Abstract
Access to lifelong combination antiretroviral therapy (cART) is expanding among HIV-infected pregnant and breastfeeding women throughout sub-Saharan Africa. For this strategy to meaningfully improve maternal HIV outcomes, retention in HIV care is essential. We developed a risk score to identify women with high likelihood of loss to follow-up (LTFU) at 6 months postpartum from HIV care, using data from public health facilities in Lusaka, Zambia. LTFU was defined as not presenting for HIV care within 60 days of the last scheduled appointment. We used logistic regression to assess demographic, obstetric, and HIV predictors of LTFU and to develop a simple risk score. Sensitivity and specificity were assessed at each risk score cut-point. Among 2,029 pregnant women initiating cART between 2009 and 2011, 507 (25%) were LTFU by 6 months postpartum. Parity, education, employment status, WHO clinical stage, duration of cART during pregnancy, and number of antenatal care visits were associated with LTFU (p-value<0.10). A risk score cut-point of 11 (42nd percentile) had 85% sensitivity (95% CI 82%, 88%) and 22% specificity (95% CI 20%, 24%) to detect women LTFU and would exclude 20% of women from a retention intervention. A risk score cut-point of 18 (69th percentile) identified the 23% of women with the highest probability of LTFU and had sensitivity 32% (95% CI 28%, 36%) and specificity 80% (95% CI 78%, 82%). A risk score approach may be useful to triage a subset of women most likely to be LTFU for targeted retention interventions.
Keywords: HIV, pregnancy, postpartum, antiretroviral, loss to follow-up
INTRODUCTION
Access to lifelong combination antiretroviral therapy (cART) is rapidly being scaled-up to HIV-infected pregnant and breastfeeding women throughout sub-Saharan Africa (SSA). In 2013, an estimated 68% of HIV-infected pregnant women received some type of antiretroviral drug during pregnancy and delivery, doubled from 33% in 2009 (UNAIDS, 2014). In countries with a generalized HIV epidemic, the World Health Organization (WHO) now recommends lifelong cART for all pregnant and breastfeeding women (Kieffer et al., 2014; WHO, 2013). This strategy, known as Option B+(Schouten et al., 2011), has become the standard of care in many SSA countries. Option B+ is recognized as a critical component for preventing mother-to-child transmission of HIV (PMTCT), improving maternal health, and reducing the risk of sexual transmission among serodiscordant couples (Ahmed, Kim, & Abrams, 2013).
For Option B+ to improve HIV outcomes, women must remain in HIV care and maintain viral suppression (Hirnschall, Harries, Easterbrook, Doherty, & Ball, 2013; Rosen & Fox, 2011; Thompson et al., 2012). Unfortunately, loss to follow-up (LTFU) during and after pregnancy is common (Shaffer, Abrams, & Becquet, 2014). In Malawi, 17% of women initiating cART during pregnancy were LTFU by 6 months after treatment initiation. Retention at 12 months was lower among pregnant women than among other adults initiating cART (Kieffer et al., 2014; Tenthani et al., 2014). In South Africa, 32% of women initiating cART during pregnancy were lost by 6 months postpartum (Phillips et al., 2014). Several factors have been associated with LTFU among pregnant women, including younger age (Boyles, Wilkinson, Leisegang, & Maartens, 2011), initiating treatment at higher CD4 counts (Tenthani et al., 2014), timing of presentation to antenatal care (ANC) (Panditrao, Darak, Kulkarni, Kulkarni, & Parchure, 2011; Westreich, Evans, Firnhaber, Majuba, & Maskew, 2012) and receiving a new HIV diagnosis during pregnancy (Kaplan, Orrell, Zwane, Bekker, & Wood, 2008). To date, strategies to identify women at time of delivery who are at high risk of LTFU are lacking.
To address this gap, we developed a risk score to identify women LTFU from HIV care by 6 months postpartum using data from public health facilities in Lusaka, Zambia. Demographic, obstetric, HIV predictors were used to develop a simple, user-friendly scoring system that could be implemented by clinicians at delivery to identify women at highest risk of LTFU for retention interventions. Since Zambia only recently implemented Option B+, our analysis focused on treatment-eligible women who initiated cART during pregnancy in the pre-Option B+ era.
METHODS
Study design and population
We conducted a retrospective cohort analysis of HIV-infected pregnant women attending public antenatal and HIV healthcare facilities in Lusaka, Zambia. Lusaka is an urban setting where over 95% of women attend at least 1 ANC visit (Central Statistical Office (CSO), 2009). Among women attending ANC, HIV testing is near universal and CD4 count screening coverage is approximately 80% (Chi et al., 2011). During the study period (2009–2011), women with a CD4 count ≤350 cells/uL were eligible for lifelong cART. Data for the present analysis were derived from two sources: (1) the Zambian Electronic Perinatal Record System (ZEPRS), which collects comprehensive obstetric information on mothers and infants through delivery (Chi et al., 2011), and (2) SmartCare, an electronic medical record system for HIV clinical information.
Women included in our analysis initiated cART during pregnancy, had a CD4 count ≤350 cells/uL (in accordance with guidelines at the time) and delivered in a public-sector facility between January 1, 2009 and November 2, 2010. Women were followed for up to 6 months after delivery. We excluded women who died during pregnancy or up to 42 days after delivery in order to exclude possible pregnancy-related deaths (World Health Organization, 2015). Ethical approval for the analysis of routinely collected clinical data was obtained from the University of Zambia Biomedical Research Ethics Committee (Lusaka, Zambia) and the University of North Carolina, Chapel Hill (Chapel Hill, NC).
Outcome and predictor definitions
We followed women from delivery through 6 months postpartum. The primary endpoint was LTFU at 6 months postpartum. LTFU was defined as the first time a woman did not present to HIV care within 60 days of the last scheduled appointment. Women were categorized as LTFU on the 61st day after a missed appointment (Chi et al., 2010). We considered both clinic visits and pharmacy refill appointments. Women who never returned to HIV care after delivery were allowed up to 30 days to schedule an appointment and were classified as LTFU 61 days after that time (91 days total of follow-up time).
Three categories of predictors for LTFU at 6 months postpartum were considered: demographic, obstetric, and HIV characteristics. We prioritized information that would be readily available to clinicians at the point of care in settings like Zambia. Demographic predictors considered were: age, level of educational, marital status and employment status. Obstetric predictors considered were: number of ANC visits, parity, low infant birthweight (LBW; <2,500 grams) and preterm birth (<37 weeks gestation). HIV predictors considered were: CD4 count, WHO clinical stage, duration of cART taken during pregnancy, new diagnosis of HIV during pregnancy (defined as a positive HIV test within 7 days of entry into ANC), body mass index (BMI), hemoglobin and self-reported tuberculosis during pregnancy. Our aim was to develop a risk score that would be easy to calculate by frontline providers; as such, candidate predictors were coded as binary or categorical. Information on predictors of LTFU such as viral load, disclosure of HIV status to a partner and distance to the clinic were not available. We conducted a sensitivity analysis additionally assessing enrollment into pre-ART care (e.g. enrolled but did not start treatment) prior to pregnancy as a candidate predictor.
Statistical analysis
The primary goal of the risk score was to create an easy to use tool for clinicians to identify women at delivery who may be at high risk of postpartum LTFU. The analysis comprised two steps. First, we developed a predictive model for LTFU at 6 months postpartum and used the beta coefficients as the basis for the risk score. We then validated the risk score by bootstrapping (Harrell, 2002).
To develop the risk score, we used logistic regression to estimate unadjusted odds ratios (ORs) for all candidate predictors. Predictors associated with LTFU at 6 months postpartum with a p-value ≤ 0.25 were identified and included in the full multivariable model. We used manual backward elimination based on likelihood ratio tests to reduce the full model to a more parsimonious final model. After the elimination of each variable, the area under the curve (AUC) was compared with the full model to determine whether the two models had comparable predictive ability. Multi-collinearity was assessed using Spearman correlations. For collinear variables, the variable with the strongest predictive power (e.g. largest coefficient) was retained in the final model. Variable elimination stopped when all predictors left in the multivariable model had a p-value ≤ 0.10, to balance retaining potentially important predictors with parsimony. Model fit was assessed using the Hosmer-Lemeshow test. A priori we specified a risk score cut-point with sensitivity > 80% and specificity >60% as optimal. These sensitivity and specificity values were selected to prioritize identifying the women most likely to be LTFU (higher sensitivity), while maintaining a reasonable ability to identify women most likely not to be LTFU.
To calculate the final risk score, beta coefficients from the final logistic regression model were multiplied by 10 and rounded to the nearest integer. Values for each person were summed to create an individual risk score. Sensitivity and specificity was assessed at each risk score cut-point. Risk score validation was carried out by bootstrapping the original data set (n=1,000) and comparing the mean, 2.5th and 97.5th percentile values for sensitivity and specificity to their respective values and 95% confidence intervals in the original data(Harrell, 2002). Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
Of 4,305 identified in ZEPRS who started cART during pregnancy, 2,982 (69%) could be linked to their SmartCare records. Demographic and obstetric characteristics were similar between women who could be linked, and those who could not. Of the 2,982 women with linked records, 925 (31%) were excluded for reasons shown in Figure 1. An additional 759 women were identified in SmartCare who initiated cART while pregnant (according to their ZEPRS record). A total of 2,265 women initiated cART during pregnancy within the study period; 2,108 (93%) had a CD4 count ≤350 and 2,034 (96%) had delivery information. Five women (0.2%) died within 42 days of delivery and were not included, for a final sample size of 2,029 women (Figure 1).
Figure 1.
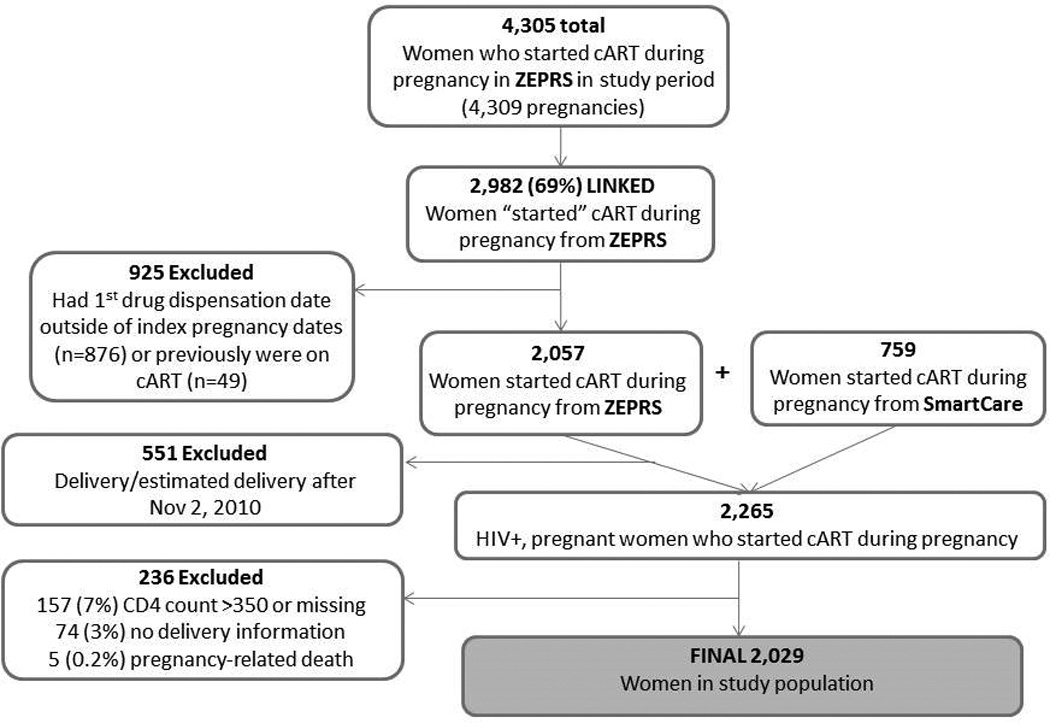
Inclusion criteria for 2, 029 HIV-infected pregnant women who initiated cART during pregnancy and had linkable records, included in the study population. ZEPRS=Zambian Electronic Perinatal Record System; SmartCare = HIV electronic medical record system.
A total of 507 (25%) women were lost to follow-up (LTFU) by 6 months postpartum; 10 (0.5%) of whom died from non-pregnancy related causes after being LTFU. Of the 507 women who were LTFU, 285 (56%) never returned to care after delivery. Among those who initially returned to care but were later LTFU, over half were lost by 18 weeks postpartum (Figure 2).
Figure 2.
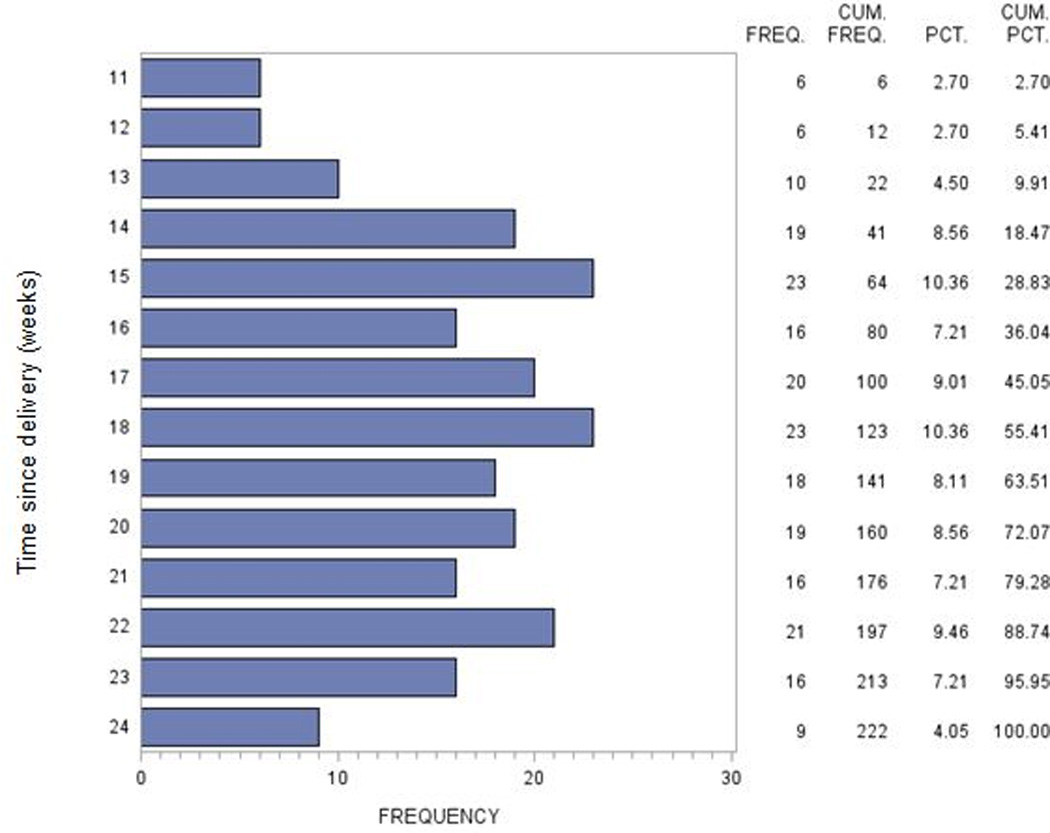
Timing of loss to follow-up among 222 women returned to HIV care after delivery and who were subsequently lost to follow-up. An additional 285 women (507 women LTFU in total) never returned to HIV care after delivery and are not included.
Overall, most women were >25 years of age (66%), married or cohabitating (93%), either unemployed or a housewife (74%) and attended only 1 or 2 ANC visits (65%) (Table 1). Most women received a HIV diagnosis (87%) during the current pregnancy. Women were overwhelmingly WHO clinical stage 1 or 2 (93%) and received ≥4 weeks of cART during pregnancy (80%).
Table 1.
Clinical characteristics of 2,029 HIV-infected women who initiated cART during pregnancy in Lusaka, Zambia 2009–2010.
| Characteristic | Lost to Follow-up at 6 months |
Remained in HIV Care at 6 months |
Total |
|---|---|---|---|
| N=507 (25.0) N(%) |
N=1522 (75.0) N(%) |
N=2,029 N (%) |
|
| Age | |||
| ≤ 25 | 201 (39.6) | 483 (31.8) | 684 (33.7) |
| >25 | 306 (60.4) | 1,037 (68.2) | 1,343 (66.3) |
| Level of education | |||
| None or primary | 211 (48.0) | 540 (40.4) | 751 (42.2) |
| Secondary or tertiary | 229 (52.1) | 798 (59.6) | 1,027 (57.8) |
| Marital status | |||
| Married or cohabitating | 450 (91.7) | 1,353 (91.6) | 1,804 (92.6) |
| Singled, divorced or widowed |
41 (8.4) | 125 (8.5) | 166 (8.4) |
| Employment status | |||
| Employed or student | 85 (19.1) | 380 (28.1) | 465 (25.8) |
| Housewife or unemployed | 361 (80.9) | 974 (71.9) | 1,335 (74.2) |
| CD4 count (cells/uL) | |||
| ≤150 | 118 (23.5) | 377 (25.1) | 495 (24.7) |
| 151–250 | 202 (40.2) | 557 (37.1) | 759 (37.9) |
| 251–350 | 118 (36.3) | 566 (37.7) | 748 (37.4) |
| WHO stage | |||
| 1 or 2 | 458 (92.9) | 1,360 (91.1) | 1,818 (92.5) |
| 3 or 4 | 35 (7.1) | 133 (8.9) | 168 (8.5) |
| Duration of antenatal cART | |||
| 1–4 weeks | 18 (27.2) | 274 (18.0) | 412 (20.3) |
| > 4 weeks | 369 (72.8) | 1,248 (82.0) | 1,617 (79.7) |
| New HIV diagnosis during pregnancy |
|||
| Yes | 439 (86.6) | 1,328 (87.3) | 1,767 (87.1) |
| No | 68 (13.4) | 194 (12.8) | 262 (12.9) |
| Body mass index (kg/m2) | |||
| <18.5 | 15 (3.2) | 46 (3.2) | 61 (3.2) |
| 18.5 –<25 | 306 (64.7) | 923 (63.4) | 1,229 (63.7) |
| 25 – <30 | 125 (26.4) | 392 (26.9) | 517 (26.8) |
| ≥30 | 27 (5.7) | 96 (6.6) | 123 (6.4) |
| Hemoglobin (g/dL) | |||
| <8 | 17 (3.7) | 45 (3.2) | 62 (3.4) |
| 8–<10 | 105 (22.9) | 357 (25.7) | 462 (25.0) |
| ≥ 10 | 337 (73.4) | 990 (71.1) | 1,327 (71.7) |
| Active tuberculosis | |||
| Yes | 10 (2.0) | 23 (1.5) | 33 (1.6) |
| No | 497 (98.0) | 1,499 (98.5) | 1,996 (98.4) |
| Number of ANC visit | |||
| 1 or 2 | 358 (70.6) | 966 (63.5) | 1,324 (65.3) |
| ≥3 | 149 (29.4) | 556 (36.5) | 704 (34.8) |
| Parity | |||
| 0 | 108 (22.0) | 252 (17.2) | 360 (18.4) |
| 1 | 126 (25.7) | 358 (24.4) | 484 (24.7) |
| 2 | 112 (5.7) | 343 (23.4) | 455 (23.3) |
| >2 | 144 (29.4) | 515 (35.1) | 659 (33.7) |
| Low birthweight (<2,500 grams) | |||
| Yes | 82 (16.5) | 204 (13.7) | 286 (14.4) |
| No | 414 (83.5) | 1287 (86.3) | 1,701 (85.6) |
| Preterm delivery (<37 weeks gestation) |
|||
| Yes | 179 (41.2) | 461 (35.6) | 640 (37.0) |
| No | 256 (58.9) | 835 (64.4) | 1,091 (63.0) |
Model development
In bivariable analyses, age, education, employment status, WHO clinical stage, duration of cART during pregnancy, hemoglobin, number of ANC visits, parity and preterm delivery predicted LTFU at 6 months postpartum (all, P < 0.25; Table 2). In the sensitivity analysis, having enrolled in pre-ART care prior to initiating treatment during pregnancy was a strong predictor of LTFU at 6 months postpartum (unadjusted OR 2.08 95% CI (1.11, 3.88)), but very few women (2%) were enrolled in pre-ART care.
Table 2.
Predictors of loss to follow-up from HIV care at 6 months postpartum among HIV-infected Zambian women initiating cART during pregnancy.
| Unadjusted | Full Model | Final Model | Risk Score | |
|---|---|---|---|---|
| Covariate | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age | ||||
| >25 | 1.00 | 1.00 | ||
| ≤ 25 | 1.41 (1.15, 1.74)a | 1.10 (0.77, 1.56) | ||
| Education | ||||
| Secondary or tertiary | 1.00 | 1.00 | 1.00 | -- |
| Primary or none | 1.36 (1.10, 1.69)a | 1.33 (1.01, 1.76) | 1.41 (1.10, 1.80)b | 3 |
| Marital status | ||||
| Married or cohabitating | 1.00 | -- | ||
| Singled, divorced or widowed | 1.18 (0.86, 1.62) | -- | ||
| Employment status | ||||
| Employed or student | 1.00 | 1.00 | 1.00 | -- |
| Housewife or unemployed | 1.66 (1.27, 2.16)a | 1.47 (1.06, 2.03) | 1.56 (1.17, 2.07)b | 4 |
| CD4 count (cells/uL) | ||||
| 251–350 | 1.00 | -- | ||
| 151–250 | 1.13 (0.89, 1.42) | -- | ||
| ≤150 | 0.97 (0.75, 1.27) | -- | ||
| WHO Clinical Stage | ||||
| 3 or 4 | 1.00 | 1.00 | 1.00 | -- |
| 1 or 2 | 1.28 (0.87, 1.89)a | 2.18 (1.26, 3.75) | 1.73 (1.10, 2.73)b | 5 |
| Duration of cART before delivery | ||||
| > 4 | 1.00 | 1.00 | 1.00 | -- |
| 1–4 | 1.70 (1.35, 2.16)a | 1.69 (1.23, 2.32) | 1.73 (1.32, 2.27)b | 5 |
| HIV diagnosis during pregnancy | ||||
| No | 1.00 | -- | ||
| Yes | 0.94 (0.70, 1.27) | -- | ||
| Body mass index (kg/m2) | ||||
| 18.5 – <25 | 1.00 | -- | ||
| <18.5 | 0.98 (0.54, 1.79) | -- | ||
| 25 – <30 | 0.96 (0.76, 1.22) | -- | ||
| ≥30 | 0.85 (0.54, 1.33) | -- | ||
| Hemoglobin (g/dL) | ||||
| ≥10 | 1.00 | 1.00 | ||
| 8–<10 | 0.86 (0.67, 1.11)a | 0.86 (0.63, 1.18) | ||
| <8 | 1.11 (0.63, 1.97) | 1.83 (0.87, 3.83) | ||
| Active tuberculosis | ||||
| No | 1.00 | -- | ||
| Yes | 1.31 (0.62, 2.77) | -- | ||
| Number of ANC visits | ||||
| ≥ 3 | 1.00 | 1.00 | 1.00 | -- |
| 1 or 2 | 1.38 (1.11, 1.72)a | 1.31 (0.97, 1.76) | 1.31 (1.02, 1.68)b | 3 |
| Parity | ||||
| >2 | 1.00 | 1.00 | -- | |
| 2 | 1.17 (0.88, 1.55) | 1.09 (0.76, 1.57) | 1.31 (0.96, 1.81)b | 3 |
| 1 | 1.26 (0.96, 1.66)a | 1.19 (0.80, 1.76) | 1.42 (1.04, 1.94)b | 3 |
| 0 | 1.53 (1.15, 2.05)a | 1.30 (0.80, 2.10) | 1.83 (1.29, 2.59)b | 6 |
| Low birthweight (<2,500 grams) | ||||
| No | 1.00 | -- | ||
| Yes | 1.25 (0.95, 1.65) | -- | ||
| Preterm delivery (<37 weeks gestation) | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.27 (1.01, 1.58)a | 1.09 (0.82, 1.44) | ||
P < 0.25,
P < 0.10
In the multivariable analysis, parity, education, employment status, WHO clinical stage, duration of cART during pregnancy, and number of ANC visits predicted LTFU at 6 months postpartum (all P < 0.10; Table 2). The strongest predictors of LTFU were primiparity, WHO clinical stage 1 or 2, and receiving only 1 to 4 weeks of cART before delivery (Table 2). Overall, discrimination in both the full (included all predictors identified in the bivariable analysis) and final (excluded age, hemoglobin, and preterm delivery) models was marginal (full model AUC 0.64; final model AUC 0.63).
Risk score performance
The risk score ranged from 3 to a maximum score of 26 (Table 2). In the bootstrapped data, mean sensitivity and specificity values were consistent with sensitivity and specificity values in the observed data, as was 95% CI coverage, indicating good internal validity of the risk score.
No risk score cut-point met our a priori targets for acceptable performance (sensitivity >80%, specificity >60%). Overall, sensitivity was approximately ≥80% for risk score cut-points below 12, indicating a reasonably good ability to accurately identify women who became LTFU. Specificity for the same range of risk scores was low (0–22%). For example, selecting a risk score cut-point of 11 resulted in 85% sensitivity (95% CI 0.82, 0.88) and 22% specificity (95% CI 0.20, 0.24) to detect women LTFU by 6 months postpartum (Figure 3a). With a 25% prevalence of LTFU in our population, the positive predictive value (PPV) for a cut-point of 11 was 0.27 (95% CI 0.24, 0.29) and the negative predictive value (NPV) was 0.81 (95% CI 0.78, 0.85; Figure 3b). Using a risk score cut-point of 11 would identify a large group of women for intervention and exclude only 411 (20%) of the 2,029 women in our study population from a retention intervention.
Figure 3.
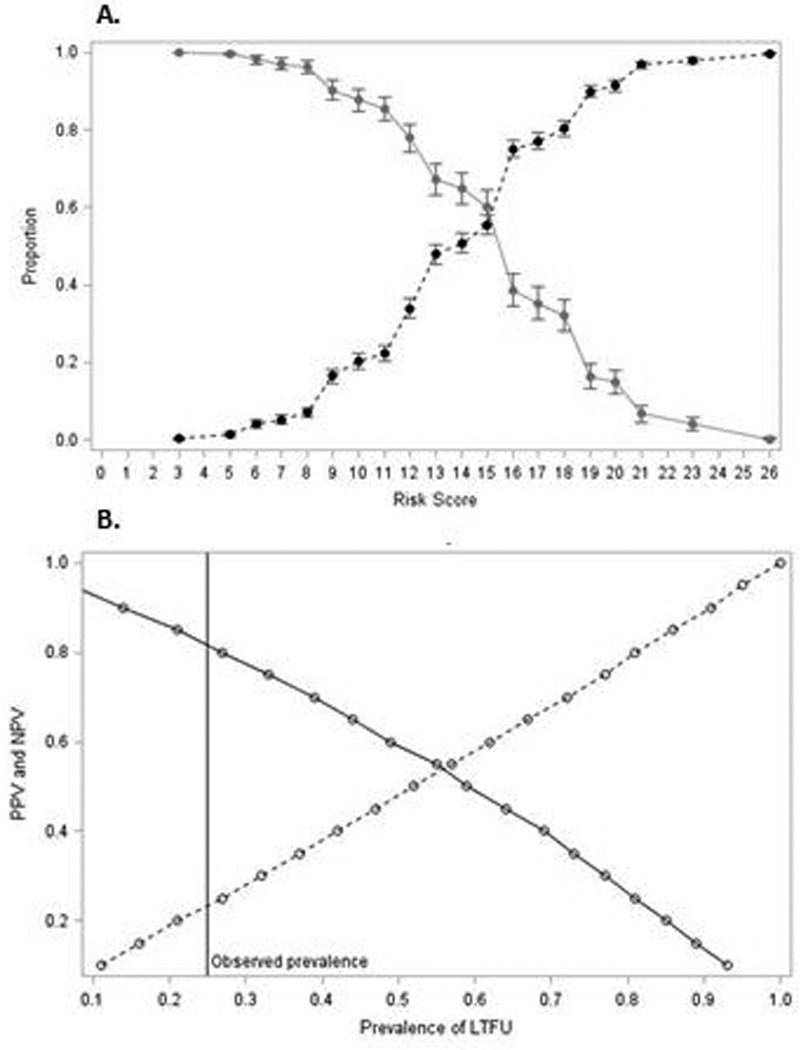
A. Sensitivity (solid line) and specificity (dotted line) at each risk score cut-off (range 3–26) to predict LTFU at 6-months postpartum among 2,029 HIV-infected Zambian women who initiated cART during pregnancy. B. Positive predictive value (PPV; dashed line) and negative predictive value (NPV; solid line) for risk score cut-point 11, as the prevalence of LTFU varies.
Alternatively, a risk score cut-point with lower sensitivity and higher specificity could be used to target a smaller group of women with higher likelihood of LTFU for an intensive retention intervention. A risk score cut-off of 18 identified the 23% of women (n=460) with the highest probability of LTFU and had sensitivity 0.32 (95% CI 0.28, 0.36) and specificity 0.80 (95% CI 0.78, 0.82). PPV improved slightly to 0.35 (95% CI 0.31, 0.40) and NPV) was 0.78 (95% CI 0.76, 0.80. Estimates of sensitivity and specificity for each risk score cut-point changed minimally when enrollment in pre-ART care was included as a predictor.
DISCUSSION
We developed a simple and easy to use risk score to identify women initiating cART during pregnancy with a high likelihood of LTFU by 6 months postpartum. No risk score cut-point met our a priori criteria for a satisfactory combination of sensitivity and specificity (sensitivity >80% and specificity >60%). PPV was also low, but will vary with the underlying prevalence of LTFU in the population (Figure 3). However, the risk score may still be useful to target a subgroup of women at delivery for retention interventions.
In our study population, a risk score cut-off of 11 identified 80% of our study population for intervention and could be used to identify a larger group of women at moderate risk of LTFU for a low-intensity intervention. Alternatively, a cut-point of 18 identified the 23% of our study population with the highest likelihood of LTFU and may be more appropriate for targeting a smaller group with a high-intensity intervention.
Our risk score is intended for use in maternity care settings in SSA, where the characteristics of women initiating cART during pregnancy and postpartum LTFU are likely to be similar to our study population. Prior to discharging women after delivery, practitioners could complete a simple checklist to assess likelihood of being LTFU (see example in Appendix). The risk score could then be used to either identify women less likely to become LTFU, so that resources are appropriately directed to those at greater risk. Alternatively, the tool could be used to identify those women at highest risk of LTFU, an approach which may be more practical in settings with limited funds for retention interventions. If adequate funds are available, it may be preferable to enroll all women in retention interventions. As with all risk scores, validation of our risk score prior to implementation is essential. If women initiating cART in our study population differ from women initiating cART in other settings, the predictive ability of the risk score may differ.
We designed our risk score to be used after delivery, in order to maximize the information available to clinicians. However, many of the factors determined at delivery (e.g. low birthweight) were not included in the final risk score, raising the possibility that ANC may be a preferable time to identify women at high risk of LTFU. Identifying women primary care setting may be more effective since it facilitates earlier intervention for women likely to become LTFU and programmatically more efficient since women may return to the same care clinic later to receive HIV treatment. Additional work is needed to explore whether the information available to clinicians during ANC is sufficient to develop a satisfactory risk score.
Predicting postpartum LTFU is difficult. In our analysis, we used data likely to be available to clinicians at the time of delivery. However, none of the characteristics strongly predicted LTFU at 6 months postpartum. To improve the predictive ability of the risk score, information on additional predictors may be necessary. Clinicians could consider asking women at delivery about whether they have disclosed their HIV status to their partner (Halperin, Pathmanathan, & Richey, 2013; Wohl et al., 2011), face structural barriers to seeking care such as transportation or financial burdens (Bogart et al., 2012; Higa, Marks, Crepaz, Liau, & Lyles, 2012) or have concerns about stigma (Bogart et al., 2012; Kempf et al., 2010). Each of these factors has been shown to predict LTFU, but such information is rarely collected as part of routine care. Further research is needed to determine whether information on social and structural barriers to care is sufficient to produce a risk score with satisfactory sensitivity and specificity.
Many of the predictors identified in our analysis have previously been associated with LTFU. We found that a lower level of education and being a housewife or unemployed predicted LTFU. Higher education, which may influence employment status, has been shown to improve cART adherence, as well as retention in care (Ayuo et al., 2013; Bardeguez et al., 2008; Panditrao et al., 2011). We also found that fewer ANC visits and shorter duration of cART during pregnancy predicted LTFU, which aligns with previous findings that late presentation to ANC is associated with postpartum LTFU (Panditrao et al., 2011). We were unable to assess whether initiating treatment with a CD4 count >350 cells/uL predicted LTFU, because of existent thresholds for CD4 eligibility during the study period. However, as Option B+ is scaled up, the need for CD4 count monitoring may become obsolete, since women will initiate treatment regardless of CD4 count. In our analysis, WHO stage 1 or 2 was associated with LTFU, suggesting that women who are otherwise asymptomatic and healthy (ie. CD4 >350 cells/uL) may be at an increased risk of LTFU (Clouse et al., 2013; Tenthani et al., 2014). As seen in other studies (Boyles et al., 2011), enrollment in pre-ART care prior to pregnancy strongly predicted LTFU; however very few women met this definition.
There was substantial LTFU in our cohort. Of the 2,029 women included in the study population, 25% (n=507) were LTFU by 6 months postpartum. In our study we followed women from delivery, rather than cART initiation, to assess LTFU after engagement with the healthcare system during pregnancy. In countries that have implemented Option B+ similar trends have been seen, with 12–24% of women reported LTFU or to have no follow-up by 6 months after cART initiation (Kieffer et al., 2014; Tenthani et al., 2014). Among women LTFU in our study, 56% (n=285) did not return to care after delivery. The high proportion of LTFU immediately following delivery has also been reported in South Africa, where among women who initiated cART during pregnancy, 64% of women LTFU never returned to care after delivery(Clouse et al., 2013). In Malawi, women who started cART while pregnant were five times as likely to never return to care, compared to non-pregnant patients initiating cART for their own health (Tenthani et al., 2014).
Improving retention in care is critical to the success of scaling up lifetime cART to pregnant and breastfeeding women (Ahmed et al., 2013; Schouten et al., 2011). Women initiating treatment during pregnancy face the dual stresses of maintaining engagement in HIV care and having a new infant to care for, making them particularly vulnerable to LTFU (Hodgson et al., 2014; Kaplan et al., 2008). In our population, over half of the women who were LTFU never returned to HIV care after delivery. It is possible that some of these women migrated from Lusaka after delivery and later returned to care. However the high proportion of women lost immediately after delivery suggests that interventions to improve postpartum retention in care may need to engage with women before delivery and focus on keeping women in care immediately following birth.
CONCLUSIONS
Maintaining engagement in care for HIV-infected women initiating cART is essential for PMTCT and improving the health of HIV-infected mothers. As Option B+ is scaled up throughout SSA, healthcare systems must balance the additional costs of providing lifelong treatment to pregnant and breastfeeding women with prevention efforts to reduce LTFU (Ciaranello et al., 2013; Kuznik et al., 2012). Risk scores offer a simple and easy tool that can be employed at delivery, when women are still engaged in care, to identify women most likely to benefit from an intervention to reduce LTFU. However, accurately predicting LTFU among postpartum women is difficult and supplementary information, including data from women initiating treatment under Option B+, may be needed to maximize risk score effectiveness.
Acknowledgments
Traineeship support for AMB was provided by the National Institutes of Health (T32 AI007001) and DW was supported by the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR; P30 AI50410).
Funding: This publication resulted (in part) from research supported by the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR), an NIH funded program P30 AI50410. Traineeship support was provided by the National Institutes of Health (T32 AI007001).
Appendix
Risk Score for Loss to Follow-up by 6 months Postpartum
Directions:
All women start with a score of 0.
Check off box correct category for each risk factor.
Sum the points for all risk factors and record the final risk score at the bottom.
| Risk Factor | Check correct box | Points |
|---|---|---|
| Education level | ||
| Secondary or tertiary | ▭ | 0 |
| Primary or none | ▭ | 3 |
| Employment status | ||
| Employed or student | ▭ | 0 |
| Housewife or unemployed | ▭ | 4 |
| WHO clinical stage (at entry into ANC) | ||
| 3 or 4 | ▭ | 0 |
| 1 or 2 | ▭ | 5 |
| Duration of cART before delivery (current pregnancy) | ||
| <4 weeks | ▭ | 0 |
| 1–4 weeks | ▭ | 5 |
| Number of ANC visits (current pregnancy) | ||
| >3 | ▭ | 0 |
| 1 or 2 | ▭ | 3 |
| Parity (number of previous deliveries) | ||
| >2 | ▭ | 0 |
| 2 | ▭ | 3 |
| 1 | ▭ | 3 |
| 0 | ▭ | 6 |
| Sum points for all checked boxes | ||
| RISK SCORE | ▭ | |
Footnotes
Conflicts of Interest: D.W. has served as an ad hoc consultant to NIH on epidemiologic methods. No overlap with current work. The other authors report no conflicts of interest.
Presentations at conferences: A version of this work was presented at the 5th International Workshop on HIV & Women, Seattle, Washington, February 21–22, 2015.
References
- Ahmed S, Kim MH, Abrams EJ. Risks and benefits of lifelong antiretroviral treatment for pregnant and breastfeeding women: a review of the evidence for the Option B+ approach. Curr Opin HIV AIDS. 2013;8(5):474–489. doi: 10.1097/COH.0b013e328363a8f2. [DOI] [PubMed] [Google Scholar]
- Ayuo P, Musick B, Liu H, Braitstein P, Nyandiko W, Otieno-Nyunya B, Wools-Kaloustian K. Frequency and factors associated with adherence to and completion of combination antiretroviral therapy for prevention of mother to child transmission in western Kenya. J Int AIDS Soc. 2013;16(1):17994. doi: 10.7448/IAS.16.1.17994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeguez AD, Lindsey JC, Shannon M, Tuomala RE, Cohn SE, Smith E, Read JS. Adherence to antiretrovirals among US women during and after pregnancy. J Acquir Immune Defic Syndr. 2008;48(4):408–417. doi: 10.1097/QAI.0b013e31817bbe80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogart LM, Chetty S, Giddy J, Sypek A, Sticklor L, Walensky RP, Bassett IV. Barriers to care among people living with HIV in South Africa: Contrasts between patient and healthcare provider perspectives. AIDS Care. 2012 doi: 10.1080/09540121.2012.729808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles TH, Wilkinson LS, Leisegang R, Maartens G. Factors influencing retention in care after starting antiretroviral therapy in a rural South African programme. PloS one. 2011;6(5):e19201. doi: 10.1371/journal.pone.0019201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Central Statistical Office (CSO), M. o. H. M., Tropical Diseases Research Centre (TDRC), University of Zambia, and Macro International Inc. Zambia Demographic and Health Survey. Vol. 2007. Calverton, Maryland, USA: 2009. Retrieved from. [Google Scholar]
- Chi BH, Cantrell RA, Mwango A, Westfall AO, Mutale W, Limbada M, Stringer JS. An empirical approach to defining loss to follow-up among patients enrolled in antiretroviral treatment programs. Am J Epidemiol. 2010;171(8):924–931. doi: 10.1093/aje/kwq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi BH, Vwalika B, Killam WP, Wamalume C, Giganti MJ, Mbewe R, Stringer JS. Implementation of the Zambia electronic perinatal record system for comprehensive prenatal and delivery care. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2011;113(2):131–136. doi: 10.1016/j.ijgo.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaranello AL, Perez F, Engelsmann B, Walensky RP, Mushavi A, Rusibamayila A, Freedberg KA. Cost-effectiveness of World Health Organization 2010 guidelines for prevention of mother-to-child HIV transmission in Zimbabwe. Clin Infect Dis. 2013;56(3):430–446. doi: 10.1093/cid/cis858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse K, Pettifor A, Shearer K, Maskew M, Bassett J, Larson B, Fox MP. Loss to follow-up before and after delivery among women testing HIV positive during pregnancy in Johannesburg, South Africa. Tropical medicine & international health : TM & IH. 2013 doi: 10.1111/tmi.12072. 10.1111/tmi.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin J, Pathmanathan I, Richey LE. Disclosure of HIV Status to Social Networks Is Strongly Associated with Increased Retention Among an Urban Cohort in New Orleans. AIDS Patient Care and STDs. 2013 doi: 10.1089/apc.2013.0037. [DOI] [PubMed] [Google Scholar]
- Harrell FE. Regression Model Strategies. 1st. New York, New York: Springer; 2002. [Google Scholar]
- Higa DH, Marks G, Crepaz N, Liau A, Lyles CM. Interventions to improve retention in HIV primary care: a systematic review of. U.S. studies. Current HIV/AIDS reports. 2012;9(4):313–325. doi: 10.1007/s11904-012-0136-6. 10.1007/s11904-012-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirnschall G, Harries AD, Easterbrook PJ, Doherty MC, Ball A. The next generation of the World Health Organization's global antiretroviral guidance. Journal of the International AIDS Society. 2013;16(1):18757. doi: 10.7448/IAS.16.1.18757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson I, Plummer ML, Konopka SN, Colvin CJ, Jonas E, Albertini J, Fogg KP. A Systematic Review of Individual and Contextual Factors Affecting ART Initiation, Adherence, and Retention for HIV-Infected Pregnant and Postpartum Women. PloS one. 2014;9(11):e111421. doi: 10.1371/journal.pone.0111421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R, Orrell C, Zwane E, Bekker LG, Wood R. Loss to follow-up and mortality among pregnant women referred to a community clinic for antiretroviral treatment. AIDS (London, England) 2008;22(13):1679–1681. doi: 10.1097/QAD.0b013e32830ebcee. 10.1097/QAD.0b013e32830ebcee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf MC, McLeod J, Boehme AK, Walcott MW, Wright L, Seal P, Moneyham L. A qualitative study of the barriers and facilitators to retention-in-care among HIV-positive women in the rural southeastern United States: implications for targeted interventions. AIDS Patient Care and STDs. 2010;24(8):515–520. doi: 10.1089/apc.2010.0065. 10.1089/apc.2010.0065. [DOI] [PubMed] [Google Scholar]
- Kieffer MP, Mattingly M, Giphart A, van de Ven R, Chouraya C, Walakira M, Simonds RJ. Lessons learned from early implementation of option b+: the elizabeth glaser pediatric AIDS foundation experience in 11 african countries. J Acquir Immune Defic Syndr. 2014;67(Suppl 4):S188–S194. doi: 10.1097/QAI.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznik A, Lamorde M, Hermans S, Castelnuovo B, Auerbach B, Semeere A, Manabe YC. Evaluating the cost-effectiveness of combination antiretroviral therapy for the prevention of mother-to-child transmission of HIV in Uganda. Bull World Health Organ. 2012;90(8):595–603. doi: 10.2471/BLT.11.095430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panditrao M, Darak S, Kulkarni V, Kulkarni S, Parchure R. Socio-demographic factors associated with loss to follow-up of HIV-infected women attending a private sector PMTCT program in Maharashtra, India. AIDS Care. 2011;23(5):593–600. doi: 10.1080/09540121.2010.516348. [DOI] [PubMed] [Google Scholar]
- Phillips T, Thebus E, Bekker LG, McIntyre J, Abrams EJ, Myer L. Disengagement of HIV-positive pregnant and postpartum women from antiretroviral therapy services: a cohort study. J Int AIDS Soc. 2014;17:19242. doi: 10.7448/IAS.17.1.19242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS medicine. 2011;8(7):e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten EJ, Jahn A, Midiani D, Makombe SD, Mnthambala A, Chirwa Z, Chimbwandira F. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011;378(9787):282–284. doi: 10.1016/S0140-6736(10)62303-3. [DOI] [PubMed] [Google Scholar]
- Shaffer N, Abrams EJ, Becquet R. Option B+ for prevention of mother-to-child transmission of HIV in resource-constrained settings: great promise but some early caution. AIDS. 2014;28(4):599–601. doi: 10.1097/QAD.0000000000000144. [DOI] [PubMed] [Google Scholar]
- Tenthani L, Haas AD, Tweya H, Jahn A, van Oosterhout JJ, Chimbwandira F, Ie DEASA. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women ('Option B+') in Malawi. AIDS. 2014;28(4):589–598. doi: 10.1097/QAD.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, Nachega JB. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Annals of Internal Medicine. 2012;156(11):817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. W-284, W-285, W-286, W-287, W-288, W-289, W-290, W-291, W-292, W-293, W-294 10.1059/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. Gap Report. Geneva, Switzerland: 2014. Retrieved from http://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2014/july/20140716prgapreport. [Google Scholar]
- Westreich D, Evans D, Firnhaber C, Majuba P, Maskew M. Prevalent pregnancy, biological sex, and virologic response to antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;60(5):489–494. doi: 10.1097/QAI.0b013e318256b310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: 2013. Retrieved from http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [PubMed] [Google Scholar]
- Wohl AR, Galvan FH, Myers HF, Garland W, George S, Witt M, Lee ML. Do social support, stress, disclosure and stigma influence retention in HIV care for Latino and African American men who have sex with men and women? AIDS and behavior. 2011;15(6):1098–1110. doi: 10.1007/s10461-010-9833-6. 10.1007/s10461-010-9833-6. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Maternal mortality ratio. 2015 Retrieved from http://www.who.int/healthinfo/statistics/indmaternalmortality/en/


