Supplemental Digital Content is available in the text.
Key Words: fibromyalgia, chronic widespread pain, central pain, sensory hypersensitivity, central amplification, central sensitivity syndromes, neuroimaging, regulatory pathway, unified diagnostic guidelines, interprofessional collaboration
Abstract
This manuscript, developed by a group of chronic pain researchers and clinicians from around the world, aims to address the state of knowledge about fibromyalgia (FM) and identify ongoing challenges in the field of FM and other chronic pain syndromes that may be characterized by pain centralization/amplification/hypersensitivity. There have been many exciting developments in research studies of the pathophysiology and treatment of FM and related syndromes that have the potential to improve the recognition and management of patients with FM and other conditions with FM-like pain. However, much of the new information has not reached all clinicians, especially primary care clinicians, who have the greatest potential to use this new knowledge to positively impact their patients’ lives. Furthermore, there are persistent misconceptions about FM and a lack of consensus regarding the diagnosis and treatment of FM. This paper presents a framework for future global efforts to improve the understanding and treatment of FM and other associated chronic pain syndromes, disseminate research findings, identify ways to enhance advocacy for these patients, and improve global efforts to collaborate and reach consensus about key issues related to FM and chronic pain in general.
We have brought together a group of chronic pain researchers and clinicians from around the world to address the state of knowledge about fibromyalgia (FM) and identify ongoing challenges in the field of fibromyalgia and other chronic pain syndromes that may be characterized by pain centralization/amplification/hypersensitivity (Sidebar 1, Supplemental Digital Content 1, http://links.lww.com/CJP/A344). There have been many exciting developments in research studies of the pathophysiology and treatment of FM and related syndromes that have the potential to improve the recognition and management of patients with FM and other conditions with FM-like pain. However, much of the new information has not reached all clinicians, especially primary care clinicians, who have the greatest potential to use this new knowledge to positively impact their patients’ lives. Furthermore, there are persistent misconceptions about FM and a lack of consensus regarding the diagnosis and treatment of FM. This paper presents a framework for future global efforts to improve the understanding and treatment of FM and other associated chronic pain syndromes, disseminate research findings, identify ways to enhance advocacy for these patients, and improve global efforts to collaborate and reach consensus about key issues related to FM and chronic pain in general.
Lesley M. Arnold, MD Professor of Psychiatry and Behavioral Neuroscience, Director, Women’s Health Research Program, University of Cincinnati College of Medicine, Cincinnati, OH
INTRODUCTION
Chronic pain constitutes a highly prevalent and burdensome condition spanning the globe. According to US statistics alone, chronic pain affects approximately 100 million adults and carries an annual national economic cost of $560 to $635 billion due to direct medical costs and lost worker productivity.1 Chronic pain, like other severe chronic conditions, represents much more than a series of biological phenomena that impact general health. Chronic pain stems from and elicits profound cognitive and emotional consequences, requiring a biopsychosocial approach to understanding and management.1
Chronic pain conditions with a predominantly nociceptive/inflammatory or neuropathic component tend to be understood by health care providers; however, chronic pain conditions with centralized phenomena are less well understood, especially in the context of a chronic pain continuum. Fibromyalgia (FM), a common chronic widespread pain disorder that can affect children and adolescents but is more frequent in adult women—is one of the conditions contributing to the pervasiveness and expense of chronic pain as a whole.2,3
FM is considered to be the prototypical central chronic pain syndrome. However, use of the term central should not suggest that peripheral nociceptive input does not contribute to a patient’s pain; rather, the patient feels more pain than typically would be expected based on the degree of nociceptive input.4 Unlike nociceptive and neuropathic pain, which are associated with identifiable tissue or nerve damage, the pain of FM is less clear but may result from neurochemical imbalances in the central nervous system (CNS) that lead to an augmentation of pain perception, typified by allodynia (pain due to a stimulus that does not usually provoke pain) and hyperalgesia (increased pain from a stimulus that usually provokes pain).4,5 Its trigger, however, is often difficult or even impossible to pinpoint. CNS factors seem to play an important role that may involve several mechanisms, including central sensitization or a decrease in descending inhibition. Similar findings of hyperalgesia and allodynia have been observed in other chronic pain states, including irritable bowel syndrome, female urethral syndrome or overactive bladder, temporomandibular joint syndrome, myofascial pain syndrome, and even osteoarthritis (OA)—suggesting that similar CNS changes that play a key role in FM are present in a number of other chronic pain conditions.4 Regardless of the pathogenesis, a diagnosis of FM is symptom based and includes the presence of widespread pain and high levels of somatic symptoms, fatigue, unrefreshed sleep, and cognitive disturbances.6
With respect to the concept of “central pain,” there is no agreement on a single name for this type of pain, and several terms are used interchangeably, including centralized, dysfunctional, pathologic, idiopathic, neuropathic-like, and FM-like pain; central amplification; central sensitization; and central sensitivity syndromes, among others.7–10 For the purposes of this white paper, we use the term centralized pain.
Despite progress in the understanding and treatment of FM and what it teaches us about pain processing, the global health care community lacks a clear understanding of where FM sits within the pain continuum relative to other peripheral chronic pain disorders such as OA or rheumatoid arthritis (RA) (which often have elements of central pain), how to study FM globally (eg, epidemiologically), and how to recognize and care for individuals with FM in different regions of the world and different health care settings. Moreover, health care professionals across the globe do not universally agree or understand how the CNS contributes to pain. To highlight these shortcomings, this white paper presents the continuing challenges confronting clinicians worldwide who manage patients with FM and other chronic pain syndromes, along with suggested practical actions to enhance the understanding of FM and the pain continuum and, ultimately, to improve patient outcomes.
Issue: There is a General Lack of Understanding of FM and Concepts Related to Centralized Pain
Challenge: Clinicians worldwide have limited awareness and understanding of FM and also face difficulty grasping new concepts regarding the pathophysiology of centralized pain. In turn, clinicians struggle with diagnosing and managing patients with FM due to inadequate education, training, or experience. In some instances, this leads to low acceptance of FM as a valid, treatable condition.
Data collected around the globe support these concerns noted. According to a survey conducted in a nationwide sample of Chinese rheumatologists, none of the 707 respondents had received any training on FM in medical school.11 Tellingly, one-third of those surveyed confused FM with muscle inflammation diseases, and 30% regarded FM solely as a psychological illness. Eighty percent of respondents reported having experience in diagnosing FM, 62% of whom had participated in continuing education programs on the disorder. Among those who had never made a diagnosis of FM, only 24% had received continuing education about FM, suggesting that lack of familiarity with FM may be associated with low diagnosis rates. In addition, nearly 80% of respondents acknowledged having difficulty in treating FM patients. The results of this study suggest that Chinese rheumatologists require further training in FM to improve their knowledge base and clinical skills.
In a survey [Survey was developed by Harris Interactive in cooperation/partnership with Pfizer Inc. and the European Network of Fibromyalgia Associations (ENFA).] of primary care physicians (PCPs), rheumatologists, neurologists, psychiatrists, and pain specialists in 6 European countries, Mexico, and South Korea, >50% of physicians reported difficulty with diagnosing FM, fewer than 50% were aware of the American College of Rheumatology (ACR) 1990 classification criteria for FM, >50% reported they had inadequate training in FM, and >30% did not consider themselves to be knowledgeable about FM.12
A cross-sectional survey of physicians belonging to the Japan College of Rheumatology and Japan Rheumatism Foundation sought to determine whether physicians’ illness perceptions correlate with their frustration or resistance to accepting new FM patients into their practice.13 The results showed that physicians might hesitate to accept patients because of a perceived difficulty in controlling the symptoms of FM. Importantly, respondents who strongly considered patient internal/psychological factors as causes of FM had significantly higher “difficult doctor-patient relationship” scores, and those who strongly considered biomedical or external factors as causes of FM had significantly lower “difficult doctor-patient relationship” scores. The study authors emphasized that “to improve the quality of consultation, physicians must continuously receive new information about the treatments and causes of FM.”
Although knowledge of FM is expanding, some doubts continue to persist as to the “legitimacy” of the disorder.11,14 Skepticism has resulted, in part, from an unclear etiology and the fact that a diagnostic test cannot confirm the presence of FM. Patients with an FM diagnosis have reported encountering dismissive attitudes from others, including disbelief, stigmatization, and lack of acceptance by their family and friends, peers and coworkers, and the health care system.15,16 Comments on numerous blogs show that patients often have no support from their spouses and friends, have been labeled “lazy” or “attention-seeking,” and are sometimes told “it’s all in your head” (eg, http://www.healingwell.com; http://www.fmnetnews.com). Such dismissiveness can have substantial impact on patients who are already distressed.14,17 Validation of the FM experience by a clinician increases the patient’s overall quality of life; conversely, invalidation can have a significant detrimental effect on not only the patient’s quality of life but degree of pain.14
Suggestion: Centralized pain plays a role in all forms of chronic pain, including nociceptive and neuropathic pain. Enhancing clinician understanding of the pathophysiology of pain, underpinned by new scientific evidence, should be a foundation for the understanding of FM and other chronic pain conditions. In addition to nociceptive and neuropathic mechanisms, this would include how pain perception can become dysfunctional in sensory processing areas of the brain (eg, centralization, amplification, or hypersensitivity) in different diseases. Thinking about the types of pain that make up a patient’s chronic pain could inform a more rational approach to both nonpharmacologic and pharmacologic treatment selection. With a stronger understanding of the science of FM, clinicians will also be better prepared to provide education and support to patients and their families.
To promote such understanding, several undertakings—ideally accomplished through a variety of educational programs and platforms—are key:
Using a simple, holistic framework, explain the key pathologic mechanisms that lead to chronic pain, and emphasize that a “lesion” need not always exist to contribute to a specific pain condition.
Frame the role and mechanisms of different pain types in conditions like FM, osteoarthritis, and low back pain within the context of emerging understanding of chronic pain and central pain pathophysiology.
Address some of the skepticism and misunderstanding pertaining to FM in particular. For example, why is skepticism for FM greater than that for migraine or other chronic pain syndromes, particularly those with centralized phenomena?
Clarify for clinicians where FM fits within the pain continuum—ranging from predominantly peripheral nociceptive, to predominantly neuropathic, to predominantly centralized pain conditions—to provide context relative to other pain conditions (Fig. 1).18
Emphasize the apparent overlap between FM and other chronic pain disorders, including RA, OA, systemic lupus erythematosus, headache, low back pain, and others (Fig. 2).18–20
Underscore that FM is not an isolated disorder by highlighting that a variety of mixed pain conditions (eg, OA, chronic back pain) have features seen in FM.
FIGURE 1.
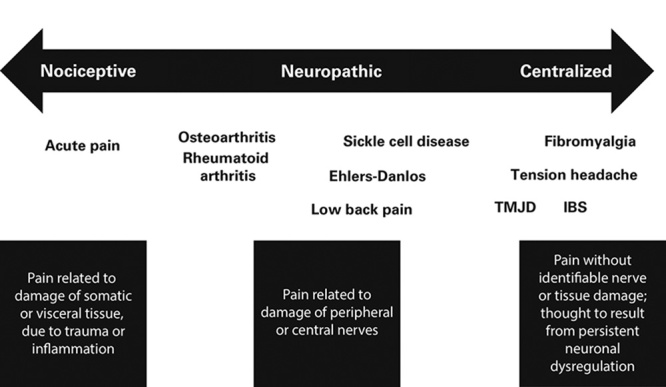
The pain continuum. Fibromyalgia (FM) is part of a larger continuum that comprises a number of clinical syndromes, some examples of which are shown here. This continuum ranges from predominantly nociceptive (peripheral), to predominantly neuropathic, to predominantly centralized pain conditions. Many experts agree that FM rests at the end of the continuum of pain processing. IBS indicates irritable bowel syndrome; TMJD, temporomandibular joint disorder. Courtesy of Don L. Goldenberg, MD.
FIGURE 2.
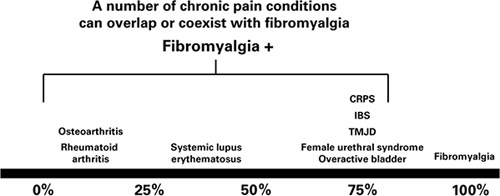
The prevalence of fibromyalgia (FM) in other chronic pain conditions. A number of chronic pain conditions can overlap or coexist with FM. It is useful to keep this in mind so that proper diagnosis and management can be undertaken. The percentages in this graphic are for illustrative purposes and are based upon the medical literature. CRPS indicates complex regional pain syndrome; IBS, irritable bowel syndrome; TMJD, temporomandibular joint disorder. Courtesy of Daniel J. Clauw, MD.
Challenge: Findings from brain neuroimaging may support a better understanding of FM, but the wealth of technical information needs to be made relevant for a primary care audience and better communicated to these providers.
There has been a surge of interest in using brain neuroimaging to study pain in a more quantitative, objective manner and to better understand the potential pathophysiology of FM. As reported in a recent systematic review, evidence from numerous imaging studies have demonstrated multiple, specific CNS changes related to central sensitization.21 However, the information can be difficult to understand. For example, it is unclear whether the CNS changes are a cause or consequence of centralized pain, and the overlap with CNS changes caused by more peripheral types of chronic pain (eg, RA) is not well defined. As a result, this information is often not adequately synthesized and disseminated to practitioners to enhance their understanding of FM and its treatment.
Suggestion: Distill the findings of seminal imaging studies into clear, simple terms and pictures to provide a better understanding of the processes underlying pain centralization/amplification/hypersensitivity, thereby enabling a better understanding of FM.
These messages should focus on the following:
Patients with FM display altered levels of key neurotransmitters (ie, increased excitatory and reduced inhibitory neurotransmitter levels),22,23 altered receptor binding,24 altered resting brain activity (ie, connectivity25,26), and differences in activation of pain-sensitive areas of the brain (Fig. 3).27,28 For example, in patients with FM, greater connectivity is observed between pain-promoting regions such as the insula and the default mode network, a network that is activated when an individual is not engaged with the external environment. Greater connectivity between the insula and the default mode network is associated with greater spontaneous clinical pain.26 These data and others imply aberrant brain neurotransmission is a hallmark of FM.26,29,30
Recent neuroimaging studies suggest that the CNS changes in patients with FM can be altered with pharmacologic and nonpharmacologic treatment.30,31 For example, an antiepileptic drug approved by the US Food and Drug Administration for the management of FM reduces glutamine/glutamate levels within the posterior insula and reduces connectivity between the posterior insula and default mode network. These changes track with changes in clinical pain.31 As such, imaging studies in diseases like FM provide a biological underpinning to better understand centralized pain. In addition, identification of functional brain “signatures” for drug action (analgesia) or disease state (neuropathic pain) could provide objective biomarkers to guide drug development.32
FIGURE 3.
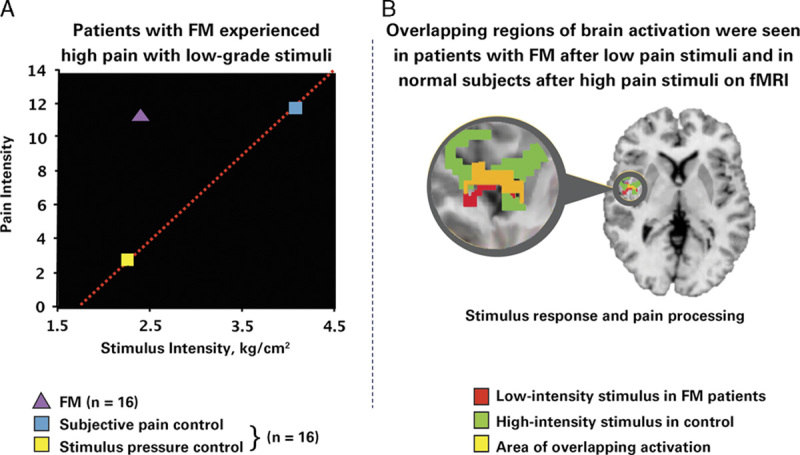
Objective evidence shows augmented pain sensitivity in individuals with fibromyalgia (FM) versus matched controls.27 A, The graph depicts mean pain ratings plotted against stimulus intensity. In FM patients, a low stimulus pressure (2.4 kg/cm2) produced a much higher pain level (mean±SD, 11.30±0.90) than in controls. However, in control subjects, a much higher stimulus pressure elicited a pain response similar to that in FM patients. B, The scan is a functional magnetic resonance image (fMRI) of the brain of a patient with FM from the same study. The imaging study demonstrated that, in patients with FM, pain processing areas of the brain are activated at a much lower level of stimulus than in control subjects. There is overlap (as indicated by the yellow area on the fMRI) between the areas activated with a low-intensity stimulus in FM patients (red area) and a high-intensity stimulus in control subjects (green area). In other words, the overlap between brain activation in FM patients receiving a low stimulus pressure and controls receiving almost twice as much pressure (ie, the amount required to cause the same amount of pain) suggests a mechanism involving central amplification of pain in the patients with FM. Because regions of brain activation in FM patients and healthy controls overlap, the pain experienced by both sets of subjects is real. Objective evidence such as this would be a valuable component of the educational framework proposed in this publication. Gracely et al.27 Reprinted with permission from John Wiley and Sons. Copyright [John Wiley and Sons, New York, NY]. All permission requests for this image should be made to the copyright holder.
Challenge: FM epidemiology data are available from different regions worldwide but are fragmented owing to different methods of data collection, patient selection, or health outcomes studied. In addition, available data are not collated in one place to facilitate learning and to guide future research to address the biggest knowledge gaps.
Reports of the prevalence of FM vary widely by geographic region for a number of reasons, including the diagnostic criteria used, differing study methodologies and designs, and different definitions of FM, among other reasons.33 In addition, even in advanced health care delivery systems, common data standards for pain and its impact on the patient (eg, function, sleep, mood) are ill defined, greatly limiting the utility of a potentially rich source of real-world data.
Suggestion: Perform a quality review of the existing data, collate reliable information, and recommend standards for gathering quality data.
As noted, several steps would be involved:
The first step is to identify and consolidate existing quality data that provide reliable insight into FM epidemiology and to determine where epidemiologic gaps currently exist. To date, widely accepted data on the epidemiology of FM include the points listed in Sidebar 2 (Supplemental Digital Content 2, http://links.lww.com/CJP/A345).18,33–44 However, the generalizability of these findings across different global regions remains to be determined, as does the collection of more consistent data regarding the prevalence of FM in different regions.
A key step is to create standards for gathering epidemiologic data to provide consistency in data collection moving forward. Toward this end, generating standard FM criteria and severity scales for use in epidemiologic and clinical studies would prove useful. Such standardization recently has been attempted by Wolfe et al45 through development of the Widespread Pain Index, Symptom Severity scale, and Fibromyalgia Survey Questionnaire,46 but global consensus among FM researchers is needed to establish such metrics as the de facto standards for epidemiologic data collection.
As quality epidemiologic data are identified or generated, efforts should be made to disseminate this information to clinicians and policymakers to underscore the breadth and magnitude of FM as a medical concern.
Ideally, with such efforts, sufficient epidemiologic data would be generated to enable the identification of different FM subgroups based on:
Trigger factors (psychosocial, trauma/abuse, infection, inflammation, sleep disorder, etc.).
Pain characteristics, including severity, perceptual pain qualities, temporal characteristics, and pain-related interference.
Overlap with other chronic pain syndromes (eg, irritable bowel syndrome, migraine, chronic fatigue syndrome, dysmenorrhea, temporomandibular joint disorder, restless legs syndrome, female urethral syndrome or overactive bladder, as well as rheumatic disorders such as OA and RA).
Genetic polymorphism associations.
Response to interventions.
Comorbid mood disorders.
Preexisting psychosocial traits, catastrophizing, education, income.
Equally important, establishing standards for data collection would also enable a more systematic exploration of centralized/amplified/hypersensitive pain in other common pain conditions.
Issue: The FM Lexicon Needs to be Framed in the Context of the Pain Continuum
Challenge: The lexicon for FM is still in flux and presents a disconnect hampering a uniform understanding and approach to FM diagnosis and interdisciplinary management.
Both the definition and understanding of FM continue to evolve, resulting in outdated and inconsistent use of sometimes arbitrary terminology.47 For example, FM was initially termed fibrositis based on early studies that described inflammatory abnormalities in the muscle or soft tissues.48 The term fibromyalgia syndrome emerged, eliminating the connotation of an underlying inflammatory connection but still implying a condition of the muscle and surrounding tissue and offering a way to compile the different symptoms of FM into one disorder. Fibromyalgia syndrome has transitioned to become just FM based on scientific evidence that FM is a distinct entity. FM is recognized by the World Health Organization with several ICD codes (ICD-9 codes: 728.79, 729.0, and 729.1; ICD-10 code: M79.7). In addition to the evolving delineation of FM, there is inconsistent use of several other terms related to the type of pain associated with FM, such as central pain, central sensitivity, centralized pain, sensory hypersensitivity, secondary FM, and so forth. The lack of common FM terminology used by clinicians undermines the willingness of some to accept or treat the disease, which poses challenges to optimizing care and outcomes for patients with FM or conditions with a predominant centralized component. In addition, opposition by certain clinicians to the term FM persists in some regions.49–51
Suggestion: A common “FM language” needs to be established.
Initial efforts toward this end include the following:
Determine whether the term FM should remain or whether an alternative term is needed (eg, central sensitization syndrome) to accurately describe the condition and the mechanisms involved in pathogenesis.
Professional associations could align on a clear, simple definition of FM (or alternative term) within the context of centralized pain; this would facilitate alignment among clinicians within different medical disciplines.
A definitive cause of FM is currently unknown and may be multifactorial. Although there is abundant useful information on FM and chronic pain syndromes, this information is fragmented. A definitive trusted source for expert opinion and guidance is needed to serve as a universal sentinel FM knowledge base.
Use clear, common language to describe the pathophysiology of FM (as discussed previously) and show where FM sits in the chronic pain continuum, to establish alignment among clinicians within different medical disciplines.
-
Information could highlight the following:
Typically, FM is characterized by chronic pain caused by alterations in sensory processing in the CNS. Although this pain is centralized, a subset of patients with FM may also present with peripheral pain generators.37
Aberrant neurochemical processing of sensory signals in the CNS may lower the threshold of pain, amplify normal sensory signals, and alter gene expression,52 thereby leading to hypersensitivity and central sensitization that result in chronic pain. Changes in the CNS that lead to FM also likely contribute to multiple associated symptoms, including sleep disturbance, fatigue, cognitive symptoms, and mood problems.18
Issue: Unified Diagnostic Guidelines for FM are Lacking
Challenge: Although there are a number of FM guidelines, no international consensus guidelines on FM diagnosis and management currently exist.
As an example, 3 different sets of ACR classification/diagnostic criteria are in use: the 1990 criteria, which review a patient’s history of widespread pain and require a tender point examination; the preliminary 2010 criteria, which provide a quantifiable measurement of chronic widespread pain and replace the tender point examination with an assessment of fatigue, waking unrefreshed, cognitive symptoms, and somatic symptoms; and the modified 2010 criteria, which rely on patient self-report of pain and a simplified listing of somatic symptoms. A recent study found that FM prevalence varies >4-fold with the application of these different criteria sets. In fact, prevalence is not only higher with the modified 2010 criteria, but a greater proportion of men are identified.33
In addition, accurate and timely diagnosis of FM currently falls far below what many would consider to be reasonable standards. In a recent survey of FM patients conducted in 6 European countries, Mexico, and South Korea, respondents reported that on average it took 2.3 years and presentation to 3.7 different physicians before they received a diagnosis of FM.53 In a separate study of 277 Brazilian women newly diagnosed with FM in a nationwide databank, analysis revealed that 74% of patients had suffered with chronic widespread pain for >3 years, 70% visited >3 doctors before a diagnosis of FM was established, and 44% experienced a lapse of >3 years between their first consultation with a medical professional and ultimately being seen by a rheumatologist.54
Clinical practice guidelines have the potential to offer much-needed assistance to practitioners tasked with diagnosing FM and delivering appropriate care; however, problems with such guidelines exist. Current guidelines to manage patients with chronic pain typically are siloed by country/region, if they exist at all.9,55–57 Even within each country/region, there can be a lack of consensus regarding the criteria used and recommendations made by such guidelines or consensus statements.44,58
Suggestion: Endeavors should be made to gain consensus on FM diagnostic and treatment guidelines from multiple stakeholders (interdisciplinary health care providers, different countries/regions) to promote widespread adoption and uptake.
Ideally, such guidelines should adhere to a number of basic principles:
The guidelines should help orient clinicians to recognize the spectrum of chronic pain syndromes, comorbid illnesses associated with FM, and the psychological and cognitive effects of FM (eg, depression, anxiety).
The guidelines should be simple and should focus on a biopsychosocial approach to FM diagnosis and management (eg, utilize a numeric rating scale to assess pain, global improvement, functional improvement, response to treatment, activities of daily living, and patient quality of life).
The guidelines should recognize that symptom intensity and functional outcomes in FM may fluctuate over time.
The guidelines should align with evidence-based classification systems for chronic pain (eg, the ACTTION-American Pain Society Pain Taxonomy).59
The guidelines should present the strength of each recommendation and indicate the quality of the scientific evidence supporting the recommendation.
The guidelines should help support clinical practice but not dictate clinical practice.
The guidelines should be written in such a way to be applicable across regions and/or to accommodate cultural differences and interprofessional differences, given the importance of multimodal approaches to care.
The guidelines should underscore the need for multimodal management of FM symptoms, including approaches that address the biological and psychosocial factors eliciting FM.
An overarching goal should be to develop streamlined approaches to reduce the office time required for clinicians to diagnose and manage FM across regions, without compromising the quality of care or patient satisfaction.
Development of such guidelines may best be accomplished through the actions of a credible global organization, as discussed next (eg, International Association for the Study of Pain [IASP]).
Issue: There is No Focused, Organized Leadership Charged With Research and Patient Care in FM and Other Chronic Pain Disorders
Challenge: No single group of physicians or other health care providers has taken ownership of FM or most other chronic pain disorders.
Patients with FM typically initially present to their primary care providers (PCPs) but thereafter consult with a variety of health care providers. In the United States and other regions, clinical services and research generally are organized along disease-specific lines. Although such an approach can be useful in disciplines such as cardiology, this organization does not lend itself to optimal care of individuals with chronic pain.
Various medical and surgical subspecialties have carved out an isolated piece of the chronic pain puzzle. Departments of anesthesiology, neurology, neurosurgery, orthopedics, and cancer take charge of treating pain etiologically associated with their diseases of interest, often without taking a more holistic view of the widespread manifestations of pain. This leads to a situation in which distinct clinical and research silos are spread across chronic pain management, which in turn hinders cross-fertilization of ideas and best practices and rejects a unifying approach to chronic pain syndromes.
This disconnect between disciplines is also reflected in national research institutes. For example, in the United States, the National Institutes of Health (NIH) has no dedicated pain institute, although there is an NIH Pain Consortium that was established to enhance pain research and promote collaboration among researchers (http://www.painconsortium.nih.gov). Some efforts have been made to collate information across institutes to better understand diseases like FM and other overlapping conditions (eg, the Workshop on Chronic Overlapping Pain Conditions; http://www.nidcr.nih.gov/NewsAndFeatures/Calendar/CalendarListing08132012.htm), but more work in this regard is necessary.
Suggestion: Promote opportunities for interprofessional collaboration on the management of FM within medical institutions.
In an ideal world, it would be preferable for PCPs to adopt the role of initial FM management, as they have done increasingly for headache and depression, with referral to a specialist when appropriate. However, this may not be a feasible approach in all countries. Opportunities for interprofessional collaboration on FM management that span a variety of medical settings may take various forms:
Develop a framework to ensure delivery of a simple, holistic, multidisciplinary care plan or pathway that enables interprofessional approaches to complex pain conditions, regardless of country or region.
Promote awareness and access to resources developed at the national level that provide practical approaches to diseases like FM. Examples of such resources include print-based articles (eg, FibroCollaborative framework for FM management),9,57 simple online tools (eg, http://fibroguide.med.umich.edu/),60 and lectures, workshops, and presentations organized by specialty societies (eg, the ACR).
Incorporate innovative diagnostic tools into daily practice (eg, treatment algorithms linked to patient electronic medical records) that facilitate optimal management of FM, including the need for referral to specialists in complex cases.
Assess the impact of educational and change initiatives on outcomes and resource utilization to further refine learning and change.
Offer grand rounds and medical conference presentations to a broad group of physicians, residents, and medical students that focus on FM and underscore the need for interdisciplinary consultation and management. This training should seek to provide guidance on when and how to refer patients with FM to other specialists, including neurologists, rheumatologists, psychologists, and others.
Ensure that other health care providers, such as nurses, physician assistants, nurse practitioners, physical therapists, and pharmacists, receive education and training on FM to help support the education and management of FM patients within primary care settings.
Arrange for pain specialists in pain management, neurology, rheumatology, psychiatry, and other departments to periodically participate in case review to offer insight into how to manage pain in complex cases.
Advocate for interdisciplinary teams of pain specialists to be collocated or embedded in primary care settings so that they can be more available for consultation during regularly scheduled PCP visits devoted to patients with FM.
Emphasize that the presence of FM can be a predictor of poorer analgesic outcomes following various pain interventions—potentially an important consideration for specialists.61,62
Challenge: Professional bodies and patient organizations are disjointed, with no recognized source of support and trusted information.
FM advocacy—as well as advocacy for other chronic pain conditions—is not organized, strong, or effective in most countries. In general, FM and related chronic pain conditions are not integrated into the chronic pain continuum, which leads to fragmented approaches to pain management. Funding agencies, pharmaceutical companies, and patient organizations tend to align themselves with specific interest groups rather than supporting broader efforts to address FM and other chronic pain disorders. Although the scientific community sometimes aligns on the goals of treating pain, different routes of distributing data and information result in fragmentation and potential confusion.
Suggestion: Establish or identify a credible global organization to serve as the flagship for FM education, advocacy, and changes to practice.
Ideally, this global organization would strive to achieve certain fundamental goals to unite the various stakeholders impacted by FM:
The organization should champion an interprofessional, comprehensive approach to chronic pain education and management by bringing together medical professionals from various specialties that deal with chronic pain, for example, pain management specialists, neurologists, rheumatologists, psychiatrists, orthopedic surgeons, gastroenterologists, gynecologists, otolaryngologists, cardiologists, pain specialists, nurse practitioners, and clinical psychologists, among others.
The organization should engage the interprofessional community to innovate solutions to increase FM understanding, learning, and change, such as through scientific meetings, a Web site, and continuing medical education programming, to ultimately improve patient outcomes.
The organization should endeavor to integrate the voice of the patient into its undertakings and offerings (eg, through “patient society days” before chapter meetings).
Of note, the IASP, which currently has >7000 members in 133 countries and 90 national chapters, may be the ideal organization to adopt this role by creating a special interest group focused on FM (http://www.iasp-pain.org/). As noted in its mission statement, IASP “brings together scientists, clinicians, health care providers, and policymakers to stimulate and support the study of pain and to translate that knowledge into improved pain relief worldwide.” Alternatively, more regional organizations already focused on FM (eg, the EFNA, http://www.enfa-europe.eu) could be bolstered and expanded to establish a worldwide presence, or a new global organization specifically focused on FM could be created.
Issue: Clear Global Regulatory Pathways for FM Treatments are Lacking
Challenge: Clear regulatory pathways are needed for FM treatment approval.
In general, approval of a therapeutic product comprises multiple stages: applying to conduct clinical trials, conducting clinical trials, applying for marketing authorization of a drug, and conducting postmarketing studies. However, there is marked variation globally in the drug-approval process within the area of chronic pain. First, not all regions have a regulatory pathway. Second, different regulatory agencies offer different approaches to pain indications.63,64 At present, draft analgesic guidances do not address FM or chronic pain syndromes. Both of these factors pose major barriers to the development of effective therapies for FM, as innovation and investment in FM conditions is contingent on regulatory pathways.
Suggestion: FM stakeholders within individual regions/countries should work to identify the regulatory pathway through which to facilitate approval of FM therapies and strengthen relations with those regulatory bodies. To emphasize the importance of these approval pathways, both pain experts and patients should be engaged in the development of regulatory requirements, and regulatory authorities from different countries should discuss and achieve consensus on these requirements.
Toward this end, several strategies may prove fruitful:
Each country’s government could develop and publish a health services plan for patients with chronic pain, including FM, and then work with regulatory authorities and other stakeholders to devise clear strategies and pathways for meeting the objectives delineated in the health services plan.
To improve acceptance of FM as a valid medical condition, each country could create a national consensus document on the diagnosis and treatment of FM (including both pharmacologic and nonpharmacologic treatment modalities), publish the document, and make it available through the Internet.
Each country could establish a “guidelines project” with its respective national medical association (eg, the American Medical Association), involving participation of medical professionals from several specialties that deal with chronic pain.
-
A primary care society or rheumatology society of each country could create a “Fibromyalgia Study Commission” that brings together specialists from other medical fields with knowledge about FM. This organization could endeavor to:
Create a Web site for doctors and another for patients containing the most relevant educational and scientific information pertinent to each group.
Translate and validate established questionnaires and other tools to gauge the impact of FM on patient function and quality of life.
Create a national databank on FM with the participation of interested physicians from academic, public, and private institutions.
Engage with the ministry of health within the country to raise awareness about the need for approval of FM therapies.
CONCLUSIONS
Currently, many clinicians worldwide struggle to understand, recognize, diagnose, and manage FM and other chronic pain syndromes, particularly within the broader context of the pain continuum, but this need not be so. By delineating the current challenges within the FM field, this white paper lays the initial groundwork for how to translate the perceived complexity of FM into meaningful action. With this foundation, we can begin to move forward to overcome some of the obstacles facing FM and other chronic pain syndromes. The intent is for this white paper and the points raised herein to serve as a call to action to stimulate key opinion leaders worldwide to unite, collaborate, and reach consensus on seminal issues pertaining to FM, thereby fostering a collective global effort to advance the field of FM specifically and of chronic pain in general.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.clinicalpain.com.
ACKNOWLEDGMENTS
The authors thank Stephen Watt, BSc, MBChB, MPhil, Andrew G. Clair, PhD, and Joanna Atkinson, MD, of Pfizer for their critical review and input during development of the manuscript.
Footnotes
A panel of international experts was convened in August 2014 by Pfizer Inc. to identify and discuss global issues and future directions in the field of fibromyalgia and chronic pain syndromes.
Editorial support was provided by SuEllen Farrell at Health and Wellness Partners of New Jersey LLC, and was funded by Pfizer Inc., New York, NY. L.M.A.: received an honorarium and support for travel to a meeting to discuss the development of this manuscript; she did not receive an honorarium for serving as an author. She currently is a consultant for Pfizer Inc., New York, NY; Daiichi Sankyo, Tokyo, Japan; Forest Laboratories, New York, NY; and Zynerba Pharmaceuticals, Devon, PA; and has received past consultancy fees from Dainippon Sumitomo Pharma, Tokyo, Japan; Innovative Med Concepts LLC (IMC), Tuscaloosa, AL; Ironwood Pharmaceuticals, Cambridge, MA; Purdue Pharma, Stamford, CT; Shire, Lexington, MA; Theravance Biopharma, South San Francisco, CA; and Toray, Tokyo, Japan. She has grants pending with Pfizer Inc., New York, NY; Eli Lilly and Company, Indianapolis, IN; Forest Laboratories, New York, NY; and Tonix Pharmaceuticals, New York, NY (money to institution); and has received past grants from Cerephex Corporation, Los Altos, CA, and Theravance Biopharma, South San Francisco, CA (money to institution). She also has received payment for lectures on behalf of Pfizer Inc., New York, NY. E.C.: received an honorarium and support for travel to a meeting to discuss the development of this manuscript; he did not receive an honorarium for serving as an author. Currently he is receiving consultancy fees and payment for lectures from Pfizer Inc., New York, NY. In addition, he is involved in Pfizer clinical trials for which fees are paid to his institution. D.J.C.: received an honorarium and support for travel to a meeting to discuss the development of this manuscript; he did not receive an honorarium for serving as an author. He is a consultant for Tonix Pharmaceuticals, New York, NY; Theravance Biopharma, South San Francisco, CA; Pfizer Inc., New York, NY; Abbott Laboratories, Abbott Park, Illinois; Samumed, San Diego, CA; Merck, Kenilworth, NJ; Eli Lilly and Company, Indianapolis, IN; UCB Pharma, Smyrna, GA; Johnson & Johnson, New Brunswick, NJ; Forest Laboratories, New York, NY; Purdue Pharma, Stamford, CT; and Zynerba Pharmaceuticals, Devon, PA. He has received grants from the National Institutes of Health, Bethesda, MD, and Eli Lilly and Company, Indianapolis, IN, and has several grants pending with the National Institutes of Health. D.L.G.: is a consultant to Pfizer Inc., New York, NY, and received an honorarium and support for travel to a meeting to discuss the development of this manuscript; he did not receive an honorarium for serving as an author. R.E.H.: received an honorarium and support for travel to a meeting to discuss the development of this manuscript; he did not receive an honorarium for serving as an author. He also has received funding including grants (past; money to institution), consulting fees or honoraria (ongoing), support for travel to meetings (past), and fees for participating in review activities (past) from Pfizer Inc., New York, NY. M.H.: received an honorarium and support for travel to a meeting to discuss the development of this manuscript; he did not receive an honorarium for serving as an author. He also has received consultancy fees (past), payment for lectures including service on speakers bureaus (current), and payment for development of educational presentations (current) from Pfizer Inc., New York, NY. T.S.J.: received an honorarium and support for travel to a meeting to discuss the development of this manuscript (money to institution); he did not receive an honorarium for serving as an author. He also has received consultancy fees from Grünenthal, Aachen Germany (ongoing); Orion Pharma, Espoo, Finland (ongoing); and Pfizer Inc., New York, NY (past). K.N.: received an honorarium and support for travel to a meeting to discuss the development of this manuscript; he did not receive an honorarium for serving as an author. He received consulting fees or honoraria and support for travel to meetings from Pfizer Inc., New York, NY (past). He also has received consultancy fees (ongoing), grants (ongoing), and speaker fees (past) from Pfizer Japan, Tokyo, Japan. S.L.S.: received an honorarium and support for travel to a meeting to discuss the development of this manuscript; he did not receive an honorarium for serving as an author. In addition, he is receiving board membership fees from Pfizer Inc., New York, NY (current). T.U.: received an honorarium and support for travel to a meeting to discuss the development of this manuscript; he did not receive an honorarium for serving as an author. He has received expert testimony fees (past), and grants (ongoing), from Pfizer Inc., New York, NY, and payment for lectures from other pharmaceutical companies. G.W.: received an honorarium and support for travel to a meeting to discuss the development of this manuscript; he did not receive an honorarium for serving as an author. He also has received payment for lectures, development of educational presentations, and meeting expenses, and has patents planned, pending, or issued with Pfizer Inc., New York, NY. S.F. has reported no conflicts of interest related to the content of this manuscript.
REFERENCES
- 1.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, et al. National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knight T, Schaefer C, Chandran A, et al. Health-resource use and costs associated with fibromyalgia in France, Germany, and the United States. Clinicoecon Outcomes Res. 2013;5:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clauw DJ, Arnold LM, McCarberg BH. FibroCollaborative. The science of fibromyalgia. Mayo Clin Proc. 2011;86:907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Association for the Study of Pain. IASP taxonomy. Available at: http://www.iasp-pain.org/Taxonomy. Accessed April 1, 2016.
- 6.Wolfe F, Walitt B. Culture, science and the changing nature of fibromyalgia. Nat Rev Rheumatol. 2013;9:751–755. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113–1122. [DOI] [PubMed] [Google Scholar]
- 8.Clauw DJ, Crofford LJ. Chronic widespread pain and fibromyalgia: what we know, and what we need to know. Best Pract Res Clin Rheumatol. 2003;17:685–701. [DOI] [PubMed] [Google Scholar]
- 9.Arnold LM, Clauw DJ, McCarberg BH. FibroCollaborative. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum. 2008;37:339–352. [DOI] [PubMed] [Google Scholar]
- 11.Mu R, Li C, Zhu JX, et al. National survey of knowledge, attitude and practice of fibromyalgia among rheumatologists in China. Int J Rheum Dis. 2013;16:258–263. [DOI] [PubMed] [Google Scholar]
- 12.Perrot S, Choy E, Petersel D, et al. Survey of physician experiences and perceptions about the diagnosis and treatment of fibromyalgia. BMC Health Serv Res. 2012;12:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homma M, Ishikawa H, Kiuchi T. Association of physicians’ illness perception of fibromyalgia with frustration and resistance to accepting patients: a cross-sectional study. Clin Rheumatol. 2016;35:1019–1027. [DOI] [PubMed] [Google Scholar]
- 14.Lobo CP, Pfalzgraf AR, Giannetti V, et al. Impact of invalidation and trust in physicians on health outcomes in fibromyalgia patients. Prim Care Companion CNS Disord. 2014;16 doi: 10.4088/PCC.14m01664. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kool MB, Van Middendorp H, Boeije HR, et al. Understanding the lack of understanding: invalidation from the perspective of the patient with fibromyalgia. Arthritis Rheum. 2009;61:1650–1656. [DOI] [PubMed] [Google Scholar]
- 16.Arnold LM, Crofford LJ, Mease PJ, et al. Patient perspectives on the impact of fibromyalgia. Patient Educ Couns. 2008;73:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kool MB, Geenen R. Loneliness in patients with rheumatic diseases: the significance of invalidation and lack of social support. J Psychol. 2012;146:229–241. [DOI] [PubMed] [Google Scholar]
- 18.Phillips K, Clauw DJ. Central pain mechanisms in the rheumatic diseases: future directions. Arthritis Rheum. 2013;65:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfe F. New American College of Rheumatology criteria for fibromyalgia: a twenty-year journey. Arthritis Care Res (Hoboken). 2010;62:583–584. [DOI] [PubMed] [Google Scholar]
- 20.Yunus MB. The prevalence of fibromyalgia in other chronic pain conditions. Pain Res Treat. 2012;2012:584573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cagnie B, Coppieters I, Denecker S, et al. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum. 2014;44:68–75. [DOI] [PubMed] [Google Scholar]
- 22.Harris RE, Sundgren PC, Craig AD, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foerster BR, Petrou M, Edden RA, et al. Reduced insular γ-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris RE, Clauw DJ, Scott DJ, et al. Decreased central µ-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000–10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen KB, Loitoile R, Kosek E, et al. Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol Pain. 2012;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Napadow V, LaCount L, Park K, et al. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gracely RH, Petzke F, Wolf JM, et al. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. [DOI] [PubMed] [Google Scholar]
- 28.Jensen KB, Kosek E, Petzke F, et al. Evidence of dysfunctional pain inhibition in fibromyalgia reflected in rACC during provoked pain. Pain. 2009;144:95–100. [DOI] [PubMed] [Google Scholar]
- 29.Emad Y, Ragab Y, Zeinhom F, et al. Hippocampus dysfunction may explain symptoms of fibromyalgia syndrome. A study with single-voxel magnetic resonance spectroscopy. J Rheumatol. 2008;35:1371–1377. [PubMed] [Google Scholar]
- 30.Becker S, Schweinhardt P. Dysfunctional neurotransmitter systems in fibromyalgia, their role in central stress circuitry and pharmacological actions on these systems. Pain Res Treat. 2012;2012:741746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119:1453–1464. [DOI] [PubMed] [Google Scholar]
- 32.Borsook D. Biomarkers for chronic pain and analgesia. Part 1: the need, reality, challenges, and solutions. Discov Med. 2011;11:197–207. [PubMed] [Google Scholar]
- 33.Jones GT, Atzeni F, Beasley M, et al. The prevalence of fibromyalgia in the general population—a comparison of the American College of Rheumatology 1990, 2010 and modified 2010 classification criteria. Arthritis Rheumatol. 2015;67:568–575. [DOI] [PubMed] [Google Scholar]
- 34.Vincent A, Lahr BD, Wolfe F, et al. Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken). 2013;65:786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams E, Daniel S, Chandran AB, et al. A population-based survey and physician assessment of the characteristics and prevalence of fibromyalgia (Abstract 137). Arthritis Rheum. 2013;65(suppl 10):S55. [Google Scholar]
- 36.Kato K, Sullivan PF, Evengård B, et al. Chronic widespread pain and its comorbidities: a population-based study. Arch Intern Med. 2006;166:1649–1654. [DOI] [PubMed] [Google Scholar]
- 37.Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547–1555. [DOI] [PubMed] [Google Scholar]
- 38.Helfenstein M, Heymann R, Feldman D. Prevalence of irritable bowel syndrome in patients with fibromyalgia [Portuguese]. Rev Bras Reumatol. 2006;46:16–23. [Google Scholar]
- 39.Hassett AL, Hilliard PE, Goesling J, et al. Reports of chronic pain in childhood and adolescence among patients at a tertiary care pain clinic. J Pain. 2013;14:1390–1397. [DOI] [PubMed] [Google Scholar]
- 40.Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. [DOI] [PubMed] [Google Scholar]
- 41.Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17:356. [DOI] [PubMed] [Google Scholar]
- 42.Ablin K, Clauw DJ. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am. 2009;35:233–251. [DOI] [PubMed] [Google Scholar]
- 43.Arnold LM, Hudson JI, Hess EV, et al. Family study of fibromyalgia. Arthritis Rheum. 2004;50:944–952. [DOI] [PubMed] [Google Scholar]
- 44.Clauw DJ.Hochberg MC, Silman AJ, Smolen JS, et al. Fibromyalgia and related syndromes. Rheumatology, 6th ed. Philadelphia, PA: Elsevier; 2015:80–1-80-12. [Google Scholar]
- 45.Wolfe F, Hassett AL, Katz RS, et al. Do we need core sets of fibromyalgia domains? The assessment of fibromyalgia (and other rheumatic disorders) in clinical practice. J Rheumatol. 2011;38:1104–1112. [DOI] [PubMed] [Google Scholar]
- 46.Häuser W, Jung E, Erbslöh-Möller B, et al. Validation of the Fibromyalgia Survey Questionnaire within a cross-sectional survey. PLoS One. 2012;7:e37504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states—maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Llewellyn LJ. A discussion on fibrositis. Proc R Soc Med. 1913;6:27–35. (Balneol Climatol Sect). [PMC free article] [PubMed] [Google Scholar]
- 49.Ehrlich GE. Pain is real; fibromyalgia isn’t. J Rheumatol. 2003;30:1666–1667. [PubMed] [Google Scholar]
- 50.Wolfe F. Fibromyalgia wars. J Rheumatol. 2009;36:671–678. [DOI] [PubMed] [Google Scholar]
- 51.Bass C, Henderson M. Fibromyalgia: an unhelpful diagnosis for patients and doctors. BMJ. 2014;348:g2168. [DOI] [PubMed] [Google Scholar]
- 52.Bjersing JL, Lundborg C, Bokarewa MI, et al. Profile of cerebrospinal microRNAs in fibromyalgia. PLoS One. 2013;8:e78762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choy E, Perrot S, Leon T, et al. A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv Res. 2010;10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rezende MC, Paiva ES, Helfenstein M, Jr, et al. EpiFibro—a nationwide databank for fibromyalgia syndrome: the initial analysis of 500 women. Rev Bras Reumatol. 2013;53:382–387. [PubMed] [Google Scholar]
- 55.de Miquel CA, Campayo J, Flórez MT, et al. Interdisciplinary consensus document for the treatment of fibromyalgia. Actas Esp Psiquiatr. 2010;38:108–120. [PubMed] [Google Scholar]
- 56.Fitzcharles MA, Ste-Marie PA, Goldenberg DL, et al. 2012 Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome: executive summary. Pain Res Manag. 2013;18:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arnold LM, Clauw DJ, Dunegan LJ, et al. FibroCollaborative. A framework for fibromyalgia management for primary care providers. Mayo Clin Proc. 2012;87:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mease PJ, Clauw DJ, Christensen R, et al. Toward development of a fibromyalgia responder index and disease activity score: OMERACT module update. J Rheumatol. 2011;38:1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fillingim RB, Bruehl S, Dworkin RH, et al. The ACTTION-American Pain Society Pain Taxonomy (AAPT): an evidence-based and multidimensional approach to classifying chronic pain conditions. J Pain. 2014;15:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams DA, Kuper D, Segar M, et al. Internet-enhanced management of fibromyalgia: a randomized controlled trial. Pain. 2010;151:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum. 2013;65:3285–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67:1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.US Food and Drug Administration (FDA). Guidance for Industry Analgesic Indications: Developing Drug and Biological Products [Draft Guidance]. Silver Spring, MD: FDA; 2014. [Google Scholar]
- 64.European Medicines Agency (EMA). Guideline on the Clinical Development of Medicinal Products Intended for the Treatment of Pain [Draft Guideline]. London, UK: EMA; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.clinicalpain.com.


