Abstract
BACKGROUND:
Children with cerebral palsy (CP) can present with severe secondary dystonia with or without associated spasticity of their extremities.
OBJECTIVE:
To assess the outcomes of combined anterior and posterior lumbar rhizotomy for the treatment of mixed hypertonia in the lower extremities of children with CP.
METHODS:
Fifty children with CP were subjected to combined anterior and posterior lumbar rhizotomies in a prospective study. Clinical outcome measurements were recorded preoperatively and were evaluated at 2, 6, and 12 months postoperatively. The operative techniques were performed by laminotomy from L1-S1, and intraoperative monitoring was used in all cases. All patients underwent intensive postoperative physiotherapy programs.
RESULTS:
Changes in muscle tone, joint range of motion, and dystonia were significant (P = .000) at postoperative assessment visits.
CONCLUSION:
This study demonstrated the potential of combined anterior and posterior lumbar rhizotomies to improve activities of daily living in children with CP and with mixed spasticity and dystonia.
ABBREVIATIONS:
BAD, Barry-Albright Dystonia Scale
CAPR, combined anterior and posterior lumbar rhizotomy
CP, cerebral palsy
ITB, intrathecal baclofen
MAS, modified Ashworth Scale
ROM, range of motion
SDR, selective dorsal rhizotomy
KEY WORDS: Botulinum toxin, Cerebral palsy, Dystonia, Habilitation, Mixed hypertonia, Rhizotomy, Spasticity
The term dystonia was first described by Oppenheim in 1911 as a movement disorder that causes sustained muscle contractions, repetitive twisting movements, and abnormal postures of the trunk, neck, face, or arms and legs.1 Dystonic movements can be either slow or rapid; they can change during different activities or postures; and they can become fixed in advanced cases.2 Children with cerebral palsy (CP) can present with severe secondary dystonia with or without spasticity in their extremities but hypotonia of their necks and trunks. For such children, combined anterior and posterior rhizotomy appears to be a reasonable option because it addresses their dystonia with anterior rhizotomies and their spasticity with conventional posterior rhizotomies.3
Secondary dystonia is associated most often with CP, but it can occur after traumatic brain injuries, strokes, or other disorders that affect the basal ganglia, usually the putamen.4 Secondary dystonia can be mild, moderate, or severe. If severe, it causes discomfort, impedes caregiving, and interferes with function.
Patients with secondary dystonia are usually treated initially with oral medications such as baclofen, levo/carbidopa, and trihexyphenidyl and with botulinum toxin injection.5
The aim of this study was to assess the outcomes of combined anterior and posterior lumbar rhizotomy (CAPR) for the treatment of handicaps and mixed hypertonia in children with CP.
METHODS
Fifty children suffering from moderate to severe mixed hypertonia resulting from CP were subjected to CAPR for management of their disabling mixed hypertonia between February 2010 and June 2014 in a prospective study performed in the Department of Neurosurgery, Ain Shams University Hospitals and Neurocare Medical Center in Cairo, Egypt. Ethics committee approval for the study design and statistical methodology was obtained.
Children were included if they had CP caused by a stationary pathology, eg, perinatal hypoxic encephalopathy, postkernicterus encephalopathy, or postencephalitic sequelae; were 4 to 18 years old; had lower-extremity mixed hypertonia in the form of moderate to severe spasticity and dystonia with a predominance of the dystonic element; had hypertonia interfering with passive range of motion (ROM), positioning, and care; and had hypertonia intractable to other treatment modalities such as tone-lowering medications, physical therapy, or botulinum toxin injections. Patients with dyskinesia or those unfit for general anesthesia were excluded from the study.
The patients underwent full preoperative clinical assessments, including history (especially perinatal, developmental history, and bowel control) and general and neurological examinations. Routine laboratory investigations and brain magnetic resonance imaging were conducted for all of the children. Tone measurement was assessed with the modified Ashworth Scale (MAS6; Table 1).
TABLE 1.
Modified Ashworth Scale6,a
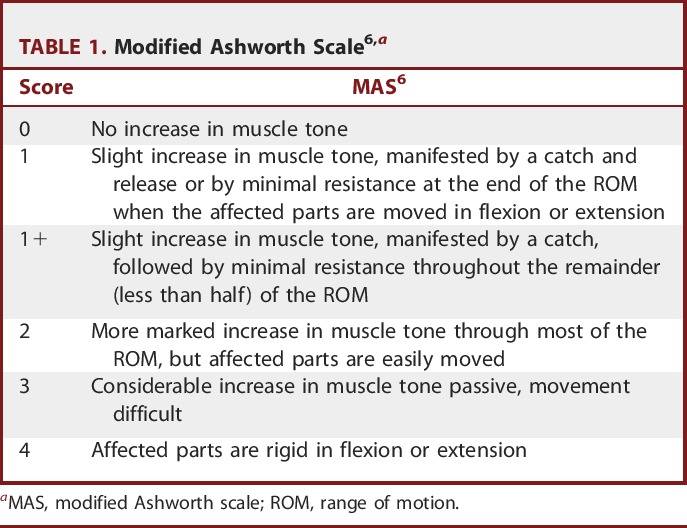
Assessment was performed for all muscle groups (hip flexors, hip adductors, knee flexors, extensors, calf planters, and dorsiflexors), and the mean of the scores was used for statistical evaluation. For statistical analysis purposes, the grade of 1± on the scale was considered a 2, and 1 was added to the remaining grades, so the grades ranged from 0 to 5.
All of the children were assessed for joint ROM preoperatively. Measurements were obtained with a manual goniometer. ROM was re-evaluated 2 months after CAPR, including in those children with limited ROM resulting from fixed muscular contractures that necessitated orthopedic interventions. Assessments was performed for all joints (hip, knee, and ankle joints), and the measured range was recorded as a percentage of the reference range for each joint (Table 2).
TABLE 2.
Published Normal Joint Range of Motion Values, in Degrees7
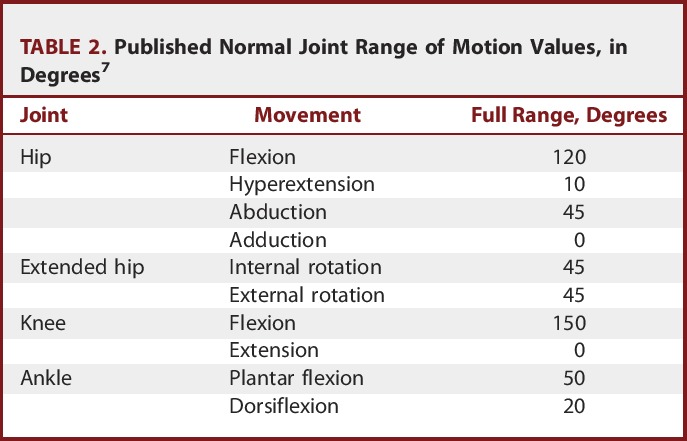
The statistical analysis of ROM values was performed in the second month postoperatively for the 2 reasons. First, our target population was suffering from mixed hypertonia, and a major proportion of them were suffering from joint or muscular contractures that affected the ROM and interfered with habilitation programs. Thus, tone management partially improved ROM in patients with contractures. Second, muscle-lengthening procedures or osteotomies produced changes in the ROM measurements, which could cause conflicts with the impact of tone management on ROM.
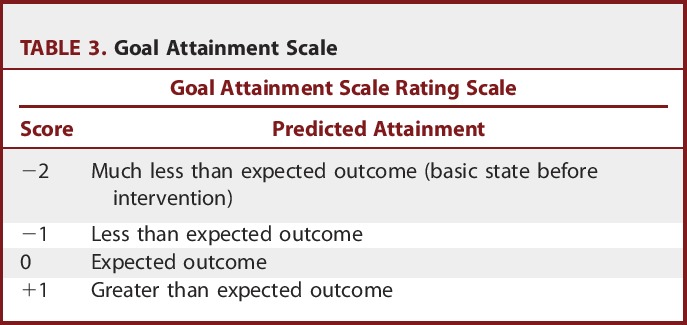
For the evaluation of the body involvement of dystonia, the Barry-Albright Dystonia Scale (BAD) was used.8 The gross motor function classification system was used for the functional grading of each child; reassessments were performed at postoperative month 12.9 The Goal Attainment Scale (Table 3) was used to evaluate the targeted functional goals after CAPR,10 and 3 goals were set for clinical evaluation: reduction of tone to MAS 1+, the ability to roll in bed, and sitting with support. For the first goal (reduction of muscle tone), scoring levels were as follows: −2, MAS = 3; −1, MAS = 2; 0, MAS = 1+; +1, MAS = 1; and +2, MAS = 0. For the second goal (rolling in bed), scoring levels were as follows: −2, the child can lift his or her head and 1 arm when attempting to roll from supine to prone; −1, the child can roll halfway from supine to prone and attain side lying; 0, the child can roll from supine to prone; +1, the child can roll from supine to prone and halfway back to supine; and +2, the child can roll from supine to prone and back to supine. For the third goal (sitting with support), scoring levels were as follows: −2, the child cannot elevate the head from the bed; −1, the child can elevate his or her head with partial trunk support; 0, the child can sit with external support; +1, the child can sit with self-support; and +2, the child can sit without support. We modified the scale by adding a −3 score to the original scale to interpret the deterioration of more than the baseline function.
TABLE 3.
Goal Attainment Scale
Outcome measurements and scales were documented in the charts for each child on presentation and at 2, 6, and 12 months postoperatively.
Statistical analysis was performed with the Statistical Program for the Social Sciences, version 19.0 (IBM SPSS Statistics for Windows, IBM Corp, Armonk, New York) as follows: Comparative tests were performed between the first-year measurements and baseline values, whereas ROMs were compared with values from the postoperative second month. The paired t test used to compare changes in mean values after 1 year of the intervention within groups with regard to quantitative variables. One-way analysis of variance with the Bonferroni adjustment as a post hoc test was used for multiple comparisons within groups. Results of comparisons were considered not significant if P > .01 and were considered significant if P ≤ .01.
Operative Technique
Anesthesia Protocol
All of the procedures were performed under general anesthesia without long-acting muscle relaxants. The fine adjustment of the depth of anesthesia and the use of drugs that do not affect neuromuscular transmission are important issues because of the frequent use of intraoperative electrophysiological stimulation and recording.
Volatile induction and maintenance of anesthesia with sevoflurane inhalational anesthetic were applied. Either the single-breath vital capacity technique or the tidal volume technique was used for induction, depending on patient response. Anesthesia was maintained with sevoflurane 1.8 to 2.5 minimal alveolar concentration, according to variations in blood pressure or heart rate compared with basal values.11
Preemptive Analgesia and Multimodal Pain Control Protocol
For perioperative analgesia, nalbuphine 0.1 to 0.2 mg/kg IM or IV was given 30 minutes before the induction of anesthesia. The maximum single dose is 20 mg, and the maximum daily dose is 160 mg in divided doses every 4 hours. Paracetamol 15 mg/kg IV infusion was administered after the induction of anesthesia and before the start of surgery, and it could be repeated every 4 to 6 hours with a maximum of 60 mg/kg, not exceeding 3 g/d.12 Antibiotic prophylaxis with ampicillin-sulbactam and ceftriaxone combinations was administered at the induction of anesthesia.
The patients were operated on in the prone position. Laminotomy from L1 to S1 was performed and kept attached to the interspinous ligament at the proximal level; then, midline durotomy was performed.
At each root level, a microsurgical technique was used to separate the anterior from posterior roots, guided by stimulation with a low-amplitude stimulus that represents the threshold for motor stimulation of anterior roots and did not cause motor response when the posterior roots were stimulated.
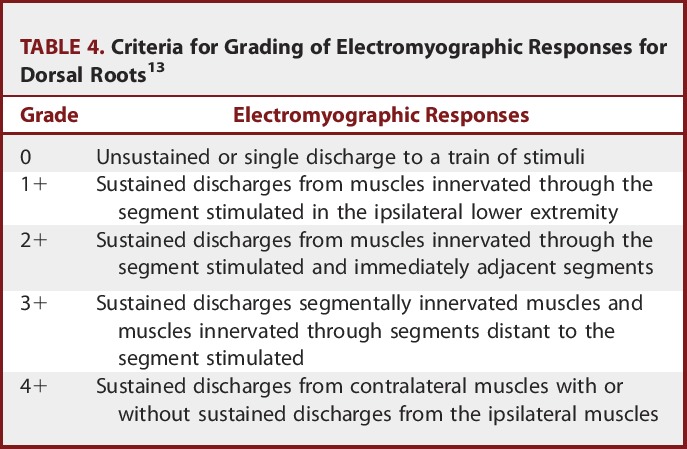
The selection criteria for dorsal root sectioning described in Park et al13 were used. First, the whole posterior root was tested with a single stimulus, and then individual rootlets of the selected roots were dissected and tested separately. Single constant square wave pulses of 0.1 millisecond in duration were applied to the rootlet at a rate of 0.5 Hz. The stimulus intensity was increased stepwise until a reflex response appeared from the ipsilateral muscles. After the reflex threshold was determined, a 50-Hz train of tetanic stimulation for 1 second was applied to the rootlet. The reflex response was then graded according to the criteria detailed in Table 4. The rootlets that produced a response of 0 were left intact. The rootlets producing 3± and 4± responses were cut, and those producing 1± and 2± responses were sometimes spared. If only 1± and 2± responses were detected, then the rootlets with the most active responses were cut. With the use of the criteria given in Table 4, sectioning of 50% to 80% of the rootlets at each level from L1 to S1 was performed: 50% at those producing 1± and 2± and 80% at those producing 3± and 4±.
TABLE 4.
Criteria for Grading of Electromyographic Responses for Dorsal Roots13
Each individual anterior root from L1 to S1 was dissected into multiple rootlets (4-6 rootlets). Sectioning of the anterior rootlets was performed according to the clinical evaluation of the severity of dystonic elements and the BAD score. A maximum of 80% of the rootlets were sectioned in severe cases (4/4 BAD score) and 50% in moderate cases (3/4 BAD score) to avoid muscular atrophy.
Intraoperative electrophysiological monitoring was used in all cases from the lower-limb muscles and sphincters for identification and sparing sphincter innervations.14
Electromyography mapping of sphincter innervation among the sacral roots enables safe division of S1 rootlets, producing optimum functional outcomes without sphincteric dysfunction. Preservation of sacral roots S2, S3, and S4 is essential for protecting bladder and sexual functions.14
The 4-channel Medtronic Xomed Inc NIM-Response 3 (Jacksonville, Florida) device and accessories were used. Two insulated electrodes were used for the stimulation of rootlets. Pairs of needle electrodes were placed in 5 muscle groups of each lower extremity: adductor longus, quadriceps (vastus lateralis), hamstrings, tibialis anterior, and gastrocnemius. The needles were spaced 3 to 5 cm apart, depending on the size of the patient. Two additional electrodes were placed in the external anal sphincter. A ground plate was placed on the calf of one limb.
All of the patients followed an inpatient intensive habilitation program for 3 to 4 weeks postoperatively, including 4 sessions per week.
RESULTS
Patient Demographics
Fifty children were enrolled in this study: 37 boys and 13 girls. The youngest child was 4 years old, and the oldest was 18 years old. The mean age was 7.92 years. All of the children were seen for all follow-up evaluation visits for up to 12 months postoperatively, and they were evaluated by the multidisciplinary team for outcome measurements.
Muscle Tone
Muscle tone was decreased after surgery. The changes in MAS after 2 months, 6 months, and 1 year were significant (Figure 1).
FIGURE 1.
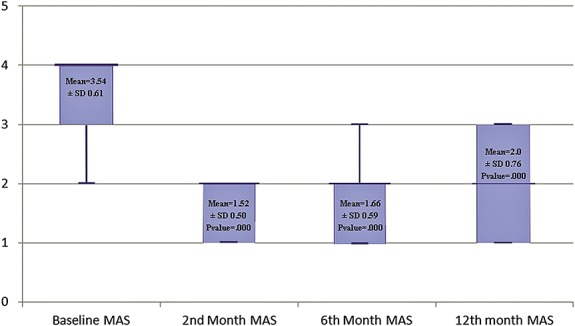
Demonstrating changes in the mean modified Ashworth scale (MAS) during the follow-up period. Color version available online only.
Joint ROM
Preoperative values were compared with the values recorded in the second month after CAPR because 64% of the children underwent orthopedic procedures at 2 months after CAPR. There was an increase in joint ROM in both lower limbs. This improvement was maintained, and results were significant at the second month postoperatively (Figure 2).
FIGURE 2.
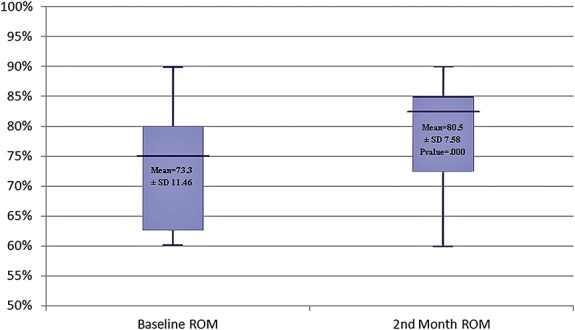
Demonstrating changes in the mean range of motion (ROM) during the follow-up period. Color version available online only.
The BAD
BAD was decreased immediately after surgery. The changes in BAD after 2 months, 6 months, and 1 year were significant (Figure 3).
FIGURE 3.
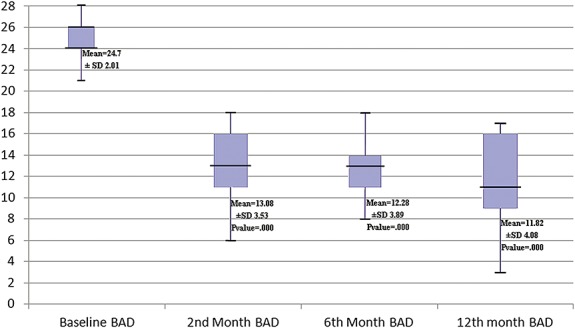
Demonstrating changes in the mean Barry-Albright Dystonia Scale (BAD) during the follow-up period. Color version available online only.
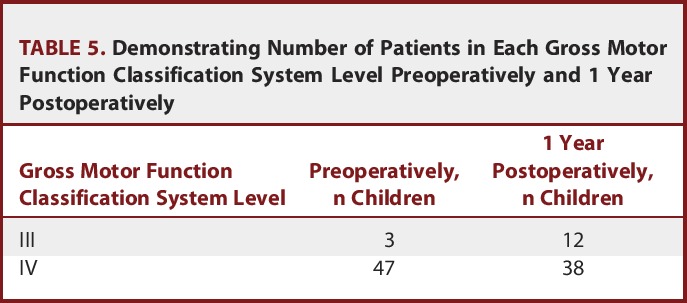
The Gross Motor Function Classification System
The gross motor function classification system showed improvement at the first-year follow-up visit with significant changes (Table 5).
TABLE 5.
Demonstrating Number of Patients in Each Gross Motor Function Classification System Level Preoperatively and 1 Year Postoperatively
The Goal Attainment Scale
The Goal Attainment Scale addressed the outcomes of the 3 predetermined goals throughout 1 year of the habilitation program.
First Goal (Tone Reduction)
Twenty-two children (44%) reached the expected goal of tone reduction (MAS 1+), 15 children (30%) reached more than the expected goal (MAS 1), and 13 children (26%) attained less than the expected goal (MAS 2).
Second Goal (Rolling in Bed)
Rolling in bed from supine to prone position was evaluated relative to baseline data. Thirty children (60%) could achieve the target goal; 5 children (10%) achieved more than the expected goal and could roll in bed from supine to prone and halfway back to supine over the side and attain side lying; 5 children (10%) could roll from supine to prone and in reverse, which is much more than expected; 4 children (8%) could attain side lying; and 6 children (12%) remained at baseline function.
Third Goal (Sitting With Support)
Achievement of a sitting posture was included in the physiotherapy programs. Ten children (20%) could achieve much more than the target and could sit alone without support; 15 children (30%) could sit with self-support; 20 children (40%) attained the goal to sit supported; and 5 children (10%) could elevate the head with partial trunk support, which was less than expected (Figure 4).
FIGURE 4.
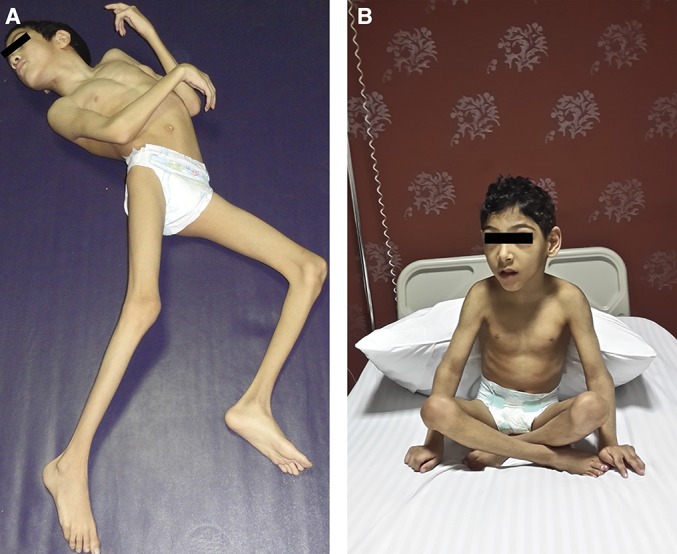
A 6-year-old boy with cerebral palsy and quadriplegia. A, preoperative dystonic posture. B, the child can sit with self-support 1 year postoperatively. Color version available online only.
Complications
Four children (8%), 2 boys and 2 girls, developed postoperative complications. One boy developed subcutaneous cerebrospinal fluid collection by the second postoperative day with no leakage through the suture line, which was managed conservatively. One girl had superficial wound dehiscence that required secondary sutures. One boy had abdominal distention caused by ileus on the first postoperative day, which was treated conservatively. One girl had urinary retention, which required urinary catheterization for 2 weeks postoperatively.
All of the children appeared for postoperative evaluation visits for up to 12 months, and 32 children (64%) were subjected to orthopedic procedures: 20 of them had multilevel muscle-lengthening procedures, and the remaining 12 children underwent osteotomies and muscle-lengthening procedures for rotational or flexion deformities.
CASE REPORTS
Case 1
A 6-year-old boy presented to the spasticity and movement disorders clinic in Ain Shams University Hospital with a history of neonatal encephalopathy resulting from obstructed labor and neonatal intensive care unit admission. He was at Gross Motor Function Classification level IV with mixed spastic dystonic diplegia; he had been on regular medications and underwent a physical habilitation program but showed no significant improvement in function. He had spasticity with a mean MAS of 3/5. His dystonia score on the BAD was 3/4 for each lower limb, and this hypertonicity impeded function and daily care. A CAPR was planned.
At surgery, osteoplastic laminotomy was performed from L1 to L5, and 50% of the posterior rootlets and 80% of the anterior rootlets from the L1-S1 roots were cut.
At 2 months postoperatively, his lower extremities were significantly improved in terms of muscle tone, he was easier to dress, and he had neither spasticity nor dystonia in either lower limb. One year later (Figure 4), he had MAS 1/5, his dystonia score for each lower limb was 1/4, and he could sit alone with self-support and performed good assisted standing with splints.
Case 2
A 9-year-old girl had CP diplegia (postkernicterus) with mixed severe spasticity and dystonia. She was at Gross Motor Function Classificationlevel IV, and she was on a regular physiotherapy program that was stopped because of a poor response and severe tightness of both hip adductor muscle groups. Plain x-ray of both hips revealed bilateral hip dislocation; she had severe spasticity with a mean MAS score of 4/5 and 5/5 for the hip adductors and a BAD score for total body involvement of 22/32.
At surgery, 80% of both the posterior and anterior roots were cut from the L1-S1 roots. Immediately postoperatively, her frequent painful dystonic spasms improved.
At 2 months after CAPR, the girl was subjected to bilateral hip varus derotation osteotomies and multilevel muscle lengthening for hip reduction and improvement of care.
At 1 year after CAPR and hip surgery, the girl had no spasticity in either lower limb (MAS 1/5) and an improved total score on the BAD to 16/32. She could sit with support and could return to her habilitation program.
DISCUSSION
The range of treatments available for children with CP has expanded considerably in recent decades, and many studies and publications have appeared on the different modalities for the treatment of pediatric hypertonia.
Rationale for CAPR
Only 1 series of 6 cases3 has addressed the issue of CAPR for the treatment of mixed hypertonia, so we must emphasize that our study on CAPR could be another step in the management of moderate to severe mixed hypertonia caused by CP in countries with special economic conditions.
The best outcomes were obtained in the setting of an experienced multidisciplinary team in which children and families were afforded the opportunity to learn about treatment options and given access to the right intervention at the right time.
Selectivity and Monitoring
In the Albright and Tyler-Kabara report of 6 cases,3 the patients were subjected to combined anterior and posterior rhizotomies for the relief of mixed hypertonia in both the upper and lower limbs. Two of them were at the cervical segments; 2 were at the lumbar segments; and 2 cases had combined anterior and posterior rhizotomies at both the cervical and lumbar segments. The parents and caregivers consistently reported less patient discomfort and improved ease of care postoperatively.
In their series of cases, these authors included only patients with severe mental effects. In addition, rhizotomies were performed nonselectively without intraoperative stimulation.3
However, our study included children with average mental function, and our goals were to improve the ease of care and to improve function, which is why intraoperative neurophysiological monitoring and electromyography recording were performed in all patients to facilitate sparing sphincteric innervation and to perform rhizotomies on selective bases.
Intraoperative stimulation and neurophysiological monitoring allow the identification of the roots that are involved in increased reflex activity and are contributing to spasticity in the lower limb muscles. In addition, it is morphologically not possible to determine which sacral roots carry significant amounts of pudendal afferent fibers, and much individual variability exists in the distribution of the sensory pudendal fibers entering the spinal cord through the dorsal sacral roots. Therefore, functional intraoperative testing is necessary to identify these roots and to facilitate sparing sphincteric innervation.14,15
Functional Performance
In this study, the effect of CAPR appeared immediately postoperatively; muscle tone was reduced and maintained throughout the follow-up period, and joint ROM improved in all of the patients, with significant changes compared with the baseline values. This outcome was consistent with the Albright case report results.16
The functional improvement as shown by the Goal Attainment Scale was highly impressive; 80% of the children could achieve rolling in bed, 20% could sit without support, and 70% could sit with support, indicating improved trunk and hip muscle control. Additionally, the parents reported obvious improvements in upper limb tone and function, which were considered suprasegmental effects of CAPR. Similar results were reported by Steinbok17 on the effect of selective dorsal rhizotomy (SDR) in children with spastic CP.
Many researchers have suggested the use of botulinum toxin A as a modality for the treatment of focal dystonia or spastic upper or lower limbs. In the Póo et al18 series of 515 children with CP, of whom 46 were children with mixed CP and 5 were children with dyskinetic CP with focal dystonia, as well as in the Camargo et al19 series of 20 children with diplegic CP, it was shown that functional improvement was temporary and was observed only during the peak effect of the drug.
In our study, we preferred surgical intervention in children with CP who suffered from regional mixed hypertonia compared with the use of the repeated multilevel botulinum toxin A injections because of the prolonged effect of surgery and the long-term functional gain. Plus, in these cases, the dose of botulinum toxin A required to control tone effectively might exceed the safe maximum dose limit.
At the same time, we recommend the use of repeated botulinum toxin A injections in cases with focal hypertonia in children who have a contraindication for surgery or general anesthesia and for the management of upper limb hypertonia.
SDR has undergone extensive investigation as a treatment option for spastic CP. There is strong evidence that SDR has long-term beneficial effects in terms of a reduction in lower-limb spasticity, an increase in the ROM, and an improvement in motor function. However, in the presence of mixed hypertonia, recurrence of tone as a result of the dystonic element always impedes functional gain.19 We believe that mixed spastic dystonic CP is not a good candidate for SDR, but it might be a candidate for CAPR or intrathecal baclofen (ITB) infusion. We agree that mixed tone disorders require a more tailored approach when treatment options are considered.
ITB therapy proved to be a good solution for severe generalized spasticity and dystonia in children with CP, and it has been associated with improved comfort and ease of care in approximately 85% of cases and with improved function in approximately 33% of children.20
In those children who did not meet the indication criteria for ITB, for example, children < 4 years old, underweight children, and those with epilepsy,21 who were in fact present in considerable numbers in our target population, ITB therapy would not be suitable. In addition, it is a costly modality, which is why CAPR was preferred more than ITB therapy for such children.
It would be worthwhile to perform a comparative multicenter study addressing the outcomes of ITB and CAPR.
Orthopedic Management After CAPR
In mixed hypertonia, the development of fixed joint contractures was frequently noticed in our target population, as was the coexistence of complex contracture-hypertonia affecting the overall joint ROM and motor function. In such circumstances, tone management comes first. This rule was adopted by our multidisciplinary team: Tone should be controlled first before proceeding to orthopedic corrections of deformities to guard against recurrence or reversal of deformities. Thirty-two children (64%) underwent different orthopedic modalities (multilevel tendon lengthening, functional tendon transfers, and correction of lever arm dysfunctions via osteotomies) at least 2 months after CAPR.
In agreement with our concept, Damiano et al22 proposed that orthopedic surgeons should work on teams including experts on tone management and preoperative and postoperative rehabilitation because orthopedic surgery does not affect motor control or balance or improve muscular strength. Orthopedic surgery could have a short-term effect on tone because the muscular tension is altered, which affects the Golgi tendon apparatus and the muscle spindle.
Boyd and Graham20 suggested a treatment algorithm closer to our concept in which orthopedic surgery was delayed until the age of approximately 7 to 9 years, with a greater focus on physical therapy and tone management in the early years. The only exception to this treatment algorithm would be if the hip were subluxed or dislocated secondary to muscle contractures. Delaying treatment has several advantages such as allowing more development of the musculoskeletal system, and it permits a “declaration” of the movement disorder because it is sometimes difficult to distinguish between severe spasticity and dystonia in very young children.
Limitations
This study was performed in children with CP resulting from stationary brain pathology that caused a mixed type of hypertonia. Children with dyskinetic signs and those with metabolic or neurodegenerative disorders were excluded from the study despite the presence of spastic dystonic features because we had no solid expectations of the outcomes owing to the progressive nature of the pathology. Because it is not a popular procedure, we were not able to enforce the evidence level of the study because of the limited number of similar published case reports. Our follow-up evaluation was limited to a 1-year period, which was short term because all of the patients were thoroughly evaluated during this period, and a significant number of patients missed follow-up visits after this period. Finally, ROM measurements in children who had muscular contractures and/or fixed joint deformities in association with mixed spastic dystonic hypertonia could not be clearly evaluated after tone management only. The improvement in ROM measurements after CAPR in those patients was limited and masked by these deformities, which is why we preferred to perform comparative analysis of the ROM measurements in the second month after CAPR and before orthopedic surgery to evaluate the impact of CAPR only on ROM improvement.
CONCLUSION
CAPR in diplegic/quadriplegic children with CP with mixed spasticity and dystonia could effectively improve muscle tone, joint ROM in lower limbs, functional gain, and ease of care. Further research, including multicenter evaluations and the examination of long-term impacts of CAPR, is required.
Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
COMMENTS
The authors have prospectively evaluated 50 children with cerebral palsy of the mixed spastic dystonic variety, many of whom were severely disadvantaged. Surgically, anterior and posterior lumbar rhizotomies were carried out with intraoperative monitoring. The results observed in their patient cohort included decreases in muscle tone and dystonia and an increase in joint range of motion. The authors conclude that anterior and posterior lumbar rhizotomies can improve activities of daily life in children with mixed spastic dystonic cerebral palsy, and from their results, this appears to be the case. This population of children is often underserved because of the lack of therapeutic options. Combining the traditional dorsal rhizotomy with sectioning of ventral rootlets will be a welcomed treatment option to those surgeons treating patients with spastic/dystonic cerebral palsy.
R. Shane Tubbs
Seattle, Washington
The authors report a prospective study of 50 children with cerebral palsy who had mixed spastic dystonic hypertonia and were treated with combined anterior and posterior lumbar rhizotomies and followed up for 1 year after surgery. This is a large series of patients having a procedure that has rarely been done. Thus, the study is important and could provide information that might allow others to determine whether they wish to treat this group of patients similarly. The results after this procedure, in a difficult population of patients to treat, were quite impressive. It is certainly something to consider as an alternative to intrathecal baclofen, which has a significant complication profile.
Paul Steinbok
Vancouver, Canada
Figure.
REFERENCES
- 1.Fahn S, Marsden CD, Calne DB. Classification and investigation of dystonia. In: Marsden CD, Fahn S, eds. Movement Disorders 2. London, UK: Butterworths; 1987:332-358. [Google Scholar]
- 2.Friedman J, Standaert DG. Dystonia and its disorders. Neurol Clin. 2001;19(3):681-705. [DOI] [PubMed] [Google Scholar]
- 3.Albright L, Tyler-Kabara EC. Combined ventral and dorsal rhizotomy for dystonic and spastic extremities. J Neurosurg. 2007;107(4 suppl):324-327. [DOI] [PubMed] [Google Scholar]
- 4.Calne DB, Lang AE. Secondary dystonia. Adv Neurol. 1988;50:9-33. [PubMed] [Google Scholar]
- 5.Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004;75(7):951-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206-207. [DOI] [PubMed] [Google Scholar]
- 7.Rothstein MJ. Assessment of joint range of motion. In: Rothstein JM, ed. Measurements in Physical Therapy. London, UK: Churchill Livingstone; 1985:86-112. [Google Scholar]
- 8.Barry M, VanSwearingen J, Albright AL. Reliability and responsiveness of the Barry-Albright dystonia scale. Dev Med Child Neurol. 1999;41(6):404-411. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum P, Palisano R, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214-223. [DOI] [PubMed] [Google Scholar]
- 10.McDougall J, King G. Goal Attainment Scaling: Description, Utility, and Applications in Pediatric Therapy Services. Resource Book/Training Manual, 2nd ed 2007. http://elearning.canchild.ca/dcd_pt_workshop/assets/planning-interventions-goals/goal-attainment-scaling.pdf. [Google Scholar]
- 11.Fernandez M, Lejus C, Rivault O, et al. Single-breath vital capacity rapid inhalational induction with sevoflurane: feasibility in children. Paediatr Anaesth. 2005;15(4):307-313. [DOI] [PubMed] [Google Scholar]
- 12.Remy C, Marret E, Bonnet F. Effect of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br J Anaesth. 2005;94(4):505-513. [DOI] [PubMed] [Google Scholar]
- 13.Park TS, Gaffney PE, Kaufman BA, Molleston MC. Selective lumbosacral dorsal rhizotomy immediately caudal to the conus medullaris for cerebral palsy spasticity. Neurosurgery. 1993;33(5):929-943. [DOI] [PubMed] [Google Scholar]
- 14.Shalash AS, Ghany WA, Desoky AE. Role of intraoperative electrophysiological monitoring during selective dorsal rhizotomy in children with spastic cerebral palsy regarding sparing of sphincter innervation. Pan Arab J Neurosurg. 2013;17(2). [Google Scholar]
- 15.Huang JC, Deletis V, Vodušek DB, Abbott R. Preservation of pudendal afferents in sacral rhizotomies. Neurosurgery. 1997;41(2):411-415. [DOI] [PubMed] [Google Scholar]
- 16.Nordmark E, Josenby AL, Lagergren J, Andersson G, Strömblad LG, Westbom L. Long-term outcomes five years after selective dorsal rhizotomy. BMC Pediatr. 2008;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinbok P. Outcomes after selective dorsal rhizotomy for spastic cerebral palsy. Childs Nerv Syst. 2001;17(1-2):1-18. [DOI] [PubMed] [Google Scholar]
- 18.Póo P, Galván-Manso M, Casartelli MJ, et al. Botulinum toxin in infantile cerebral palsy. Rev Neurol. 2008;47(suppl 1):S21-S24. [PubMed] [Google Scholar]
- 19.Camargo CH, Teive HA, Zonta M, et al. Botulinum toxin type A in the treatments of lower-limb spasticity in children with cerebral palsy. Arq Neuropsiquiatr. 2009;67:62-68. [DOI] [PubMed] [Google Scholar]
- 20.Boyd R, Graham HK. Botulinum toxin A in the management of children with cerebral palsy: indications and outcome. Eur J Neurol. 1997;4(supp 2):S15. [Google Scholar]
- 21.Albright AL, Ferson SS. Intrathecal baclofen therapy in children. Neurosurg Focus. 2006;21(2):e3. [DOI] [PubMed] [Google Scholar]
- 22.Damiano DL, Alter KE, Chambers H. New clinical and research trends in lower extremity management for ambulatory children with cerebral palsy. Phys Med Rehabil Clin N Am. 2009;20(3):469-491. [DOI] [PMC free article] [PubMed] [Google Scholar]



