Abstract
Background
Stereotactic body radiation therapy (SBRT) for localized prostate cancer has potential advantages over traditional radiotherapies. We compared national trends in utilization, complications, and costs of SBRT to traditional radiotherapies.
Methods
We identified men who underwent SBRT, intensity-modulated radiation therapy (IMRT), brachytherapy, and proton beam therapy as primary treatment for prostate cancer during 2004 and 2011 from Surveillance, Epidemiology, and End Results Program (SEER)-Medicare linked data. Temporal trend of therapy utilization was assessed using Cochran-Armitage test. Two-year outcomes were compared using chi-square test. Median treatment costs were compared using Kruskal Wallis test.
Results
542 men received SBRT, 9,647 brachytherapy, 23,408 IMRT and 800 proton beam therapy. There was significant increase in SBRT and proton beam utilization (p<0.001), whereas brachytherapy utilization decreased (p<0.001). A higher proportion of SBRT and brachytherapy subjects had low grade (Gleason≤6 vs. ≥7) cancer compared to IMRT and proton therapy (54.0%, 64.2% vs 35.2%, 49.6%, respectively; p<0.001). SBRT compared to brachytherapy and IMRT was associated with equivalent gastrointestinal toxicity but more erectile dysfunction at two-year follow-up (p<0.001). SBRT was associated with more urinary incontinence compared to IMRT and proton therapy but less compared to brachytherapy (p<0.001, respectively). Median cost of SBRT was $27,145 compared to $17,183 for brachytherapy, $37,090 for IMRT and $54,706 for proton beam therapy (p<0.001).
Conclusions
Utilization of SBRT and proton therapy for localized prostate cancer has increased over time. Despite men of lower stage undergoing SBRT, SBRT was associated with greater toxicity but lower healthcare costs compared to IMRT and proton therapy.
Keywords: prostate cancer, radiotherapy, radiosurgery, proton therapy, stereotactic body radiation therapy, health care costs, toxicity
Introduction
Stereotactic body radiation therapy (SBRT) is a form of radiosurgery that comprises the delivery of highly conformal hypofractionated radiation to a well-defined target. SBRT is considered to offer advantages over traditional radiation therapy, insofar as it enables delivery of high radiation doses over fewer fractionations, thereby curtailing the overall duration of treatment, compared to traditional external beam approaches such as intensity-modulated radiation therapy (IMRT).1 Additionally, radiobiologic evidence suggests that a hypofractionated approach presents the potential for therapeutic equipoise without additional normal tissue toxicity.2 The combination of promising data and aggressive marketing of SBRT with technologies, such as Cyberknife® (Accuray, Inc., Sunnyvale, CA), has resulted in the utilization of SBRT for the treatment of prostate cancer in both the localized and metastatic settings.3–7
While SBRT may confer advantages by way of a shorter treatment course and lower costs,8 there has been concern regarding the toxicity of SBRT resulting from high radiation dose delivery to adjacent normal tissues.9 Furthermore, the optimal candidate for prostate cancer SBRT therapy remains unknown due to the absence of randomized trials.
Given the perceived rapid adoption of SBRT and the concerns regarding its toxicity, it is crucial that stakeholders, including men diagnosed with prostate cancer, practitioners, payors, health systems and policy makers understand the rate of diffusion, patterns of care, outcomes and healthcare costs of this particular technology. Because of the paucity in research regarding utilization and outcomes of SBRT and the perception that prostate cancer is a litmus test for healthcare reform, we set out to assess temporal, geographic, and treatment variation in the utilization of SBRT for primary prostate cancer therapy using a nationally representative cohort. Additionally, we aimed to characterize the complications following SBRT compared to traditional radiation therapies. Herein, we report the current trends of SBRT utilization within the United States amongst elderly men diagnosed with prostate cancer.
Methods
Data Source
We used the most recent release of Surveillance, Epidemiology, and End Results Program (SEER) and Medicare linked data with incident cancers through 2011 and Medicare follow-up through 2012. SEER is a nationally representative, population-based cancer registry that collects incidence, treatment, and mortality data.10 Successful linkage with Medicare hospital and physician claims is achieved for more than 90% of subjects.11 SEER identifies 28% of all incident cancer cases in the United States, and Medicare insures approximately 97% of all Americans aged ≥65 years. The study was approved by the Weill Cornell Medical College Institutional Review Board.
Study Population
We identified men aged 65 years or older who underwent either SBRT or traditional radiotherapy (IMRT, brachytherapy, proton beam therapy or combination therapy) as primary treatment for localized prostate cancer during 2004 through 2011. Patients who were diagnosed prior to 2004 were excluded. We included men with a primary diagnosis of prostate cancer without evidence of metastases and without history of non-prostate malignancy. Primary treatment was defined as treatment within 6 months of initial prostate cancer diagnosis in men with no prior surgical or radiation therapy. Combination therapy was defined as receipt of multiple radiotherapies within 6 months. To ensure complete capture of claim data, we included only patients who were continuously enrolled in Medicare Part A and Part B and not enrolled in health maintenance organization from one year prior to diagnosis through death or last available record in 2012.
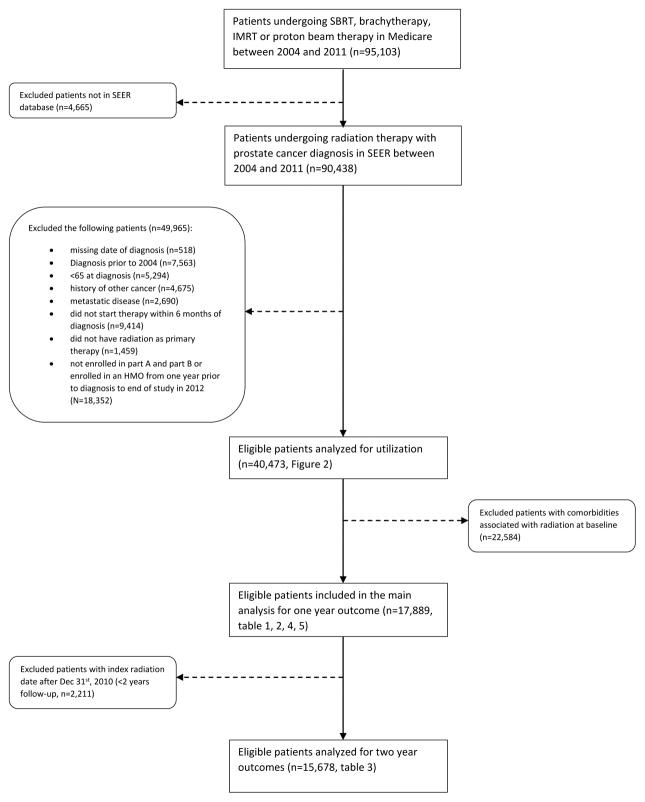
Subjects who underwent SBRT (n=542) were identified using International Classification of Disease-9 (ICD-9) codes 92.30–92.33, 92.39 and Common Procedural Terminology-4 (CPT-4) codes 77373, 77435, G0339, and G0340. Men who underwent intensity modulated radiation therapy (IMRT) (n=23,408), brachytherapy (n=9,647), proton beam therapy (n=800), or combination therapies (n=6,076) during the study period were identified consistent with prior methods.12 To accurately address the association between radiation therapy and associated complications, our final study population did not include patients exhibiting comorbidities associated with radiation therapy complications prior to their index radiation. Patient selection process is depicted in Figure 1.
Figure 1.
Flow chart of patient selection
All subjects were followed-up for at least one year after initiation of radiation therapy, with end of study being December 2012. A total of 17,889 of subjects undergoing SBRT (n=237), brachytherapy (n=4,136), IMRT (n=10,715), proton beam therapy (n=363) and combination therapy (n=2,438) were analyzed for one year outcomes. Analysis of two-year outcomes was performed in a subgroup of patients with sufficient follow-up time.
Study Outcomes
Trends in utilization of primary radiation therapies were characterized along with complications including urinary incontinence, non-incontinence genitourinary morbidity, erectile dysfunction, gastrointestinal morbidity, and hip fracture consistent with prior studies.12 We identified complications from Medicare inpatient, outpatient, home health agency records and carrier files within one year and two years following initial therapy (Supplemental Material). Use of androgen deprivation therapy (ADT) from 3 months to one year and two years following primary radiation therapy was also identified accordingly.
Health care expenditures were derived by summing the total amount paid by Medicare for inpatient, outpatient and physician services during the year before and the year following prostate cancer diagnosis consistent with validated methodology.13 We then subtracted the one year pre-diagnosis expenditures from the one year post-diagnosis expenditures. The difference was considered to equal the costs relating to radiation therapy, associated treatments and laboratory tests within one year. Subjects who switched therapy at follow-up were excluded from cost analysis to ensure accurate estimate of expenditures specific to a single radiation therapy. Costs were adjusted to 2012 dollars using 2015 Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds. For each therapy, total annual expenditures in the United States were calculated according to previously validated methods.13
Independent Variables
Socio-demographic characteristics included age at diagnosis, race, marital status, socioeconomic status (census tract measure of high school education and median household income), geographic region (SEER registry), and population density. Cancer characteristics included tumor grade and clinical stage according to the American Joint Committee on Cancer (AJCC) tumor, node, and metastasis (TNM) staging system.14 Comorbidities were assessed within 12 months prior to treatment from Medicare inpatient, outpatient, physician and home health agency records using algorithms validated by Elixhauser.15 Treatment variables included year of initiation of radiation therapy, time between diagnosis and index treatment, and concurrent ADT, defined as ADT administered within 3 months prior to or 3 months following the initiation of radiation therapy.
Statistical Analysis
We compared characteristics, comorbidities and outcome measures between subjects who received SBRT, brachytherapy, IMRT and proton beam therapy as primary therapy for prostate cancer. Differences by treatment were compared using percentage of event count and chi-square test. Temporal trend of a single radiation therapy was assessed using Cochran-Armitage trend test. We reported overall and stratified median costs by year of diagnosis. Differences in overall median costs were examined using Kruskal Wallis test. A p-value < 0.05 was considered statistically significant. All analyses were performed using SAS v9.3.
Results
Utilization
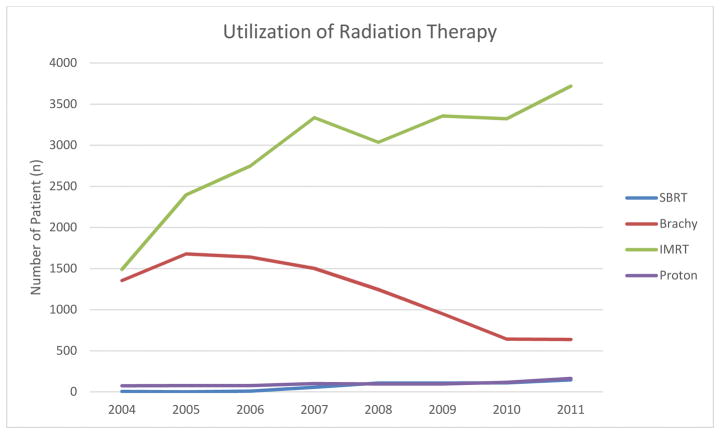
From 2004 to 2011, 542 received SBRT, 9,647 patients received brachytherapy, 23,408 patients received IMRT and 800 patients received proton beam therapy as primary treatment for prostate cancer. There was an increase in SBRT utilization from less than 0.4% to 2.7% among all radiation therapies (Figure 2). Similarly, we observed an increase in IMRT and proton beam therapy and decrease in brachytherapy over time within each type of radiation therapy. (p<0.001, respectively).
Figure 2.
Utilization of radiation therapy for prostate cancer patients between 2004 and 2011
Baseline Characteristics
Sociodemographic characteristics for all men without baseline genitourinary or gastrointestinal comordbidity who underwent radiation therapy are presented in Table 1. Men undergoing SBRT and IMRT were more likely to be aged equal to or greater than 75 years at diagnosis compared to brachytherapy, and proton beam (35.0%, 46.1%, 33.6%, and 33.6%, respectively; p<0.001). A lower proportion of men who underwent SBRT, brachytherapy and proton beam were black (7.2%, 6.9% and 3.0%, respectively) compared to IMRT (10.3%; p<0.001). SBRT was associated with greater utilization in metropolitan areas (94.9% vs 80.2% vs 81.7% vs 89.8%) and the Northeast region (54.9% vs 15.6% vs 25.8% vs <4.7%) compared to brachytherapy, IMRT and proton beam therapy (p<0.001). In addition, a higher proportion of men who underwent SBRT had median household annual income greater than $60,000 compared to competing radiotherapies (p<0.001).
Table 1.
Baseline demographics and comorbidities of patients undergoing radiation therapy between 2004 and 2011
| SBRT (N=237) | Brachytherapy (N=4136) | IMRT (N=10715) | Proton beam therapy (N=363) | Combination therapy (N=2438) | p-value | |
|---|---|---|---|---|---|---|
| Race | ||||||
| White | 207(87.3%) | 3630(87.8%) | 8861(82.7%) | 337(92.8%) | 2041(83.7%) | <0.001 |
| Black | 17(7.2%) | 284(6.9%) | 1108(10.3%) | 11(3.0%) | 243(10.0%) | |
| Other | 13(5.5%) | 222(5.4%) | 746(7.0%) | 15(4.1%) | 154(6.3%) | |
| Age at diagnosis | ||||||
| 65–69 | 58(24.5%) | 1188(28.7%) | 2065(19.3%) | 120(33.1%) | 641(26.3%) | <0.001 |
| 70–74 | 96(40.5%) | 1560(37.7%) | 3712(34.6%) | 121(33.3%) | 948(38.9%) | |
| >=75 | 83(35.0%) | 1388(33.6%) | 4938(46.1%) | 122(33.6%) | 849(34.8%) | |
| High school education % | ||||||
| <75/unknown | 26(11.0%) | 914(22.2%) | 2598(24.4%) | 56(15.5%) | 541(22.3%) | <0.001 |
| 75–84 | 45(19.0%) | 1005(24.4%) | 2637(24.7%) | 97(26.8%) | 541(22.3%) | |
| 85–89 | 56(23.6%) | 783(19.0%) | 2130(20.0%) | 76(21.0%) | 492(20.2%) | |
| 90+ | 110(46.4%) | 1416(34.4%) | 3296(30.9%) | 133(36.7%) | 857(35.3%) | |
| Median household Income, $ | ||||||
| <35,000/unknown | 29(12.2%) | 925(22.5%) | 2590(24.3%) | 71(19.6%) | 450(18.5%) | <0.001 |
| 35,000–44,99 | 30(12.7%) | 883(21.4%) | 2248(21.1%) | 86(23.8%) | 425(17.5%) | |
| 45,000–59,99 | 55(23.2%) | 1020(24.8%) | 2634(24.7%) | 92(25.4%) | 589(24.2%) | |
| >=60,000 | 123(51.9%) | 1290(31.3%) | 3189(29.9%) | 113(31.2%) | 967(39.8%) | |
| Region* | ||||||
| Northeast | 130(54.9%) | 644(15.6%) | 2767(25.8%) | NR | 588(24.1%) | <0.001 |
| South | NR | 1227(29.7%) | 2632(24.6%) | 23(6.3%) | 890(36.5%) | |
| West | 63(26.6%) | 1880(45.5%) | 3913(36.5%) | 323(89.0%) | 819(33.6%) | |
| Midwest | NR | 385(9.3%) | 1403(13.1%) | NR | 141(5.8%) | |
| Population density** | ||||||
| Metro | 225(94.9%) | 3317(80.2%) | 8752(81.7%) | 326(89.8%) | 2112(86.6%) | <0.001 |
| Non-Metro | 12(5.1%) | 819(19.8%) | 1959(18.3%) | 37(10.2%) | 326(13.4%) | |
| Marital status | ||||||
| Married | 188(79.3%) | 3134(75.8%) | 7498(70.0%) | 287(79.1%) | 1839(75.4%) | <0.001 |
| Other/Unknown | 49(20.7%) | 1002(24.2%) | 3217(30.0%) | 76(20.9%) | 599(24.5%) | |
| Gleason Score | ||||||
| ≤6 | 128(54.0%) | 2657(64.2%) | 3769(35.2%) | 180(49.6%) | 652(26.7%) | <0.001 |
| 7–10 | NR | 1430(34.6%) | 6791(63.4%) | NR | 1755(72.0%) | |
| Unknown | NR | 49(1.2%) | 155(1.4%) | NR | 31(1.3%) | |
| Clinical stage | ||||||
| T1 | 162(68.4%) | 2639(63.8%) | 6132(57.2%) | 223(61.4%) | 1420(58.2%) | <0.001 |
| T2–T4 | NR | NR | 4536(42.3%) | NR | NR | |
| Tx/Unknown | NR | NR | 47(0.4%) | NR | NR | |
| Comorbidities | ||||||
| Coronary artery disease | 61(25.7%) | 1197(28.9%) | 3361(31.4%) | 83(22.9%) | 787(32.3%) | <0.001 |
| Hypertension | 160(67.5%) | 2989(72.3%) | 7796(72.8%) | 211(58.1%) | 1808(74.2%) | <0.001 |
| Congestive heart failure | 12(5.1%) | 245(5.9%) | 816(7.6%) | 16(4.4%) | 155(6.4%) | <0.001 |
| Diabetes | 66(27.8%) | 1040(25.1%) | 3240(30.2%) | 70(19.3%) | 698(28.6%) | <0.001 |
| Chronic pulmonary disease | 45(19.0%) | 759(18.4%) | 1963(18.3%) | 57(15.7%) | 470(19.3%) | 0.54 |
| Obesity | 14(5.9%) | 148(3.6%) | 422(3.9%) | NR | 86(3.5%) | 0.06 |
| Anemia | 37(15.6%) | 499(12.1%) | 1663(15.5%) | 55(15.2%) | 379(15.5%) | <0.001 |
| Peripheral vascular disease | 35(14.8%) | 531(12.8%) | 1835(17.1%) | 29(8.0%) | 395(16.2%) | <0.001 |
| Cardiovascular disease | 21(8.9%) | 335(8.1%) | 1093(10.2%) | 16(4.4%) | 228(9.4%) | <0.001 |
| Concurrent ADT | 30(12.7%) | 781(18.9%) | 5174(48.3%) | 60(16.5%) | 1152(47.3%) | <0.001 |
Region categorization: northeast: Connecticut and New Jersey; south, Atlanta, rural Georgia, Kentucky, Louisiana and greater Georgia; west: San Francisco, Hawaii, New Mexico, Seattle, Utah, San Jose, Los Angeles, and greater California; and midwest: Detroit and Iowa.
Population density categorization using SEER urban/rural code: metropolitan: big metro, metro; non-metropolitan: urban, less urban and rural.
p-value is obtained from Kruskal Wallis test.
NR=not reportable for blinding purpose
In terms of prostate cancer characteristics at diagnosis, SBRT was the second most likely group to have indolent cancer of Gleason score less than or equal to 6 (54.0% vs. 64.2% vs. 35.2% vs 49.6%) compared to brachytherapy, IMRT and proton beam therapy. Moreover, men undergoing SBRT were more likely to have low clinical stage (T1 vs ≥T2) cancer (68.4% vs 63.8% vs 57.2% vs 61.4%) and less likely to undergo concurrent ADT (12.7% vs 18.9% vs 48.3% vs 16.5%) compared to men receiving brachytherapy, IMRT and proton beam therapy.
Complications
One-year outcomes following therapy are presented in Table 2. SBRT was associated with highest rate of erectile dysfunction (16.0% vs 11.4% vs 7.3% vs 4.7%, respectively; p<0.001), and lowest ADT use within one year of initial treatment (5.5% vs 6.9% vs 28.6% vs 9.1%) compared to brachytherapy, IMRT and proton beam therapy. Moreover, SBRT and brachytherapy were associated with higher risk of urinary incontinence (15.6% vs 32.2% vs 13.1% vs 6.9%) compared to IMRT and proton therapy. On the other hand, SBRT and proton beam therapy were associated with lower risk of non-incontinence urinary toxicity (9.7% and 5.2%) compared to brachytherapy and IMRT (25.1% and 9.8%).
Table 2.
Complications and use of androgen deprivation therapy (ADT) within one year of therapy
| SBRT (n=237) | Brachytherapy (n=4,136) | IMRT (n=10,715) | Proton beam Therapy (n=363) | Therapy (n=2,438) | p-value | |
|---|---|---|---|---|---|---|
| Gastrointestinal | 48(20.3%) | 814(19.7%) | 2018(18.8%) | 59(16.3%) | 476(19.5%) | 0.44 |
| Urinary non-incontinence | 23(9.7%) | 1038(25.1%) | 1053(9.8%) | 19(5.2%) | 623(25.6%) | <0.001 |
| Urinary incontinence | 37(15.6%) | 1330(32.2%) | 1399(13.1%) | 25(6.9%) | 802(32.9%) | <0.001 |
| Erectile dysfunction | 38(16.0%) | 471(11.4%) | 777(7.3%) | 17(4.7%) | 239(9.8%) | <0.001 |
| Hip fracture | NR | NR | 59(0.6%) | NR | 20(0.8%) | 0.01 |
| ADT | 13(5.5%) | 285(6.9%) | 3064(28.6%) | 33(9.1%) | 567(23.3%) | <0.001 |
NR=not reported for blinding purpose.
Two-year outcomes following therapy are presented in Table 3. Within two years of follow-up, SBRT was also associated with highest rate of erectile dysfunction (23.3% vs 18.8% vs 12.3% vs 10.8%), and lowest ADT use (7.4% vs 7.7% vs 29.5% vs 9.5%) compared to brachytherapy, IMRT and proton beam therapy. Similarly, SBRT and brachytherapy were associated with higher risk of urinary incontinence (23.9% vs 38.6% vs 19.9% vs 14.4%) compared to IMRT and proton therapy. Non-incontinence urinary toxicity was lower among patients receiving SBRT and proton beam therapy (14.8% vs 10.8%) than patients receiving brachytherapy and IMRT (30.7% vs 15.4%).
Table 3.
Complications and use of androgen deprivation therapy (ADT) within two years of therapy
| SBRT (n=176) | Brachytherapy (n=3,885) | IMRT (n=9,148) | Proton beam Therapy (n=306) | Combination Therapy (n=2,172) | p-value | |
|---|---|---|---|---|---|---|
| Gastrointestinal | 69(39.2%) | 1493(38.4%) | 3433(37.5%) | 124(40.5%) | 859(39.5%) | 0.37 |
| Urinary non-incontinence | 26(14.8%) | 1191(30.7%) | 1405(15.4%) | 33(10.8%) | 737(33.9%) | <0.001 |
| Urinary incontinence | 42(23.9%) | 1501(38.6%) | 1824(19.9%) | 44(14.4%) | 906(41.7%) | <0.001 |
| Erectile dysfunction | 41(23.3%) | 729(18.8%) | 1129(12.3%) | 33(10.8%) | 384(17.7%) | <0.001 |
| Hip fracture | NR | 25(0.6%) | 104(1.1%) | NR | 30(1.4%) | 0.02 |
| ADT | 13(7.4%) | 301(7.7%) | 2701(29.5%) | 29(9.5%) | 542(25.0%) | <0.001 |
NR=not reported for blinding purpose.
Costs
Across all years, median cost of SBRT was $27,145 compared to $17,183 for brachytherapy, $37,090 for IMRT and $54,706 for proton beam therapy (p<0.001) (Table 4). Estimated national expenditures in 2011 for SBRT were $15,574,407 compared to $52,878,347 for brachytherapy, $565,084,893 for IMRT, and $39,625,507 for proton therapy (Table 5). Estimated national expenditures for SBRT throughout the study period were $109,203,228.
Table 4.
Median cost of primary radiation therapy, stratified by year of diagnosis
| Year of diagnosis | $
|
||||
|---|---|---|---|---|---|
| SBRT | Brachytherapy | IMRT | Proton beam therapy | p-value | |
| Overall Median | 27,145 | 17,183 | 37,090 | 54,706 | <.0001* |
| 2004 | 43,669 | 21,683 | 40,484 | 44,897 | |
| 2005 | 33,644 | 19,817 | 38,250 | 50,108 | |
| 2006 | 32,120 | 17,568 | 38,440 | 66,908 | |
| 2007 | 26,257 | 16,138 | 36,693 | 68,610 | |
| 2008 | 26,607 | 14,808 | 36,027 | 50,624 | |
| 2009 | 28,609 | 14,719 | 36,345 | 49,669 | |
| 2010 | 24,878 | 13,590 | 35,910 | 58,759 | |
| 2011 | 26,535 | 14,274 | 36,319 | 59,510 | |
p-value is obtained from Kruskal Wallis test
Table 5.
Estimates of national expenditures due to radiotherapy, 2004–2011
| 2011
|
||||
|---|---|---|---|---|
| Therapy | Utilization of Radiotherapy from Our Cohort (%) | Estimated No. of Radiotherapy in the US | Mean Cost of Radiotherapy ($) | Total Expenditures in the US ($) |
| SBRT | 2.72 | 590 | 26417 | 15,574,407 |
| Brachytherapy | 11.96 | 2592 | 20398 | 52,878,347 |
| IMRT | 69.85 | 15140 | 37324 | 565,084,893 |
| Proton Therapy | 3.06 | 663 | 59744 | 39,625,507 |
| 2004–2011
|
||||
|---|---|---|---|---|
| Therapy | Utilization of Radiotherapy from Our Cohort (%) | Estimated No. of Radiotherapy in the US | Mean Cost of Radiotherapy ($) | Total Expenditures in the US ($) |
| SBRT | 1.34 | 3746 | 29150 | 109,203,228 |
| Brachytherapy | 23.82 | 66594 | 20213 | 1,346,060,726 |
| IMRT | 57.84 | 161704 | 39720 | 6,422,877,573 |
| Proton Therapy | 1.98 | 5536 | 54132 | 299,648,000 |
Discussion
The therapeutic and cost advantages of SBRT, bolstered by direct-to-consumer advertising, have led to adoption of this technology for the treatment of prostate cancer.7 SBRT patients require far fewer therapy sessions compared to conventional IMRT, and while long-term data regarding oncologic efficacy and toxicity are lacking, short- and moderate-term results are encouraging.5 Indeed, the American Society for Radiation Oncology (ASTRO) and the National Comprehensive Cancer Network (NCCN) both note the efficacy of SBRT the moderate-term, while cautioning that long-term results are awaited.16,17 As we anticipate long-term data from ongoing trials, it is crucial that physicians and regulators attempt to characterize the rate of diffusion and real-world implementation of this novel technology in order to inform future research and policy.
Our study has several important findings. It is the first to demonstrate a significant difference in patient selection for SBRT compared to other therapies, as SBRT patients had significantly less aggressive prostate cancer than those receiving other therapies. Men who underwent SBRT were more likely to have low grade (Gleason 6) prostate cancer compared to those who underwent IMRT. Additionally, SBRT patients were also more likely to have T1 disease compared to those who underwent alternate therapies, which is consistent with the composition of cohorts studied in early trials of SBRT for prostate cancer.18,19 These differences in patient selection may be indicative of a general wariness among radiation oncologists and urologists to treat higher stage prostate cancer with SBRT given limited data regarding long-term oncologic efficacy and efficacy in high risk cancer.20,21
Second, we characterized comparative utilization of SBRT relative to competing radiotherapies for primary treatment of prostate cancer in a population-based sample, which has not been previously described. Yu et al. compared toxicity of SBRT and IMRT in a national sample of Medicare beneficiaries, however brachytherapy and proton therapy were excluded and temporal trends in utilization were not described. We found a significant concurrent increase in utilization of SBRT, IMRT, and proton therapy, whereas brachytherapy utilization decreased substantially. With recent data showing increased utilization of active surveillance in low risk prostate cancer, the current uptrend in utilization of SBRT may represent overtreatment of low risk cancer.22 Thus, as diffusion of this technology continues, it is critical that practitioners and policy makers attempt to identify the precise role for SBRT in the prostate cancer treatment algorithm.
We also found that the majority of SBRT is performed in metropolitan areas, consistent with prior findings.9 Additionally, the Northeast region of the United States had greatest uptake of SBRT, which may be due to a higher concentration of institutions with SBRT capabilities in this region.23 Regional imbalance in utilization of surgical therapies for prostate cancer has been previously demonstrated in 1993 by Lu-Yao et al. who found geographic variation in radical prostatectomy rates with lowest utilization in the New England and Mid-Atlantic regions.24 Interestingly, this geographic variation persists more than two decades later, though with higher rather than lower volume of SBRT in these regions. Men who underwent SBRT had higher median income than men who underwent non-SBRT therapies, which is consistent with prior studies demonstrating higher income among men who pursue prostate cancer therapy with newer technologies.12,25
Third, SBRT was associated with more urinary incontinence and erectile dysfunction at two-years compared to IMRT. This is consistent with Yu et al. who found a significantly higher rate of genitourinary toxicity with SBRT.9 In contrast, multiple prior single-institution studies revealed comparable rates of short- and long-term genitourinary toxicities following SBRT compared to other therapies.5,20,26–28 Differences between these studies and the population-based studies likely result from length of follow-up, cohort selection, and definition of toxicity. Furthermore, “not all prostate SBRT or IMRT regimens are created equal,” and single-institution studies may have unique protocols that minimize morbidity.29 We also found equivalent gastrointestinal toxicity among SBRT subjects, despite previous predictions that the rectal-sparing approach of SBRT would likely result in reduction of gastrointestinal toxicity.30
Finally, we found that SBRT was less costly than IMRT and proton therapy. The rapid adoption of new technologies despite the absence of cost effectiveness studies has raised concerns about increased healthcare spending in the treatment of prostate cancer.13 However, multiple prior studies have shown that SBRT is less costly compared to IMRT and proton therapy, and this is reaffirmed by the current study.9,31–34 Despite higher rates of toxicity, Yu et al. suggested that SBRT may still be cost effective for both the patient and payor.9 The current study adds to the body of literature supporting the cost effectiveness of SBRT. Proton therapy generated almost double the costs of SBRT, thus negating the reduced costs associated with lower toxicity compared to traditional therapy.
Our findings must be interpreted within the context of our study design. First, the study included a relatively small population of subjects that underwent SBRT, which was smaller than the cohort studied by Yu et al.9 We excluded men with baseline genitourinary and gastrointestinal comorbidity, and we likely failed to capture a substantial cohort of subjects who were treated in non-SEER regions, particularly those high-volume areas in the Northeast where SBRT appears most popular. However, through linkage to SEER, we are able to identify differences in tumor characteristics by treatment type, a novel finding. Additionally, the findings of our study may only be generalizable to elderly Americans. Second, while we reported major toxicities according to previously validated methods, we were unable to capture toxicities that did not prompt intervention or the generation of billing codes, which leads to an underestimation of toxicity. However, this limitation should remain balanced across all therapies and therefore should enable accurate assessment of relative toxicity among the therapies. Additionally, bias may exist in patient and physician reporting of morbidity; the extent to which this bias may vary between different therapies is unknown.12
Conclusions
Utilization of SBRT and proton therapy for the treatment of primary prostate cancer has increased over time. Those who underwent SBRT were less likely to have high stage cancer or clinically significant prostate cancer and experienced higher toxicity from therapy compared to those who underwent IMRT. With higher toxicity but lower costs compared to alternate therapies, the precise role of SBRT in the treatment algorithm for prostate cancer requires further definition. Randomized trials are needed to compare the long-term toxicities and oncologic outcomes of these therapies in a prospective fashion.
Supplementary Material
Acknowledgments
Source of Funding: The study was funded in part through UO1 grant (NIH-1U01FD004494-01) from National Institutes of Health and US Food and Drug Administration. AS received the funding for establishing the FDA’s Medical Device Epidemiology Network’s (MDEpiNet) Science and Infrastructure Center.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Disclosures: Paul L. Nguyen has consulted for Ferring Pharmaceuticals, Medivation Inc., and GenomeDx.
Author Contributions
JAH: Conceptualization, project administration, writing – original draft, writing – review and editing,
AS: Conceptualization, data curation, funding acquisition, methodology, project administration,
WCH: Data curation, formal analysis
JM: Data curation, formal analysis
TJD: Methodology, formal analysis, writing – review and editing
PLN: Writing – review and editing
EBG: Writing – review and editing
JK: Writing – review and editing
JCH: Conceptualization, methodology, project administration, supervision, writing – original draft, writing –review and editing
References
- 1.Tan TJ, Siva S, Foroudi F, et al. Stereotactic body radiotherapy for primary prostate cancer: a systematic review. J Med Imaging Radiat Oncol. 2014;58:601–11. doi: 10.1111/1754-9485.12213. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DJ. Toward optimal external-beam fractionation for prostate cancer. Int J Radiat Oncol Biol Phys. 2000;48:315–6. doi: 10.1016/s0360-3016(00)00591-5. [DOI] [PubMed] [Google Scholar]
- 3.Katz AJ, Kang J. Stereotactic body radiotherapy as treatment for organ confined low-and intermediate-risk prostate carcinoma, a 7-year study. Front Oncol. 2014;4:240. doi: 10.3389/fonc.2014.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzam G, Lanciano R, Arrigo S, et al. SBRT. An Opportunity to Improve Quality of Life for Oligometastatic Prostate Cancer. Front Oncol. 2015;5:101. doi: 10.3389/fonc.2015.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen LN, Suy S, Uhm S, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol. 2013;8:58. doi: 10.1186/1748-717X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaeger TE. Accuray company advertising successful prostate cancer treatments with CyberKnife (CK) Int J Radiat Oncol Biol Phys. 2009;73:638–9. doi: 10.1016/j.ijrobp.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Stieber VW. Ethically problematic tactic: advertising CyberKnife as a therapeutic modality to patients (and underinformed clinicians) is inaccurate. Int J Radiat Oncol Biol Phys. 2009;73:638. doi: 10.1016/j.ijrobp.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 8.Meier R. Dose-Escalated Robotic SBRT for Stage I–II Prostate Cancer. Front Oncol. 2015;5:48. doi: 10.3389/fonc.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu JB, Cramer LD, Herrin J, et al. Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: comparison of toxicity. J Clin Oncol. 2014;32:1195–201. doi: 10.1200/JCO.2013.53.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8:1117–21. [PubMed] [Google Scholar]
- 11.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 12.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–20. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–24. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng L, Montironi R, Bostwick DG, et al. Staging of prostate cancer. Histopathology. 2012;60:87–117. doi: 10.1111/j.1365-2559.2011.04025.x. [DOI] [PubMed] [Google Scholar]
- 15.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Oncology ASfR. Stereotactic Body Radiation Therapy (SBRT) Model Policy. 2013. [Google Scholar]
- 17.Mohler JL, Kantoff PW, Armstrong AJ, et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw. 2014;12:686–718. doi: 10.6004/jnccn.2014.0072. [DOI] [PubMed] [Google Scholar]
- 18.Yeoh EE, Botten RJ, Butters J, et al. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase III randomized trial. Int J Radiat Oncol Biol Phys. 2011;81:1271–8. doi: 10.1016/j.ijrobp.2010.07.1984. [DOI] [PubMed] [Google Scholar]
- 19.Aluwini S, van Rooij P, Hoogeman M, et al. CyberKnife stereotactic radiotherapy as monotherapy for low- to intermediate-stage prostate cancer: early experience, feasibility, and tolerance. J Endourol. 2010;24:865–9. doi: 10.1089/end.2009.0438. [DOI] [PubMed] [Google Scholar]
- 20.King CR, Brooks JD, Gill H, et al. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:877–82. doi: 10.1016/j.ijrobp.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 21.Katz A, Kang J. Stereotactic body radiotherapy with or without external beam radiation as treatment for organ confined high-risk prostate carcinoma: a six year study. Radiat Oncol. 2014;9:1. doi: 10.1186/1748-717X-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA. 2015;314:80–2. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 23.Tipton K, Launders JH, Inamdar R, et al. Stereotactic body radiation therapy: scope of the literature. Ann Intern Med. 2011;154:737–45. doi: 10.7326/0003-4819-154-11-201106070-00343. [DOI] [PubMed] [Google Scholar]
- 24.Lu-Yao GL, McLerran D, Wasson J, et al. An assessment of radical prostatectomy. Time trends, geographic variation, and outcomes. The Prostate Patient Outcomes Research Team. JAMA. 1993;269:2633–6. doi: 10.1001/jama.269.20.2633. [DOI] [PubMed] [Google Scholar]
- 25.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302:1557–64. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 26.Oliai C, Lanciano R, Sprandio B, et al. Stereotactic body radiation therapy for the primary treatment of localized prostate cancer. J Radiat Oncol. 2013;2:63–70. doi: 10.1007/s13566-012-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen LN, Suy S, Wang H, et al. Patient-reported urinary incontinence following stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer. Radiat Oncol. 2014;9:148. doi: 10.1186/1748-717X-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz AJ, Kang J. Quality of Life and Toxicity after SBRT for Organ-Confined Prostate Cancer, a 7-Year Study. Front Oncol. 2014;4:301. doi: 10.3389/fonc.2014.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuller DB. Regarding relative toxicities of stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer. J Clin Oncol. 2014;32:3455–6. doi: 10.1200/JCO.2014.56.5572. [DOI] [PubMed] [Google Scholar]
- 30.Budaus L, Bolla M, Bossi A, et al. Functional outcomes and complications following radiation therapy for prostate cancer: a critical analysis of the literature. Eur Urol. 2012;61:112–27. doi: 10.1016/j.eururo.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 31.Sher DJ, Parikh RB, Mays-Jackson S, et al. Cost-effectiveness analysis of SBRT versus IMRT for low-risk prostate cancer. Am J Clin Oncol. 2014;37:215–21. doi: 10.1097/COC.0b013e31827a7d2a. [DOI] [PubMed] [Google Scholar]
- 32.Amin NP, Sher DJ, Konski AA. Systematic review of the cost effectiveness of radiation therapy for prostate cancer from 2003 to 2013. Appl Health Econ Health Policy. 2014;12:391–408. doi: 10.1007/s40258-014-0106-9. [DOI] [PubMed] [Google Scholar]
- 33.Parthan A, Pruttivarasin N, Davies D, et al. Comparative cost-effectiveness of stereotactic body radiation therapy versus intensity-modulated and proton radiation therapy for localized prostate cancer. Front Oncol. 2012;2:81. doi: 10.3389/fonc.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laviana AA, Ilg AM, Veruttipong D, et al. Utilizing time-driven activity-based costing to understand the short- and long-term costs of treating localized, low-risk prostate cancer. Cancer. 2015 doi: 10.1002/cncr.29743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.