Abstract
We report herein a simple, metal- and additive-free, photoinduced borylation of haloarenes, including electron-rich fluoroarenes, as well as arylammonium salts directly to boronic acids. This borylation method has a broad scope and functional group tolerance. We show that it can be further extended to boronic esters, and carried out on gram scale as well as under flow conditions.
Aromatic boronic acids find increasingly important roles in the areas of synthetic organic chemistry,1 catalysis,2 molecular self-assembly,3 carbohydrate analysis,4 molecular sensing,5 materials science6 and medicinal chemistry.7 While the reaction of organomagnesium and organolithium reagents with trialkyl borates, first described by Khotinsky (RMgX)8 and Letsinger (RLi),9 remains in use, transition metal-catalyzed C–X-10 and C–H-borylation11 reactions have recently emerged as efficient alternatives that bypass air- and moisture-sensitive intermediates. In addition, transition metal-free, base-mediated borylation of iodo- and bromoarenes to arylboronic esters,12 as well as the Sandmeyer-type borylation of anilines13 and electrophilic borylation of electron-rich arenes14 have been recently developed. These methods can be advantageous, since the use of heavy metals, expensive and air-sensitive catalysts and ligands can be avoided. However, the practicality and scalability of these methods may be limited by the narrow substrate scope and high molecular weight of the boron reagents.
Photochemical activation of molecules enables reactivity patterns that can be difficult to achieve from the ground states.15 A multitude of homolytic and heterolytic pathways that can be accessed from singlet and triplet excited states affords numerous synthetic possibilities. A number of photoinduced processes have been successfully adapted in the chemical industry (e.g. the Toray PNC process,16 rose oxide17 and vitamin D18 syntheses), pointing to the significant potential of photoinduced reactions.19
Photoinduced Ar–X bond dissociation is a key step in a number of important photochemical carbon–carbon and carbon–heteroatom bond-forming reactions, including nucleophilic substitution,20 arylation,21 alkylation,22 and photocyclization23 reactions.
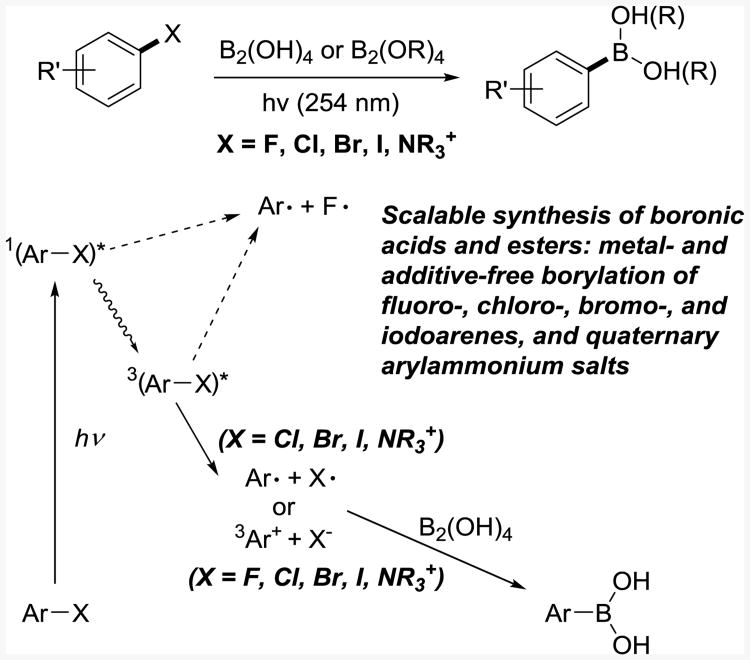
We report herein that haloarenes, including electron rich fluoroarenes, as well as aryltrimethylammonium salts readily react with tetrahydroxydiboron (first introduced by Molander and Dreher for the Pd-, and later Ni-catalyzed borylations24) and other diboron reagents to give aromatic boronic acids and esters under ultraviolet light in the absence of catalysts and additives in a scalable and practical manner in common grade solvents and without deoxygenation of the reaction mixtures (Figure 1).
Figure 1. Photoinduced borylation.
Initial experiments showed that a reaction of bromobenzene with tetrahydroxydiboron in methanol under ultraviolet irradiation (λ = 254 nm) produced phenylboronic acid (1) in 92% yield after 3 h at 15 °C. The optimal temperature was 10–20 °C, with increased amounts of benzene observed at higher temperatures. The reaction did not proceed in the dark (see Table S1 in the SI) and showed a good quantum yield (Φ = 0.34).
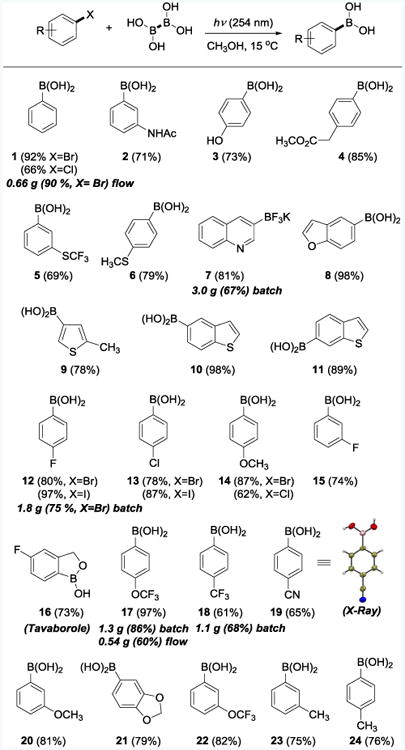
The preparative scope of the reaction was explored with a series of substituted bromoarenes. A diverse set of functional groups is well tolerated, including amide (2, 71%), phenol (3, 73%), arylacetate ester (4, 85%), thioether (5, 79% and 6, 69%), and other functionalities (7-25). Both electron-releasing and electron-withdrawing substituents can be very well accommodated. Nitrogen-, oxygen-, and sulfur-containing heteroarylboronic acids were also prepared in high yields, as shown for products 7–11. The boronic acids can be directly converted into the corresponding organotrifluoroborates as a part of the isolation procedure. For example, the borylation reaction of 3-bromoquinoline was followed by treatment of the reaction mixture with potassium difluoride25 to give the corresponding trifluoroborate 11 in 81% yield.
Iodoarenes and chloroarenes were also successfully converted to boronic acids. The reactions with iodoarenes were very fast and produced the boronic acids (12 and 13) in high yields within 1 h. Chloroarenes reacted slower than bromoarenes (66% of 1 and 62% of 14 after 24 h). These observations are in accord with the increase in the bond dissociation energies (BDE) of the C6H5–X bonds (C–I 272, C–Br 336, C–Cl 400 kJ·mol-1).26 The differential reactivity of C–X bonds can be used for the chemoselective and high-yielding synthesis of haloarylboronic acids, as shown for 12, 13, 15 and 16.
Boronic acids find increasing use in medicine,7 as exemplified by the fungal leucyl-tRNA synthetase inhibitor tavaborole (16, Table 1) that has recently been approved for treatment of onychomycosis.27 Using the photoinduced borylation, 16 was prepared from the corresponding bromoarene in 73% yield after a simple work-up. Tavaborole was previously prepared using air- and moisture-sensitive reagents (NaH, n-BuLi, and B(OiPr)3).28 The successful synthesis of 16 also indicates that an ortho-substituent is well-tolerated during the borylation event. Furthermore, good quantum efficiency of the borylation reaction enabled practical gram-scale syntheses of several representative boronic acids (12, 17, 18 on 1.1–1.8 g scales) and trifluoroborate 7 (3 g) using simple quartz test-tubes and low intensity UV sources without deoxygenation of the reaction solutions.
Table 1. Scope of the Photoinduced Reaction of Haloarenes with Tetrahydroxydiborona.
|
Bromoarenes were used unless specified otherwise. Reaction conditions for small scale experiments: haloarene (0.6–1 mmol), tetrahydroxydiboron (1–1.6 mmol), CH3OH (5–6 mL), 15 °C, 3–24 h, UV lamp (254 nm); then treatment with KHF2 for ArBF3K.
Preliminary experiments also show that the reaction can be translated to a continuous flow process (1, 90% and 17, 60%). Reaction mixtures typically remained homogeneous. Monitoring of the borylation reactions by 11B NMR indicated formation of trimethyl borate that may arise from solvolysis of B–X bond-containing inorganic byproducts of the reaction.
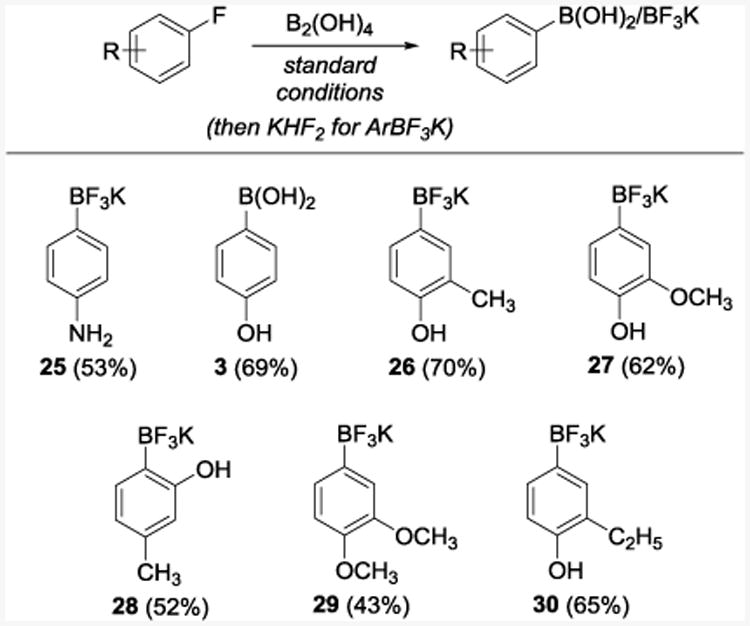
Fluorobenzene proved resistant to the borylation in line with the high BDE of the C6H5-F bond (526 kJ·mol-1)26 that exceeds the energy of absorbed light (469 kJ·mol-1). Remarkably, 4-fluoroaniline readily produced (4-aminophenyl)boronic acid that was isolated as trifluoroborate 25 in 53% yield, indicating that certain Ar–F bonds can be harnessed for the synthesis of boronic acids without the use of transition metals.29 Similarly, 4-fluorophenol reacted smoothly to give boronic acid 3 in 69% yield (Table 2). Other fluoroarenes bearing electron-releasing substituents are also competent substrates (26–30). The procedure is particularly suitable for borylation of unprotected fluoroanilines and fluorophenols that have so far eluded C–F borylation. The reactivity observed for 4-fluoroanline and other similar fluoroarenes in the photoinduced borylation reaction is consistent with the formation of the triplet aryl cation via the heterolysis of the C–F bond. This process is favored for the electron rich haloarenes and, unlike Ar–F homolysis, is greatly facilitated in polar protic solvents, as previously described by Albini, Fagnoni and Protti.30 Indeed, the yield of the reaction of 4-fluorophenol with B2pin2 was solvent-dependent.
Table 2. Borylation of Fluoroarenesa.
|
Reaction conditions: see footnote for Table 1.
No reaction was observed in hexane after 2 h, and only 5% of the borylation product was formed in acetonitrile, while 20% of the borylation product was produced in methanol. This observation is in line with the experimental and computational data reported for the photoinduced heterolysis of 4-fluorophenol.30 Although B2(OR)4 generally behave as electrophiles, complexation with Lewis bases (e.g., fluoride, alkoxides, N-heterocyclic carbenes, 4-methylpyridine, and phosphines)31 produces nucleophilic sp2-sp3 Lewis base–diboron adducts that react with organic electrophiles in the absence of transition metal catalysts.32 It is possible that the photoheterolytically-produced fluoride ion forms a transient nucleophilic sp2-sp3 B2(OR)4F- complex that then reacts with the triplet cation.33 Interestingly, B2pin2F- adduct was proposed as an intermediate in the borylation of arenediazonium salts,13c and [NMe4][B2pin2F] was subsequently shown to effect the diazonium borylation.31d The photoinduced borylation of fluoroarenes is the first example of metal-free and direct conversion of fluoroarenes to arylboronic acids.
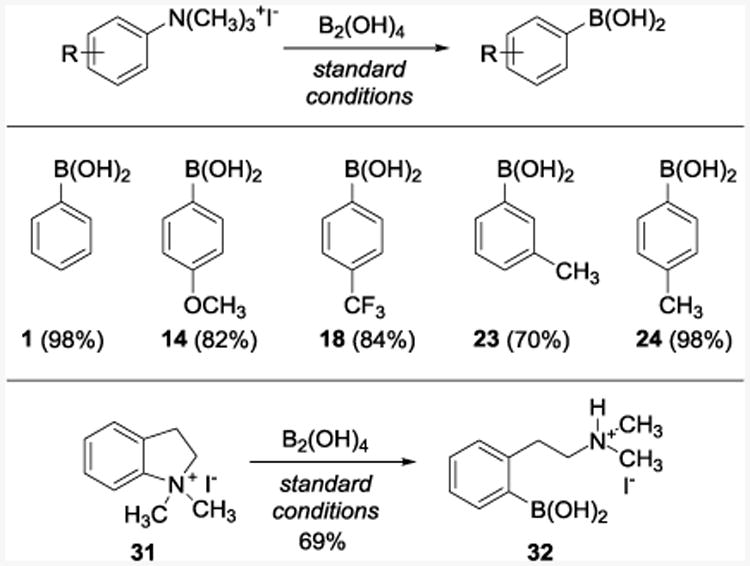
Encouraged by the outcome of the experiments with haloarenes, we turned our attention to the borylation of other carbon–heteroatom bonds. In particular, we focused on the Ar–N-borylation, since few methods are known for this transformation.13,34 Interestingly, aryltrimethylammonium iodides reacted rapidly and produced the corresponding boronic acids 1, 14, 18, 23, and 24 in 70–98% yields. In addition, ring scission of the cyclic salt 31 afforded boronic acid 32 in 69% yield (Table 3).
Table 3. Borylation of Quaternary Arylammonium Saltsa.
|
Reaction conditions: see footnote for Table 1.
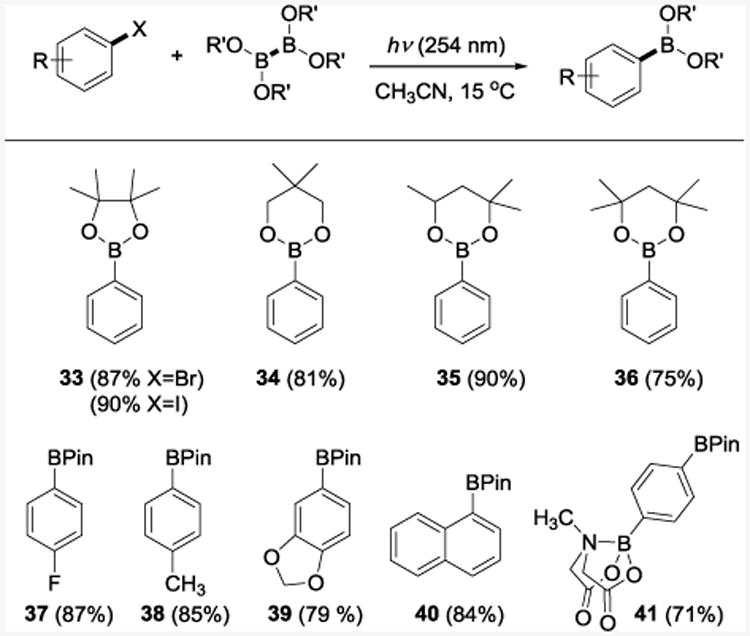
We next evaluated several diboron esters as counterparts in the reaction with haloarenes (Table 4). The borylation reactions with diboron esters derived from pinacol, neopentyl glycol, 1,1,3-trimethylethylene glycol, and 1,1,4,4,-tetramethylethylene glycol delivered the corresponding phenylboronic esters 33–36 in high yields using acetonitrile as a solvent. The reaction scope was further tested with several bromoarenes with varied substitution patterns. In all cases the corresponding borolanes 37–40 were produced in excellent yields without any noticeable phototransposition.35 Interestingly, the MIDA boronate ester 41 was also prepared, indicating that bifunctional borylated arenes can be efficiently accessed using this method.
Table 4. Photoinduced Reaction of Haloarenes with Diboronic Estersa.
|
Bromoarenes were used unless specified otherwise. Reaction conditions: haloarene (0.5–1 mmol), diboron ester (1–2 mmol), CH3CN (6 mL), 15 °C, 4–14 h, UV lamp (254 nm). Bpin = 4,4,5,5-tetramethyl-1,3,2-dioxaborolane.
Although further work is necessary to clarify the mechanism, it is possible that homolytic substitution36 in the diboron reagents and sp2-sp3 Lewis base–diboron adducts by a photogenerated aryl radical and aryl triplet cation can be responsible for the observed reactivity.33
In summary, this paper describes a simple, scalable, metal- and additive-free method of photoinduced borylation of haloarenes and quaternary arylammonium salts. The borylation produces aromatic boronic acids and esters in good to excellent yields under mild conditions and with formation of innocuous, easy-to-remove byproducts. The reaction is distinguished by a broad scope that includes electron-rich fluoroarenes.
Supplementary Material
Acknowledgments
Financial support by the Welch Foundation (AX-1788), the NSF (CHE-1455061), the Max and Minnie Tomerlin Voelcker Fund, and UTSA is gratefully acknowledged. Mass spectroscopic analysis was supported by a grant from the NIMHD (G12MD007591).
Footnotes
Supporting Information: Experimental and spectral details for all new compounds and all reactions reported. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes: The authors declare no competing financial interests.
References
- 1.(a) Suzuki A, Brown HC. Organic Syntheses Via Boranes. Vol. 3 Aldrich Chemical Company; Milwakee: 2003. [Google Scholar]; (b) Hall DG, editor. Boronic Acids. 2nd. Wiley-VCH; Weinheim: 2011. [Google Scholar]; (c) Gutekunst WR, Baran PS. Chem Soc Rev. 2011;40:1976–1991. doi: 10.1039/c0cs00182a. [DOI] [PubMed] [Google Scholar]
- 2.(a) (b) Corey EJ. Angew Chem, Int Ed. 2002;41:1650–1667. doi: 10.1002/1521-3773(20020517)41:10<1650::aid-anie1650>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]; (c) Dimitrijević E, Taylor MS. ACS Catal. 2013;3:945–962. [Google Scholar]; (d) Ishihara K. Top Organomet Chem. 2015;49:243–270. [Google Scholar]; (e) Taylor MS. Acc Chem Res. 2015;48:295–305. doi: 10.1021/ar500371z. [DOI] [PubMed] [Google Scholar]
- 3.(a) Fujita N, Shinkai S, James TD. Chem Asian J. 2008;3:1076–1091. doi: 10.1002/asia.200800069. [DOI] [PubMed] [Google Scholar]; (b) Mastalerz M. Angew Chem, Int Ed. 2010;49:5042–5053. doi: 10.1002/anie.201000443. [DOI] [PubMed] [Google Scholar]
- 4.(a) James TD, Shinkai S. Top Curr Chem. 2002;218:159–200. [Google Scholar]; (b) Jelinek R, Kolusheva S. Chem Rev. 2004;104:5987–6015. doi: 10.1021/cr0300284. [DOI] [PubMed] [Google Scholar]; (c) Pal A, Berube M, Hall DG. Angew Chem, Int Ed. 2010;49:1492–1495. doi: 10.1002/anie.200906620. [DOI] [PubMed] [Google Scholar]; (d) Hansena JS, Christensena JB, Petersena JF, Hoeg-Jensenb T, Norrild JC. Sens Actuators, B. 2012;161:45–79. [Google Scholar]
- 5.(a) Wade CR, Broomsgrove AEJ, Aldridge S, Gabbaï FP. Chem Rev. 2010;110:3958–3984. doi: 10.1021/cr900401a. [DOI] [PubMed] [Google Scholar]; (b) Wu X, Li Z, Chen XX, Fossey JS, James TD, Jiang YB. Chem Soc Rev. 2013;42:8032–8048. doi: 10.1039/c3cs60148j. [DOI] [PubMed] [Google Scholar]; (c) Guan Y, Zhang Y. Chem Soc Rev. 2013;42:8106–8121. doi: 10.1039/c3cs60152h. [DOI] [PubMed] [Google Scholar]; (d) You L, Zha D, Anslyn EV. Chem Rev. 2015;115:7840–7892. doi: 10.1021/cr5005524. [DOI] [PubMed] [Google Scholar]; (e) Wu J, Kwon B, Liu W, Anslyn EV, Wang P, Kim JS. Chem Rev. 2015;115:7893–7943. doi: 10.1021/cr500553d. [DOI] [PubMed] [Google Scholar]
- 6.(a) Lorbach A, Huebner A, Wagner M. Dalton Trans. 2012;41:6048–6063. doi: 10.1039/c2dt30118k. [DOI] [PubMed] [Google Scholar]; (b) Liu J, Lavigne JJ. In: Boronic Acids. 2nd. Hall DG, editor. Wiley-VCH; Weinheim: 2011. [Google Scholar]; (c) Jäkle F. Top Organomet Chem. 2015;49:297–325. [Google Scholar]
- 7.(a) Trippier PC, McGuigan C. Med Chem Commun. 2010;1:183–198. [Google Scholar]; (b) Yang W, Gao W, Wang B. Biological and Medicinal Applications of Boronic Acids. In: Hall DG, editor. Boronic Acids. Wiley-VCH; Weinheim: 2011. [Google Scholar]; (c) Ban HS, Nakamura H. Chem Rec. 2015;15:616–635. doi: 10.1002/tcr.201402100. [DOI] [PubMed] [Google Scholar]
- 8.Khotinsky E, Melamed M. Ber Dtsch Chem Ges. 1909;42:3090–3096. [Google Scholar]
- 9.Letsinger RL, Skoog IH. J Org Chem. 1953;18:895–897. [Google Scholar]
- 10.(a) Ishiyama T, Murata M, Miyaura N. J Org Chem. 1995;60:7508–7510. [Google Scholar]; (b) Murata M, Watanabe S, Masuda Y. J Org Chem. 1997;62:6458–6459. doi: 10.1021/jo971143f. [DOI] [PubMed] [Google Scholar]; (c) Ishiyama T, Miyaura N. J Organomet Chem. 2000;611:392–402. [Google Scholar]; (d) Ishiyama T, Miyaura N. In: Boronic Acids. 2nd. Hall DG, editor. Wiley-VCH; Weinheim: 2011. [Google Scholar]; (e) Chow WK, Yuen OY, Choy PY, So CM, Lau CP, Wong WT, Kwong FY. RSC Adv. 2013;3:12518–12539. [Google Scholar]
- 11.(a) Cho JY, Tse MK, Holmes D, Maleczka RE, Smith MR. Science. 2002;295:305–308. doi: 10.1126/science.1067074. [DOI] [PubMed] [Google Scholar]; (b) Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem Rev. 2010;110:890–931. doi: 10.1021/cr900206p. [DOI] [PubMed] [Google Scholar]; (c) Hartwig JF. Acc Chem Res. 2012;45:864–873. doi: 10.1021/ar200206a. [DOI] [PubMed] [Google Scholar]; (d) Stahl T, Muether K, Ohki Y, Tatsumi K, Oestreich M. J Am Chem Soc. 2013;135:10978–10981. doi: 10.1021/ja405925w. [DOI] [PubMed] [Google Scholar]
- 12.(a) Yamamoto E, Izumi K, Horita Y, Ito H. J Am Chem Soc. 2012;134:19997–20000. doi: 10.1021/ja309578k. [DOI] [PubMed] [Google Scholar]; (b) Nagashima Y, Takita R, Yoshida K, Hirano K, Uchiyama M. J Am Chem Soc. 2013;135:18730–18733. doi: 10.1021/ja409748m. [DOI] [PubMed] [Google Scholar]; (c) Zhang J, Wu HH, Zhang J. Eur J Org Chem. 2013:6263–6266. [Google Scholar]; (d) Yamamoto E, Izumi K, Horita Y, Ukigai S, Ito H. Top Catal. 2014;57:940–945. [Google Scholar]; (e) Bose SK, Marder TB. Org Lett. 2014;16:4562–4565. doi: 10.1021/ol502120q. [DOI] [PubMed] [Google Scholar]; (f) Yamamoto E, Ukigai S, Ito H. Chem Sci. 2015;6:2943–2951. doi: 10.1039/c5sc00384a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Uematsu R, Yamamoto E, Maeda S, Ito H, Taketsugu T. J Am Chem Soc. 2015;137:4090–4099. doi: 10.1021/ja507675f. [DOI] [PubMed] [Google Scholar]; (h) Bose SK, Deissenberger A, Eichhorn A, Steel PG, Lin Z, Marder TB. Angew Chem, Int Ed. 2015;54:11843–11847. doi: 10.1002/anie.201505603. [DOI] [PubMed] [Google Scholar]
- 13.(a) Mo F, Jiang Y, Qiu D, Zhang Y, Wang J. Angew Chem, Int Ed. 2010;49:1846–1849. doi: 10.1002/anie.200905824. [DOI] [PubMed] [Google Scholar]; (b) Yu J, Zhang L, Yan G. Adv Synth Catal. 2012;354:2625–2628. [Google Scholar]; (c) Zhu C, Yamane M. Org Lett. 2012;14:4560–4563. doi: 10.1021/ol302024m. [DOI] [PubMed] [Google Scholar]; (d) Qiu D, Jin L, Zheng Z, Meng H, Mo F, Wang X, Zhang Y, Wang J. J Org Chem. 2013;78:1923–1933. doi: 10.1021/jo3018878. [DOI] [PubMed] [Google Scholar]; (e) Qiu D, Zhang Y, Wang J. Org Chem Front. 2014;1:422–425. [Google Scholar]; (f) Erb W, Hellal A, Albini M, Rouden J, Blanchet J. Chem Eur J. 2014;20:6608–6612. doi: 10.1002/chem.201402487. [DOI] [PubMed] [Google Scholar]; (g) Zhao CJ, Xue D, Jia ZH, Wang C, Xiao J. Synlett. 2014;25:1577–1584. [Google Scholar]
- 14.(a) De Vries TS, Prokofjevs A, Vedejs E. Chem Rev. 2012;112:4246–4282. doi: 10.1021/cr200133c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ingleson MJ. Synlett. 2012;23:1411–1415. [Google Scholar]; (c) Ingleson MJ. Top Organomet Chem. 2015;49:39–71. [Google Scholar]; (d) Warner AJ, Lawson JR, Fasano V, Ingleson MJ. Angew Chem, Int Ed. 2015;54:11245–11249. doi: 10.1002/anie.201505810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Schutt L, Bunce NG. In: CRC Handbook of Organic Photochemistry and Photobiology. 2nd. Horspool WM, Lenci F, editors. CRC Press; Boca Raton: 2004. [Google Scholar]; (b) Albini A, Fagnoni M, editors. Handbook of Synthetic Photochemistry. Wiley-VCH; Weinheim: 2010. [Google Scholar]; (c) Albini A, Fagnoni M. Photochemically-Generated Intermediates in Synthesis. John Wiley & Sons, Inc; Hoboken: 2013. [Google Scholar]
- 16.(a) Pape M. Pure Appl Chem. 1975;41:535–558. [Google Scholar]; (b) Weissermal K, Arpe HJ. Industrial Organic Chemistry. 3th. VCH; Weinheim: 1997. p. 254. [Google Scholar]
- 17.Ravelli D, Protti S, Neri P, Fagnoni M, Albini A. Green Chem. 2011;13:1876–1884. [Google Scholar]
- 18.(a) Zhu GD, Okamura WH. Chem Rev. 1995;95:1877–1952. [Google Scholar]; (b) Labler L. Ullmann's Encyclopedia of Industrial Chemistry. 7th. Wiley-VCH; Weinheim: 2011. Vitamins 3, Vitamin D. [Google Scholar]
- 19.For recent examples, see: Yoshimura A, Takamachi Y, Han LB, Ogawa A. Chem Eur J. 2015;21:1–5. doi: 10.1002/chem.201502425.Brimioulle R, Bauer A, Bach T. J Am Chem Soc. 2015;137:5170–5176. doi: 10.1021/jacs.5b01740.Mukhina OA, Kutateladze AG. J Am Chem Soc. 2016 doi: 10.1021/jacs.5b12690.
- 20.(a) Bunnett JF. Acc Chem Res. 1978;11:413–420. [Google Scholar]; (b) Karapire C, Siddik I. In: CRC Handbook of Organic Photochemistry and Photobiology. 2nd. Horspool WM, Lenci F, editors. CRC Press; Boca Raton: 2004. [Google Scholar]; (c) Uyeda C, Tan YC, Fu GC, Peters JC. J Am Chem Soc. 2013;135:9548–9552. doi: 10.1021/ja404050f. [DOI] [PubMed] [Google Scholar]; (d) Tan YC, Munoz-Molina JM, Fu GC, Peters JC. Chem Sci. 2014;5:2831–2835. [Google Scholar]; (e) Li L, Liu W, Zeng H, Mu X, Cosa G, Mi Z, Li CJ. J Am Chem Soc. 2015;137:8328–8331. doi: 10.1021/jacs.5b03220. [DOI] [PubMed] [Google Scholar]
- 21.(a) Dichiarante V, Fagnoni M, Albini A. Angew Chem Int Ed. 2007;46:6495–6498. doi: 10.1002/anie.200701462. [DOI] [PubMed] [Google Scholar]; (b) Buden ME, Guastavino JF, Rossi RA. Org Lett. 2013;15:1174–1177. doi: 10.1021/ol3034687. [DOI] [PubMed] [Google Scholar]; (c) Ruch J, Aubin A, Erbland G, Fortunato A, Goddard JP. Chem Comm. 2016;52:2326–2329. doi: 10.1039/c5cc08927a. [DOI] [PubMed] [Google Scholar]
- 22.(a) Mella M, Coppo P, Guizzardi B, Fagnoni M, Freccero M, Albini A. J Org Chem. 2001;66:6344–6352. doi: 10.1021/jo010469s. [DOI] [PubMed] [Google Scholar]; (b) Guizzardi B, Mella M, Fagnoni M, Albini A. Chem Eur J. 2003;9:1549–1555. doi: 10.1002/chem.200390178. [DOI] [PubMed] [Google Scholar]; (c) Zheng XL, Yang L, Du WY, Ding AS, Guo H. Chem - Asian J. 2014;9:439–442. doi: 10.1002/asia.201301371. [DOI] [PubMed] [Google Scholar]
- 23.(a) Grimshaw J, de Silva AP. Chem Soc Rev. 1981;10:181–203. [Google Scholar]; (b) Park YT, Song NW, Hwang CG, Kim KW, Kim D. J Am Chem Soc. 1997;119:10677–10683. [Google Scholar]; (c) Lu SC, Zhang XX, Shi ZJ, Ren YW, Li B, Zhang W. Adv Synth Cat. 2009;351:2839–2844. [Google Scholar]
- 24.For Pd- and Ni-catalyzed borylation with tetrahydroxydiboron, see: Molander GA, Trice SLJ, Dreher SD. J Am Chem Soc. 2010;132:17701–17703. doi: 10.1021/ja1089759.Molander GA, Trice SLJ, Kennedy SM, Dreher SD, Tudge MT. J Am Chem Soc. 2012;134:11667–11673. doi: 10.1021/ja303181m.Molander GA, Trice SLJ, Kennedy SM. J Org Chem. 2012;77:8678–8688. doi: 10.1021/jo301642v.Molander GA, Trice SL, Kennedy SM. Org Lett. 2012;14:4814–4817. doi: 10.1021/ol302124j.Molander GA, Cavalcanti LN, Garcia-Garcia C. J Am Chem Soc. 2013;78:6427–6439. doi: 10.1021/jo401104y.
- 25.(a) Vedejs E, Chapman RW, Fields SC, Lin S, Schrimpf MR. J Org Chem. 1995;60:3020–3027. [Google Scholar]; (b) Molander GA, Ellis N. Acc Chem Res. 2007;40:275–286. doi: 10.1021/ar050199q. [DOI] [PubMed] [Google Scholar]; (c) Darses S, Genet JP. Chem Rev. 2008;108:288–325. doi: 10.1021/cr0509758. [DOI] [PubMed] [Google Scholar]; (d) Molander GA, Jean-Gérard L. In: Boronic Acids. 2nd. Hall DG, editor. Wiley-VCH; Weinheim: 2011. [Google Scholar]
- 26.Luo YR. Comprehensive Handbook of Chemical Bond Energies. CRC Press; Boca Raton, FL: 2007. [Google Scholar]
- 27.(a) Baker SJ, Zhang YK, Akama T, Lau A, Zhou H, Hernandez V, Mao W, Alley MRK, Sanders V, Plattner JJ. J Med Chem. 2006;49:4447–4450. doi: 10.1021/jm0603724. [DOI] [PubMed] [Google Scholar]; (b) Rock FL, Mao W, Yaremchuk A, Tukalo M, Crépin T, Zhou H, Zhang YK, Hernandez V, Akama T, Baker SJ, Plattner JJ, Shapiro L, Martinis SA, Benkovic SJ, Cusack S, Alley MRK. Science. 2007;316:1759–1761. doi: 10.1126/science.1142189. [DOI] [PubMed] [Google Scholar]
- 28.Gunasekera DS, Gerold DJ, Aalderks NS, Chandra JS, Maanu CA, Kiprof P, Zhdankin VV, Reddy MVR. Tetrahedron. 2007;63:9401–9405. [Google Scholar]
- 29.For Ni-catalyzed borylation of fluoroarenes, see: Liu XW, Echavarren J, Zarate C, Martin R. J Am Chem Soc. 2015;137:12470–12473. doi: 10.1021/jacs.5b08103.Niwa T, Ochiai H, Watanabe Y, Hosoya T. J Am Chem Soc. 2015;137:14313–14318. doi: 10.1021/jacs.5b10119.
- 30.(a) Freccero M, Fagnoni M, Albini A. J Am Chem Soc. 2003;125:13182–13190. doi: 10.1021/ja036000r. [DOI] [PubMed] [Google Scholar]; (b) Fagnoni M, Albini A. Acc Chem Res. 2005;38:713–721. doi: 10.1021/ar0402356. [DOI] [PubMed] [Google Scholar]; (c) Dichiarante V, Fagnoni M. Synlett. 2008:787–800. [Google Scholar]; (d) Raviola C, Ravelli D, Protti S, Albini A, Fagnoni M. Synlett. 2015;26:471–478. [Google Scholar]
- 31.(a) Nguyen P, Dai C, Taylor NJ, Power WP, Marder TB, Pickett NL, Norman NC. Inorg Chem. 1995;34:4290–4291. [Google Scholar]; (b) Clegg W, Dai C, Lawlor FJ, Marder TB, Nguyen P, Norman NC, Pickett NL, Power WP, Scott AJ. J Chem Soc Dalton Trans. 1997:839–846. [Google Scholar]; (c) Kleeberg C, Crawford AG, Batsanov AS, Hodgkinson P, Apperley DC, Cheung MS, Lin Z, Marder TB. J Org Chem. 2012;77:785–789. doi: 10.1021/jo202127c. [DOI] [PubMed] [Google Scholar]; (d) Pietsch S, Neeve EC, Apperley DC, Bertermann R, Mo F, Qiu D, Cheung MS, Dang L, Wang J, Radius U, Lin Z, Kleeberg C, Marder TB. Chem Eur J. 2015;21:7082–7098. doi: 10.1002/chem.201500235. [DOI] [PubMed] [Google Scholar]
- 32.(a) Lee KS, Zhugralin AR, Hoveyda AH. J Am Chem Soc. 2009;131:7253–7255. doi: 10.1021/ja902889s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bonet A, Gulyas H, Fernandez E. Angew Chem, Int Ed. 2010;49:5130–5134. doi: 10.1002/anie.201001198. [DOI] [PubMed] [Google Scholar]; (c) Bonet A, Pubill-Ulldemolins C, Bo C, Gulyas H, Fernandez E. Angew Chem, Int Ed. 2011;50:7158–7161. doi: 10.1002/anie.201101941. [DOI] [PubMed] [Google Scholar]; (d) Wu H, Radomkit S, O'Brien JM, Hoveyda AH. J Am Chem Soc. 2012;134:8277–8285. doi: 10.1021/ja302929d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Bonet A, Sole C, Gulyas H, Fernandez E. Org Biomol Chem. 2012;10:6621–6623. doi: 10.1039/c2ob26079d. [DOI] [PubMed] [Google Scholar]; (f) Sanz X, Lee GM, Pubill-Ulldemolins C, Bonet A, Gulyas H, Westcott SA, Bo C, Fernandez E. Org Biomol Chem. 2013;11:7004–7010. doi: 10.1039/c3ob41328d. [DOI] [PubMed] [Google Scholar]; (g) Palau-Lluch G, Sanz X, La Cascia E, Civit MG, Miralles N, Cuenca AB, Fernandez E. Pure Appl Chem. 2015;87:181–193. [Google Scholar]; (h) Dewhurst RD, Neeve EC, Braunschweig H, Marder TB. Chem Comm. 2015;51:9594–9607. doi: 10.1039/c5cc02316e. [DOI] [PubMed] [Google Scholar]
- 33.See Scheme S1 and further discussion in the SI.
- 34.(a) Tobisu M, Nakamura K, Chatani N. J Am Chem Soc. 2014;136:5587–5590. doi: 10.1021/ja501649a. [DOI] [PubMed] [Google Scholar]; (b) Hu J, Sun H, Cai W, Pu X, Zhang Y, Shi Z. J Org Chem. 2016;81:14–24. doi: 10.1021/acs.joc.5b02557. [DOI] [PubMed] [Google Scholar]
- 35.Cameron KS, Pincock AL, Pincock JA, Thompson A. J Org Chem. 2004;69:4954–4960. doi: 10.1021/jo040123s. [DOI] [PubMed] [Google Scholar]
- 36.(a) Davies AG, Roberts BP, Scaiano JC. J Chem Soc B. 1971:2171–2176. [Google Scholar]; (b) Nozaki K, Oshima K, Utimoto K. Bull Chem Soc Jpn. 1991;64:403–409. [Google Scholar]; (c) Brown HC, Midland MM. Angew Chem, Int Ed Engl. 1972;11:692–700. [Google Scholar]; (d) Ollivier C, Renaud P. Chem Rev. 2001;101:3415–3434. doi: 10.1021/cr010001p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.