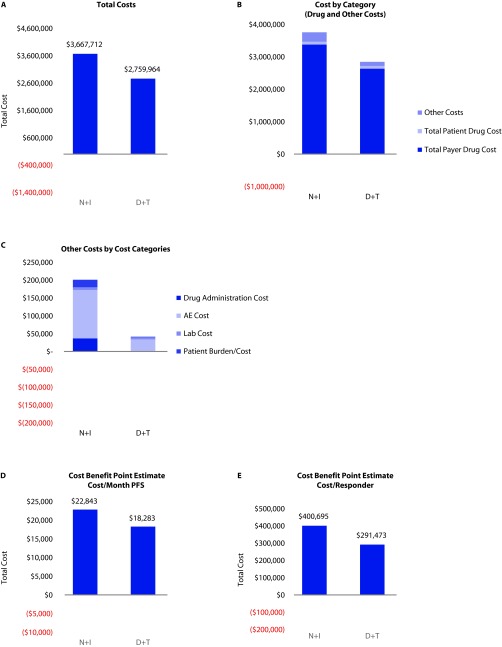
Budget impact results: Total societal costs (payer and all patients’ perspective) were $3,667,712 for the N+I regimen and $2,759,964 for the D+T regimen. Similarly, total costs per patient were $201,117 for the D+T regimen and $267,264 for the N+I regimen.
Cost-benefit results: Similar to the payer perspective, the total cost per month of PFS for the D+T regimen was $18,283/PFS month and $22,843/PFS month for N+I. The total cost/ORR was $291,473/responder for D+T and $400,695/responder for N+I (Figure 2).
Figure 2.
Total payer and societal costs.
*All model outputs are based on a sample size of 13.7 patients for each regimen.
Other costs to patients: adverse events and patient time
The most frequently experienced grade 3 and 4 AEs were 9.8% (colitis), 9.6% (diarrhea), 8.1% (AST), 7.1% (ALT) for N+I, and 5.4% (Pyrexia) for D+T. All other AEs were <5% for both regimens.
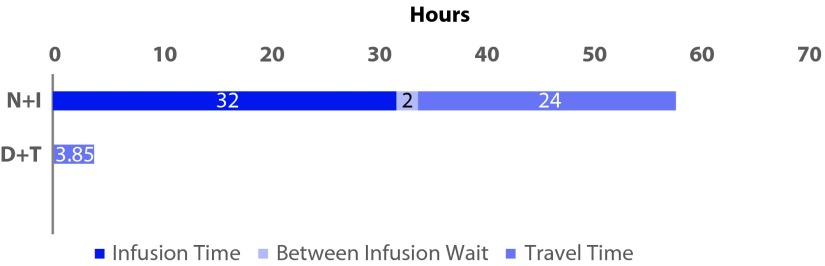
Total patient-burden time (per patient) captures travel time for drug administration, as well as the time required for the administration. Time burdens reflected the fact that D+T is orally administered, while N+I infusion is administered clinically. Total time taken was 3.9 hours for D+T and 55.9 hours for N+I (Figure 3). The 55.9 hours reflects infusion time (31 hours), time between infusions (2 hours) and travel time of N+I. D+T travel time was 3.9 hours.
Figure 3.
Patient burden time (time lost per patient over the course of the treatment regimen).
Sensitivity analysis
There were several sensitivity analyses executed as part of this analysis:
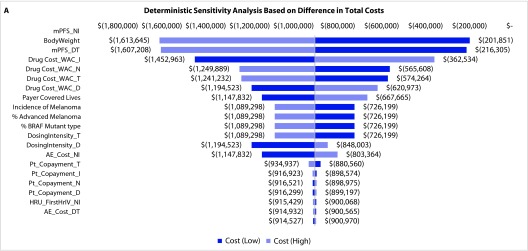
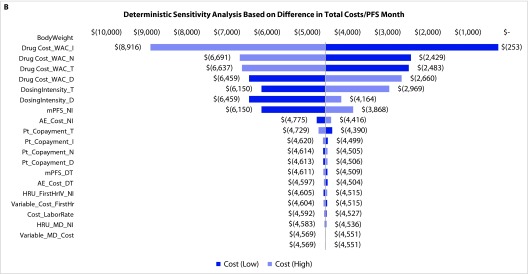
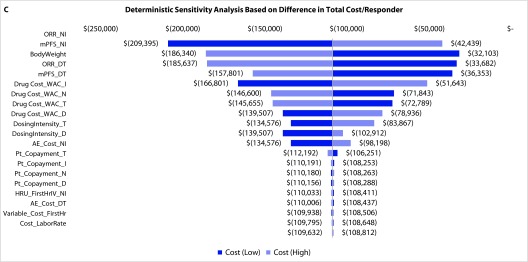
Deterministic sensitivity analyses: The DSA on the total costs of N+I and D+T regimens found that the model was most sensitive to the estimated inputs for mPFS, body weight, and drug costs (Figure 4a). For estimated total costs per month of PFS, the model was most sensitive to estimated body weight and drug costs (Figure 4b). For cost per ORR, DSA revealed the greatest sensitivity to ORR, mPFS, and body weight (Figure 4c).
Figure 4.
Deterministic and probabilistic sensitivity analysis.
*All model outputs are based on a sample size of 13.7 patients for each regimen.
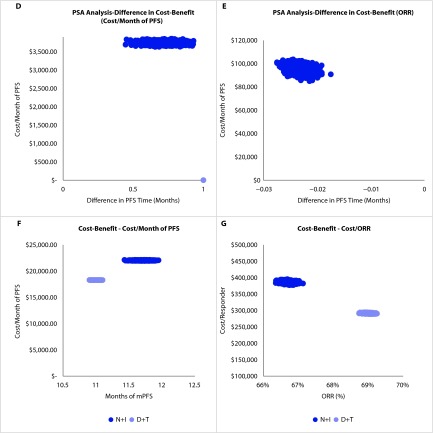
Probabilistic sensitivity analyses: The PSA was conducted on the cost-benefit results. Figure 4d shows that in 100% of model simulations D+T provided a lower cost/month of PFS than N+I. Similarly, Figure 4e shows that in 100% of model simulations D+T has a lower cost/ORR than N+I. These PSA results indicate that the model results are fairly robust.
Bootstrapping of PFS and ORR: Figure 4f displays the bootstrapped data for each mPFS used to calculate the respective cost-benefit. The N+I cost/month of PFS was more widely dispersed than D+T due to the greater level of uncertainty as a result of the smaller sample size. For the same reason, the dispersion of ORR/responder was much wider than for D+T (Figure 4g).
Other sensitivity analyses analyzing other key model inputs: In the real world setting, time on treatment (TOT) may be longer than mPFS, given that some patients continue to benefit from therapy despite disease progression. For patients treated with D+T, Long et al. reported 29% of patients continued therapy for at least 15 days, which results in underestimation of mPFS in this study [6]. To understand the impact of potentially longer TOT, we conservatively assumed 29% of patients would have an additional 30 days of therapy, and 71% of patients would receive therapy for the duration of PFS. This resulted in a slight increase in cost-benefit for D+T from $18,283/month of PFS to $18,824/month of PFS but still lower than the $22,843/month of PFS estimated for treatment with N+I. A similar trend was seen for the cost/responder result.
For patients treated with N+I, TOT may also be longer or shorter than mPFS time. Shorter TOT for N+I was evaluated in a sensitivity analysis. We assumed that patients discontinuing N+I continued to respond for 4 weeks, based on the estimated 2–4-week half-life of the immunotherapy agents [16,17]. To understand the potential impact of this possibility on model results, the number of discontinuing N+I patients who continue to respond was estimated to be 26%, because 38% of responders discontinue due to AEs, and 67% of patients respond [9,15]. Including this discontinuation rate, cost/month of PFS improved slightly for N+I from $22,843 to $22,309/month of PFS. Doubling the post-discontinuation response to 8 weeks resulted in a further small improvement for the cost-benefit of N+I ($18,283/month of PFS), although it remained higher than D+T. A similar result was seen for the cost/responder results.
In our base case, we assumed a mean body weight of advanced melanoma patient of 80 kg [22]. Cost per month of PFS and cost per responder were equal for the two regimens when reducing the body weight to 63 kg for cost per PFS and 57 kg for cost per responder.
As stated previously, the ORR, PFS, and bootstrapping sample inputs utilized in this model were derived from the COMBI-d trial. Alternatively, from the societal perspective, utilizing data from a second phase III RCT of D+T (COMBI-v trial; mPFS=12.6; ORR=66%; n=352) would have resulted in lower costs per PFS month ($18,255 for COMBI-v vs $18,283 for COMBI-d) as a result of longer mPFS, and higher costs per responder ($348,501 for COMBI-v vs $291,473 for COMBI-d), due to lower ORR found in the trial. Similar results were observed for the payer perspective [6,34].
Discussion
This study evaluated the payer and societal costs associated with N+I and D+T as first-line therapies in the treatment of BRAF V600 mutation-positive advanced melanoma. Total costs, total costs by category, cost/month of PFS, and cost/responder were generally lower for D+T compared to N+I therapy, from both a payer and societal perspective. Drug costs were lower for the orally administered D+T regimen than the N+I regimen, which requires infusions every 2–3 weeks.
As stated earlier, the rates for grade 3 and 4 AEs used in the analysis were reported in RCTs [5,6,9,10]. In these trials, most grade 3 or 4 AEs occurred in less than 5% of patients receiving either N+I or D+T. However, more prevalent grade 3 or 4 AEs – those that occurred in more than 5% of patients – include colitis, diarrhea, aspartate aminotransferase, and alanine aminotransferase for N+I and pyrexia for D+T. The D+T and N+I trials used different definitions for what constitutes an AE. In the D+T trials AEs were reported regardless of causality, whereas in the N+I trial AEs were reported as treatment-related AEs. The prescribing information for nivolumab reports AEs regardless of causality for N+I which was found to be 72% overall compared with 54% and 68.7% as reported in the trials [9,10,16]. Less frequent and less costly grade 3 and 4 AEs for the D+T regimen reinforced these cost advantages in addition to the conservative AE rate assumptions.
While the sensitivity analysis of AE management did not substantially impact costs in the model, it is worth noting that AEs tended to be $102,052 costlier for patients on N+I therapy. This difference suggests the need for a fuller analysis of AEs. Our study was hampered by the lack of data on AEs that occur after progression or late in the treatment journey. However, some evidence suggests the possibility of AEs occurring after progression and beyond [36]. This limitation may be better addressed in the future as real-world data on N+I and D+T usage become available.
The literature does not report TOT for the two regimens which can be used to calculate the treatment cost appropriately, so mPFS was used as a proxy. Sensitivity analyses were executed to evaluate the impact of potential overestimation and underestimation of TOT. Given the potential for N+I patients discontinuing therapy and continuing to accrue benefit, using the mPFS may have overestimated the TOT. However, the impact on model results was found to be minimal. Using mPFS may have been an underestimate for D+T. Long and colleagues reported 29% of patients may receive an additional 15 or more days of therapy. Assuming 30 additional days of D+T therapy, costs would increase by 1.03 fold. A minimal increase in cost was observed when using this adjustment on all cost drivers for D+T. These results suggest using mPFS as a proxy for TOT is a reasonable assumption.
The DSA found body weight to be a sensitive input for the model. Our base assumption used 80 kg as the average weight for melanoma patients as reported by Ouellet et al [22]. Body weight influences costs of N+I because the regimen is dosed based on body weight. However, body weight has no impact on the cost of D+T since this regimen uses the same dosing for all patients, regardless of body weight. Lower-weight patients would therefore have lower costs for N+I relative to D+T. The sensitivity analysis found the cost benefit of the two regimens to be the same when the body weight was 63 kg for the cost per PFS or 57 kg for cost per responder. Thus, N+I is estimated to generate lower costs per unit of benefit for patients weighing less than 57 kg.
While the discussion above focuses on average costs and benefits, the two regimens also differ in their variance. Figure 4f demonstrates the dispersion of mPFS for N+I is greater than for D+T. Dispersion of mPFS for D+T would be even less when using the COMBI-v data which was based on 352 patients, or if the COMBI-d and COMBI-v trials were pooled, thereby boosting the sample size to 763 patients. The wider mPFS dispersion for the N+I regimen resulted in a wider dispersion in cost benefit for N+I. Thus, payers and clinicians need to consider the differences in uncertainty across the two choices alongside the differences in average outcomes. Risk-averse decision makers may value the greater certainty of D+T, while risk-preferring agents might prefer to gamble on the possibility of greater upside with N+I therapy. This is mainly due to relatively small sample size (102) of N+I in BRAF+ patients.
From the patient’s perspective, we found considerable differences in the time burden across the two regimens. N+I requires patients to spend approximately 2 hours per month receiving and traveling for infusions. In contrast, D+T requires a trip to the pharmacy and oral self-administration. Whether a patient lives in a rural or urban area may significantly influence distance to a pharmacy or infusion clinic and thus travel time. In addition to travel time, patients are likely to experience different AE profiles. These AEs likely lead to a reduction in patient well-being and also in the time they have available for work and leisure. We do not quantify these losses in the model, but evidence in the literature suggests there may be effects. For example, systemic intravenous corticosteroid therapy is often administered for grade 4 dermatologic AEs [37]. This would require time off of work, away from the family, or other activities of daily living. However, with absent estimates around lost time due to N+T or D+T AEs, it is not possible to estimate their effect on patient burden.
Finally, our analysis evaluated use of COMBI-d and COMBI-v RCT data. From this analysis we can conclude the model results are consistent, regardless of the data set used, further substantiating the robustness and credibility of the model results.
Limitations
A simplifying model assumption was used to exclude the BRAF mutation testing, as it was assumed to be similar across all patients. Including this test would marginally increase the total costs for both regimens. Furthermore, the frequency of lab tests/monitoring was not clearly stated in package inserts; therefore, the model may be overestimating costs, although the impact on cost results are negligible. Although the N+I point estimates for ORR and PFS data were for BRAF+ mutant melanoma patients, the Kaplan–Meier data available for bootstrapping is not specific to BRAF+ mutant type patients. However, the point estimates used were included in the range found from the dispersion of benefits from the bootstrapping analysis. Recently, N+I’s package inserts were updated with complete (8.9%) and partial response rates (41%), giving an ORR of 49.9% [38]. The authors chose to use the ORR from Larkin et al. [10], as the rates in the package insert were not specific to BRAF V600 mutation-positive advanced melanoma [15,38]. Furthermore, the model used mPFS as a proxy for TOT, which may underestimate or overestimate the treatment cost. The model also includes the societal perspective where we used conservative published values of patient out of pocket costs, transportation costs, travel time, and the value of a patient’s time (hourly wage). Although none of these parameters were shown in the sensitivity analyses to have significant impact on the model results, these figures may vary more than what was permitted within the sensitivity analysis. Model results could be underestimated with respect to the patient burden. The cost benefits chosen for analysis were cost per month of PFS and cost per responder, as these benefits, in the authors’ experience, interest US payers the most. Alternative cost benefits could have been selected for analysis, such as cost per AE, cost per hour spent on receiving therapy, and others, which could have produced similar or different results.
In conclusion, the model presented in this study was used to analyze the clinical and economic benefit of using combination therapies in advanced melanoma patients. This analysis suggests D+T therapy is associated with less patient time and lower costs relative to N+I to gain similar PFS and ORR benefits. Sensitivity analyses indicated the model was most sensitive to estimates of ORR, mPFS, and body weight. The exact impact of these inputs on costs and cost benefits is not certain, therefore future prospective studies comparing N+I and D+T are needed to validate the results of this analysis.
Abbreviations:
- AE
adverse event
- CTLA-4
anticytotoxic, T lymphocyte–associated antigen 4
- DSA
deterministic sensitivity analysis
- D+T
Dabrafenib + Trametinib
- HRU
health resource utilization
- mPFS
median progression free survival
- N+I
nivolumab + ipilimumab
- NCCN
National Comprehensive Cancer Network
- ORR
overall response rate
- OS
overall survival
- PD-1
programmed death 1
- PFS
progression-free survival
- PSA
probabilistic sensitivity analysis
- RCT
randomized clinical trial
- TOT
time on treatment
Footnotes
Dabrafenib + trametinib (Taflinar + Mekinist; Novartis Pharmaceuticals Corporation, Hanover, NJ USA) and ipilimumab + nivolumab (Yervoy+Opdivo; Bristol-Myers Squibb, New York, New York USA) are registered trademarks.
Contributions: Ivar S. Jensen programmed the model and codrafted the manuscript with Emily Zacherle, who also conducted the targeted literature search in support of the model. All authors were instrumental in the conceptual design and participated extensively in review and editing of the model and manuscript.
Potential conflicts of interest: Jie Zhang is an employee of Novartis Corporation and owns NVS stock options. Ivar S. Jensen, Emily Zacherle, and Christopher M. Blanchette have provided consulting services to Novartis. Wes Yin has provided consulting services to Precision Health Economics. The International Committee of Medical Journal Editors’ (ICMJE) Potential Conflicts of Interests form for the author is available for download at: http://www.drugsincontext.com/wp-content/uploads/2016/07/dic.212297-COI.pdf.
Funding Declaration: This study was funded by Novartis Pharmaceuticals Corporation (Hanover, NJ USA).
Correct attribution: Copyright © 2016 Jensen IS, Zacherle E, Blanchette CM, Zhang J, Yin W. http://dx.doi.org/10.7573/dic.212297. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 3.0.
Article URL: http://www.drugsincontext.com/evaluating-cost-benefits-of-combination-therapies-for-advanced-melanoma
Provenance: Submitted; externally peer reviewed
References
- 1. NCCN.org [Internet]. National Comprehensive Cancer Network Clinical Practices Guidelines in Oncology: Melanoma Version 2.2016 [cited 2016 Mar 20 ]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/melanoma_blocks.pdf. [DOI] [PubMed]
- 2.Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, Hughes TM, Thompson JF, Scoyler RA, Kefford RF. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29(10):1239–46. doi: 10.1200/JCO.2010.32.4327. http://dx.doi.org/10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 3. Seer.cancer.gov [Internet]. SEER Stat Fact Sheets: Melanoma of the Skin [Cited June 16, 2016]. Available from: http://seer.cancer.gov/statfacts/html/melan.html.
- 4.Hauschild AJ, Grob J, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH, JR, Kaempgen E, Martin-Algarra S, Karaszewska B, Mauch C, Chiarion-Sileni V, Martin AM, Swann S, Haney P, Mirakhur B, Guckert ME, Goodman V, Chapman PB. Dabrafenib in Braf-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. The Lancet. 2012;379(9839):358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 5.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, Chiarion-Sileni V, Drucis K, Krajsova I, Hauschild A, Lorigan P, Wolter P, Long GV, Flaherty K, Nathan P, Ribas A, Martin AM, Sun P, Crist W, Legos J, Rubin SD, Little SM, Schadendorf D. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Eng J Med. 2015 Jan 1;372:30–39. doi: 10.1056/NEJMoa1412690. http://dx.doi.org/10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 6.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de BF, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, Chiarion-Sileni V, Lebbe C, Mandalà M, Millward M, Arance A, Bondarenko I, Haanen JB, Hansson J, Utikal J, Ferraresi V, Kovalenko N, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, DeMarini DJ, Irani JG, Swann S, Legos JL, Jin F, Mookerjee B, Flaherty K. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double -blind, phase 3 randomised controlled trial. Lancet. 2015 Aug 1;386:444–51. doi: 10.1016/S0140-6736(15)60898-4. http://dx.doi.org/10.1016/S0140-6736[15]60898-4. [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van dE, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Eng J Med. 2010 Aug 19;363:711–23. doi: 10.1056/NEJMoa1003466. http://dx.doi.org/10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Eng J Med. 2011 Jun 5;364:2517–26. doi: 10.1056/NEJMoa1104621. http://dx.doi.org/10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 9.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD. Nivolumab and Ipilimumab versus Ipilimumab in untreated melanoma. N Engl J Med. 2015 May 21;372:2006–17. doi: 10.1056/NEJMoa1414428. http://dx.doi.org/10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Eng J Med. 2015 Jul 2;373:23–34. doi: 10.1056/NEJMoa1504030. http://dx.doi.org/10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selby M, Engelhardt J, Lu LS, Quigley M, Wang C, Chen B, Korman AJ. Antitumor activity of concurrent blockade of immune checkpoint molecules CTLA-4 and PD-1 in preclinical models [abstract] J Clin Oncol. 2013 May 20;31 abstr 3061. Poster presented at 2013 ASCO Annual Meeting. Available at: http://meetinglibrary.asco.org/content/113653-132. [Google Scholar]
- 12.Schnipper LE, Davidson NE, Wollins DS, Tyne Courtney, Blayney DW, Blum D, Dicker AP, Ganz PA, Hoverman R, Langodon R, Lyman GH, Meropol NJ, Mulvey T, Newcomer L, Peppercorn J, Polite B, Raghavan D, Rossi G, Saltz L, Schrag D, Smith TJ, Yu PP, Hudis CA, Schilsky RL. American society of clinical oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015 Jun 22; doi: 10.1200/JCO.2015.61.6706. http://jco.ascopubs.org/cgi/doi/10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drugabacus.org [Internet]. Evidence driven drug pricing project [Cited June 16, 2016]. Available from: http://www.drugabacus.org/
- 14. NCCN.org [Internet] NCCN Clinical Practice Guidelines in Oncology [NCCN Guidelines] with NCCN Evidence Blocks [Cited June 16, 2016]. Available from: https://www.nccn.org/evidenceblocks/default.aspx.
- 15.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Hogg D, Hill A, Carlino MS, Wolter P, Lebbé C, Schachter J, Thomas L, Hassel JC, Lorigan P, Walker D, Jiang J, Hodi FS, Wolchok JD. Efficacy and Safety in Key Patient Subgroups of Nivolumab Alone or Combined with Ipilimumab versus Ipilimumab alone in treatment-naive patients with advanced melanoma [Checkmate 067] EJC. 2015 Sep;51:S664–S65. http://dx.doi.org/10.1016/S0959-8049[16]31822-6. [Google Scholar]
- 16. BMS.com [Internet]. OPDIVO® [nivolumab] Package Insert [revised 2015; Oct cited 2015 Nov 6 ]. Available from: http://packageinserts.bms.com/pi/pi_opdivo.pdf.
- 17. BMS.com [Internet]. YERVOY® [ipilimumab] Package Insert [revised 2015 Oct; cited 2015 Nov 6 ]. Available from: http://packageinserts.bms.com/pi/pi_yervoy.pdf.
- 18. FDA.gov [Internet]. TAFINLAR [dabrafenib] Label [revised 2013 May; cited 2015 Nov 6 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/202806s000lbl.pdf.
- 19. FDA.gov [Internet]. MEKINIST [trametinib] Label [revised 2013 May; cited 2015 Nov 6 ]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204114s000lbl.pdf.
- 20.Elkin EB, Weinstein MC, Winer EP, Kuntz KM, Schnitt SJ, Weeks JC. Her-2 Testing and Trastuzumab Therapy for Metastatic Breast Cancer: a Cost-Effectiveness Analysis. J Clin Oncol. 2004 Mar 1;22:854–63. doi: 10.1200/JCO.2004.04.158. http://dx.doi.org/10.1200/JCO.2004.04.158. [DOI] [PubMed] [Google Scholar]
- 21.Gebhart F. Rite aid 15 minute rx guarantee [Internet]. 2011 May 15 [cited 2016 Jan 6 ]. Available from: http://drugtopics.modernmedicine.com/drugtopics/news/modernmedicine/modern-medicine-news/rite-aid-offers-15-minute-rx-guarantee.
- 22.Ouellet D, Gibiansky E, Leonowens C, O’Hagan A, Haney P, Switzky J, Goodman VL. Population pharmacokinetics of dabrafenib, a BRAF inhibitor: effect of dose, time, covariates, and relationship with its metabolites. J Clin Pharm. 2014 Jan 1;54:696–706. doi: 10.1002/jcph.263. http://dx.doi.org/10.1002/jcph.263. [DOI] [PubMed] [Google Scholar]
- 23. DeArment E [Internet]. Rx impact: community pharmacy brings innovation to patient care. Pharmacists are face of health care community [cited 2015 Nov 8 ]. Available from: http://www.nacds.org/pdfs/pr/2012/rximpact-0312.pdf.
- 24.Ma Q, Zhao Z, Barber BL. Hospital costs of adverse events in patients with metastatic melanoma [abstract]. Value in Health. 2014 May 17[3]: A82. http://dx.doi.org/10.1016/j.jval.2014.03.477.
- 25.Arondekar B, Curkendall SM, Monberg M, Mirakhur B, Oglesby AK, Lenhart GM, Meyer NM. Economic burden associated with adverse events in patients with metastatic melanoma. JMCP. 2015 Feb;21(2):158–64. doi: 10.18553/jmcp.2015.21.2.158. http://dx.doi.org/10.18553/jmcp.2015.21.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lilly E, Burke M, Kluger H, Choi J. Pregabalin for the treatment of painful hand-foot skin reaction associated with Dabrafenib. JAMA Dermatol. 2015 Jan;151(1):102–3. doi: 10.1001/jamadermatol.2014.2455. http://dx.doi.org/10.1001/jamadermatol.2014.2455. [DOI] [PubMed] [Google Scholar]
- 27.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. JNCI. 2011 Jan 19;103:117–28. doi: 10.1093/jnci/djq495. http://dx.doi.org/10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. BLS.gov [Internet] Consumer Price Index – All Urban Consumers Medical Care Item U.S. Bureau of Labor Statistics. U.S. Bureau of Labor Statistics [cited 2015 Nov 8 ]. Available from: http://www.bls.gov/news.release/cpi.t01.htm.
- 29. Cigna [Internet]. Affordable Care Act cost sharing limits [cited 2016 Mar 24 ]. Available from: http://www.cigna.com/health-care-reform/cost-sharing-fact-sheet.
- 30. Awcc.state.ar.us [Internet]. 2015. Clinical Diagnostic Laboratory Fee Schedule. American Medical Association. [cited 2015 Nov 8 ]. Available from: http://www.awcc.state.ar.us/rule30misc/2015_lab_schedule.pdf.
- 31. Micromedexsolutions.com [Internet]. Micromedex Solutions. Truven Health Products [cited 2015 Nov 8 ]. Available from: http://www.micromedexsolutions.com/home/dispatch.
- 32. BLS.gov [Internet] Table B-3 Average Hourly and Weekly Earnings of All Employees on Private Nonfarm Payrolls by Industry Sector, Seasonally Adjusted. U.S. Bureau of Labor Statistics. U.S. Bureau of Labor Statistics [cited 2015 Nov 7 ]. Available from: http://www.bls.gov/news.release/empsit.t19.htm.
- 33. EIA.gov [Internet]. U.S. Energy Information Administration – EIA – Independent Statistics and Analysis. Gasoline and Diesel Fuel Update. US Energy Information Administration [cited 2015 Nov 8 ]. Available from: http://www.eia.gov/petroleum/gasdiesel/
- 34.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroyakovskiy D, Lichinitser M, Dummer R, Grange F, Mortier L, Chiarion-Sileni V, Drucis K, Krajsova I, Hauschild A, Mookerjee B, Legos J, Schadendorf D. Two Year Estimate of overall survival in COMBI-v, a randomized, open-label, phase III study comparing the combination of dabrafenib [D] and trametinib [T] with vemurafenib [Vem] as first line therapy in patients with unresectable or metastatic BRAF V600 E/K mutation-positive cutaneous melanoma. Presented at ECCO-ORG.EU [2015]
- 35. Surveillance, Epidemiology, and End Results [SEER] Program [ www.seer.cancer.gov] SEER*Stat Database: Incidence – SEER 9 Regs Research Data, Nov 2014 Sub [1973–2012] <Katrina/Rita Population Adjustment> – Linked To County Attributes – Total U.S., 1969–2013 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission.
- 36.Weber JS, Dummer R, de Pril V, Lebbe C, Hodi S. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab. Cancer. 2013 May 1;119:1675–82. doi: 10.1002/cncr.27969. http://dx.doi.org/10.1002/cncr.27969. [DOI] [PubMed] [Google Scholar]
- 37.Tarhini A. Immune-mediated adverse events associated with Ipilimumab CTLA-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica. 2013 Jan 1;1:1–19. doi: 10.1155/2013/857519. http://dx.doi.org/10.1155/2013/857519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. BMS.com [Internet]. OPDIVO® [nivolumab] Package Insert [Revised 2016 Jan; cited 2016 Mar 24 ]. Available from: http://packageinserts.bms.com/pi/pi_opdivo.pdf.
- 39.Schadendorf Dirk, Flaherty Keith T, Hersey Peter, Nathan Paul, Garbe Claus, Milhem Mohammed M, Demidov Lev V, Hassel Jessica C, Rutkowski Piotr, Mohr Peter, Dummer Reinhard, Trefzer Uwe, Larkin James M, Utikal Jochen, Dreno Brigitte, Nyakas Marta, Middleton Mark R, Becker Juergen C, Casey Michelle, Carver Jennifer, Ouellet Daniele, Martin Anne-Marie, Wu Frank S, Patel Kiran, Robert Caroline. Overall survival update on METRIC [ NCT01245062], a randomized phase 3 study to assess efficacy of trametinib compared with chemotherapy in patients with BRAF V600E/K mutation–positive [+] advanced or metastatic melanoma. Presented at SMR 2013 Congress.