Abstract
Vascular tumors are among the most common neoplasms in infants and children; 5%–10% of newborns present with or develop lesions within the first 3 months of life. Most are benign infantile hemangiomas that typically regress by 5 years of age; other vascular tumors include congenital tufted angiomas (TAs), kaposiform hemangioendotheliomas (KHEs), and childhood lobular capillary hemangiomas (LCHs). Some of these lesions can become locally invasive and unresponsive to pharmacologic intervention, leading to significant complications. Recent investigation has revealed that activating mutations in HRAS, KRAS, NRAS, GNAQ, and GNA11 can cause certain types of rare childhood vascular tumors, and we have now identified causal recurrent somatic activating mutations in GNA14 by whole-exome and targeted sequencing. We found somatic activating GNA14 c.614A>T (p.Gln205Leu) mutations in one KHE, one TA, and one LCH and a GNA11 c.547C>T (p.Arg183Cys) mutation in two LCH lesions. We examined mutation pathobiology via expression of mutant GNA14 or GNA11 in primary human endothelial cells and melanocytes. GNA14 and GNA11 mutations induced changes in cellular morphology and rendered cells growth-factor independent by upregulating the MAPK pathway. Our findings identify GNA14 mutations as a cause of childhood vascular tumors, offer insight into mechanisms of oncogenic transformation by mutations affecting Gaq family members, and identify potential targets for therapeutic intervention.
Main Text
Childhood vascular tumors are a heterogeneous group of lesions. Most are benign infantile hemangiomas (IHs [MIM: 602089]); other entities include congenital tufted angiomas (TAs [MIM: 607859]), kaposiform hemangioendotheliomas (KHEs [MIM: 141000]), and childhood lobular capillary hemangiomas (LCHs [MIM: 140850]), also known as pyogenic granulomas.1 Congenital hemangiomas are divided into rapidly involuting (RICH [MIM: 602089]) and non-involuting (NICH [MIM: 602089]) subtypes and, along with TAs, LCHs, and KHEs, are distinguished from IHs by their lack of glucose transporter 1 (GLUT-1) immunoreactivity.1, 2 These lesions can rarely be associated with Kasabach-Merritt syndrome (MIM: 141000), a potentially fatal complication characterized by consumptive thrombocytopenia and coagulopathy.3 Diagnosis of vascular tumors remains challenging given the histological overlap, and although beta-blockers and steroids are efficacious for a large number of lesions, serious side effects including hypotension, hypoglycemia, and bradycardia can be encountered.4, 5, 6, 7 Surgery remains the most effective intervention for refractory lesions.
Postzygotic somatic mutations have been found in specific classes of vascular tumors: activating mutations in HRAS (HRas proto-oncogene, GTPase [MIM: 190020]), KRAS (KRAS proto-oncogene, GTPase [MIM:190070]), and NRAS (neuroblastoma RAS viral oncogene homolog [MIM: 164790]) and downstream effectors, including BRAF (B-Raf proto-oncogene, serine/threonine kinase [MIM: 164757]), are found in NICHs and in up to 10% of sporadic LCHs.8, 9 Recently, we and others discovered that activating mutations in GNA11 (G protein subunit alpha 11 [MIM: 600998]) and GNAQ (G protein subunit alpha q [MIM: 139313]) are present in RICHs, NICHs, and placental chorangiocarcinomas.10, 11 Other vascular lesions, including port-wine stains (PWSs [MIM: 163000]) and Sturge-Weber syndrome (SWS [MIM: 185300]), harbor GNAQ mutations, and changes in residue Arg183 are consistently present in lesions from individuals with SWS.12, 13 Activated GNA11 and GNAQ mediate VEGFR2 phosphorylation in human umbilical vein endothelial cells (HUVECs), inducing cell proliferation.14, 15 GNA11 and GNAQ mutations leading to p.Gln209Pro (c.626A>C) and p.Arg183Cys (c.547C>T) changes are also detected in nearly 50% of primary uveal melanomas, in 83% of blue nevi, and in affected tissues of the mosaic disorder phakomatosis pigmentovascularis, which features capillary malformations, dermal melanocytosis, nevus spilus, and nevus of Ota.16, 17
We studied a cohort of congenital vascular tumors without evidence of GLUT-1 immunoreactivity, which included four TAs and three KHEs. TAs and KHEs share clinical and histological features and are considered to be part of the same neoplastic spectrum.18 In addition, we examined 21 sporadic LCHs arising in childhood. The study protocol was approved by the Yale Human Investigation Committee, and subjects’ written consent was obtained prior to participation. Genomic DNA was isolated from lesions or unaffected epidermis via laser capture microdissection and then extracted with the DNeasy Blood & Tissue Kit (QIAGEN). DNA from blood or saliva was obtained via a standard phenol-chloroform protocol. All lesions were screened via Sanger sequencing for mutations across all exons of HRAS, KRAS, NRAS, BRAF, GNAQ, and GNA11.
An identical somatic GNA11 mutation, c.547C>T (p. Arg183Cys) (GenBank: NM_002067.4), was found in two LCHs (2/21 [10%]; subjects V104 and V105; Table 1 and Figure S1). This mutation has not been previously identified in vascular tumors, although somatic mutation c.548G>A (p.Arg183Gln) in paralog GNAQ is common in capillary malformations and PWSs and has been found in a single case of secondary LCH arising within a PWS.8, 12, 19 A recent survey of 16 congenital hemangiomas identified 12/16 (75%) lesions with GNA11 and GNAQ mutations, but all were Pro or Leu substitutions at Gln209.10
Table 1.
Somatic GNA14 and GNA11 Mutations Cause Vascular Tumors
| Sample | Age (Years) | Sex | Site | Diagnosis | Somatic Mutation |
No. of Reads in Tissue |
No. of Reads in Blood |
p Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Ref. | Non-ref. | Ref. | Non-ref. | |||||||
| V101 | 0 | male | neck | KHE | GNA14 c.614A>T (p.Gln205Leu) | 81 | 8 | 96 | 0 | 2.4 × 10−3 |
| V102 | 0 | male | abdomen | TA | GNA14 c.614A>T (p.Gln205Leu) | NAa | NAa | NAa | NAa | NA |
| V103 | 2 | male | cheek | LCH | GNA14 c.614A>T (p.Gln205Leu) | NAa | NAa | NAa | NAa | NA |
| V104 | 12 | male | scalp | LCH | GNA11 c.547C>T (p.Arg183Cys) | 55 | 16 | NAb | NAb | NA |
| V105 | 2 | male | cheek | LCH | GNA11 c.547C>T (p.Arg183Cys) | NAa | NAa | NAa | NAa | NA |
Abbreviations are as follows: KHE, kaposiform hemangioendothelioma; LCH, lobular capillary hemangioma; NA, not applicable; non-ref., non-reference; ref., reference; and TA, tufted angioma.
Mutation identified by Sanger sequencing.
Exome sequencing performed on tumor only.
We further analyzed subjects without a detected mutation. In these subjects, we employed whole-exome sequencing (WES), performed by the Yale Center for Genome Analysis, by using barcoded libraries from sheared genomic DNA (Table S1). We pooled tumor DNA to run four barcoded samples per lane and pooled blood samples to run six barcoded samples per lane and ran all on the Illumina HiSeq 2000 with 75 bp paired-end reads. Using the Burrows-Wheeler Aligner (BWA-MEM), we aligned reads to the hg19 human reference sequence (UCSC Genome Browser) and trimmed them to targeted intervals with Picard.20, 21 We employed a Perl script to remove PCR duplicates, recalibrated the resulting BAM files according to the Genome Analysis Toolkit (GATK) Best Practices, and called blood and tissue variants with the GATK Haplotype Caller.21 We used MuTect to identify all single-nucleotide variants (SNVs), deletions, and insertions and used ANNOVAR to annotate all variants for functional impact.22, 23 Data were filtered for damaging mutations (missense and nonsense mutations, splice sites, and indels) that (1) had at least three non-reference reads in tissue and zero, one, or two non-reference reads in blood and (2) were absent in 1000 Genomes (May 2011 release), the National Heart, Lung, and Blood Institute (NHLBI) Exome Sequencing Project (ESP) Exome Variant Server (release ESP6500), and the Exome Aggregation Consortium (ExAC) Browser control set of >134,000 exomes (v.0.3). We used a one-tailed Fisher’s exact test to compare blood and tissue read numbers and excluded variants with a p value greater than 1.0 × 10−2 (Table S2). We examined the remaining mutations with the Broad Institute Integrative Genomics Viewer (IGV) to screen for variants resulting from alignment error.24
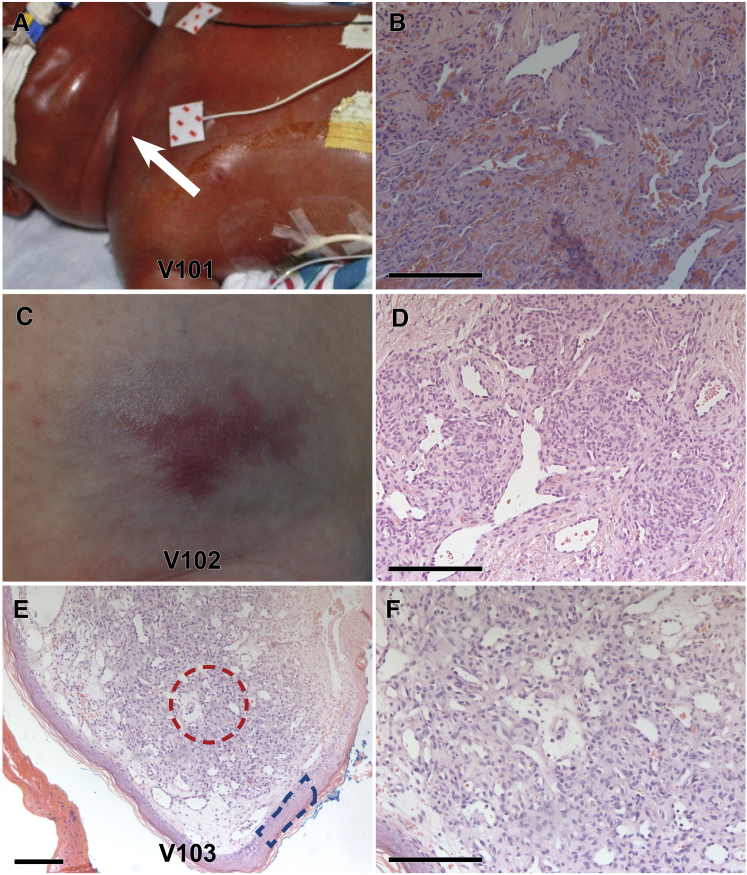
Our first subject, V101, was a male infant who had a diffuse right supraclavicular mass complicated by Kasabach-Merritt syndrome, which was identified as a KHE by histologic examination (Figure 1).25 WES identified in GNA14 (G protein subunit alpha 14 [MIM: 604397]) a single heterozygous somatic SNV, c.614A>T (p.Gln205Leu), which was confirmed by Sanger sequencing (Table 1 and Figure S2). There was no evidence of copy-number variation, loss of heterozygosity, or secondary mutation (Figure S2). Subsequent Sanger sequencing of all seven exons of GNA14 in the remaining two KHEs, four TAs, and 19 LCHs without mutations in HRAS, KRAS, NRAS, BRAF, GNAQ, or GNA11 identified the same heterozygous GNA14 c.614A>T (p.Gln205Leu) somatic mutation (GenBank: NM_004297.3) in one TA (V102; Figure 1, Table 1, and Figure S2) and one sporadic LCH (V103; Figure 1, Table 1, and Figure S2). V102 was a male infant who presented at 5 weeks of age with a 5.5 × 3.5 cm, firm, reddish-blue abdominal lesion with prominent draining vessels. Doppler ultrasound revealed fast-flow and high-resistance vascular signals, and a punch biopsy diagnosed the lesion as a TA. By 26 months of age, the lesion had markedly regressed and appeared as a slightly depressed, atrophic telangiectatic patch. V103 was an archival specimen of a sporadic LCH from the cheek of a 2-year-old boy. Our discovery of three independent lesions with a recurrent GNA14 mutation identifies GNA14 as a driver of childhood vascular tumors (Table 1).
Figure 1.
Somatic Activating Mutations in GNA14 Cause Congenital and Sporadic Vascular Tumors
(A–D) Subjects V101 (A and B) and V102 (C and D) presented with a KHE (A, white arrow) and a TA, respectively. (B and D) Histology at 20× magnification demonstrates infiltrating nodules of spindled endothelial cells and intervening fibrous bands.
(E and F) 10× (E) and 20× (F) histology of V103, a sporadic lobular capillary hemangioma from the cheek of a 2-year-old male, demonstrates encapsulated lobules of proliferating vessels.
For samples where blood was unavailable (V102 and V103), sequencing was compared between laser capture microdissection of unaffected epidermis (E, blue box) and tumor (E, red circle). Scale bars represent 150 μm.
GNA14, GNA11, and GNAQ encode Gα subunits that form a heterotrimer with Gβ and Gγ subunits and bind to the cytosolic side of inactive seven-transmembrane G-protein-coupled receptors (GPCRs).26, 27 GPCRs bind a diverse array of ligands that activate the receptor, promoting the exchange of bound GDP to GTP on the Gα subunit. This facilitates the release of the G protein heterotrimer from the GPCR, as well as the dissociation of the activated Gα subunit from the β/γ dimer, leading to downstream activation of phospholipases, calcium channels, and other cellular pathways, including AKT and MAPK.26, 27
Given that α subunit hydrolysis of GTP initiates the signaling cascade upon interaction with the GPCR, the properties of the α subunit define the functional characteristics of G proteins.28 The Gα subunits are divided into four main families according to sequence homology: Gαs, Gαq, Gαi, and Gα12 and Gα13.29 Multiple members are within each family, and GNA14, GNA11, GNAQ, GNA15, and GNA16 belong to the Gαq family. Protein alignment of GNA14 to GNA11 and GNAQ shows close conservation, such that GNA14 Gln205 aligns with Gln209 of GNA11 and GNAQ and GNA14 Arg179 aligns with Arg183 of GNA11 and GNAQ, suggesting that mutations affecting GNA14 Arg179 could also cause vascular tumors (Figure S3). Of the 78 GNA14 mutations listed in the Catalogue of Somatic Mutations in Cancer (COSMIC), one (c.536G>A [p.Arg179Gln]) was reported in malignant melanoma.30
To assess the molecular consequence of GNA14 and GNA11 mutations, we used Phyre231 to perform structural modeling for GNA14 and GNA11 and visualized them in Chimera32 (Figure S4). Using an established structure of GNAQ as a template,33 we inferred the structure of the GNA14 and GNA11 GTPase domain, which shares 92% homology with that of GNAQ (BLASTp). This revealed that all mutated residues fall at positions necessary for stabilizing the interaction between the GTPase catalytic domain and GTP. The GNAQ amide group of Arg183, which corresponds to Arg179 of GNA14 and Arg183 of GNA11, stabilizes the leaving γ-phosphate to facilitate hydrolysis.34 GNAQ Gln209, corresponding to Gln205 of GNA14 and Gln209 of GNA11, lies in the switch II region and coordinates the critical nucleophilic water moiety responsible for hydrolysis of the γ-phosphate.28, 34 Compared to their native states, GNA14 p.Arg179Cys and GNA11 p.Arg183Cys increase the molecular distance of the side chains from the γ-phosphate by 3.766 and 3.002 Å, respectively, whereas GNA14 p.Gln205Leu and GNA11 p.Gln209Pro are moved away from the water molecule by 5.016 and 2.862 Å, respectively, destabilizing hydrolysis (Figure S4). Both substitutions are thus expected to generate a constitutively active GTP-bound subunit.28, 34
To examine the pathobiology of the GNA14 and GNA11 mutations causing vascular tumors, we performed lentiviral transduction of primary HUVECs and primary newborn human melanocytes (NBMELs). Given the prevalence of GNA11 and GNAQ mutations in uveal melanoma, we considered melanocytes an important comparator. NBMELs were isolated from human foreskin as previously described,35 and HUVECs were purchased from the Yale Vascular Biology and Therapeutics Core and maintained in EGM-2 (Lonza). To analyze mutations identified within our cohort and those predicted to be damaging via comparison of amino acid conservation at mutated sites between GNA14 and GNA11, we generated wild-type GNA14 and GNA11, GNA14 c.614A>T (p.Gln205Leu), GNA14 c.[535C>T; 537A>T] (p.Arg179Cys), GNA11 c.626A>C (p.Gln209Pro), and GNA11 c.547C>T (p.Arg183Cys) via the QuickChange Site-Directed Mutagenesis Kit (Agilent) in the pLVX-Puro lentiviral expression vector (Clontech). Lentiviral particles were packaged in 293T cells with ViraPower Lentiviral Packaging Mix (Thermo Fisher Scientific) according to the manufacturer’s instructions and concentrated by centrifugation with the Amicon Ultra-15 Centrifugal Filter Unit with Ultracel-100 (UFC910024, EMD Millipore) prior to adsorption. After 2 days, the growth medium was supplemented with puromycin at 5 μg/ml (NBMELs) or 2 μg/ml (HUVECs) for 72 hr. Afterwards, NBMELs and HUVECs were maintained at a lower puromycin concentration of 1 and 0.5 μg/ml, respectively.
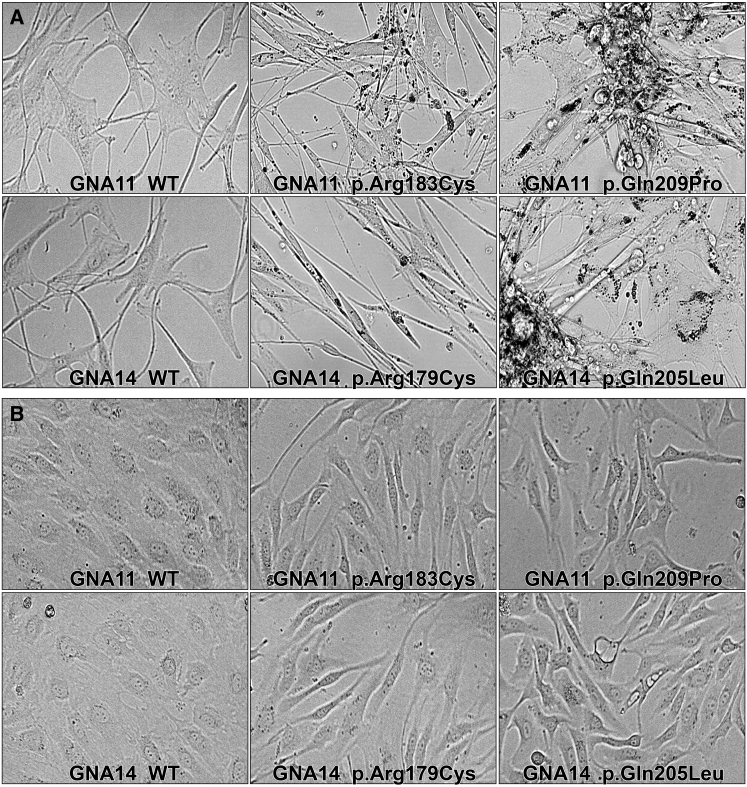
Less than 72 hr after transduction, both HUVECs and NBMELs expressing GNA14 or GNA11 mutations demonstrated dramatic changes in morphology in comparison to wild-type controls (Figure 2). NBMELs with GNA11 p.Arg183Cys and GNA14 p.Arg179Cys became elongated, whereas those with GNA11 p.Gln209Pro and GNA14 p.Gln205Leu grew over each other in piles. Further, large black granules surrounding the nucleus were noted in all mutant NBMELs (Figure 2). Electron microscopy with a FEI Tecnai transmission electron microscope at 80 kV accelerating voltage revealed these foci to be large vacuolar structures resembling autophagosomes containing degraded melanin or melanosomes; indeed, cell pellets of NBMELs with GNA14 and GNA11 mutations were lighter in color than those of wild-type and untransduced parental controls (Figure S5).36 Amounts of tyrosinase and Pmel17, markers of melanogenesis and intact melanosomes, respectively, were also found to be reduced in response to the mutations (Figure S5).37, 38 NBMELs transduced with KRAS c.35G>A (p.Gly12Asp) and BRAF c.1799T>A (p.Val600Glu) did not exhibit these black foci (Figure S6). HUVECs with GNA14 p.Arg179Cys and p.Gln205Leu and GNA11 p.Arg183Cys and p.Gln209Pro also lost organized cobblestone morphology characteristic of normal HUVECs and became markedly elongated, whereas cells transduced with wild-type constructs and vector controls showed normal morphology (Figure 2). HUVECs with KRAS p.Gly12Asp and both wild-type and p.Val600Glu BRAF were elongated and dysplastic (Figure S6).
Figure 2.
NBMELs and HUVECs Expressing Mutant GNA14 and GNA11 Demonstrate Morphological Changes
(A) 40× magnification of NBMELs with GNA11 p.Arg183Cys or GNA14 p.Arg179Cys and GNA11 p.Gln209Pro or GNA14 p.Gln205Leu demonstrate elongation and black foci. Melanocytes with GNA11 p.Arg183Cys or GNA14 p.Arg179Cys are more spindled and dispersed than those with GNA11 p.Gln209Pro or GNA14 p.Gln205Leu, which exhibit significant piling.
(B) HUVECs with activating mutations in GNA14 and GNA11 lose the normal cobblestone morphology seen in wild-type cells and exhibit elongation and disarray.
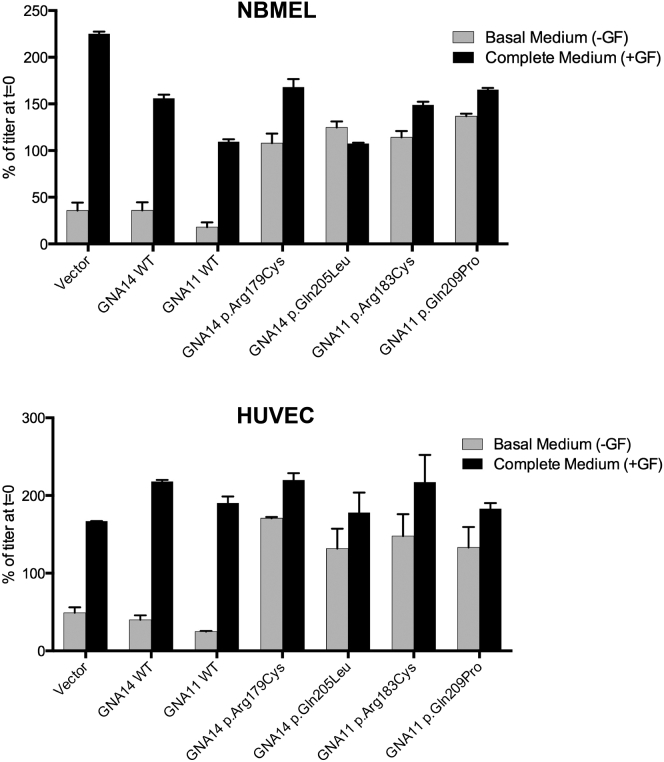
Given the morphologic changes in cells expressing GNA14 and GNA11 mutations, we considered the possibility that the cells were developing features of oncogenic transformation, one in vitro hallmark of which is prolonged survival and proliferation in the absence of growth factors.39, 40, 41 Transduced NBMELs and HUVECs were cultured in either basal medium without growth factors required for their proliferation (NBMELs: IBMX, dbcCAMP, and bFGF; HUVECs: EGM-2 Bullets) or complete medium and were assessed for cell viability after 48 hr with the CellTiter-Glo Luminescent Cell Viability Assay (Promega). NBMELs and HUVECs expressing wild-type GNA14 and GNA11 and parental cells ceased growth or died in medium without growth factors, whereas NBMELs and HUVECs with GNA14 p.Arg179Cys and p.Gln205Leu and GNA11 p.Arg183Cys and p.Gln209Pro were successfully maintained and passaged in both conditions, suggesting the acquisition of growth-factor independence (Figure 3).
Figure 3.
Activating Mutations in GNA14 and GNA11 Render NBMELs and HUVECs Growth-Factor Independent
Mutant NBMELs (top) and HUVECs (bottom) are able to survive in basal medium depleted of growth factors.
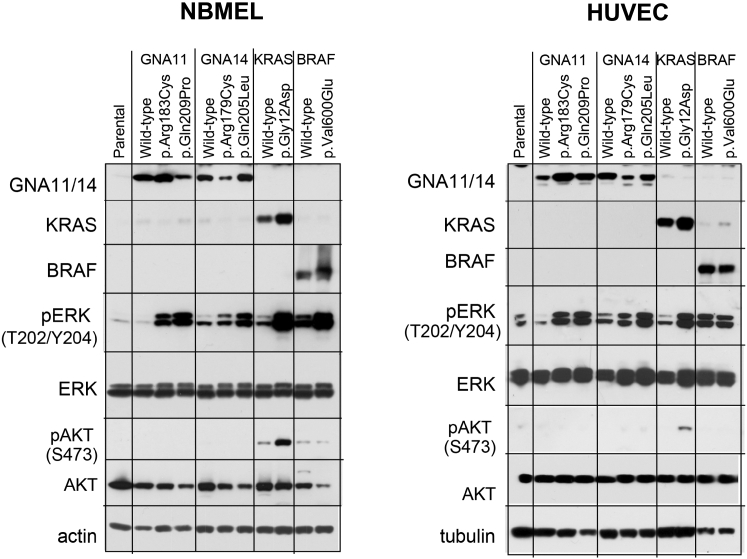
Assessment of the activity of the MAPK pathway showed significant upregulation of activated ERK1/2 in HUVECs and NBMELs expressing mutant but not wild-type GNA14 or GNA11 and no evidence of AKT activation (Figure 4). In contrast, KRAS p.Gly12Asp showed increased amounts of p-AKT (Figure 4). These findings suggest that the GNA14 and GNA11 mutations we found in KHEs, TAs, and LCHs induce changes in cellular morphology and growth-factor independence via MAPK activation.
Figure 4.
GNA14 and GNA11 Mutations Upregulate the MAPK Pathway in NBMELs and HUVECs Independently of AKT Activation
Activating mutations in GNA14 and GNA11 upregulate phosphorylation of ERK1/2 in NBMELs (left) and HUVECs (right). GNA14 p.Arg179Cys and GNA11 p.Arg183Cys induce lower p-ERK levels in both cell types than do GNA14 p.Gln205Leu and GNA11 p.Gln209Pro. The membranes were probed with antibodies against GNA11 (D-17, Santa Cruz), GNA14 (SAB4300771, Sigma-Aldrich), BRAF (AF3424, R&D), KRAS (3B10-2F2 WH0003845M1, Sigma-Aldrich), pERK p44/42K T202/Y204 (E10, 9106, Cell Signaling Technology), ERK (4695, Cell Signaling Technology), phospho AKT S473 (9271, Cell Signaling Technology), AKT (9272, Cell Signaling Technology), and β-actin (A1978, Sigma-Aldrich) or tubulin (T5168 Sigma-Aldrich) as controls for protein loading.
To date, mutations in GNA14 have not been associated with disease, although increased amounts of GNA14 were observed within pulmonary artery endothelial and smooth muscle cells in subjects with pulmonary arterial hypertension, as well as within the endothelial cells of human placentas from pre-eclamptic pregnancies.42, 43 A multiple-candidate-gene approach for surveying hypertension susceptibility genes among a cohort of 3,305 hypertensive and 3,827 normotensive control subjects identified a significant positive association between GNA14 and RGS20 (regulator of G-protein signaling 20 [MIM: 607193]) and hypertension, further implicating GNA14 in vascular biology.44 Recently, consistent with our findings, constitutive activation of GNA14 was shown to increase RAS activation and downstream phosphorylation of ERK.45
Vascular phenotypes resulting from mutations affecting Gαq family members range from vascular stains, such as PWSs and capillary malformations, to frank tumors. Our identification of GNA14 mutations in KHEs, TAs, and LCHs—three distinct classes of vascular tumors—also highlights the pleiotropy of Gαq variants, as seen in GNAQ and GNA11 mutations causing LCHs, PWSs, and NICHs.10, 11, 12, 16, 19 Similarly, activating mutations in HRAS, KRAS, and NRAS also demonstrate phenotypic heterogeneity within vascular anomalies, giving rise to both LCHs and NICHs.8, 9 It remains unclear how identical somatic mutations can give rise to distinct clinical phenotypes. Although GNA15 and GNA16 mutations have not yet been identified, they could, in theory, contribute to vascular pathology.
In summary, we have identified a recurrent somatic activating GNA14 mutation in two congenital vascular tumors and one LCH and found GNA11 c.547C>T (p.Arg183Cys) in two LCHs. We have shown that mutations in GNA14 and GNA11 induce changes in cellular morphology and render cells growth-factor independent via MAPK activation. These findings and recent discoveries that activating mutations in HRAS, KRAS, NRAS, and BRAF also drive vascular tumors raise the possibility that inhibition of the MAPK pathway could be an effective therapeutic approach in congenital hemangiomas and infantile vascular lesions unresponsive to conventional therapy.
Acknowledgments
We thank Xinran Liu for assistance with electron-microscopy images, Elaine Zhou, Jing Zhou, Rong-Hua Hu, and Jonathan Levinsohn for technical assistance, Jordan Pober and William Chang for insightful correspondence on vascular biology, and members of the Yale Center Mendelian Genomics, including Richard P. Lifton, Shrikant M. Mane, and Kaya Bilguvar. This study was supported by a Doris Duke Charitable Foundation Clinical Scientist Development Award to K.A.C. and by the Yale Center for Mendelian Genomics (NIH U54 HG006504). Y.H.L. was supported by the Medical Scientist Training Program at Yale University (NIH/NIGMS T32 GM007205) and is a recipient of the Clinical Mentorship Award from the Doris Duke Charitable Foundation. R.H., A.B., and R.S. are supported by Yale SPORE in Skin Cancer, funded by the NIH National Cancer Institute under award number 1 P50 CA121974 (R.H.).
Published: July 28, 2016
Footnotes
Supplemental Data include six figures and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.06.010.
Accession Numbers
The accession number for the sequencing data reported in this paper is dbGAP: phs000744.
Web Resources
1000 Genomes, http://www.1000genomes.org
COSMIC, http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/
ExAC Browser, http://exac.broadinstitute.org/
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
OMIM, http://www.omim.org/
UCSC Genome Browser, http://genome.ucsc.edu
Supplemental Data
References
- 1.North P.E., Waner M., Buckmiller L., James C.A., Mihm M.C., Jr. Vascular tumors of infancy and childhood: beyond capillary hemangioma. Cardiovasc. Pathol. 2006;15:303–317. doi: 10.1016/j.carpath.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 2.North P.E., Waner M., Mizeracki A., Mihm M.C., Jr. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum. Pathol. 2000;31:11–22. doi: 10.1016/s0046-8177(00)80192-6. [DOI] [PubMed] [Google Scholar]
- 3.O’Rafferty C., O’Regan G.M., Irvine A.D., Smith O.P. Recent advances in the pathobiology and management of Kasabach-Merritt phenomenon. Br. J. Haematol. 2015;171:38–51. doi: 10.1111/bjh.13557. [DOI] [PubMed] [Google Scholar]
- 4.Chiu Y.E., Drolet B.A., Blei F., Carcao M., Fangusaro J., Kelly M.E., Krol A., Lofgren S., Mancini A.J., Metry D.W. Variable response to propranolol treatment of kaposiform hemangioendothelioma, tufted angioma, and Kasabach-Merritt phenomenon. Pediatr. Blood Cancer. 2012;59:934–938. doi: 10.1002/pbc.24103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drolet B.A., Frommelt P.C., Chamlin S.L., Haggstrom A., Bauman N.M., Chiu Y.E., Chun R.H., Garzon M.C., Holland K.E., Liberman L. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128–140. doi: 10.1542/peds.2012-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain V., Roychoudhury S., Chadha R., Puri A. Variable response to propranolol therapy for infantile hemangiomas. Indian J. Dermatol. 2012;57:126–127. doi: 10.4103/0019-5154.94281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulliken J.B., Enjolras O. Congenital hemangiomas and infantile hemangioma: missing links. J. Am. Acad. Dermatol. 2004;50:875–882. doi: 10.1016/j.jaad.2003.10.670. [DOI] [PubMed] [Google Scholar]
- 8.Groesser L., Peterhof E., Evert M., Landthaler M., Berneburg M., Hafner C. BRAF and RAS Mutations in Sporadic and Secondary Pyogenic Granuloma. J. Invest. Dermatol. 2015 doi: 10.1038/JID.2015.376. Published online September 29, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Lim Y.H., Douglas S.R., Ko C.J., Antaya R.J., McNiff J.M., Zhou J., Choate K.A., Narayan D. Somatic Activating RAS Mutations Cause Vascular Tumors Including Pyogenic Granuloma. J. Invest. Dermatol. 2015;135:1698–1700. doi: 10.1038/jid.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayturk U.M., Couto J.A., Hann S., Mulliken J.B., Williams K.L., Huang A.Y., Fishman S.J., Boyd T.K., Kozakewich H.P., Bischoff J. Somatic Activating Mutations in GNAQ and GNA11 Are Associated with Congenital Hemangioma. Am. J. Hum. Genet. 2016;98:789–795. doi: 10.1016/j.ajhg.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funk, T., Lim, Y.H., Kulungowski, A.M., Prok, L., Crombleholme, T.M., Choate, K.A., and Bruckner, A.L. Symptomatic Congenital Hemangioma and Congenital “Hemangiomatosis” Associated with a Somatic Activating Mutation in GNA11. J. Am. Med. Assoc. Dermatology. Published online July 20, 2016. http://dx.doi.org/10.01/jamadermatol.2016.2365. [DOI] [PubMed]
- 12.Nakashima M., Miyajima M., Sugano H., Iimura Y., Kato M., Tsurusaki Y., Miyake N., Saitsu H., Arai H., Matsumoto N. The somatic GNAQ mutation c.548G>A (p.R183Q) is consistently found in Sturge-Weber syndrome. J. Hum. Genet. 2014;59:691–693. doi: 10.1038/jhg.2014.95. [DOI] [PubMed] [Google Scholar]
- 13.Shirley M.D., Tang H., Gallione C.J., Baugher J.D., Frelin L.P., Cohen B., North P.E., Marchuk D.A., Comi A.M., Pevsner J. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N. Engl. J. Med. 2013;368:1971–1979. doi: 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng H., Zhao D., Yang S., Datta K., Mukhopadhyay D. Heterotrimeric G alpha q/G alpha 11 proteins function upstream of vascular endothelial growth factor (VEGF) receptor-2 (KDR) phosphorylation in vascular permeability factor/VEGF signaling. J. Biol. Chem. 2003;278:20738–20745. doi: 10.1074/jbc.M209712200. [DOI] [PubMed] [Google Scholar]
- 15.Sivaraj K.K., Li R., Albarran-Juarez J., Wang S., Tischner D., Grimm M., Swiercz J.M., Offermanns S., Wettschureck N. Endothelial Gαq/11 is required for VEGF-induced vascular permeability and angiogenesis. Cardiovasc. Res. 2015;108:171–180. doi: 10.1093/cvr/cvv216. [DOI] [PubMed] [Google Scholar]
- 16.Thomas A.C., Zeng Z., Rivière J.B., O’Shaughnessy R., Al-Olabi L., St-Onge J., Atherton D.J., Aubert H., Bagazgoitia L., Barbarot S. Mosaic Activating Mutations in GNA11 and GNAQ Are Associated with Phakomatosis Pigmentovascularis and Extensive Dermal Melanocytosis. J. Invest. Dermatol. 2016;136:770–778. doi: 10.1016/j.jid.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Raamsdonk C.D., Griewank K.G., Crosby M.B., Garrido M.C., Vemula S., Wiesner T., Obenauf A.C., Wackernagel W., Green G., Bouvier N. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu C.Y., Hsiao C.H., Chiu H.C. Transformation between Kaposiform hemangioendothelioma and tufted angioma. Dermatology (Basel) 2003;206:334–337. doi: 10.1159/000069947. [DOI] [PubMed] [Google Scholar]
- 19.Couto J.A., Huang L., Vivero M.P., Kamitaki N., Maclellan R.A., Mulliken J.B., Bischoff J., Warman M.L., Greene A.K. Endothelial Cells from Capillary Malformations Are Enriched for Somatic GNAQ Mutations. Plast. Reconstr. Surg. 2016;137:77e–82e. doi: 10.1097/PRS.0000000000001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van der Auwera G.A., Carneiro M.O., Hartl C., Poplin R., Del Angel G., Levy-Moonshine A., Jordan T., Shakir K., Roazen D., Thibault J. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinformatics. 2013;43:11.10.1–11.10.33. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cibulskis K., Lawrence M.S., Carter S.L., Sivachenko A., Jaffe D., Sougnez C., Gabriel S., Meyerson M., Lander E.S., Getz G. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol. 2013;31:213–219. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorvaldsdóttir H., Robinson J.T., Mesirov J.P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malhotra Y., Yang C.S., McNamara J., Antaya R.J. Congenital kaposiform hemangioendothelioma with Kasabach-Merritt phenomenon successfully treated with low-dose radiation therapy. Pediatr. Dermatol. 2014;31:595–598. doi: 10.1111/pde.12090. [DOI] [PubMed] [Google Scholar]
- 26.Hubbard K.B., Hepler J.R. Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell. Signal. 2006;18:135–150. doi: 10.1016/j.cellsig.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Wettschureck N., Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 28.Heydorn A., Ward R.J., Jorgensen R., Rosenkilde M.M., Frimurer T.M., Milligan G., Kostenis E. Identification of a novel site within G protein alpha subunits important for specificity of receptor-G protein interaction. Mol. Pharmacol. 2004;66:250–259. doi: 10.1124/mol.66.2.250. [DOI] [PubMed] [Google Scholar]
- 29.Strathmann M.P., Simon M.I. G alpha 12 and G alpha 13 subunits define a fourth class of G protein alpha subunits. Proc. Natl. Acad. Sci. USA. 1991;88:5582–5586. doi: 10.1073/pnas.88.13.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forbes S.A., Beare D., Gunasekaran P., Leung K., Bindal N., Boutselakis H., Ding M., Bamford S., Cole C., Ward S. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43:D805–D811. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley L.A., Mezulis S., Yates C.M., Wass M.N., Sternberg M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 33.Taylor V.G., Bommarito P.A., Tesmer J.J. Structure of the Regulator of G Protein Signaling 8 (RGS8)-Gαq Complex: MOLECULAR BASIS FOR Gα SELECTIVITY. J. Biol. Chem. 2016;291:5138–5145. doi: 10.1074/jbc.M115.712075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimple A.J., Bosch D.E., Giguère P.M., Siderovski D.P. Regulators of G-protein signaling and their Gα substrates: promises and challenges in their use as drug discovery targets. Pharmacol. Rev. 2011;63:728–749. doi: 10.1124/pr.110.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krauthammer M., Kong Y., Ha B.H., Evans P., Bacchiocchi A., McCusker J.P., Cheng E., Davis M.J., Goh G., Choi M. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat. Genet. 2012;44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drochmans P. Electron microscope studies of epidermal melanocytes, and the fine structure of melanin granules. J. Biophys. Biochem. Cytol. 1960;8:165–180. doi: 10.1083/jcb.8.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonhardt R.M., Vigneron N., Hee J.S., Graham M., Cresswell P. Critical residues in the PMEL/Pmel17 N-terminus direct the hierarchical assembly of melanosomal fibrils. Mol. Biol. Cell. 2013;24:964–981. doi: 10.1091/mbc.E12-10-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boswell H.S., Nahreini T.S., Burgess G.S., Srivastava A., Gabig T.G., Inhorn L., Srour E.F., Harrington M.A. A RAS oncogene imparts growth factor independence to myeloid cells that abnormally regulate protein kinase C: a nonautocrine transformation pathway. Exp. Hematol. 1990;18:452–460. [PubMed] [Google Scholar]
- 40.Rodeck U. Growth factor independence and growth regulatory pathways in human melanoma development. Cancer Metastasis Rev. 1993;12:219–226. doi: 10.1007/BF00665954. [DOI] [PubMed] [Google Scholar]
- 41.Wang L., Damania B. Kaposi’s sarcoma-associated herpesvirus confers a survival advantage to endothelial cells. Cancer Res. 2008;68:4640–4648. doi: 10.1158/0008-5472.CAN-07-5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lei W., Chen P., Yue Y., He Y., Shui X., Li G., Zhang L., Huang S., Chen C. Subcellular distribution patterns and elevated expression of GNA11 and GNA14 proteins in the lungs of humans with pulmonary arterial hypertension. Cell Biol. Int. 2014;38:1041–1049. doi: 10.1002/cbin.10292. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y.J., Zou Q.Y., Li Y., Li H.H., Wu Y.M., Li X.F., Wang K., Zheng J. Expression of G-protein subunit α-14 is increased in human placentas from preeclamptic pregnancies. J. Histochem. Cytochem. 2014;62:347–354. doi: 10.1369/0022155414521213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kohara K., Tabara Y., Nakura J., Imai Y., Ohkubo T., Hata A., Soma M., Nakayama T., Umemura S., Hirawa N. Identification of hypertension-susceptibility genes and pathways by a systemic multiple candidate gene approach: the millennium genome project for hypertension. Hypertens. Res. 2008;31:203–212. doi: 10.1291/hypres.31.203. [DOI] [PubMed] [Google Scholar]
- 45.Kwan D.H., Yung L.Y., Ye R.D., Wong Y.H. Activation of Ras-dependent signaling pathways by G(14) -coupled receptors requires the adaptor protein TPR1. J. Cell. Biochem. 2012;113:3486–3497. doi: 10.1002/jcb.24225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.